Highlights
-
•
Buparvaquone inhibits proliferation of Neospora caninum at nanomolar concentrations.
-
•
In vitro, the drug acts mainly parasitostatic.
-
•
Parasiticidal effects occur at µmolar concentrations after extended periods of time.
-
•
Buparvaquone acts slowly as evidenced by transmission electron microscopy.
-
•
Buparvaquone prevents clinical signs of acute neosporosis in mice.
Keywords: Neospora caninum, Neosporosis, Buparvaquone, Electron microscopy, Cerebral infection, Real time PCR
Graphical Abstract

Abstract
The naphthoquinone buparvaquone is currently the only drug used against theileriosis. Here, the effects of buparvaquone were investigated in vitro and in an experimental mouse model for Neospora caninum infection. In 4-day proliferation assays, buparvaquone efficiently inhibited N. caninum tachyzoite replication (IC50 = 4.9 nM; IC100 = 100 nM). However, in the long term tachyzoites adapted and resumed proliferation in the presence of 100 nM buparvaquone after 20 days of cultivation. Parasiticidal activity was noted after 9 days of culture in 0.5 µM or 6 days in 1 µM buparvaquone. TEM of N. caninum infected fibroblasts treated with 1 µM buparvaquone showed that the drug acted rather slowly, and ultrastructural changes were evident only after 3–5 days of treatment, including severe alterations in the parasite cytoplasm, changes in the composition of the parasitophorous vacuole matrix and a diminished integrity of the vacuole membrane. Treatment of N. caninum infected mice with buparvaquone (100 mg/kg) either by intraperitoneal injection or gavage prevented neosporosis symptoms in 4 out of 6 mice in the intraperitoneally treated group, and in 6 out of 7 mice in the group receiving oral treatment. In the corresponding controls, all 6 mice injected intraperitoneally with corn oil alone died of acute neosporosis, and 4 out of 6 mice died in the orally treated control group. Assessment of infection intensities in the treatment groups showed that, compared to the drug treated groups, the controls showed a significantly higher parasite load in the lungs while cerebral parasite load was higher in the buparvaquone-treated groups. Thus, although buparvaquone did not eliminate the parasites infecting the CNS, the drug represents an interesting lead with the potential to eliminate, or at least diminish, fetal infection during pregnancy.
1. Introduction
Apicomplexan parasites are responsible for a variety of diseases in humans, pets and/or farm animals, and are thus of high medical and economic importance. Those most relevant for farm animals are Babesia, Besnoitia, Cryptosporidium, Eimeria, Neospora, Sarcocystis, Theileria, and Toxoplasma, all causing diseases of great socio-economic impact worldwide (Müller and Hemphill, 2013). Neospora caninum is phylogenetically closely related to Toxoplasma gondii, but distinct from Toxoplasma with regard to several biological features including the life cycle, host range and pathogenicity (Hemphill et al., 2006, 2013). Canids, namely dogs, wolves, dingoes and coyotes, represent definitive hosts of N. caninum. Besides cattle, sheep, goats, and many more species have been reported as intermediate hosts (Buxton et al., 2002; Dubey, 2003). Three infective stages of N. caninum have been identified to date. These are (i) tachyzoites, which represent the disease-causing and rapidly proliferating stage; (ii) slowly replicating bradyzoites that form tissue cysts; and (iii) sporozoites, the end products of a sexual process, which takes place in the intestine of the definitive host followed by sporulation in the environment. Although a sylvatic cycle for N. caninum has been demonstrated (Rosypal and Lindsay, 2005; Gondim, 2006), its importance as reservoir for the transmission to domestic animals has not been definitely elucidated.
Infection of pregnant cattle with N. caninum causes annual losses of around 1.3 billion US dollars through abortion, stillbirth, or birth of weak offspring (Reichel et al., 2012). In addition, N. caninum infection can result in birth of clinically healthy, but persistently infected calves, which in turn then vertically transmit the parasite to the next generation. Control options to limit the economic impact of neosporosis that have been proposed and modeled include (i) testing and culling of seropositive animals, (ii) discontinued breeding with offspring from seropositive cows, (iii) vaccination of susceptible and infected animals, and (iv) chemotherapeutic treatment of calves from seropositive cows (Häsler et al., 2006a, 2006b). However, the most effective option is not always the most economic one and a detailed economic study has to be made specifically for each case before deciding on a control strategy (Larson et al., 2004; Häsler et al., 2006a; Reichel and Ellis, 2006). In addition, none of the control strategies studied to date have reached a seroprevalence of zero because of the existence of the horizontal transmission. In order to eliminate N. caninum, it would be necessary to control both the vertical transmission within a herd as well as the horizontal transmission through oocyst shedding by canids (Häsler et al., 2008). In this context, treatment options for dogs would also represent an interesting aspect (Monney and Hemphill, 2014).
In general, chemotherapeutic treatment of seropositive animals has not been regarded as economically interesting, since to date no effective and safe drugs are available on the market, and because of the long period of treatment during which milk or meat from drug treated animals remains unacceptable (Dubey et al., 2007). Nevertheless, experimental studies have revealed potentially interesting effects of several compounds in vitro and in laboratory animal models in vivo (Müller and Hemphill, 2011). Other publications reported on further promising drug candidates including toltrazurilsulfone (ponazuril) (Kritzner et al., 2002; Strohbusch et al., 2009), artemisone (Mazuz et al., 2012), di-cationic diamidine derivatives (Debache et al., 2011; Schorer et al., 2012), miltefosine (Debache and Hemphill, 2012), organometallic ruthenium complexes (Barna et al., 2013) and bumped kinase inhibitors (Ojo et al., 2014).
Buparvaquone (2-((4-tert-butylcyclohexyl)methyl)-3-hydroxy-1,4-naphthoquinone; BPQ) is a hydroxynaphthoquinone related to parvaquone. Developed in the 1980s, BPQ has been extensively tested for veterinary use against Theileria annulata (Tropical theileriosis), Theileria parva (East Coast Fever) (McHardy et al., 1985; Dhar et al., 1986; Wilkie et al., 1998) and Theileria sergenti (Minami et al., 1985), both in laboratory studies and in field trials. BPQ kills T. parva in vitro with an IC50 of 10 nM (McHardy et al., 1985), and 150 nM of BPQ are sufficient to induce parasiticidal activity in Theileria-infected bovine macrophage cell lines (Hostettler et al., 2014). Moreover, activities of BPQ against a number of infectious diseases other than theileriosis have been described, including malaria (Martin et al., 1973), cryptosporidiosis (Kayser et al., 2001), leishmaniasis (Croft et al., 1992), and Pneumocystis carinii pneumonia in AIDS and immunosuppressed patients (Kaneshiro et al., 2001). The mechanism of action of the drug has not been fully elucidated. In analogy to atovaquone and other 1,4-naphthoquinones, BPQ most likely acts via a mechanism involving the inhibition of Complex III (b-c1 complex) as postulated to be the case in Theileria, Plasmodium, Eimeria and Toxoplasma (Hudson et al., 1985; Fry and Pudney, 1992). In the particular case of P. carinii there seems to be more than one mechanism of action of the drug. The effect of BPQ and other naphthoquinones on the ubiquinone (Q10) biosynthesis has also been described (Kaneshiro et al., 2001, 2006).
In the case of neosporosis, with no vaccine and no drug currently on the market, drug repurposing could be a valuable approach to identify compounds that limit the effects of the disease, and these could be rather rapidly implemented. BPQ represents an obvious candidate, as this compound is already used in cattle. In this study, we report the inhibitory effects of BPQ treatment of N. caninum in vitro and in an experimental mouse infection model.
2. Materials and methods
2.1. Tissue culture media, biochemicals, and drugs
If not otherwise stated, all tissue culture media were purchased from Gibco-BRL (Zürich, Switzerland), and biochemical reagents were from Sigma (St. Louis, MO). Kits for molecular biology were purchased from Qiagen (Hilden, Germany). Buparvaquone (McHardy and Morgan, 1985) was provided by Cross Vetpharm Group Limited (Dublin, Ireland), and was kept as 1.5 mM stock solution in dimethyl sulfoxide (DMSO) at −20 °C.
2.2. Host cell cultivation and parasite cultures
Human foreskin fibroblasts (HFF) were maintained in Dulbecco's Modified Eagle Medium (DMEM), and Vero cells were cultured in RPMI-1640 medium, both with phenol red supplemented with 10% heat inactivated and sterile filtrated fetal calf serum (FCS), 50 U of penicillin/ml, and 50 µg streptomycin/ml (=culture medium). They were cultured at 37 °C and 5% CO2 in tissue culture flasks (Sarstedt, Sevelen, Switzerland). Cultures were passaged at least once a week. Transgenic beta-galactosidase expressing-N. caninum (Nc-beta-gal) tachyzoites (used for screening purposes) and the N. caninum Liverpool (Nc-Liv) isolate were maintained by serial passages in Vero cells in the same medium (Schorer et al., 2012; Barna et al., 2013). Tachyzoites were harvested by scraping off the infected cell layer with a rubber cell scraper, followed by repeated passages through a 25-gauge needle at 4 °C, and separation from cell debris on a Sephadex-G25 column (Hemphill et al., 1996). Purified tachyzoites were used to infect HFF monolayers or for in vivo experiments as described below.
2.3. Alamar blue cytotoxicity assay
Viability assessment of HFF upon drug exposure was done as previously reported (Küster et al., 2012). Flat bottomed 96 well plates (Gibco-BRL) were seeded with 1 × 103 HFF per well in phenol-red free culture medium and grown at 37 °C and 5% CO2 to confluence before adding BPQ or DMSO as a solvent control. The cells were further cultured at 37 °C and 5% CO2 for 5 days. Subsequently, medium was removed and 0.2 ml PBS containing resazurin (10 mg/ml) were added per well, and the fluorescence at 590 nm wavelength (FI590) was measured at various time points using an Enspire multilabel counter (2300 EnSpire™ Multilabel Reader, Perkin-Elmer, Turku, Finland).
2.4. Measurement of the toxicity of BPQ against N. caninum tachyzoites
Assays based on a transgenic N. caninum strain expressing beta-galactosidase (Nc-beta-gal) were used (Barna et al., 2013). Flat bottomed 96 well plates (Sarstedt) were seeded with 5 × 103 HFF cells per well in culture medium and cultured at 37 °C and 5% CO2 until they had grown to confluence. Prior to the drug treatment, the old medium was removed and 100 µl of fresh culture medium containing 1 × 103 freshly harvested N. caninum Nc-beta-gal tachyzoites were distributed per well. Immediately thereafter, BPQ was added at desired amounts in 100 µl culture medium. Negative controls received the corresponding amounts of DMSO. The cultures were further maintained at 37 °C and 5% CO2. After 96 h, the plates were centrifuged at 500 rpm for 5 min, medium was removed, and 200 µl PBS was added to each well. After a second centrifugation (500 rpm, 5 min), PBS was removed and 100 µl PBS containing 0.05% Triton X-100 and 0.5 mM chlorophenol red-beta-D-galactopyranoside (CPRG; Roche Diagnostics, Rotkreuz, Switzerland) was added. CPRG was hydrolyzed by beta-galactosidase, causing the release of chlorophenol red, which was measured at (A570) at various time points in a VersaMax 96 well multiplate reader (Bucher Biotec, Basel, Switzerland). The initial velocity (ΔA570/min) was proportional to the number of tachyzoites.
To analyze effects of BPQ on extracellular tachyzoites and its ability to inhibit host cell invasion, Nc-beta-gal tachyzoites were purified as described and incubated in medium containing 0.5 µM BPQ or DMSO as a solvent control for 2 h followed by three washes with medium (i.e. centrifugation at 2300 × g for 5 min at 4 °C). The pellet was resuspended in medium and seeded at a density of 5 × 105 tachyzoites per well on confluent HFF layers grown in a 96-well-plate. After a subsequent incubation at 37 °C for 24 h, the beta-galactosidase-assay was performed as described.
In order to explore the effects of BPQ with regard to an already established infection, HFF monolayers were infected as above, and BPQ (100 nM) was added at the time point of adding the tachyzoites, after 72 h, or after 90 h of culture. After 96 h, the plates were centrifuged at 200 × g for 5 min, medium was removed, and beta-galactosidase activity was measured at (A570) as described above.
2.5. Assessment of parasiticidal activities of BPQ
HFF (5 × 105/flask) were inoculated into a T25 tissue culture flask, and after 2–4 h, fibroblasts were infected with 1 × 106 N. caninum Nc-Liv tachyzoites. After 1 day, the medium was substituted by fresh culture medium, or medium containing 100 nM, 500 nM, 1 µM or 2.5 µM of BPQ as indicated in Table 1. Drug-containing media were removed and substituted by normal media without compound on days 3, 6 or 9. During these time periods, the flasks were microscopically checked on a daily basis for signs of re-emerging parasite replication and parasitophorous vacuole formation. As soon as patches of destroyed HFF monolayers appeared as plaques due to tachyzoite egress, this was considered proof of regrowth and the experiment was terminated.
Table 1.
Long-term BPQ treatments of N. caninum tachyzoites grown in human fibroblasts. HFF monolayers cultured in T25 tissue culture flasks were infected with N. caninum Nc-Liv tachyzoites. Treatments were carried out for 3, 6, or 9 days in the presence of various concentrations of BPQ. Culture post-treatment took place in the absence of compounds for a maximum time span of 35 days.
| BPQ concentration (µM) | Duration of treatment (days) | Culture post-treatment (days)* | Parasitical effect (yes/no) |
|---|---|---|---|
| 0 | No treatment | 3 | No |
| 0.1 | 20** | n.d. | No |
| 0.5 | 3 | 3 | No |
| 6 | 10 | No | |
| 9 | 35*** | Yes | |
| 1.0 | 3 | 9 | No |
| 6 | 35*** | Yes | |
| 2.5 | 3 | 10 | No |
| 6 | 35*** | Yes |
Numbers indicate the numbers of days of culture in the absence of BPQ following treatment until re-emergence of parasite proliferation was evident by the occurrence of plaques detected by light microscopy.
Parasite proliferation was observed after 20 days continuous culture in the presence of 0.1 µM BPQ.
No reappearence of Neospora after culture in the absence of BPQ, indicating parasiticidal effect.
2.6. Transmission electron microscopy
HFF (5 × 105/flask) were inoculated into a T25 tissue culture flask and were infected with 5 × 105 N. caninum (Nc-Liv) tachyzoites. From 24 h p.i. infection onwards, the cultures were treated with 1 µM BPQ, control cultures were treated with the corresponding amount of DMSO. After 1, 2 and 5 days of continuous culture in the presence of BPQ, monolayers were washed with 100 mM sodium cacodylate buffer (pH 7.3) and fixed with cacodylate buffer containing 2.5% glutaraldehyde for 10 min. Cells were collected using a rubber cell scraper, centrifuged for 10 min at 1200 rpm at room temperature, the supernatant was removed, and infected cells were fixed further in 2.5% glutaraldehyde/cacodylate at 4 °C overnight. Postfixation was carried out in 2% OsO4 in cacodylate buffer for 2 h at room temperature, followed by extensive washing of the fixed material in water. After pre-staining in saturated uranyl acetate for 1 h at room temperature, specimens were dehydrated in a graded series of ethanol (30%, 50%, 70%, 90%, 3 × 100%) and embedded in Epon 820 epoxy resin, with two resin changes during 48 h. Polymerization of the resin was achieved at 65 °C for 24 h. Ultrathin (80 to 90 nm) sections were cut on a Reichert and Jung ultramicrotome, sections were loaded onto 300-mesh copper grids (Plano GmbH), and samples were stained with uranyl acetate and lead citrate (Kropf et al., 2012). Specimens were viewed on a Phillips 400T transmission electron microscope operating at 80 kV.
2.7. Effects of BPQ treatment of host cells prior to invasion by N. caninum tachyzoites
To study whether pretreatment of host cells prior to invasion had any effect on parasite proliferation, confluent HFF grown in 6-well plates were treated with 1 µM BPQ in medium for 1 h or 5 h, and controls were exposed to the corresponding amounts of DMSO. Subsequently, the drug-containing medium was removed and monolayers were washed 4 times with Hank's Balanced Salt Solution, and were infected with 1 × 106Nc-Liv tachyzoites in 5 ml medium without any drug or solvent. After 2 days, cells were collected with a cell scraper, centrifuged, washed once more in PBS, and the pellet was stored at −20 °C prior to quantification of N. caninum proliferation by N. caninum-specific real time PCR as outlined below.
2.8. In vivo effects of BPQ in N. caninum infected mice
Twenty five female Balb/c mice between 8 and 9 weeks of age were purchased from Charles River Laboratories (Sulzheim, Germany) and were maintained in a common room under controlled temperature and a 14 h dark/10 h light cycle according to the standards set up by the animal welfare legislation of the Swiss Veterinary Office. The experimental protocol was approved by the Commission for Animal Experimentation of the Canton of Bern, Switzerland (Animal license No. BE115/14). Mice were randomly caged into four experimental groups as outlined in Table 2. Enzyme-linked immunosorbent assay (ELISA) was carried out to ensure that mice were serologically Neospora-negative (Debache et al., 2010). On day 0, all mice were infected by intraperitoneal (i.p.) injection of 2 × 106 freshly purified N. caninum tachyzoites. After 48 h, mice received BPQ (100 mg/kg) as suspension in corn oil either by i.p. injection of a volume of 100 µl or by oral application of 100 µl by gavage. The control groups obtained the corresponding amount of the solvent only, either i.p. or orally (see Table 2). The treatments were performed 5 times on a daily basis. If not indicated otherwise, mice were inspected twice daily for clinical signs (ruffled coat, apathy, hind limb paralysis) until day 21 post infection (p.i.), at which time they were euthanized. Mice exhibiting clinical signs prior to day 21 p.i. were euthanized earlier. From each animal the brain and pieces of lung were recovered and processed for DNA-extraction (Debache et al., 2010; Monney et al., 2011).
Table 2.
Effects of BPQ treatments on survival of N. caninum infected mice. On day 0, Balbc mice were infected by intraperitoneal injection of 2 × 106 freshly purified N. caninum Nc-Liv tachyzoites. After 48 h, mice received buparvaquone (BPQ; 100 mg/kg) as suspension in corn oil either by intraperitoneal injection of a volume of 100 µl (i.p.), or orally by application of 100 µl by gavage (or). The control groups obtained the corresponding amount of the solvent only. The treatments were performed 5 times on a daily basis.
| Group | No. of mice | Mortalitya | Time of death (day p.i.) |
|---|---|---|---|
| BPQ ip | 6 | 2 | 7/7 |
| Control i.p. | 6 | 6 | 6/6/7/7/7/7 |
| BPQ p.o | 7 | 1 | 19b |
| Control p.o | 6 | 4 | 7/8/9/9 |
Number of mice killed upon severe acute disease signs.
Euthanized due to neurological signs of disease.
2.9. DNA extraction and assessment of cerebral parasite burden by real time PCR
DNA purification from in vitro cultures, brain and lung tissues was performed employing the DNeasy Blood & Tissue Kit (Qiagen, Basel, Switzerland) according to the standard protocol suitable for animal tissues. The DNA concentrations in all samples were determined using the QuantiFluor dsDNA System (Promega, Madison, WI) according to the manufacturer's instructions and adjusted to 5 ng/µl with sterile DNAse free water. The assessments of N. caninum tachyzoite loads were performed using the Rotor-Gene 6000 real-time PCR machine (Corbett Research, Qiagen). The parasite counts were calculated by interpolation from a standard curve with DNA equivalents from 1000, 100 and 10 parasites included in each run (Schorer et al., 2012; Ojo et al., 2014).
2.10. Statistics
IC50 values were calculated after the logit-log-transformation of the relative growth (RG; control = 1) according to the formula ln[RG/(1-RG)] = a × ln(drug concentration) + b and subsequent regression analysis by the corresponding software tool contained in the Excel software package (Microsoft, Seattle, WA, USA). Survival analysis of in vivo experiments was done via Kaplan–Meier estimator followed by regression analysis according to Cox and by Log-Rank analysis. Statistical analysis of the parasite burdens in brain and lungs was done by two-way ANOVA followed by pairwise t-tests with Bonferroni correction. These analyses were performed using the software package R (R Core Team, 2012).
3. Results
3.1. Effects of BPQ treatment on N. caninum proliferation in human fibroblasts
The effects of BPQ treatment were investigated in 4-day proliferation assays (Fig. 1A). HFF monolayers were infected with N. caninum tachyzoites expressing beta-galactosidase and BPQ was added at different concentrations. Measurement of beta-galactosidase activity showed that BPQ profoundly impaired N. caninum proliferation, with an IC50 (the concentration that inhibited the proliferation of tachyzoites by 50%) of 4.9 nM (Fig. 1A). Tachyzoite proliferation was entirely blocked at 100 nM. In contrast, BPQ had no effect on the viability of HFF monolayers (IC50 > 50 µM; data not shown).
Fig. 1.
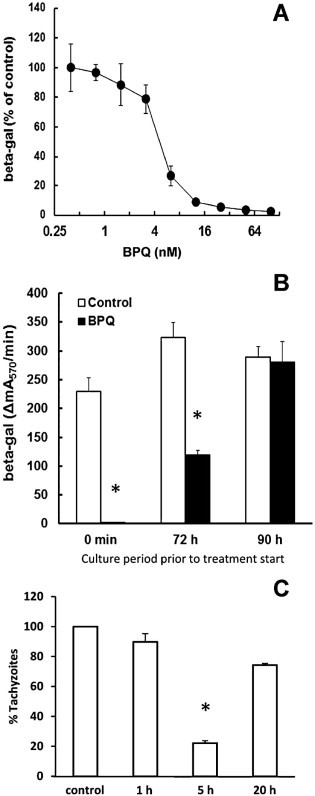
Inhibition of N. caninum proliferation by buparvaquone. (A) HFF were grown to confluence in a 96-well plate, treated with buparvaquone (BPQ) at various concentrations or DMSO as a solvent control and infected with N. caninum tachyzoites (103 per well) expressing the Escherichia coli beta-galactosidase gene. After 3 days, beta-galactosidase activity resulting from intracellular tachyzoites was determined. The activity is given as percentage of the solvent control. Mean values ± SE correspond to four replicates. (B) HFF were grown to confluence in a 96-well plate, infected with N. caninum tachyzoites (103 per well) expressing the E. coli beta-galactosidase gene. After different time points, the infected cells were treated with 100 nM buparvaquone (BPQ) or with DMSO as a solvent control. After 4 days, beta-galactosidase activity resulting from intracellular tachyzoites was determined. The activity is given as percentage of the solvent control. Mean values ± SE correspond to four replicates. The values marked by an asterisk are significantly different from the corresponding control value (two-sided t-test, P < 0.01). (C) HFF grown in 6 well plates were treated with 1 µM BPQ for 1 or 5 h, washed with PBS, and infected with 2 × 105N. caninum tachyzoites/well. After 4 days, cells were harvested and subjected to quantitative real time PCR. Results are shown as % proliferation in relation to untreated controls. Mean values ± SE correspond to four replicates. The values marked by asterisks are significantly different from the corresponding control value (two-sided t-test, P < 0.01).
In a next experiment, we investigated whether BPQ could also exert anti-parasitic activity in an already established infection (Fig. 1B). HFF monolayers were infected with N. caninum tachyzoites and BPQ (100 nM) was added at the time point of infection, or alternatively after 72 h or 90 h of culture. Parasites were harvested after 96 h p.i. Beta-galactosidase activity measurements confirmed that 100 nM BPQ completely inhibited tachyzoite proliferation during these 4 days, and a pronounced effect was also noted when the compound was added after 72 h. Addition of BPQ at 90 h p.i. (6 h prior to harvesting of cells) did not have a significant effect on tachyzoite proliferation.
When extracellular tachyzoites were treated with BPQ followed by removal of BPQ prior to infection using the experimental set-up as described, the treatment had no significant effects on further proliferation. After 24 h p.i., the beta-galactosidase activities (ΔmA570/min) were 5.3 ± 0.2 for BPQ treated tachyzoites vs. 4.6 ± 0.3 for control tachyzoites.
In an additional experiment HFF monolayers were pretreated with BPQ for 1, 5 and 20 h, respectively, and monolayers were subsequently infected with N. caninum tachyzoites in the absence of BPQ and cultured for a period of 2 days. At the end of the experiment, assessment of parasite replication by real-time PCR showed that a 1 h pretreatment did not have a pronounced negative impact on tachyzoite proliferation, while incubation of host cells with BPQ for 5 h prior to infection had a negative effect on parasite growth (Fig. 1C).
In order to investigate whether treatment of N. caninum infected HFF with BPQ had parasiticidal effects or acted only parasitostatically, infected HFF were exposed to different concentrations of BPQ for 3–9 days as indicated in Table 1. At defined time points, the drug pressure was released and cultures were maintained in medium without BPQ and were inspected daily by light microscopy for the presence of tachyzoites and tachyzoite proliferation. We found that the continuous presence of 100 nM BPQ in N. caninum-infected HFF did not exert parasiticidal activity in the long term, since parasite-induced lysis of host cells started to occur after 20 days, indicating that tachyzoites could adapt to this concentration of BPQ (Table 1). Treatments at 0.5 µM BPQ for 3 and 6 days did also not exert parasiticidal activity, while 9 days were sufficiently long to eliminate all viable N. caninum tachyzoites (no growth observed after 35 days without BPQ). When treatments were performed at 1 or 2.5 µM BPQ, 6 days were sufficient to exert parasiticidal activity.
3.2. Transmission electron microscopy of BPQ-treated N. caninum tachyzoites
N. caninum infected HFF were treated with 1 µM BPQ, and samples were fixed and processed for TEM after 1, 3 and 5 days, respectively. BPQ treatment induced distinct ultrastructural changes in N. caninum tachyzoites, although surprisingly these changes occurred not immediately but only after several days of drug treatment. Control specimens fixed at 48 h and not exposed to drug treatment are shown in Fig. 2A and at higher magnification in Fig. 2B. Intracellular tachyzoites reside in the cytoplasm of their host cell within a parasitophorous vacuole (PV), surrounded by a PV membrane. Tachyzoites exhibited typical features such as a posterior nucleus, anterior apical complex with conoid, micronemes and rhoptries, and electron-dense granules and mitochondria were clearly visible. The matrix of the PV is composed of a tubular network. Frequently, tachyzoites undergoing replication by endodyogeny could be identified.
Fig. 2.

Short-term (up to 24 h) BPQ treatment of N. caninum tachyzoites cultured in human fibroblasts does not induce structural alterations. (A) Low magnification view of a non-treated N. caninum-infected HFF fixed and processed at day 4 p.i. Numerous tachyzoites reside within a parasitophorous vacuole (PV) just adjacent to the host cell nucleus (hcnu). (B) A higher magnification view of (A). The PV matrix is composed of a tubular network (PVtn). The tachyzoite nucleus (nu), mitochondrium (mito) and secretory organelles such as rhoptries (rho), micronemes (mic) and dense granules (dg) are easily identified. The white arrows in A and B point toward a zoite undergoing endodyogeny. (C) N. caninum tachyzoites that were exposed to 0.5 µM BPQ for 24 h. No structural alterations were visible, con = conoid. Bar in A = 3.8 µm; B = 0.9 µm; C = 1.0 µm.
Specimens of N. caninum tachyzoites treated with 1 µm BPQ during 24 h did not exhibit noticeable ultrastructural alterations (Fig. 2C). On the other hand, slight but distinct changes in parasite morphology were noticeable in cultures treated for 3 days (Fig. 3A, B), mostly with regard to an increased formation of cytoplasmic vacuoles. However, no alterations that would indicate parasite death, such as nuclear condensation or severe changes in the parasite cytoplasm were noted at this stage. Few parasites exhibited peripheral accumulation of electron dense material, and in many instances (see Fig. 3B) the PV was not readily identifiable anymore, indicating that these tachyzoites could be located freely in the cytoplasm rather than inside a vacuole. After 5 days of BPQ treatment, dramatic alterations in tachyzoites as well as their host cells became evident (Fig. 3C–F): larger vacuoles containing numerous tachyzoites had disappeared. The majority of parasites that could be identified were single tachyzoites, often exhibiting increased vacuolization and nuclear condensation indicating cell death, and some associated with either electron-dense accumulations at their periphery (Fig. 3C). Alternatively there were visible PVs that appeared either empty or filled with less electron-dense material of unknown nature (Fig. 3D). In addition, many lipid droplets were found in the vicinity of BPQ-treated parasites. Few tachyzoites (less than 1%) were found exhibiting a largely intact ultrastructure, at least on the section plane visualized (see tachyzoite marked with an arrow in Fig. 3D). However, there was no clearly defined PV delineated by a PV membrane visible in most BPQ-treated specimens. In addition, host cells contained large numbers of PVs, which contained remnants of BPQ-treated parasites with no discernible ultrastructure, and which were either surrounded by membrane stacks (Fig. 3E) or single membranes (Fig. 3D).
Fig. 3.
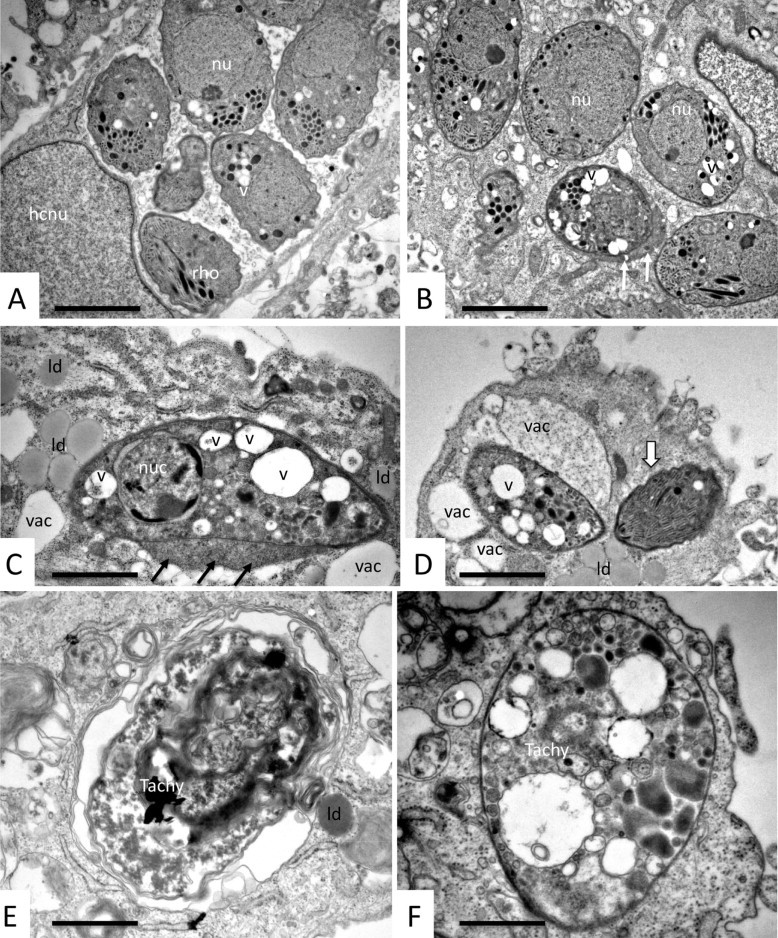
Longer-term BPQ treatment (24–120 h) of N. caninum tachyzoites cultured in human fibroblasts alters the ultrastructure of N. caninum tachyzoites. (A and B) Typical specimens fixed and processed after 72 h of drug treatment. Tachyzoites were found either in large parasitophorous vacuoles (A), or the parasitophorous vacuole was not discernible (B), and some tachyzoites had developed electron-dense accumulations at their periphery (white arrows in B). In any case, tachyzoites exhibited an increased cytoplasmic vacuolization (v). hcnu = host cell nucleus, nu = tachyzoite nucleus, rho = rhoptries. Ultrastructural changes were more evident after 120 h of BPQ treatment. In about 80% of cases parasites were still recognizable, but clearly damaged (C, D), with condensed nucleus (nuc), aberrant cytoplasmic organization with large vacuoles (v). There is no parasitophorous vacuole visible, but electron dense material accumulating at the tachyzoite periphery (black arrows in C), vacuolization in the host cell cytoplasm adjacent to the parasites (vac), and formation of many lipid droplets (ld). (E and F) Examples of completely distorted tachyzoite residues (Tachy), either packed into a vacuole and surrounded by several membranes (E), or as a single entity delineated by a still intact plasma membrane only (F). Bars in A and B = 1.5 µm, C = 1.7 µm, D = 1.6 µm, E and F = 0.9 µm.
3.3. Effects of BPQ treatment in experimentally infected mice
The N. caninum Nc-Liv isolate is highly virulent. Experimental infection in non-drug-treated control mice (receiving corn oil only either by i.p. injection or orally by gavage) lead to mortality of 10 out of the 12 control animals. On the other hand, BPQ treatment exhibited a profound protective effect, no matter whether the drug was applied i.p. or orally, with an overall mortality in only 3 out of total 13 animals from the two BPQ treated groups (see Table 2). Most animals that succumbed to infection did so during the acute phase of disease, meaning between day 6 and 9 p.i. They exhibited symptoms such as ruffled coat, rounded back and apathy, reaching a clinical score that required euthanasia. The only exception was one mouse in the group receiving oral BPQ treatment, which was euthanized at day 19 p.i. due to hind limb paralysis and circular movements, indicating neuronal impairment.
Survival analysis was done via Kaplan–Meier estimator (Fig. 4) followed by regression analysis according to Cox and by Log-Rank analysis. For i.p. treated mice, both Cox and Log-Rank-tests gave P values <0.05. For orally treated mice, the P values were slightly higher, but below 0.1. Since the survival curves of orally and i.p. treated mice showed the same tendencies, both applications could be combined. In this case, the survival analysis gave P values <0.02 for both tests. BPQ prevented neosporosis symptoms in 4 out of 6 mice in the i.p. treated group, and in 6 out of 7 mice in the group receiving oral treatment. In the corresponding control groups, all 6 mice injected i.p. with corn oil alone died of acute neosporosis, but only 4 out of 6 mice died in the orally treated control group. This indicates that i.p. application is somewhat more incriminating than oral application, which is not surprising as it represents an invasive procedure.
Fig. 4.
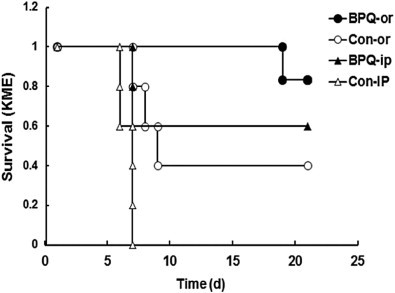
Mice infected with N. caninum and subsequently treated with buparvaquone (PBQ) have a higher survival rate than control mice. Survival ratio of the mice as listed in Table 2 expressed as Kaplan–Meier-Estimator (KME); or = oral application of buparvaquone (BPQ) or solvent (control); i.p. = intraperitoneal application.
Analysis of the parasite load in the brain and in the lungs was performed by quantitative PCR. Parasite loads in the lungs were significantly lower (2-way-ANOVA followed by pairwise t-tests with Bonferroni correction) in the two BPQ-treated groups compared to the corresponding control groups (Fig. 5A). On the other hand, the infection intensity in the brain was higher in the BPQ-treated groups compared to the cerebral parasite load in the controls. This effect was more pronounced in the orally treated animals (Fig. 5B).
Fig. 5.

Mice treated with buparvaquone (BPQ) have a lower parasite load in lungs, but not in brains. Balbc mice were infected with Nc-Liv tachyzoites and subsequently treated with buparvaquone or solvent as detailed in Table 2. After euthanasia, lungs and brains were collected and the amount of tachyzoites was determined by quantitative PCR. (A) Boxplots of tachyzoites in lungs; (B) boxplots of tachyzoites in brains. Values superscribed by different letters are significantly different (P < 0.05; two-way-ANOVA followed by pairwise-t-tests with Bonferroni correction).
4. Discussion
BPQ, currently known as the only active drug against theileriosis, is also highly active against N. caninum in vitro. In short term (4-day) proliferation assays, BPQ was active with an IC50 of 4.9 nM, which is one of the lowest values reported so far for drugs that were assessed against N. caninum in vitro. The IC50 for miltefosine was 5 µM (Debache and Hemphill, 2012), and two dicationic compounds (the arylimidamides DB745 and DB750) inhibited N. caninum proliferation in vitro with IC50s of 80–160 nM (Debache et al., 2011; Schorer et al., 2012). More recently, the bumped kinase inhibitor (BKI) 1294, which is targeted against the calcium dependent protein kinase 1 (CDPK1), was shown to inhibit N. caninum in vitro proliferation with an IC50 of 30 nM (Ojo et al., 2014). For all three compound groups, in vivo treatments in experimentally infected mice resulted in various beneficial effects such as reduced mortality and lower cerebral parasite burdens. Recently, 2 organometallic ruthenium compounds were reported to exhibit similarly low IC50 values of 6–12 nM (Barna et al., 2013), but they have not been assessed in vivo. For most other compounds that were reported to act against N. caninum in vitro and in vivo, such as artemisone (Mazuz et al., 2012) and ponazuril (Darius et al., 2004), no IC50 values have been reported.
Inhibition of N. caninum tachyzoite proliferation occurred even as BPQ was added after 72 h p.i., but conversely, extracellular tachyzoites were not affected by this compound. Prophylactic treatment of HFF monolayers for one hour, followed by infection with N. caninum tachyzoites and culture in the absence of BPQ, did not affect these parasites, but pre-treatment for 5 h reduced intracellular proliferation by 80% compared to controls. This indicates that BPQ, due to its lipophilic nature, was not taken up efficiently by HFF in the short term (1 h), but after 5 h the compound has reached the intracellular space of HFF monolayers, and then exerted its activity and inhibited tachyzoite proliferation. In any case, these results show that the efficacy of BPQ was exerted only upon intracellular parasites, this in contrast to what has been observed for other compounds such as DB745 (Schorer et al., 2012) and ruthenium based organometallic drugs (Barna et al., 2013). On the other hand, the BKI 1294 clearly inhibited host cell invasion and did not affect intracellular tachyzoite replication. However, as shown by electron microscopy, the BKI 1294 interfered in the formation of daughter zoites during endodyogeny, resulting in large multinucleated complexes that were trapped within the host cells and eventually died (Ojo et al., 2014).
Despite its low IC50 value, BPQ treatment did not clear an infection in vitro immediately. On the contrary, at 100 nM BPQ, a concentration that inhibited the parasite replication in 4-day assay by 100%, long-term treatments (up to 20 days) gave rise to N. caninum tachyzoites that had obviously adapted to BPQ, and this resulted in recurrence of parasite replication. As reported earlier, N. caninum tachyzoites also adapted readily to other experimental compounds such as pentamidine derivatives (Schorer et al., 2012) (Schorer et al., 2012) and ruthenium-based drugs (Barna et al., 2013), with the ability to resume proliferation at concentrations that were up to 20 times higher than the corresponding IC50s of non-adapted parasites. Similar findings were obtained for T. gondii tachyzoites (Kropf et al., 2012). How this drug adaptation in N. caninum is achieved has not been investigated so far. However, adaptation could be accompanied by an increased expression of genes coding for components of the detoxification machinery, such as e.g. ATP-binding cassette (ABC) transporters, which represent an important family of membrane proteins such as P-glycoprotein involved in drug resistance and other biological activities in the closely related Toxoplasma (Schmid et al., 2009), and might also be relevant in Neospora. Another potential adaptation mechanism could involve the reduced uptake of drugs, due to the downregulation of transporter activities (such as the adenosine transporters which are involved in diamidine uptake in trypanosomes (Matovu et al., 2003; Witola et al., 2004)). Down regulation of the expression of such transporters could, besides limiting drug uptake, also limit the uptake of other, potentially essential molecules, and this could contribute to the initially reduced proliferation. It is not known whether the ability to drug adaptation also occurs in vivo. However, the fact that N. caninum has an outstanding adaptive potential to cell culture changes even in the absence of drugs has been noted by others earlier (Perez-Zaballos et al., 2005).
Only after prolonged treatments at concentrations in the micromolar range – thus three orders of magnitude above the IC50 – a complete parasite clearance could be observed in vitro. This can be explained by the fact that a small number of tachyzoites remained fully intact after BPQ treatment. Upon early release of drug pressure, these would be the tachyzoites to restart proliferation. At this moment we cannot say whether these parasites represent a stage that resembles the classical cyst-forming bradyzoites, as this would require studies on expression of bradyzoite-specific genes such as BAG1 (Vonlaufen et al., 2002), SAG4 (Fernandez-Garcia et al., 2006), or BSR4 (Risco-Castillo et al., 2007). However, TEM observations did not indicate that the formation of a cyst wall, or cyst wall-like structure, was initiated during BPQ treatments, as previously observed for sodium nitroprusside treated N. caninum-infected keratinocytes and Vero cell cultures (Vonlaufen et al., 2002, 2004).
When applied in mice, BPQ showed a beneficial effect in preventing acute neosporosis. The Nc-Liv isolate is highly virulent, known to establish a rapid systemic infection, which can be visualized by parasite loads in a number of organs such as lungs, kidney, spleen and liver (Alaeddine et al., 2005) and is responsible for early death of infected animals shortly after infection (Collantes-Fernandez et al., 2006). In our experiment, the low parasite burden of BPQ treated mice in the lungs correlates well with the survival rate. Conversely, the cerebral parasite burden in brain tissues was increased in BPQ treated mice that exhibited a much longer survival time. However, it is important to note that high parasite loads in the lungs were measured in animals that mostly died already at days 6–8 p.i. Earlier studies have shown that at later time points, e.g. in animal surviving 3–4 weeks, parasite loads in lungs are significantly reduced, even in the absence of drug treatment (Alaeddine et al., 2005; Collantes-Fernandez et al., 2006).
Thus, it is conceivable that (i) the start of BPQ treatment at 48 h p.i. did not eliminate 100% of tachyzoites, with some parasites remaining viable and still infective (similar to the in vitro situation; see above); (ii) as a consequence BPQ did not completely prevent tachyzoites from crossing the blood–brain border since parasites already had 48 h prior to treatment to reach the CNS; and (iii) with the brain representing an immune-privileged site even low numbers of parasites establishing a cerebral infection underwent proliferation without causing extensive immunopathology. Thus, the treatment with BPQ early after inoculation of N. caninum tachyzoites could potentially be a suitable method to establish an infection in mice that is localized exclusively in the brain. Upon release of the drug pressure and subsequent pregnancy, this infection could become systemic and then be vertically transmitted to the offspring, thus mimicking the situation in cattle. This may constitute a suitable tool for studies on the biology of cerebral Neospora infection and/or control options (including vaccination) that aim to prevent endogenous transplacental N. caninum infection in chronically infected animals, for which a good and reliable mouse model is not yet available despite considerable efforts that have been undertaken (Jiménez-Ruiz et al., 2013).
The in vivo experiments show that BPQ applied as a suspension in corn oil inhibited the outbreak of acute neosporosis in N. caninum infected mice, but further studies are required to improve efficacy. BPQ has a low aqueous solubility and high lipophilicity, properties that hamper absorption. One option could be to employ the more hydrophilic BPQ-3-phosphate as a prodrug, which was superior to BPQ in reducing the liver parasite burden in Leishmania donovani-infected mice, and to employ other vehicles such as isopropyl-myristate and polyethylene-glycol 400 (Garnier et al., 2007), which could increase solubility and bioavailability. In addition, novel oxime derivatives of BPQ and O-methyl-BPQ were evaluated in vitro against L. donovani, and showed promising activities, thus they could also provide valuable alternatives. However, there are no reports on in vivo activity of these compounds (Mäntylä et al., 2004). Another possibility includes application of a recently developed formulation that contains BPQ coupled to solid lipid particles. Studies in rats demonstrated an altered biodistribution and pharmacokinetics of the drug, and an increased half-life of up to 20 h (Soni et al., 2014). In addition, one should take into account to combine BPQ with other compounds that efficiently cross the blood–brain barrier and limit cerebral infection, such as the previously studied calcium dependent kinase inhibitor 1294 (Ojo et al., 2014). These compounds could act synergistically and clear both acute disease as well as CNS invasion.
In conclusion, we have demonstrated the in vitro characteristics of BPQ activities in N. caninum tachyzoites, and shown that application of this compound in mice prevents acute neosporosis, but the compound does apparently not cross the blood–brain barrier in sufficient amounts to kill the parasites in the CNS. However, further investigations will show whether this drug is capable of preventing infection of the fetus in pregnant mice. Pharmacokinetic studies of BPQ in cattle have shown that the maximum concentration in blood plasma of a marketed BPQ formulation (Butalex™) injected intramuscularly was around 0.229 µg/ml, corresponding to 700 nM, and a mean time to reach this concentration was 2.6 h (Muraguri et al., 2006). Whether these properties are sufficient to prevent N. caninum recrudescence in pregnant animals and to protect the fetus from infection remains to be investigated.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
The authors wish to thank Sabrina Sonda (University of Zürich, Switzerland) and David Sibley (Washington University, St. Louis, Missouri, USA) for the transgenic N. caninum strain expressing beta-galactosidase. This work was financed through the National Science Foundation (grant No. 310030_146162/1) and the Vetsuisse Faculty of the University of Bern.
References
- Alaeddine F., Keller N., Leepin A., Hemphill A. Reduced infection and protection from clinical signs of cerebral neosporosis in C57BL/6 mice vaccinated with recombinant microneme antigen NcMIC1. J. Parasitol. 2005;91:657–665. doi: 10.1645/GE-401R. [DOI] [PubMed] [Google Scholar]
- Barna F., Debache K., Vock C.A., Kuster T., Hemphill A. In vitro effects of novel ruthenium complexes in Neospora caninum and Toxoplasma gondii tachyzoites. Antimicrob. Agents Chemother. 2013;57:5747–5754. doi: 10.1128/AAC.02446-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D., McAllister M.M., Dubey J.P. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002;18:546–552. doi: 10.1016/s1471-4922(02)02414-5. [DOI] [PubMed] [Google Scholar]
- Collantes-Fernandez E., Arnaiz-Seco I., Burgos B.M., Rodriguez-Bertos A., Aduriz G., Fernandez-Garcia A. Comparison of Neospora caninum distribution, parasite loads and lesions between epidemic and endemic bovine abortion cases. Vet. Parasitol. 2006;142:187–191. doi: 10.1016/j.vetpar.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Croft S.L., Hogg J., Gutteridge W.E., Hudson A.T., Randall A.W. The activity of hydroxynaphthoquinones against Leishmania donovani. J. Antimicrob. Chemother. 1992;30:827–832. doi: 10.1093/jac/30.6.827. [DOI] [PubMed] [Google Scholar]
- Darius A.K., Mehlhorn H., Heydorn A.O. Effects of toltrazuril and ponazuril on the fine structure and multiplication of tachyzoites of the NC-1 strain of Neospora caninum (a synonym of Hammondia heydorni) in cell cultures. Parasitol. Res. 2004;92:453–458. doi: 10.1007/s00436-003-1063-7. [DOI] [PubMed] [Google Scholar]
- Debache K., Hemphill A. Effects of miltefosine treatment in fibroblast cell cultures and in mice experimentally infected with Neospora caninum tachyzoites. Parasitology. 2012;139:934–944. doi: 10.1017/S0031182012000066. [DOI] [PubMed] [Google Scholar]
- Debache K., Guionaud C., Alaeddine F., Hemphill A. Intraperitoneal and intra-nasal vaccination of mice with three distinct recombinant Neospora caninum antigens results in differential effects with regard to protection against experimental challenge with Neospora caninum tachyzoites. Parasitology. 2010;137:229–240. doi: 10.1017/S0031182009991259. [DOI] [PubMed] [Google Scholar]
- Debache K., Guionaud C., Kropf C., Boykin D., Stephens C.E., Hemphill A. Experimental treatment of Neospora caninum-infected mice with the arylimidamide DB750 and the thiazolide nitazoxanide. Exp. Parasitol. 2011;129:95–100. doi: 10.1016/j.exppara.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Dhar S., Malhotra D.V., Bhushan C., Gautam O.P. Chemotherapy of Theileria annulata infection with buparvaquone. Vet. Rec. 1986;119:635–636. [PubMed] [Google Scholar]
- Dubey J.P. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 2003;41:1–16. doi: 10.3347/kjp.2003.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Schares G., Ortega-Mora L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007;20:323–367. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Garcia A., Risco-Castillo V., Zaballos A., Alvarez-Garcia G., Ortega-Mora L.M. Identification and molecular cloning of the Neospora caninum SAG4 gene specifically expressed at bradyzoite stage. Mol. Biochem. Parasitol. 2006;146:89–97. doi: 10.1016/j.molbiopara.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Fry M., Pudney M. Site of action of the anti-malarial hydroxynaphtoquinone, 2[trans-4-(4'chlorophenyl)cyclohexyl]-3-hadroxy-1,4-naphtoquinone (566C80) Biochem. Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- Garnier T., Mantyla A., Jarvinen T., Lawrence M.J., Brown M.B., Croft S.L. Topical buparvaquone formulations for the treatment of cutaneous leishmaniasis. J. Pharm. Pharmacol. 2007;59:41–49. doi: 10.1211/jpp.59.1.0006. [DOI] [PubMed] [Google Scholar]
- Gondim L.F. Neospora caninum in wildlife. Trends Parasitol. 2006;22:247–252. doi: 10.1016/j.pt.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Häsler B., Regula G., Stark K.D., Sager H., Gottstein B., Reist M. Financial analysis of various strategies for the control of Neospora caninum in dairy cattle in Switzerland. Prev. Vet. Med. 2006;77:230–253. doi: 10.1016/j.prevetmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Häsler B., Stark K.D., Sager H., Gottstein B., Reist M. Simulating the impact of four control strategies on the population dynamics of Neospora caninum infection in Swiss dairy cattle. Prev. Vet. Med. 2006;77:254–283. doi: 10.1016/j.prevetmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Häsler B., Stark K., Gottstein B., Reist M. Epidemiological and financial considerations for the control of Neospora caninum on Swiss dairy farms. Schweiz. Arch. Tierheilkd. 2008;150:273–280. doi: 10.1024/0036-7281.150.6.273. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Gottstein B., Kaufmann H. Adhesion and invasion of bovine endothelial cells by Neospora caninum. Parasitology. 1996;112(Pt 2):183–197. doi: 10.1017/s0031182000084754. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Vonlaufen N., Naguleswaran A. Cellular and immunological basis of the host-parasite relationship during infection with Neospora caninum. Parasitology. 2006;133:261–278. doi: 10.1017/S0031182006000485. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Debache K., Monney T., Schorer M., Guionaud C., Alaeddine F. Proteins mediating the Neospora caninum-host cell interaction as targets for vaccination. Front. Biosci. 2013;5:23–36. doi: 10.2741/e593. [DOI] [PubMed] [Google Scholar]
- Hostettler I., Müller J., Stephens C.E., Haynes R., Hemphill A. A quantitative reverse-transcriptase PCR assay for the assessment of drug activities against intracellular Theileria annulata schizonts. Int. J. Parasitol. Drugs Drug Resist. 2014;4:201–209. doi: 10.1016/j.ijpddr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A.T., Randall A.W., Fry M., Ginger C.D., Hill B., Latter V.S. Novel anti-malarial hydroxynaphtoquinones with potent broad spectrum anti-protozoal activity. Parasitology. 1985;90:45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ruiz E., Álvarez-García G., Aguado-Martínez A., Ortega-Mora L.M. Mice congenitally infected with low-to-moderate virulence Neospora caninum isolates exhibited clinical reactivation during the mating period without transmission to the next generation. Exp. Parasitol. 2013;134:244–248. doi: 10.1016/j.exppara.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Kaneshiro E.S., Sul D., Basselin M., Kayser O. Pneumocystis carinii synthesizes four ubiquinone homologs: inhibition by atovaquone and bupravaquone but not by stigmatellin. J. Eukaryot. Microbiol. 2001;Suppl:172S–173S. doi: 10.1111/j.1550-7408.2001.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Kaneshiro E.S., Basselin M., Merali S., Kayser O. Ubiquinone synthesis and its regulation in Pneumocystis carinii. J. Eukaryot. Microbiol. 2006;53:435–444. doi: 10.1111/j.1550-7408.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- Kayser O., Waters W.R., Woods K.M., Upton S.J., Keithly J.S., Kiderlen A.F. Evaluation of in vitro activity of aurones and related compounds against Cryptosporidium parvum. Planta Med. 2001;67:722–725. doi: 10.1055/s-2001-18357. [DOI] [PubMed] [Google Scholar]
- Kritzner S., Sager H., Blum J., Krebber R., Greif G., Gottstein B. An explorative study to assess the efficacy of toltrazuril-sulfone (ponazuril) in calves experimentally infected with Neospora caninum. Ann. Clin. Microbiol. Antimicrob. 2002;1:4. doi: 10.1186/1476-0711-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf C., Debache K., Rampa C., Barna F., Schorer M., Stephens C.E. The adaptive potential of a survival artist: characterization of the in vitro interactions of Toxoplasma gondii tachyzoites with di-cationic compounds in human fibroblast cell cultures. Parasitology. 2012;139:208–220. doi: 10.1017/S0031182011001776. [DOI] [PubMed] [Google Scholar]
- Küster T., Lense N., Barna F., Hemphill A., Kindermann M.K., Heinicke J.W. A new promising application for highly cytotoxic metal compounds: η6-areneruthenium(II) phosphite complexes for the treatment of alveolar echinococcosis. J. Med. Chem. 2012;55:4178–4188. doi: 10.1021/jm300291a. [DOI] [PubMed] [Google Scholar]
- Larson R.L., Hardin D.K., Pierce V.L. Economic considerations for diagnostic and control options for Neospora caninum-induced abortions in endemically infected herds of beef cattle. J. Am. Vet. Med. Assoc. 2004;224:1597–1604. doi: 10.2460/javma.2004.224.1597. [DOI] [PubMed] [Google Scholar]
- Mäntylä A., Rautio J., Nevalainen T., Keski-Rahkonen P., Vepsälainen J., Järvinen T. Design, synthesis and in vitro evaluation of novel water-soluble prodrugs of buparvaquone. Eur. J. Pharm. Sci. 2004;23:151–158. doi: 10.1016/j.ejps.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Martin Y.C., Bustard T.M., Lynn K.R. Relationship between physical properties and antimalarial activities of 1,4-naphthoquinones. J. Med. Chem. 1973;16:1089–1093. doi: 10.1021/jm00268a005. [DOI] [PubMed] [Google Scholar]
- Matovu E., Stewart M.L., Geiser F., Brun R., Maser P., Wallace L.J. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell. 2003;2:1003–1008. doi: 10.1128/EC.2.5.1003-1008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazuz M.L., Haynes R., Shkap V., Fish L., Wollkomirsky R., Leibovich B. Neospora caninum: in vivo and in vitro treatment with artemisone. Vet. Parasitol. 2012;187:99–104. doi: 10.1016/j.vetpar.2011.12.020. [DOI] [PubMed] [Google Scholar]
- McHardy N., Morgan D.W. Treatment of Theileria annulata infection in calves with parvaquone. Res. Vet. Sci. 1985;39:1–4. [PubMed] [Google Scholar]
- McHardy N., Wekesa L.S., Hudson A.T., Randall A.W. Antitheilerial activity of BW720C (buparvaquone): a comparison with parvaquone. Res. Vet. Sci. 1985;39:29–33. [PubMed] [Google Scholar]
- Minami T., Nakano T., Shimizu S., Shimura K., Fujinaga T., Ito S. Efficacy of naphthoquinones and imidocarb dipropionate on Theileria sergenti infections in splenectomized calves. Nihon Juigaku Zasshi. 1985;47:297–300. doi: 10.1292/jvms1939.47.297. [DOI] [PubMed] [Google Scholar]
- Monney T., Hemphill A. Vaccines against neosporosis: what can we learn from the past studies? Exp. Parasitol. 2014;140:52–70. doi: 10.1016/j.exppara.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Monney T., Rutti D., Schorer M., Debache K., Grandgirard D., Leib S.L. RecNcMIC3-1-R is a microneme- and rhoptry-based chimeric antigen that protects against acute neosporosis and limits cerebral parasite load in the mouse model for Neospora caninum infection. Vaccine. 2011;29:6967–6975. doi: 10.1016/j.vaccine.2011.07.038. [DOI] [PubMed] [Google Scholar]
- Muraguri G.R., Ngumi P.N., Wesonga D., Ndungu S.G., Wanjohi J.M., Bang K. Clinical efficacy and plasma concentrations of two formulations of buparvaquone in cattle infected with East Coast fever (Theileria parva infection) Res. Vet. Sci. 2006;81:119–126. doi: 10.1016/j.rvsc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Müller J., Hemphill A. Drug target identification in intracellular and extracellular protozoan parasites. Curr. Top. Med. Chem. 2011;11:2029–2038. doi: 10.2174/156802611796575876. [DOI] [PubMed] [Google Scholar]
- Müller J., Hemphill A. In vitro culture systems for the study of apicomplexan parasites in farm animals. Int. J. Parasitol. 2013;43:115–124. doi: 10.1016/j.ijpara.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Ojo K.K., Reid M.C., Kallur Siddaramaiah L., Müller J., Winzer P., Zhang Z. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neosporosis therapy. PLoS ONE. 2014;9:e92929. doi: 10.1371/journal.pone.0092929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Zaballos F.J., Ortega-Mora L.M., Alvarez-Garcia G., Collantes-Fernandez E., Navarro-Lozano V., Garcia-Villada L. Adaptation of Neospora caninum isolates to cell-culture changes: an argument in favor of its clonal population structure. J. Parasitol. 2005;91:507–510. doi: 10.1645/GE-381R1. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2012. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Reichel M.P., Ellis J.T. If control of Neospora caninum infection is technically feasible does it make economic sense? Vet. Parasitol. 2006;142:23–34. doi: 10.1016/j.vetpar.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Reichel M.P., Alejandra Ayanegui-Alcérreca M., Gondim L.F., Ellis J.T. What is the global economic impact of Neospora caninum in cattle – the billion dollar question. Int. J. Parasitol. 2012;43:133–142. doi: 10.1016/j.ijpara.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Risco-Castillo V., Fernandez-Garcia A., Zaballos A., Aguado-Martinez A., Hemphill A., Rodriguez-Bertos A. Molecular characterisation of BSR4, a novel bradyzoite-specific gene from Neospora caninum. Int. J. Parasitol. 2007;37:887–896. doi: 10.1016/j.ijpara.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Rosypal A.C., Lindsay D.S. The sylvatic cycle of Neospora caninum: where do we go from here? Trends Parasitol. 2005;21:439–440. doi: 10.1016/j.pt.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Schmid A., Sauvage V., Escotte-Binet S., Aubert D., Terryn C., Garnotel R. Molecular characterization and expression analysis of a P-glycoprotein homologue in Toxoplasma gondii. Mol. Biochem. Parasitol. 2009;163:54–60. doi: 10.1016/j.molbiopara.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Schorer M., Debache K., Barna F., Monney T., Müller J., Boykin D.W. Di-cationic arylimidamides act against Neospora caninum tachyzoites by interference in membrane structure and nucleolar integrity and are active against challenge infection in mice. Int. J. Parasitol. Drugs Drug Resist. 2012;2:109–120. doi: 10.1016/j.ijpddr.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni M.P., Shelkar N., Gaikwad R.V., Vanage G.R., Samad A., Devarajan P.V. Buparvaquone loaded solid lipid nanoparticles for targeted delivery in theleriosis. J. Pharm. Bioallied Sci. 2014;6:22–30. doi: 10.4103/0975-7406.124309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohbusch M., Muller N., Hemphill A., Krebber R., Greif G., Gottstein B. Toltrazuril treatment of congenitally acquired Neospora caninum infection in newborn mice. Parasitol. Res. 2009;104:1335–1343. doi: 10.1007/s00436-009-1328-x. [DOI] [PubMed] [Google Scholar]
- Vonlaufen N., Muller N., Keller N., Naguleswaran A., Bohne W., McAllister M.M. Exogenous nitric oxide triggers Neospora caninum tachyzoite-to-bradyzoite stage conversion in murine epidermal keratinocyte cell cultures. Int. J. Parasitol. 2002;32:1253–1265. doi: 10.1016/s0020-7519(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Vonlaufen N., Guetg N., Naguleswaran A., Muller N., Bjorkman C., Schares G. In vitro induction of Neospora caninum bradyzoites in vero cells reveals differential antigen expression, localization, and host-cell recognition of tachyzoites and bradyzoites. Infect. Immun. 2004;72:576–583. doi: 10.1128/IAI.72.1.576-583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie G.M., Brown C.G., Kirvar B.E., Thomas M., Williamson S.M., Bell-Sakyi L.J. Chemoprophylaxis of Theileria annulata and Theileria parva infections of calves with buparvaquone. Vet. Parasitol. 1998;78:1–12. doi: 10.1016/s0304-4017(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Witola W.H., Inoue N., Ohashi K., Onuma M. RNA-interference silencing of the adenosine transporter-1 gene in Trypanosoma evansi confers resistance to diminazene aceturate. Exp. Parasitol. 2004;107:47–57. doi: 10.1016/j.exppara.2004.03.013. [DOI] [PubMed] [Google Scholar]


