Graphical Abstract
Keywords: Teladorsagia circumcincta, Combination anthelmintics, Reversion, Resistance
Highlights
-
•
Best practise parasite management strategies were implemented on commercial farms.
-
•
Strategies included refugia and almost exclusive use of combination anthelmintics.
-
•
Aim was to maintain productivity and prevent resistance developing further.
-
•
Efficacy of three drench family's was assessed on a yearly basis.
-
•
Resistance did not get worse and reversion to susceptibility maybe occurring.
Abstract
Maintaining production and economic viability in the face of resistance to multiple anthelmintic actives is a challenge for farmers in many countries. In this situation, most farmers in New Zealand rely on the use of combination products, containing multiple actives with similar spectra of activity, in order to maintain control. However, there are concerns that use of combinations, once resistance has already developed to the individual actives, could rapidly lead to complete failure of all actives. This study followed seven farms, previously diagnosed with resistance to at least two classes of anthelmintic, which were implementing a tailored programme of ‘best practice parasite management’. The aim was to ascertain whether the programmes, which included the almost exclusive use of combination anthelmintics, were able to prevent resistance from developing further. Strategies implemented on each farm varied, but had consistent underlying principles i.e. to avoid over-use of anthelmintics; to minimise parasite challenge to susceptible stock; to maintain refugia of susceptibility and to ensure that only effective anthelmintics were used. Annual faecal egg count reduction tests (FECRT) were undertaken in lambs on all farms to monitor anthelmintic efficacy over 5 years. The efficacy of albendazole, ivermectin and levamisole was calculated and the changes in efficacy against Teladorsagia circumcincta assessed. Overall, there was a significant improvement in the effectiveness of both levamisole and ivermectin against T. circumcincta, and a positive but non-significant trend in efficacy of albendazole, i.e. there was evidence for reversion towards susceptibility. Hence, the almost exclusive use of combination anthelmintics, integrated with other resistance management strategies, did not result in further resistance development despite all farms exhibiting resistance to multiple actives at the outset. What-is-more, the measured increases in anthelmintic efficacy suggests that adoption of best practice management strategies may extend the useful life of anthelmintics even after resistance has been diagnosed.
1. Introduction
Anthelmintic resistance in nematode parasites of grazing animals is an expanding problem and is of major concern in many countries (Kaplan, 2004; Waghorn et al., 2006; Besier, 2007; Sutherland and Leathwick, 2011). As anthelmintics are generally the cornerstone of most parasite control programmes, the impact of parasites which are resistant to these drugs can be considerable (Besier, 2007; Sutherland et al., 2010; Miller et al., 2012; Stromberg et al., 2012). In order to retain the effectiveness of anthelmintics for as long as possible, it is essential that strategies are developed and implemented which can slow or prevent the development of resistance (Besier, 2007; Nielsen et al., 2014). With respect to nematode parasites of sheep, there has been extensive research over many years aiming to increase our understanding of the factors influencing the development of resistance, and its management once present (Barnes et al., 1995; Leathwick et al., 2009; Leathwick and Besier, 2014). As a result, for sheep at least, there is an array of established approaches for managing resistance (Leathwick and Besier, 2014).
Historically, one of the main strategies promoted to slow the development of resistance was to alternate (rotate) the class of anthelmintic used on an approximately annual basis (Donald et al., 1980; Prichard et al., 1980; Waller et al., 1989; Coles and Roush, 1992). This recommendation was based on the expectation that if there was a fitness cost associated with being resistant then rotating anthelmintics would allow the opportunity for reversion towards susceptibility to occur in the period when alternative drugs were used. Also, it was considered that by not exposing individual worms to more than one class of anthelmintic, selection for resistance to multiple actives was less likely to occur (Prichard et al., 1980). The practice of rotating different anthelmintic classes annually was widely adopted in some countries (Kettle et al., 1983; Waller et al., 1989; Lawrence et al., 2007; Sargison et al., 2007) and is still promoted today in some countries and classes of animals (McMahon et al., 2013a). However, in the interim resistance has continued to develop and resistance to multiple anthelmintic classes is now well established on some farms (van Wyk et al., 1997; Waghorn et al., 2006; Le Jambre et al., 2010; Torres-Acosta et al., 2012; McMahon et al., 2013b; Geurden et al., 2014).
An alternative approach to drug use was suggested by early modelling studies (Smith, 1990; Barnes et al., 1995) which showed that simultaneously using multiple actives, with similar spectra of activity, had the potential to dramatically slow the development of resistance. Subsequently, commercial interests developed and marketted such combination anthelmintic products and these are now used extensively in some countries, but are still not available in others (Bartram et al., 2012; Geary et al., 2012). Subsequent modelling and empirical studies have continued to support the use of combinations to slow the development of resistance (Learmount et al., 2012; Leathwick, 2012; Leathwick et al., 2012). However, both modelling and empirical studies indicate that when the frequency of resistance alleles is already high in a population, combinations lose much of their ability to slow the further development of resistance (Leathwick et al., 2012). This has been used as an argument against the use of combinations (Coles and Roush, 1992) and a perception has developed that their use, once resistance to multiple anthelmintic classes has already established, will result in the further and rapid escalation of resistance to all of the different classes. This perception has created a dilemma for those farmers and their advisors who, prior to the release of new anthelmintic actives (monepantel (Kaminsky et al., 2008) and derquantel (Little et al., 2010)) found themselves in a situation where no single active product would work effectively on their farm and the only products which could be used to maintain control were combinations.
Between 2010 and 2013, a four year extension programme was run to implement and evaluate parasite and resistance management programmes on commercial sheep and beef farms throughout New Zealand (Rhodes et al., 2011). One of the principle aims of this programme was to implement strategies which would maintain or improve on-farm productivity whilst preventing any further increase in the resistance status of parasites on the farms. Given that most of the sheep farms enrolled in this programme already had significant resistance to at least two anthelmintic classes, routine use of combination anthelmintics was inevitable. This situation, therefore, presented an opportunity to follow the progress of anthelmintic resistance on a number of farms under integrated resistance management programmes which included, along with other refugia and management practices, the almost exclusive use of combination anthelmintics.
2. Materials and methods
2.1. The best practice parasite management programme
The Best Practice Parasite Management programme (BPPMP) was an industry-funded programme which aimed to develop and implement parasite and resistance management programmes on a diverse range of farms throughout the country and to evaluate their performance over time (Rhodes et al., 2011). Details of the programme will be published elsewhere, but briefly the intent was to design a parasite management programme specifically to fit the requirements of each farm and farmer, in order to maintain or improve production and profitability, whilst ensuring that the efficacy of anthelmintics did not decline from its initial level.
The programme involved an annual visit to the farm by the farm veterinarian, a parasitologist and an agricultural consultant. A whole-of-farm approach was taken such that at each visit a comprehensive review was undertaken of farm practices and events (e.g. purchase and sale of stock, livestock management, fertiliser applications, and crops grown) over the previous 12 month period along with a detailed review of the current animal health programme. Modifications to the plan were then discussed, informed by any monitoring data, and changes agreed upon for implementation in the following year along with a programme to monitor parasite levels (through regular faecal nematode egg counts (FEC) and coprocultures). Over the following 12 months the plan was implemented by the farmer with assistance from the veterinarians.
2.2. Resistance management strategies
The suite of parasite control and resistance management strategies implemented varied depending on the characteristics of each farm, so no two farms applied the same set of strategies in the same way. There were, however, some consistent underlying principles which can be grouped as:
-
1.
use effective anthelmintic products i.e. on the basis of annual efficacy tests, select products which will achieve high efficacy against all worm species (at least 95%). Because all seven farms were known to have resistance to at least two anthelmintic classes, this required the use of combination products and/or new actives.
-
2.
avoid over-use of anthelmintics i.e. maintain a structured preventive programme of treatments to lambs (Vlassoff and Brunsdon, 1981) with all other treatments on the basis of demonstrated need (i.e. signs of illthrift or FEC) (Leathwick and Besier, 2014).
-
3.
do not administer anthelmintic treatments at intervals shorter than 28 days to allow for some limited contamination of pastures with susceptible genotypes after the pre-patent period of new infection.
-
4.
minimise or eliminate the use of anthelmintics with persistent activity (Leathwick and Besier, 2014).
-
5.
administer a single treatment, containing a new anthelmintic class, to lambs in late summer to remove resistant genotype worms which have accumulated over previous treatments (Leathwick and Hosking, 2009).
-
6.
maximise the opportunities for retention of unselected genotypes (i.e. to maintain refugia). The principal method used to achieve this was to minimise the treatment of adult sheep and ensure that treated lambs and untreated ewes grazed over the same pastures as much as possible. Where this was not practical other approaches were used (Leathwick et al., 2008; Leathwick and Besier, 2014).
-
7.
maximise the use of integrated grazing (cattle, deer, sheep) and crops to minimise parasite challenge to susceptible stock, and adjust anthelmintic treatment schedules accordingly.
-
8.
ensure that anthelmintic treatments did not coincide with a shift to pastures likely to have low numbers of infective larvae unless other strategies were in place to ensure adequate refugia (e.g. treated lambs followed by untreated adult sheep) (Waghorn et al., 2009).
-
9.
effective quarantine procedures to prevent the introduction of resistant genotypes with stock transferred onto the farm (Leathwick and Besier, 2014).
Following each annual visit by the advisory team, throughout the course of the programme, each farmer was allocated a subjective scoring for their level of adoption of practices within each of the above categories (Table 1).
Table 1.
The anthelmintics used, the annual number of treatments to lambs and the mean interval between these treatments, along with a qualitative scoring on the level of adoption by farmers of best practice parasite management practices, for each of the 7 farms in the study.
| Factor | Farm | ||||||
|---|---|---|---|---|---|---|---|
| B | D | H | K | M | P | W | |
| Actives used (use effective products) | AL AD ABL |
ABL Mon |
BL ABL Mon |
ABL Mon |
ABL Mox Mon |
ABL Mon |
ABL AD |
| Average annual number of treatments to lambs | 9 | 7 | 10 | 7 | 8 | 7 | 7 |
| Average interval between treatments (days) | 28 | 29 | 30 | 28 | 29 | 28 | 39 |
| Avoid over-use of anthelmintics | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓ | ✓✓✓ | ✓✓✓ |
| No long acting treatments | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓ | ✓ | ✓✓✓ |
| Use a new active in summer | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| Maintain ‘Refugia’ of susceptibility | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓ | ✓✓✓ | ✓✓ |
| Integrated grazing | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓✓ | ✓✓✓ |
| No treat and shift | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓ | ✓✓ | ✓✓✓ |
| Effective quarantine | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ |
✓, low level of adoption; ✓✓, medium level of adoption; ✓✓✓, high level of adoption.
AL, Abamectin, levamisole; AD, Abamectin, derquantel; ABL, Abamectin, levamisole, oxfendazole/albendazole; Mon, Monepantel; BL, Albendazole, levamisole; Mox, Moxidectin.
2.3. Monitoring anthelmintic efficacy
An annual faecal egg count reduction test (FECRT) was undertaken on all farms to monitor anthelmintic efficacy over time. Where feasible this was continued after the programme finished, so that anthelmintic efficacy was monitored for five years on most farms.
Each year, a mob of at least 60 lambs was set aside before weaning, and these animals were monitored for FEC and remained untreated with anthelmintics until the mean count from 10 samples reached approximately 750 eggs per g faeces (epg). Where-ever possible the test was conducted on lambs which had never been treated previously with anthelmintic, thereby removing the possibility that previous treatments would bias the result by not completely removing all worm genotypes. The veterinarian was sent a kit containing everything needed to carry out the test including the anthelmintics, which ensured that all tests were conducted using the same anthelmintic products, which in most cases came from the same container. Faecal samples were couriered to a central laboratory for processing so that all processing was carried out by the same personnel.
Each FECRT comprised 4 treatment groups each of at least 12 animals. On day 0 all animals were weighed, sampled for FEC and then administered either; albendazole at 5.0 mg/kg live weight (Albendazole, Merial New Zealand Ltd), levamisole at 7.5 mg/kg live weight (Levicare Hi Mineral, Merial New Zealand Ltd), ivermectin at 0.2 mg/kg live weight (Ivomec Liquid for sheep & goats, Merial New Zealand Ltd) or were left as an untreated control. Seven to ten days after treatment a second faecal sample was collected from all animals.
Samples were kept cool and couriered over night to the laboratory. Faecal egg counts were performed using a modified McMaster method (Lyndal-Murphy, 1993) where one egg seen equated to 50 epg. Faeces from the post-treatment samples was pooled by treatment group and cultured at 22 °C for 14 days before undergoing baermannisation to extract the third stage larvae (L3) (Hendrix, 1998). Individual L3 (N = 96) from each culture were identified to species using a multiplexed PCR technique with primers designed to target unique sequence motifs present in the second internal transcribed spacer region of the ribosomal DNA of each of the larval species (Bisset et al., 2014; Waghorn et al., 2014). The proportion of each nematode species in the culture from the control samples was used to apportion FEC to species in the pre-test treated groups. Similarly, FEC in the post-treatment samples was apportioned to species based on the proportion of each species in the post-treatment samples (McKenna, 1996). These numbers were then used to calculate the efficacy (reduction in FEC) of treatment for each species of nematode using the equation below (Presidente, 1985; Waghorn et al., 2006). For the purpose of this study anthelmintic resistance was defined as a <95% reduction in FEC (McKenna, 1994). Ninety-five per cent confidence intervals around the efficacy were calculated using the formulae of Lyndal-Murphy et al. (2010).
2.4. Statistical analysis
Resistance was mostly commonly identified in Teladorsagia circumcincta, and this was the only species in which resistance to ivermectin was identified. Therefore, this was the only nematode species for which resistance to all three of the tested anthelmintics was found. Seven farms were identified as having resistance to two or more anthelmintics in this nematode species at the beginning of the BPPM programme, and as the aim was to examine any possible worsening of resistance over the course of the programme, analysis of the efficacy data was restricted to this species.
Separate analyses were conducted for each of the three actives. Linear regression, weighted by the apportioned FEC (so efficacy figures based on low egg counts had less influence than those based on higher numbers) was used. Only linear regression models were explored due to some farms not having data for all 5 years (thus non-linear models have not been considered). Since the slopes and intercepts associated with the linear regression model fitted for each of the seven farms may be different, the ‘Random coefficient regression models’ (Raja, 1984) were utilised. Modelling was undertaken using the lmer function of the package lme4 of the R software (R Core Team, 2014).
3. Results
Overall, all farmers made consistent efforts to adopt most of the resistance management practices worked out with their advisors (Table 1). The majority of anthelmintic treatments were with combination products, the only exceptions being a strategic annual treatment with the new active monepantel on 5 farms and a single annual treatment with moxidectin for control of Haemonchus contortus on farm M. Although, the detail of how strategies were implemented invariably differed between farms the efforts made by the farmers resulted in scores of 3 (on a scale of 0–3) for most resistance management practices (Table 1).
At the start of the BPPMP, three of the 10 sheep farms had T. circumcincta resistant to two classes of anthelmintic and four had populations resistant to all 3 classes. Several farms had serious resistance to all 3 actives. For example, Farm M had efficacies for albendazole, levamisole and ivermectin of 0%, 70% and 0%, respectively, whilst Farm K had efficacies of 40%, 88% and 10% for the same actives (Table 2).
Table 2.
Efficacy data for Teladorsagia circumcincta against each active tested (A – Albendazole, B – Levamisole and C – Ivermectin) with 95% confidence intervals (CI) for the 7 farms over the 4 years of the project and the one follow up year. The faecal egg counts allocated to T. circumcincta based on the proportion of this species found in the control cultures and used as a covariant in the analysis are also given.
| Year of test | ||||||
|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | ||
| (A) Albendazole | ||||||
| Farm B | % efficacy | 51 | 86 | 92 | NT | 71 |
| 95% CI | 12–73 | 79–91 | 89–93 | 9–92 | ||
| FEC | 175 | 1101 | 875 | 73 | ||
| Farm D | % efficacy | 96 | 98 | 100 | 100 | 100 |
| 95% CI | 95–97 | 97–99 | 97–100 | 96–100 | 98–100 | |
| FEC | 625 | 153 | 118 | 30 | 86 | |
| Farm H | % efficacy | 96 | 100 | 98 | 95 | 98 |
| 95% CI | 93–98 | 97–100 | 94–99 | 93–96 | 96–99 | |
| FEC | 21 | 114 | 25 | 35 | 72 | |
| Farm K | % efficacy | 40 | NT | 100 | 67 | 96 |
| 95% CI | −30–72 | 95–100 | 55–77 | 94–98 | ||
| FEC | 84 | 44 | 242 | 265 | ||
| Farm M | % efficacy | −9 | 70 | 92 | 97 | 83 |
| 95% CI | −55–24 | 53–81 | 69–98 | 94–99 | 59–92 | |
| FEC | 53 | 596 | 160 | 1007 | 75 | |
| Farm P | % efficacy | 87 | 53 | 73 | 53 | NT |
| 95% CI | 78–92 | 24–71 | 61–80 | 25–69 | ||
| FEC | 88 | 48 | 30 | 23 | ||
| Farm W | % efficacy | 92 | NT | 97 | 56 | 91 |
| 95% CI | 91–93 | 85–99 | 31–73 | 68–97 | ||
| FEC | 285 | 32 | 102 | 104 | ||
| (B) Levamisole | ||||||
| Farm B | % efficacy | 27 | 92 | 98 | NT | 56 |
| 95% CI | −1–48 | 85–95 | 97–99 | 17–79 | ||
| FEC | 175 | 1101 | 875 | 73 | ||
| Farm D | % efficacy | 75 | 99 | 96 | 98 | 98 |
| 95% CI | 50–87 | 98–100 | 93–98 | 97–100 | 90–100 | |
| FEC | 625 | 153 | 118 | 30 | 86 | |
| Farm H | % efficacy | 91 | 98 | 100 | 98 | 94 |
| 95% CI | 64–98 | 85–100 | 93–100 | 75–100 | 78–98 | |
| FEC | 21 | 114 | 25 | 35 | 72 | |
| Farm K | % efficacy | 88 | NT | 100 | 95 | 94 |
| 95% CI | 80–93 | 96–100 | 84–99 | 84–98 | ||
| FEC | 84 | 44 | 242 | 265 | ||
| Farm M | % efficacy | 70 | 99 | 98 | 100 | 77 |
| 95% CI | 8–90 | 93–100 | 76–100 | 99–100 | 50–88 | |
| FEC | 53 | 596 | 160 | 1007 | 75 | |
| Farm P | % efficacy | 36 | 94 | 96 | 89 | NT |
| 95% CI | −19–66 | 47–99 | 90–98 | 66–96 | ||
| FEC | 88 | 48 | 30 | 23 | ||
| Farm W | % efficacy | 78 | NT | 99 | 86 | 92 |
| 95% CI | 65–86 | 98–100 | 59–95 | 78–97 | ||
| FEC | 285 | 32 | 102 | 104 | ||
| (C) Ivermectin | ||||||
| Farm D | % efficacy | 93 | 98 | 100 | 100 | 100 |
| 95% CI | 79–97 | 95–99 | 98–100 | 96–100 | 97–100 | |
| FEC | 625 | 153 | 118 | 30 | 86 | |
| Farm H | % efficacy | 90 | 100 | 33 | 97 | 95 |
| 95% CI | 52–98 | 98–100 | −131–75 | 91–99 | 76–99 | |
| FEC | 21 | 114 | 25 | 35 | 72 | |
| Farm K | % efficacy | 10 | NT | 79 | 54 | 95 |
| 95% CI | −155–68 | 28–94 | 15–76 | 84–98 | ||
| FEC | 84 | 44 | 242 | 265 | ||
| Farm M | % efficacy | −128 | 64 | 66 | 97 | 71 |
| 95% CI | −237–−55 | 45–77 | 50–77 | 93–98 | 53–80 | |
| FEC | 53 | 596 | 160 | 1007 | 75 | |
| Farm P | % efficacy | 82 | 72 | 100 | 90 | NT |
| 95% CI | 42–95 | −95–96 | 96–100 | 74–96 | ||
| FEC | 88 | 48 | 30 | 23 | ||
| Farm W | % efficacy | 58 | NT | 99 | 97 | 67 |
| 95% CI | 29–75 | 93–100 | 73–100 | 12–88 | ||
| FEC | 285 | 32 | 102 | 104 | ||
NT, not tested.
Across the 7 farms, the efficacy of albendazole against T. circumcincta averaged 66% in year one, ranging from 0 to 96%. At the end of the 5 years the average efficacy had increased to 90% and ranged from 71 to 100%. Although the regression had a positive slope of 3.8, this was not significant (p = 0.17) (Fig. 1a).
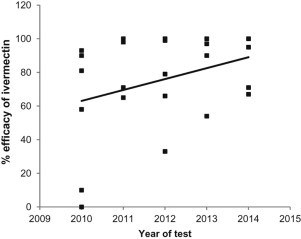
Fig. 1.

Efficacy of (a) albendazole, (b) levamisole and (c) ivermectin against Teladorsagia circumcincta as measured by faecal egg count reduction on 7 sheep farms over 5 years.
The efficacy of levamisole against T. circumcincta also averaged 66% in year one with a range of 27 to 91% across the 7 farms. By year 5 the average efficacy was 85% with a range of 56 to 98%. The slope of the linear regression was 5.4 which was significant with p = 0.04 (Fig. 1b).
The efficacy of ivermectin against T. circumcincta averaged 55% in year one with a range of 0 to 93%. By year 5 the average had increased to 86% and the range reduced to 67 to 100%. With a positive slope of 8.6 this increase in efficacy was also significant with p = 0.01 (Fig. 1c). Farm B was not included in the ivermectin analysis due to high efficacy with this active throughout the study resulting in no variance.
In the final FECRT, three farms were still classified as resistant to all three actives; one farm was resistant to two actives; two farms were resistant to one active and one farm had no resistance to the three actives. The efficacy of the actives on the three farms still showing triple resistance had increased, in some cases dramatically. For example, Farm M had final efficacies for albendazole, levamisole and ivermectin of 83%, 77% and 71%, respectively, whilst Farm K had efficacies of 96%, 94% and 95% for the same actives (Table 2).
4. Discussion
The purpose of this study was to monitor the efficacy (i.e. level of resistance) in T. circumcincta under best practice parasite management in order to ascertain whether the strategies implemented were able to prevent resistance from becoming any worse. On the seven farms which started the programme with resistance to at least two classes of anthelmintic, not only did resistance not get any worse, but overall there was a significant improvement in the effectiveness of both levamisole and ivermectin i.e. there was evidence for some reversion towards susceptibility. This result was unexpected for a number of reasons. Firstly, as resistance was already well advanced on all these farms, it seemed reasonable to expect that any initial fitness cost associated with resistant genotypes would have been eroded by re-association of resistance and fitness traits during the selection process (Kelly et al., 1978; Martin et al., 1988; Maingi et al., 1990; Prichard, 1990). Secondly, all these farms routinely used combination anthelmintics throughout the BPPMP, and so there seemed little opportunity for any release from selection which would be an expected requirement for reversion to occur (Prichard, 1990). Thirdly, on some farms the efficacy levels were so low at the start of the BPPMP that a further rapid decline in efficacy might have been expected under continued selection (Coles and Roush, 1992). Clearly this did not happen.
A recent modelling study may offer at least a partial explanation for these findings (Leathwick, 2013). This study compared the effect of using drugs in combination with an annual rotation, under different levels of fitness cost associated with resistant genotypes, and assumed that fitness costs were additive when worms carried multiple sets of resistance genes i.e. that worms carrying multiple sets of resistance genes were less fit than those resistant to just one drug. The results indicated that reversion was more likely to occur (i.e. at lower fitness costs) when drugs were used in combination than in rotation because the combination removed all resistant worm genotypes except those simultaneously carrying multiple sets of resistance genes, which were the least fit. Further, it was found that the size of the worm population in refugia was also important, with resistance being delayed more and reversion occurring at lower fitness costs when a larger proportion of the worm population was not exposed to treatment. There is evidence to support a fitness cost in being resistant, studies on T. colubriformis and H. contortus have shown reduced establishment in resistant isolates (Maclean et al., 1987; Maingi et al., 1990). Scott and Armour (1991) and Maingi et al. (1990) also found that the proportion of viable eggs being produced by resistant isolates of H. contortus was much less than that produced by susceptible isolates.
The BPPMP applied a number of resistance management strategies to every farm (Table 1) and the set of strategies was invariably different, either in composition or in the manner in which they were applied. Therefore, it is not possible to determine which strategies contributed, or had the greatest impact, on the results reported here. Indeed, it is likely that the different mixtures of strategies implemented on different farms contributed to the variability in degree of reversion seen on different farms. However, given that along with the use of effective combination anthelmintics, substantial efforts were also made to avoid unnecessary treatments and ensure the maximum refugia consistent with production targets, it seems highly likely that the complex of strategies was effective rather than any individual approach. The results are, however, consistent with the hypothesis raised by Leathwick (2013) that reversion to susceptibility can occur when combination anthelmintics are used, especially when high levels of refugia are also maintained.
In Australia and New Zealand, farmers are often required to use combination anthelmintics just to maintain control, unless they can afford the additional cost of using products containing new actives (McKenna, 2010; Leathwick and Besier, 2014). The results of this study offer some encouragement for these farmers and their long-term future in that the use of combination anthelmintics against worm populations which were already demonstrating well developed resistance to multiple actives did not result in any escalation towards total treatment failure. In fact, for all the farmers involved in this study, the efficacy of the double or triple combination anthelmintics which they were using actually increased over the duration of the BPPMP. Clearly, this would have increased their ability to maintain worm control and improved the long-term sustainability of their farming operation. On some farms this increase in efficacy was substantial e.g. Farm M, where efficacy increased from 0% for two drugs in year one to around 80% by the 5th year for all the drugs. It must, however, be emphasised that in this study a suite of changes were made to achieve this goal and that just switching to use of combination drenches, without addressing other issues such as the size of the selection pressure and maintaining refugia of susceptibility, may not produce the same result.
The key finding from this study is that when anthelmintics containing multiple actives were used against worm populations with varying levels of resistance to two or more of these actives, resistance did not get worse. What-is-more, evidence was found for significant reversion towards susceptibility. It must be noted, however, that combination anthelmintics were not used in isolation from other resistance management strategies, and so it cannot be assumed that combinations alone would have achieved this outcome. The results are consistent with the findings of a recent modelling study which indicated that reversion towards susceptibility is feasible when using anthelmintics in combination if individuals resistant to multiple actives are less fit than those resistant to only one (Leathwick, 2013). Work is currently underway to determine whether differences in fitness between isolates and genotypes might explain these results.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
Tony Rhodes, PGG Wrightson Consulting, was the driving force behind the BPPM programme from which the data for this study were derived, along with Richard Shaw and Ian Sutherland made helpful comments on a draft manuscript. Aspects of this study were supported by funding from MAF SFF (07/030), Beef + Lamb NZ (14AR02 and 07WS/04), AGMARDT (Grant No: 923) and MSD (83647). Preparation of this manuscript was supported by a grant from Beef + Lamb New Zealand. Funders played no role in the design, analysis or interpretation of the results.
References
- Barnes E.H., Dobson R.J., Barger I.A. Worm control and anthelmintic resistance: adventures with a model. Parasitol. Today. 1995;11:56–63. doi: 10.1016/0169-4758(95)80117-0. [DOI] [PubMed] [Google Scholar]
- Bartram D.J., Leathwick D.M., Taylor M.A., Geurden T., Maeder S.J. The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet. Parasitol. 2012;186:151–158. doi: 10.1016/j.vetpar.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Besier B. New anthelmintics for livestock: the time is right. Trends Parasitol. 2007;23:21–24. doi: 10.1016/j.pt.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bisset S.A., Knight J.S., Bouchet C.L.G. A multiplex PCR-based method to identify strongylid parasite larvae recovered from ovine faecal cultures and/or pasture samples. Vet. Parasitol. 2014;200:117–127. doi: 10.1016/j.vetpar.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Coles G.C., Roush R.T. Slowing the spread of anthelmintic resistant nematodes of sheep and goats in the United Kingdom. Vet. Rec. 1992;130:505–510. doi: 10.1136/vr.130.23.505. [DOI] [PubMed] [Google Scholar]
- Donald A.D., Waller P.J., Dobson R.J., Axelsen A. The effect of selection with levamisole on benzimidazole resistance in Ostertagia spp. of sheep. Int. J. Parasitol. 1980;10:381–389. doi: 10.1016/0020-7519(80)90039-9. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Hosking B.C., Skuce P.J., von Samson-Himmelstjerna G., Maeder S., Holdsworth P. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) Guideline: anthelmintic combination products targeting nematode infections of ruminants and horses. Vet. Parasitol. 2012;190:306–316. [Google Scholar]
- Geurden T., Hoste H., Jacquiet P., Traversa D., Sotiraki S., Frangipane di Regalbono A. Anthelmintic resistance and multidrug resistance in sheep gastro-intestinal nematodes in France, Greece and Italy. Vet. Parasitol. 2014;201:59–66. doi: 10.1016/j.vetpar.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Hendrix C.M. second ed. Mosby Inc; St Louis MO, USA: 1998. Common Laboratory Procedures for Diagnosing Parasitism, Diagnostic Veterinary Parasitology; pp. 239–277. [Google Scholar]
- Kaminsky R., Ducray P., Jung M., Clover R., Rufener L., Bouvier J. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kelly J.D., Whitlock H.V., Thompson H.G., Hall C.A., Martin I.C., Le Jambre L.F. Physiological characteristics of free-living and parasitic stages of strains of Haemonchus contortus, susceptible or resistant to benzimidazole anthelmintics. Res. Vet. Sci. 1978;25:376–385. [PubMed] [Google Scholar]
- Kettle P., Vlassoff A., Reid Y., Horton C. A survey of nematode control measures used by milking goat farmers and of anthelmintic resistance on their farms. N. Z. Vet. J. 1983;31:139–143. doi: 10.1080/00480169.1983.34999. [DOI] [PubMed] [Google Scholar]
- Lawrence K.E., Leathwick D.M., Rhodes A.P., Jackson R., Heuer C., Pomroy W.E. Management of gastrointestinal nematode parasites on sheep farms in New Zealand. N. Z. Vet. J. 2007;55:228–234. doi: 10.1080/00480169.2007.36773. [DOI] [PubMed] [Google Scholar]
- Le Jambre L.F., Martin P.J., Johnston A. Efficacy of combination anthelmintics against multiple resistant strains of sheep nematodes. Anim. Prod. Sci. 2010;50:946–952. [Google Scholar]
- Learmount J., Taylor M.A., Bartram D.J. A computer simulation study to evaluate resistance development with a derquantel–abamectin combination on UK sheep farms. Vet. Parasitol. 2012;187:244–253. doi: 10.1016/j.vetpar.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M. Modelling the benefits of a new class of anthelmintic in combination. Vet. Parasitol. 2012;186:93–100. doi: 10.1016/j.vetpar.2011.11.050. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M. Managing anthelmintic resistance – parasite fitness, drug use strategy and the potential for reversion towards susceptibility. Vet. Parasitol. 2013;198:145–153. doi: 10.1016/j.vetpar.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Besier R.B. The management of anthelmintic resistance in grazing ruminants in Australasia – strategies and experiences. Vet. Parasitol. 2014;204:44–54. doi: 10.1016/j.vetpar.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Hosking B.C. Managing anthelmintic resistance: modelling strategic use of a new anthelmintic class to slow the development of resistance to existing classes. N. Z. Vet. J. 2009;57:203–207. doi: 10.1080/00480169.2009.36902. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Miller C.M., Atkinson D.S., Haack N.A., Waghorn T.S., Oliver A.-M. Managing anthelmintic resistance: untreated adult ewes as a source of unselected parasites, and their role in reducing parasite populations. N. Z. Vet. J. 2008;56:184–195. doi: 10.1080/00480169.2008.36832. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Hosking B.C., Bisset S.A., McKay C.H. Managing anthelmintic resistance: is it feasible in New Zealand to delay the emergence of resistance to a new anthelmintic class? N. Z. Vet. J. 2009;57:181–192. doi: 10.1080/00480169.2009.36900. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Waghorn T.S., Miller C.M., Candy P.M., Oliver A.M.B. Managing anthelmintic resistance – use of a combination anthelmintic and leaving some lambs untreated to slow the development of resistance to ivermectin. Vet. Parasitol. 2012;187:285–294. doi: 10.1016/j.vetpar.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Little P., Hodge A., Watson T., Seed J., Maeder S. Field efficacy and safety of an oral formulation of the novel combination anthelmintic, derquantel-abamectin, in sheep in New Zealand. N. Z. Vet. J. 2010;58:121–129. doi: 10.1080/00480169.2010.67513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyndal-Murphy M. Anthelmintic resistance in sheep. In: Corner L.A., Bagust T.J., editors. Australian Standard Diagnostic Techniques for Animal Diseases. Melbourne, Australia; CSIRO: 1993. pp. 1–17. [Google Scholar]
- Lyndal-Murphy M., Rogers D., Ehrlich W.K., James P.J., Pepper P.M. Reduced efficacy of macrocyclic lactone treatments in controlling gastrointestinal nematode infections of weaner dairy calves in subtropical eastern Australia. Vet. Parasitol. 2010;168:146–150. doi: 10.1016/j.vetpar.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Maclean J.M., Lewis D., Holmes P.H. The pathogenesis of benzimidazole-resistant and benzimidazole-susceptible strains of Trichostrongylus colubriformis in the Mongolian gerbil (Meriones unguiculatus) J. Helminthol. 1987;61:179–189. doi: 10.1017/s0022149x00009962. [DOI] [PubMed] [Google Scholar]
- Maingi N., Scott M.E., Prichard R.K. Effect of selection pressure for thiabendazole resistance on fitness of Haemonchus contortus in sheep. Parasitology. 1990;100:327–335. doi: 10.1017/s0031182000061345. [DOI] [PubMed] [Google Scholar]
- Martin P.J., McKenzie J.A., Stone R.A. The inheritance of thiabendazole resistance in Trichostrongylus colubriformis. Int. J. Parasitol. 1988;18:703–709. doi: 10.1016/0020-7519(88)90109-9. [DOI] [PubMed] [Google Scholar]
- McKenna P.B. Criteria for diagnosing anthelmintic resistance by the faecal egg count reduction test. N. Z. Vet. J. 1994;42:153–154. doi: 10.1080/00480169.1994.35808. [DOI] [PubMed] [Google Scholar]
- McKenna P.B. Potential limitations of the undifferentiated faecal egg count reduction test for the detection of anthelmintic resistance in sheep. N. Z. Vet. J. 1996;44:73–75. doi: 10.1080/00480169.1996.35938. [DOI] [PubMed] [Google Scholar]
- McKenna P.B. Update on the prevalence of anthelmintic resistance in gastrointestinal nematodes of sheep in New Zealand. N. Z. Vet. J. 2010;58:172–173. doi: 10.1080/00480169.2010.67520. [DOI] [PubMed] [Google Scholar]
- McMahon C., Barley J.P., Edgar H.W.J., Ellison S.E., Hanna R.E.B., Malone F.E. Anthelmintic resistance in Northern Ireland (II): variations in nematode control practices between lowland and upland sheep flocks. Vet. Parasitol. 2013;192:173–182. doi: 10.1016/j.vetpar.2012.10.022. [DOI] [PubMed] [Google Scholar]
- McMahon C., Bartley D.J., Edgar H.W.J., Ellison S.E., Barley J.P., Malone F.E. Anthelmintic resistance in Northern Ireland (I): prevalence of resistance in ovine gastrointestinal nematodes, as determined through faecal egg count reduction testing. Vet. Parasitol. 2013;195:122–130. doi: 10.1016/j.vetpar.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Miller C.M., Waghorn T.S., Leathwick D.M., Candy P.M., Oliver A.-M.B., Watson T.G. The production cost of anthelmintic resistance in lambs. Vet. Parasitol. 2012;186:376–381. doi: 10.1016/j.vetpar.2011.11.063. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Reinemeyer C.R., Donecker J.M., Leathwick D.M., Marchiondo A.A., Kaplan R.M. Anthelmintic resistance in equine parasites – current evidence and knowledge gaps. Vet. Parasitol. 2014;204:55–63. doi: 10.1016/j.vetpar.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Presidente P.J.A. Methods for detection of resistance to anthelmintics. In: Anderson N., Waller P.J., editors. Resistance in Nematodes to Anthelmintic Drugs. CSIRO Australia and Australian Wool Corporation Technical Publication; Glebe, Australia: 1985. pp. 13–27. [Google Scholar]
- Prichard R.K. Anthelmintic resistance in nematodes: extent, recent understanding and future directions for control and research. Int. J. Parasitol. 1990;20:515–523. doi: 10.1016/0020-7519(90)90199-w. [DOI] [PubMed] [Google Scholar]
- Prichard R.K., Hall C.A., Kelly J.D., Martin I.C., Donald A.D. The problem of anthelmintic resistance in nematodes. Aust. Vet. J. 1980;56:239–251. doi: 10.1111/j.1751-0813.1980.tb15983.x. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Raja J.S. Linear regression model with random coefficients. Biom. J. 1984;26:817–820. [Google Scholar]
- Rhodes A.P., Leathwick D.M., Pomroy W.E. 2011. Best Practice Parasite Management: an approach to working with farmers to best manage multiple anthelmintic resistance. Proceedings of the 40th Annual Conference, The Society of Sheep and Beef Cattle Veterinarians of the NewZealand Veterinary Association, p. 2.16.11. [Google Scholar]
- Sargison N.D., Wilson D.J., Bartley D.J., Penny C.D., Jackson F. Haemonchosis and teladorsagiosis in a Scottish sheep flock putatively associated with the overwintering of hypobiotic fourth stage larvae. Vet. Parasitol. 2007;147:326–331. doi: 10.1016/j.vetpar.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Scott E., Armour J. Effect of development of resistance to benzimidazoles, salicylanilides and ivermectin on the pathogenicity and survival of Haemonchus contortus. Vet. Rec. 1991;128:346–349. doi: 10.1136/vr.128.15.346. [DOI] [PubMed] [Google Scholar]
- Smith G. A mathematical model for the evolution of anthelmintic resistance in a direct life cycle nematode parasite. Int. J. Parasitol. 1990;20:913–921. doi: 10.1016/0020-7519(90)90030-q. [DOI] [PubMed] [Google Scholar]
- Stromberg B.E., Gasbarre L.C., Waite A., Bechtol D.T., Brown M.S., Robinson N.A. Cooperia punctata: effect on cattle productivity? Vet. Parasitol. 2012;183:284–291. doi: 10.1016/j.vetpar.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Leathwick D.M. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 2011;27:176–181. doi: 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Bailey J., Shaw R.J. The production costs of anthelmintic resistance in sheep managed within a monthly preventive drench program. Vet. Parasitol. 2010;171:300–304. doi: 10.1016/j.vetpar.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Torres-Acosta J.F.J., Mendoza-de-Gives P., Aguilar-Caballero A.J., Cuéllar-Ordaz J.A. Anthelmintic resistance in sheep farms: update of the situation in the American continent. Vet. Parasitol. 2012;189:89–96. doi: 10.1016/j.vetpar.2012.03.037. [DOI] [PubMed] [Google Scholar]
- van Wyk J.A., Malan F.S., Randles J.L. How long before resistance makes it impossible to control some field strains of Haemonchus contortus in South Africa with any of the modern anthelmintics? Vet. Parasitol. 1997;70:111–122. doi: 10.1016/s0304-4017(96)01147-8. [DOI] [PubMed] [Google Scholar]
- Vlassoff A., Brunsdon R.V. Control of gastro-intestinal nematodes: advantages of a preventive anthelmintic drenching programme for lambs on pasture. N. Z. J. Exp. Agric. 1981;9:221–225. [Google Scholar]
- Waghorn T.S., Leathwick D.M., Rhodes A.P., Lawrence K.E., Jackson R., Pomroy W.E. Prevalence of anthelmintic resistance on sheep farms in New Zealand. N. Z. Vet. J. 2006;54:271–277. doi: 10.1080/00480169.2006.36710. [DOI] [PubMed] [Google Scholar]
- Waghorn T.S., Miller C.M., Oliver A.M.B., Leathwick D.M. Drench-and-shift is a high-risk practice in the absence of refugia. N. Z. Vet. J. 2009;57:359–363. doi: 10.1080/00480169.2009.64723. [DOI] [PubMed] [Google Scholar]
- Waghorn T.S., Knight J., Leathwick D. The distribution and anthelmintic resistance status of Trichostrongylus colubriformis, T. vitrinus and T. axei in lambs in New Zealand. N. Z. Vet. J. 2014;62:152–159. doi: 10.1080/00480169.2013.871193. [DOI] [PubMed] [Google Scholar]
- Waller P.J., Donald A.D., Dobson R.J., Lacey E., Hennessy D.R., Allerton G.R. Changes in anthelmintic resistance status of Haemonchus contortus and Trichostrongylus colubriformis exposed to different anthelmintic selection pressures in grazing sheep. Int. J. Parasitol. 1989;19:99–110. doi: 10.1016/0020-7519(89)90027-1. [DOI] [PubMed] [Google Scholar]


