Abstract
Butterflies of the genus Heliconius are well known for their peculiar habits of utilizing pollen as a source of amino acids. Saliva plays a major role in the process of extracting amino acids and proteins from the pollen grains. In this investigation, we obtained samples of saliva from adult Heliconius melpomene by placing pumpkin pollen or fine glass-beads on the proboscis, which stimulates the butterflies to release saliva. Proteolytic activity was determined in the saliva by an insoluble protein-dye that turns blue when cleaved by proteases. Its extinction value was measured with a spectrophotometer at 595 nm. Both the saliva sampled with pollen and the saliva obtained from inert glass-beads exhibit proteolytic activity demonstrating that the saliva contains proteases. The proteolytic activity of the pollen/saliva samples was higher than that of the glass-bead/saliva samples, which we attribute to the stimulating effects of pollen, such as taste, smell, and texture, and not to proteases which might have been liberated from the pollen. This is indicated by the fact that pollen samples without saliva showed only a negligible indication for proteolytic activity. In general, females exhibit higher proteolytic activities than males, presumably due to their greater amino acid investment in reproduction. We present here first evidence for the existence of proteases in the saliva of a butterfly species and suggest that these enzymes are crucial for the use of amino acids and proteins from pollen in Heliconius butterflies.
Keywords: Pollen-feeding, Pollen, Enzyme, Proteolytic activity, Pre-oral digestion
1. Introduction
Heliconius butterflies supplement their nectar diet in a special manner by feeding on pollen. These neotropical butterflies share this remarkable behaviour only with the closely related Laparus doris (L.), which formerly was assigned to the genus Heliconius (Gilbert, 1972, 1991;Brown, 1981).
Gilbert (1972) was the first to describe the pollen collecting activity of Heliconius butterflies in which the butterfly removes pollen grains from the anthers of a blossom with typical jerky movements of the extended proboscis. The pollen grains stick to the proboscis and gradually form a wet lump. When sufficient pollen has accumulated the butterfly discharges a clear liquid from the tip of the proboscis. At first, it was assumed that this liquid was regurgitated nectar, however, experiments with red dyed sugar solutions (C. Boggs, personal communication) indicate that it is most likely saliva. Discharge of saliva was observed in other nymphalids which use it to liquefy solid or viscous substances such as dried nectar (Kirbach, 1884; Wigglesworth, 1972; Knopp and Krenn, 2003). The wet pollen lump is agitated for several hours by uncoiling and recoiling of the proboscis, which enable the release of amino acids and proteins from the pollen grains (Gilbert, 1972). Prior to this study it was assumed that nitrogen resources used for reproduction and maintenance in Lepidoptera were obtained exclusively during the larval stages. Since those investigations it is a fact that at least in the genus Heliconius the imagines use pollen as an additional source of nitrogen. However, using stable isotopes O’Brien et al. (2003) found that only essential amino acids gained from pollen were transferred to eggs, so that these amino acids are the limited resource rather than nitrogen. Thus, the utilization of pollen is crucial for Heliconius butterflies and has far-reaching implications for their biology. For example, their larval stage is short compared to that of other butterflies. Since only Heliconius butterflies are able to obtain amino acids from pollen grains as adults the short larval stage is advantageous since caterpillars of tropical butterflies are particularly endangered by predation and parasitism (Gilbert, 1972, 1991). The imagines of Heliconius are known to have one of the longest active life-spans (6 months and more) of butterflies which is another advantage of pollen feeding (Gilbert, 1972). Females collect more pollen than males under natural conditions (Gilbert, 1972; Boggs et al., 1981) resulting in constant egg-production for the duration of their life (Gilbert, 1972). The ability of adult Heliconius to produce cyanoglucosides from amino acid precursors (Nahrstedt and Davis, 1983; Gilbert, 1991) is another important consequence of pollen-use, which helps to protect them from predators and might be a precondition for the evolution of Müllerian mimicry in Heliconius (Gilbert, 1991).
Adaptations to pollen feeding were found in the mouthpart morphology (Krenn and Penz, 1998) and in the time pattern of the feeding behaviour (Penz and Krenn, 2000). In Heliconius, discharging saliva through the food canal of the proboscis is a necessary precondition for this kind of extra-oral pollen digestion (Eberhard and Krenn, 2005). However, the anatomy and histology of the salivary glands of Heliconius do not differ from those in other nymphalids (Eberhard and Krenn, 2003), which were found to use saliva to liquefy dried sucrose solutions (Knopp and Krenn, 2003).
Insect saliva is generally admixed to food (Ribeiro, 1995) and serves both to lubricate the mouthparts and to moisten dry food. Moreover, it contains enzymes which commence the digestion process (Chapman, 1998). In general, the presence of particular enzymes in saliva is dependent on diet; the two most commonly found enzymes in insect saliva are amylase and invertase (Wigglesworth, 1972; Chapman, 1998). To digest sucrose either one of the enzymes α-glucosidase or β-fructosidase is necessary (Terra, 1990). In adult Lepidoptera for example, β-fructosidase is found in the salivary glands of the adult moth Heliothis zea, while β-fructosidase and α-glucosidase occur in the ventriculus (Burton, 1975). A study on the nectarvorous hawk moth Erinnyis ello (L.) showed the presence of amylase in the salivary glands and the presence of trypsin, aminopeptidase and other enzymes in the midgut (Terra, 1990).
To explain the underlying mechanism of the amino acid release from pollen the first hypothesis assumed that the collected pollen grains were induced to undergo pre-germination (Gilbert, 1972) and the release of free amino acids is a part of that process. Free amino acids are also released into a plain sucrose solution by means of a passive diffusion process hence germination of pollen is not absolutely necessary for amino acid release from pollen (Erhardt and Baker, 1990). O’Brien et al. (2003) showed that the pollen processing behaviour increase amino acid release from pollen beyond the effect of soaking pollen in distilled water alone. This might be a hint that a compound in the saliva of Heliconius enhance the amino acid release. Using stable isotopes the amino acids were shown to be transferred to the developing eggs (O’Brien et al., 2003).
Gilbert (1972) found that the enzymes from the pollen lumps obtained from Heliconius proboscises differed from the enzymes in the crop and gut contents. He concluded that the enzymes found in the moist pollen lumps were derived from the pollen grains. The possibility that the detected amino-peptidases could be derived from the saliva was not taken into consideration. Evidence for the existence of proteases in the saliva of adult butterflies has been lacking so far. Therefore, we focus on the question whether saliva of adult Heliconius contains enzymes such as proteases which could be responsible for release of amino acids from the pollen grains. To answer this, we compared the proteolytic activity of saliva samples obtained with both pollen grains as natural food and glass-beads to act as an inert carrier for saliva.
2. Material and methods
2.1. Breeding of the investigated species
Pupae of Heliconius melpomene were purchased from London Pupae Supplies—The Granary Manor Farm (Oxford, UK). The butterflies were kept in a green house of the Department of Ecophysiology and Functional Anatomy of Plants of the University of Vienna at temperatures between 25 and 30 °C and with a relative humidity between 50% and 90%. The caterpillars were provided with Passiflora caerulea L. (Passifloraceae) as a food source. The butterflies were individually marked on their hind wings no later than 24 h after hatching from the chrysalis. The imagines were fed with Butterfly Nectar (The Birding Company, Yarmouth, ME), which contains dextrose, sucrose, fructose, sodium salts, orchid extract, and trace minerals, as well as pumpkin pollen (Cucurbita pepo L. conar. citrullina var. styriaca, Cucurbitaceae). Since pumpkin pollen is comparatively large (cross-sections about 150 μm), the pollen grains cannot pass into the gastrointestinal tract, because the inflow-slits between the galeal linking structures on the tip-region of the proboscis leading into the food canal are only about 10 μm broad (H.W. Krenn, unpublished data).
2.2. Weighing of individuals
To calculate a rate for the body size of Heliconius, the individuals were weighed with a Mettler PM400 laboratory balance. All individuals were weighted twice. First, after the individuals were killed by deep freezing (fresh weight) and second, after several days of drying (dry weight) in a drier at 40 °C. The number of individual specimens totalled 16; 6 females and 10 males. After the investigations the sex of the individuals was determined by preparation.
2.3. Saliva samples
Saliva samples were obtained from a total number of 23 animals (7 females, 10 males, and 6 undetermined) and used to test for differences in proteolytic activity between the males and females. The samples taken from the specimens, which were not sex-determined, were used only to standardize the tests. For the preparation of the saliva samples of Heliconius it was not possible to measure the volume of the droplets of saliva released by the butterflies. As soon as a droplet became visible, it was rinsed off the proboscis into a small vessel. It is quite probable that greater amounts of saliva would contain more proteases than smaller ones. Thus, it was important to take multiple samples from each individual in all categories, since the mean value would better represent the true average amount of proteases in the saliva samples of the investigated individuals.
2.4. Extraction of saliva samples
The saliva samples were gained by providing the butterflies with pumpkin pollen, as an equivalent to their natural pollen sources, and with small glass-beads (Sigma® with diameters of 106 μm and finer). Previously, Gilbert (1972) showed in choice experiments with pollen and pollen-sized glass-beads that Heliconius attempt to manipulate glass-beads in the same way as pollen. The glass-beads thus served as an inert carrier medium for the butterflies’ saliva. They were placed on the proboscis of H. melpomene using a needle moistened with distilled water. The butterflies responded immediately with emission of a droplet of saliva from the tip of the proboscis. The mixture of glass-beads and saliva was rinsed off the proboscis into a small vessel with a syringe containing 0.1 mL distilled water. The samples were frozen at −40 °C. Samples collected by means of fresh or frozen pumpkin pollen were conducted in the same manner. Pollen collected directly from flowers of C. pepo and stored in a refrigerator at +4 °C for 1–2 days before use is referred to here as fresh pollen; pollen stored in a freezer at −40 °C for up to several weeks and defrosted prior to use is termed frozen pollen. Frozen pollen was used when fresh pollen was not available. To standardize the saliva samples, several samples from each category (glass-beads, fresh and frozen pollen) were taken from each individual on different days. The period of sampling commenced one or two days after emergence of the imago from the chrysalis. Generally, individuals were sampled daily in alternating fashion using either pollen or glass-beads. The sampling period was completed after 12 samples per category were obtained or when the individual had deceased.
2.5. Different categories of saliva samples
We tested different categories of saliva samples (termed here pollen/saliva and glass-bead/saliva) for signs of proteolytic activity and for potential differences in the mean extinction rates between the categories and sexes. Each individual was sampled more than once for each method of preparation (fresh pollen, frozen pollen, glass-beads) to reduce variability caused by the sampling technique since the amount of saliva per sample could not be standardised. The values for each category were thus averaged for each individual. The number of samples obtained per animal and category ranged from 2 to 12 due to the different life spans of individuals. Variation in life span was also the reason why it was not possible to obtain saliva samples for each category from each individual. Since there were no relevant differences between fresh or frozen pollen which has been tested in preexaminations, these sample categories were combined.
2.6. Testing for proteolytic activity in the saliva samples
To detect proteolytic activity in the saliva samples, we modified the method described in Naiem et al. (1999). If proteases are present in saliva, the protein-dye-complex of Hide Powder Azure (HPA) from Calbiochem® would be cleaved, and the dye turns the saliva sample to a colour of blue, depending on the amount of proteases in the saliva. The intensity of the colour was measured in a spectrophotometer at a wavelength of 595 nm to obtain the extinction value. Thus, the extinction indirectly expresses the amount of proteases in the saliva.
2.7. Choice of the buffer
For the photometric analyses, the best results were achieved with a phosphate-borate-buffer with the following compounds: 16 g NaOH, 5.25 mL H3PO4 (85%), 10.5 g citric acid and 3.54 g boric acid dissolved in 1 L distilled water. Several pH levels were tested to determine the pH optimum for the reaction. The optimal buffer was determined to be a 1:30 diluted phosphate-borate solution with a pH-value of 9.5.
2.8. Photometric analysis
The steps for analyses of the saliva samples were as follows: the frozen saliva samples were defrosted and centrifuged at 0 °C (Hettich Rotanta/RP) for 10 min at 2600g in order to precipitate the pollen grains or glass beads on the bottom of the vessels. The following steps were carried out in ice water in order to ensure continuous cooling: 80 μL was pipetted from each sample into a small vessel with 12.5 mg HPA as substrate and combined with 420 μL phosphate-borate-buffer. In each procedure, one blank test of the phosphate-borate-buffer (“buffer 0”), and two substrate blank tests (“HPA 0”) were prepared. The buffer blank contained 420 μL phosphate-borate-buffer and 80 μL distilled water, the substrate blank additionally contained 12.5 mg HPA. All samples were incubated at a temperature of 32 °C for 90 min in a water bath while being mechanically agitated (120 times/min). Immediately after incubation the reaction was stopped by addition of 500 μL ice cold phosphate-borate-buffer. After filtration and transfer into a cuvette the extinction value was measured with a spectrophotometer (PM6 of Zeiss) at a wave length of 595 nm, which is the optimum for HPA. The “buffer 0” sample was used as the blank sample for calibration. From the measured extinction value both the corresponding “HPA 0-value” (mean from 2 measurements) and the corresponding “sample 0-values” were subtracted. “Sample 0-values” were either the “pollen/saliva 0-value” or the “glass-bead/saliva 0-value”. The “pollen/saliva 0-sample” contained 80 μL of the pollen/saliva sample in 420 μL buffer solution instead of distilled water and no HPA, and correspondingly the “glass-bead/saliva 0-sample” contained 80 μL of the glass-bead/saliva sample. Both samples were treated as the others described above. The “pollen/saliva 0-value” was represented by the mean of 12 measurements and amounted to an extinction of 0.0102±0.0098. The “glass-bead/saliva 0-value” amounted to 0.0030±0.0064; in contrast to the pollen/saliva samples they were transparent thus only 5 measurements were required. These “0-values” were subtracted from the corresponding measured extinction values.
2.9. Analysis of data
The data were analysed with SPSS 11.0 for Windows. The non-parametric Mann–Whitney-U-test was performed to detect statistically significant differences and, if so, then a Bonferroni-correction was performed. The significance level was set at p<0.05.
3. Results
As shown in this study, the saliva of adult Heliconius butterflies contains proteases. Proteolytic activity was found in female and male saliva samples that were obtained either with fresh and frozen pollen or with glass-beads. The extinction values of the fresh pollen/saliva samples (0.0969±0.0236, n = 8) and the frozen pollen/saliva samples (0.1111±0.0271, n = 16) showed no significant difference (p = 0.192). Therefore, they were combined together in the following analysis.
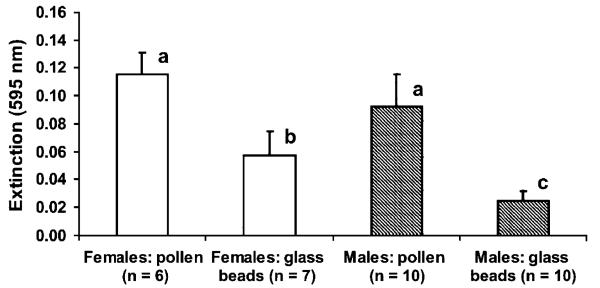
The saliva samples obtained from females using glass-beads exhibited significantly (p = 0.004) less proteolytic activity than samples collected using pollen (glass-bead/saliva samples: 0.0573±0.0175, n = 7; pollen/saliva samples: 0.1155±0.0158, n = 6) (Table 1 and Fig. 1). Likewise, male glass-bead/saliva samples were significantly (p ≤ 0.0001) lower than the pollen/saliva samples (glass-beads: 0.0252±0.0067, n = 10; pollen: 0.0928±0.0228, n = 10) (Table 1 and Fig. 1).
Table 1.
Proteolytic activity of saliva samples
| Sex | Category | mv±sd | max | min | n | ns |
|---|---|---|---|---|---|---|
| Female | Pollen | 0.1155±0.0158 | 0.1426 | 0.0953 | 6 | 55 |
| Female | Glass-beads | 0.0573±0.0175 | 0.0757 | 0.0260 | 7 | 71 |
| Male | Pollen | 0.0928±0.0228 | 0.1374 | 0.0584 | 10 | 83 |
| Male | Glass-beads | 0.0252±0.0067 | 0.0361 | 0.0161 | 10 | 108 |
Mean extinction values (mv), standard deviations (sd), maxima (max), minima (min), number of sampled individuals (n), and total number of saliva samples (ns) from two categories for female and male Heliconius melpomene. The total number of saliva samples represents the pooled samples resulting in one data-point per individual for the analysis. The category “pollen” means that the saliva samples were collected with the aid of either fresh or frozen pumpkin pollen (for analysis both were combined); “glass-beads” refer to saliva samples collected by means of glass-beads.
Fig. 1.
Average extinction values in two categories of samples indicate the presence of proteases in the saliva of H. melpomene. “Pollen” means that the saliva samples were collected either with fresh or frozen pollen (for the analysis both were combined), whereas “glass-beads” refer to saliva samples collected by means of fine glass-beads which serve as an inert carrier for butterfly saliva. Different letters indicate a significant difference (Mann–Whitney-U-test plus Bonferroni-correction; the significant level was set at p<0.05).
Glass-bead/saliva samples of females showed a significant (p = 0.008) higher proteolytic activity than that of males, however female and male pollen/saliva samples were not significantly different (p = 0.168) (Table 1 and Fig. 1).
Higher proteolytic activities in female vs. male saliva cannot be explained by differences in body weight. The average fresh weight of females, used for saliva analyses, was 0.1390±0.0229 g (n = 6) (dry weight: 0.0338±0.0089 g, n = 6). The corresponding male average fresh and dry weights were 0.1188±0.0079 g (n = 10) and 0.0326±0.0053 g (n = 10). Neither the fresh weight (p = 0.073) nor the dry weight (p = 0.958) comparison between males and females showed any significant difference.
To discount the possibility that after incubation of pollen the resulting extinction value was caused by proteases derived from the pollen grains themselves, we tested frozen pumpkin pollen which had never come in contact with Heliconius-saliva in the same way described above. This pollen had only minimal extinction values (<0.0098) therefore proteases from the pollen grains cannot have played a considerable role in our analyses.
4. Discussion
The results yield the first evidence of proteases in the saliva of an adult butterfly. We conclude that the proteases in the saliva of H. melpomene are a substantial aid in digesting proteins derived from pollen grains. This kind of pollen feeding, as it occurs in the genus Heliconius, is unique in the order of Lepidoptera and seems to be a key innovation in the evolution of these neotropical butterflies.
Proteolytic activity was detected in all saliva samples, regardless whether they were obtained with the aid of pollen or glass beads. It remains to be explained why the amount of proteases is higher in the pollen/saliva samples than in the glass-bead/saliva samples. Even if the used frozen pollen was non-viable it has no influence on the results basically. One explanation for the differences in proteolytic activity between the pollen/saliva samples and glass-bead/saliva samples may be that the pumpkin pollen itself releases proteases. Since proteolytic activity of native pollen without butterfly saliva was very low, it cannot have played a considerable role in this study. However, we cannot exclude the possibility that substances in the saliva might stimulate pollen to release proteases. If so, this would also be an innovative adaptation to pollen feeding. Another explanation concerns itself with a possibly methodogical problem. The amount of pollen on the proboscises appeared similar for all individuals and sexes although it was not possible to measure the amount exactly. The same is true for the amount of glass-beads. Nonetheless, it is our impression that the amount of glass-beads applied to the proboscises was slightly less than the amount of applied pollen. Since the volume of the secreted saliva may be dependent on the amount of pollen or glass-beads on the proboscis, it is likely that the pollen/saliva samples would contain more saliva and therefore more proteases which should have been detected as a higher extinction in this category of samples. A more reasonable explanation for the differences between the pollen/saliva samples and the glass-bead/saliva samples is that, in contrast to glass-beads, pollen grains by virtue of their odour, taste or texture should provide a more realistic stimulus in Heliconius, one in which greater amounts of saliva and therefore more proteases are released.
The various species of Heliconius visit a wide range of plants in their natural habitats, however, some species such as H. melpomene (L.) seem to prefer the pollen of Psiguria and Gurania, both of which belong to the Cucurbitaceae (Gilbert, 1975; Boggs et al., 1981; Estrada and Jiggins, 2002). The most probable reason for this preference lies in the relatively large size of the pollen-grains of these plants (Boggs et al., 1981). Therefore, we selected pumpkin pollen to serve as natural stimulus in this study.
O’Brien et al. (2003) found that amino acids released by corn pollen soaked in distilled water were predominantly proline. Based on their results they suggested that the investigated species, Heliconius charitonia, was able to enhance the release of amino acids from pollen beyond that caused by soaking alone. Fresh pollen grains from pumpkin (C. pepo L. var. styriaca) placed in water rapidly release the bulk of their contents through their apertures, in fact the pollen grains actually burst open (Eberhard, unpublished data). In summary, it seems that neither a sugar solution nor germination of pollen (Erhardt and Baker, 1990) is absolutely necessary for amino acid release from pollen grains as it was assumed by Gilbert (1972), although germination in itself may enhance or accelerate the release. In conclusion, our results indicate that proteases in the saliva of Heliconius are responsible for enhancing the amino acid release from pollen.
A greater amount of proteases was found in female saliva than in male saliva; the difference is significant however only for saliva collected by means of glass-beads and not for samples collected by means of pollen. The difference cannot be attributed to different body weight, since both sexes exhibited very similar body weights. One reason for the different proteolytic activity might be related to the fact that females collect more pollen in their natural habitats than males (Gilbert, 1972; Boggs et al., 1981). In female Heliconius, pollen feeding greatly increases and extends egg production (Dunlap-Pianka et al., 1977) and amino acids and proteins derived from pollen are transferred to eggs (O’Brien et al., 2003). Moreover, it is clear that female investment in egg production is greater than male investment in spermatophore production (Gilbert, 1972; Boggs et al., 1981). Thus, even if we assume that no proteases are released from the pollen grains themselves during pollen manipulation by Heliconius, we must conclude that either the amount or the concentration of the released saliva differ between the sexes. The former possibility is the more likely since Heliconius secrete fluid until the collected pollen is fully suspended. Thus in females, the larger pollen masses require greater volumes to suspend. In other insects such as honeybees large differences exist between female and male salivary enzyme production and gut enzyme production, such that old workers produce amylase in their hypopharyngeal glands while drones do not (Hrassnigg and Crailsheim, 2005; Hrassnigg et al., 2005). Furthermore, pollen consumption differs tremendously between workers and drones (Szolderits and Crailsheim, 1993).
The discovery of proteases in the saliva of Heliconius is a first step toward better understanding the mechanism of nutrient release during pollen processing behaviour and poses numerous questions for future research. It would be essential to determine if closely related Heliconiines, e.g. Neruda and Eueides, also possess proteases in their saliva. If not, it would be self-evident that salivary proteases are a novel trait associated with the evolution of pollen feeding. Also, it would be interesting to make an analysis of the full composition of Heliconius saliva. Perhaps one of the most important issues in context with pollen feeding is to figure out what happens with the pollen grains on the proboscis of Heliconius. This could give evidence as to how pollen contents are released. Heliconius may also utilize other pollen ingredients, like starch. If so we would expect that the enzymatic composition of Heliconius saliva should be accordingly adapted. Many other questions can be and must be posed else to reveal the evolution of this extraordinary pollen feeding behaviour in butterflies.
Acknowledgements
We are grateful to the Department of Ecophysiology and Functional Anatomy of Plants (University of Vienna) for kindly granting us permission to use their greenhouse, J. Plant for linguistic help, and L. Gilbert and a second anonymous reviewer for helpful comments and advices. The study was funded by The Austrian Science Fund (FWF-Projekt 18425-B03).
References
- Boggs CL, Smiley JT, Gilbert LE. Patterns of pollen exploitation by Heliconius butterflies. Oecologia (Berlin) 1981;48:284–289. doi: 10.1007/BF00347978. [DOI] [PubMed] [Google Scholar]
- Brown KS. The biology of Heliconius and related genera. Annual Review of Entomology. 1981;26:427–456. [Google Scholar]
- Burton RL. Carbohydrate digestion in the adult moth Heliothis zea. Journal of Insect Physiology. 1975;21:1855–1857. [Google Scholar]
- Chapman RF. The Insects: Structure and Function. fourth ed Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Dunlap-Pianka H, Boggs CL, Gilbert LE. Ovarian dynamics in Heliconiine butterflies: programmed senescence versus eternal youth. Science. 1977;197:487–490. doi: 10.1126/science.197.4302.487. [DOI] [PubMed] [Google Scholar]
- Eberhard SH, Krenn HW. Salivary glands and salivary pumps in adult Nymphalidae. Zoomorphology. 2003;122:161–167. [Google Scholar]
- Eberhard SH, Krenn HW. Anatomy of the oral valve in nymphalid butterflies and a functional model for fluid uptake in Lepidoptera. Zoologischer Anzeiger. 2005;243:306–312. [Google Scholar]
- Erhardt A, Baker I. Pollen amino acids—an additional diet for a nectar feeding butterfly? Plant Systematics and Evolution. 1990;169:111–121. [Google Scholar]
- Estrada C, Jiggins CD. Patterns of pollen feeding and habitat preference among Heliconius species. Ecological Entomology. 2002;27:448–456. [Google Scholar]
- Gilbert LE. Pollen feeding and reproductive biology of Heliconius butterflies. Proceedings of the National Academy of Science, USA. 1972;69:1403–1407. doi: 10.1073/pnas.69.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LE. Ecological consequences of a coevolved mutualism between butterflies and plants. In: Gilbert LE, Raven PH, editors. Coevolution of Animals and Plants. University of Texas Press; Austin: 1975. pp. 210–240. [Google Scholar]
- Gilbert LE. Biodiversity of a central American Heliconius community: pattern, process and problems. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW, editors. Plant–Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Wiley; New York: 1991. pp. 403–427. [Google Scholar]
- Hrassnigg N, Crailsheim K. Differences in drone and worker physiology in honeybees (Apis mellifera) Apidologie. 2005;36:255–277. [Google Scholar]
- Hrassnigg N, Brodschneider R, Fleischmann PH, Crailsheim K. Unlike nectar foragers, honeybee drones (Apis mellifera) are not able to utilize starch as fuel for flight. Apidologie. 2005;36:547–557. [Google Scholar]
- Kirbach P. Über die Mundwerkzeuge der Schmetterlinge. Archiv fuer Naturgeschichte. 1884;50:78–119. [Google Scholar]
- Knopp MCN, Krenn HW. Efficiency of fruit juice feeding in Morpho peleides (Nymphalidae, Lepidoptera) Journal of Insect Behavior. 2003;16:67–77. [Google Scholar]
- Krenn HW, Penz CM. Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): a search for anatomical adaptations to pollen-feeding behavior. International Journal of Insect Morphology and Embryology. 1998;27:301–309. [Google Scholar]
- Nahrstedt A, Davis R. Occurrence, variation and biosynthesis of the cyanogenic glucosides linamarin and lotaustralin in species of the Heliconiini (Insecta: Lepidoptera) Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology. 1983;75:65–73. [Google Scholar]
- Naiem E-S, Hrassnigg N, Crailsheim K. Nurse bees support the physiological development of young bees (Apis mellifera L.) Journal of Comparative Physiology B. 1999;169:271–279. [Google Scholar]
- O’Brien DM, Boggs CL, Fogel ML. Pollen feeding in the butterfly Heliconius charitonia: isotopic evidence for essential amino acid transfer from pollen to eggs. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2003;270:2631–2636. doi: 10.1098/rspb.2003.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penz CM, Krenn HW. Behavioral adaptations to pollen-feeding in Heliconius butterflies (Nymphalidae, Heliconiinae): an experiment using Lantana flowers. Journal of Insect Behavior. 2000;13:865–880. [Google Scholar]
- Ribeiro JMC. Insect saliva: function, biochemistry and physiology. In: Chapman RF, de Boer G, editors. Regulatory Mechanism in Insect Feeding. Chapman & Hall; New York: 1995. pp. 74–97. [Google Scholar]
- Szolderits MJ, Crailsheim K. A comparison of pollen consumption and digestion in honeybee (Apis mellifera carnica) drones and workers. Journal of Insect Physiology. 1993;39:877–881. [Google Scholar]
- Terra WR. Evolution of digestive systems of insects. Annual Review of Entomology. 1990;35:181–200. [Google Scholar]
- Wigglesworth VB. The Principles of Insect Physiology. seventh ed Wiley; New York: 1972. [Google Scholar]