Abstract
Background
Age-related differences in white matter (WM) tract microstructure have been well-established across the lifespan. In the present cross-sectional study we examined whether these differences are associated with neurocognitive performance from childhood to late adulthood.
Methods
Diffusion tensor imaging was performed in 296 healthy subjects aged 8–68 years (mean=29.6, SD=14.6). The corpus callosum, two projection tracts, and five association tracts were traced using probabilistic tractography. A neurocognitive test battery was used to assess speed of processing, attention, spatial working memory, verbal functioning, visual learning and executive functioning. Linear mediation models were used to examine whether differences in WM tract fractional anisotropy (FA) were associated with neurocognitive performance, independent of the effect of age.
Results
From childhood to early adulthood, higher FA of the cingulum bundle and inferior fronto-occipital fasciculus (IFOF) was associated with higher executive functioning and global cognitive functioning, respectively, independent of the effect of age. When adjusting for speed of processing, FA of the IFOF was no longer associated with performance in the other cognitive domains with the exception of visual learning. From early adulthood to late adulthood, WM tract FA was not associated with cognitive performance independent of the age effect.
Conclusions
The cingulum bundle may play a critical role in protracted maturation of executive functioning. The IFOF may play a key role in maturation of visual learning, and may act as a central ‘hub’ in global cognitive maturation by subserving maturation of processing speed.
Keywords: white matter, diffusion tensor imaging, development, aging, cognition, executive functioning
Introduction
Brain white matter (WM) tracts are critical to coordinated activity between brain regions. Diffusion tensor imaging (DTI) studies have demonstrated tract-specific differences in WM microstructure across the lifespan, for example substantially lower fractional anisotropy (FA), a putative measure of neural fiber coherence, diameter and myelination (1, 2), in childhood than early adulthood and higher FA in early adulthood than late adulthood (3–5). Investigating the neurocognitive correlates of these age-related differences in WM microstructure may provide insights relevant to the onset and course of neurocognitive disorders in adolescence and adulthood.
To date, published DTI studies have not included both children and older adults to examine the relationships between WM tract microstructure and cognitive performance across the lifespan. In a prior study and meta-analysis, we identified robust age-related FA differences across adolescence, particularly in the superior longitudinal fasciculus (SLF), which were associated with verbal working memory and verbal fluency independent of the effect of age (6). Other DTI studies have observed further associations between WM microstructure and cognitive performance from childhood to adulthood, independent of the effect of age, in the SLF (7–10), splenium of the corpus callosum (CC) (7, 11), genu of CC (7, 12), and the inferior fronto-occipital fasciculus (IFOF)/ inferior longitudinal fasciculus (ILF) (8). Similarly, from adulthood to old age, DTI studies have found associations between WM microstructure and cognitive performance, independent of the effect of age, in the SLF (13), splenium of CC (14, 15), genu of CC (15, 16), IFOF (14, 17), ILF (13, 14, 18), and cingulum (13). The relationships between WM tract microstructure and cognition observed across adulthood do not necessarily correspond to the relationships observed across adolescence.
Most prior work included relatively small samples sizes that did not evaluate children and older adults collectively. Thus, these samples were limited in their ability to detect trends that are comparable to the age-related WM differences reported in more general DTI studies across the lifespan (3–5). Another limitation has been the use of voxelwise analyses (e.g., 8, 12, 13), regions of interest (11, 16), or atlas-based masks (4, 10), as opposed to a priori defined and anatomically valid, individually traced WM tracts. In addition, most studies included one or few neurocognitive tests and/ or few WM tracts, limiting their ability to examine the specificity of WM tracts to different neurocognitive domains.
In the current study, we employed probabilistic tractography (19) to identify nine anatomically valid WM tracts in the brain and examined their relationship to age and neurocognitive performance across multiple domains from childhood to late adulthood. Importantly, this allowed us to investigate the neurocognitive correlates of the major WM tracts in relation to their unique aging patterns. We hypothesized that (1) WM tract FA and neurocognitive performance show pronounced differences between childhood and early adulthood, and thereafter moderate differences between early adulthood and late adulthood; (2) differences in WM tract FA are associated with neurocognitive performance from childhood to late adulthood, independent of the effect of age. As a secondary aim, we compared different modeling approaches of age-related WM differences, and examined the effect of using different models on the relationships between age, WM tract FA and cognition. To our knowledge, this is the first DTI study to report on these relationships across multiple cognitive domains from childhood to late adulthood.
Methods
Participants
Two-hundred and ninety-six healthy individuals (52% male) between the ages of 8.1 and 68.1 years (mean 29.6±14.6; median 25.9) were recruited through local advertisements and by word of mouth. Written informed consent was obtained from participants or from a parent or guardian if the participant was a minor; all minors provided assent. Participants had no history of a DSM-IV axis I major mood or psychotic disorder as assessed by structured diagnostic interview (20, 21). Other exclusion criteria included: (1) intellectual or learning disability; (2) medications with known adverse cognitive effects; (3) MRI contraindications; (4) pregnancy; (5) significant medical illness that could affect brain structure. Mean IQ as estimated from the Wide Range Achievement Test 3 (Reading subtest) was 103±11.5 (data missing for 2 subjects) (22). Handedness was determined using the Edinburgh Handedness Inventory (23); median laterality quotient was 0.74 (range −1 to 1). Please see table 1 for demographic characteristics. This study was approved by the Institutional Review Board of the North Shore – Long Island Jewish Health System.
Table 1.
Demographic Characteristics by Age Group.
| 8–18 years (n=83) |
18–28 years (n=83) |
28–38 years (n=39) |
38–48 years (n=51) |
48–58 years (n=27) |
58–68.1 years (n=13) |
Difference1 | |
|---|---|---|---|---|---|---|---|
| SES2 | 2.10 | 2.25 | 2.37 | 2.62 | 2.54 | 2.70 | F=2.08 (5), p = 0.068 |
| Sex, M, %3 | 49.4 | 38.6 | 59.0 | 68.6 | 51.9 | 76.9 | X2=15.88 (5), p=0.007 |
| Race, %4 | |||||||
| Caucasian | 49.4 | 55.4 | 41.0 | 37.3 | 74.1 | 84.6 | X2=30.46 (5), p=0.028 |
| African-American | 27.7 | 24.1 | 28.2 | 47.1 | 18.5 | 7.7 | |
| Hispanic | 8.4 | 14.5 | 17.9 | 5.9 | 3.7 | 7.7 | |
| Asian | 6.0 | 3.6 | 10.3 | 2.0 | 3.7 | 0 | |
| Other | 8.4 | 2.4 | 2.6 | 7.8 | 0 | 0 | |
| Laterality quotient5 | 0.64 | 0.79 | 0.83 | 0.80 | 0.77 | 0.44 | F=2.78 (5), p=0.030 |
Statistical differences in categorical data were assessed using Pearson Chi-Squared Test and Fisher’s Exact Test with Monte Carlo sampling for counts lower than 5, and in continuous data using univariate analysis of variance.
Social Economic Status (SES) was determined using the Hollingshead Two Factor Index of Social Position (24); for minors this was based on the head of household. SES is scored as I, II, III, IV or V, where a lower score indicates higher SES. Pair-wise post-hoc tests (Games Howell test) indicated lower SES in the 8–18 group compared to the 38–48 group (p=0.063); all other pair-wise post-hoc tests p>0.050. Data missing for 1 participant 8–18 years, 4 participants 18–28 years, 1 participant 28–38 years, 4 participants 38–48 years, 1 participant 48–58 years, and 3 participants 58–68.1 years.
Post-hoc pair-wise Chi-Squared Tests indicated that there were significantly fewer males in the 8–18 group than the 38–48 group, and in the 18–28 group than the 28–38, 38–48 and 58–68.1 groups (all pair-wise comparisons p<0.050).
Post-hoc pair-wise Chi-Squared Tests indicated that there were significantly fewer Caucasians in the 8–18 group than the 48–58 and 58–68.1 groups, in the 18–28 group than the 58–68.1 group, in the 28–38 group than the 48–58 and 58–68.1 groups, in the 38–48 group than the 48–58 and 58–68.1 groups, and significantly more Caucasians in the 18–28 group than the 38–48 group (all pair-wise comparisons p<0.050).
Determined using the Edinburgh Handedness Inventory (23). While the analysis of variance indicated significant between group differences, pair-wise post-hoc tests (Games Howell test) did not indicate significant differences between the age groups (all pair-wise comparisons p>0.050).
DTI Acquisition and Preprocessing
All subjects received a DTI exam at the North Shore University Medical Center, Manhasset, NY, on a GE Signa HDx 3.0 T system (General Electric, Milwaukee, WI). The sequence included volumes with diffusion gradients applied along 31 non-parallel directions (b=1000 s/mm2) and 5 volumes without diffusion weighting (TR=14 s, TE=min., matrix=128×128, FOV=240 mm). Each volume consisted of 51 contiguous 2.5-mm axial slices acquired parallel to the anterior-posterior commissural line using a ramp sampled, double spin-echo, single shot echo-planar imaging method.
All scans were reviewed by a radiologist and all images were visually inspected to ensure that no gross abnormalities were evident. Image processing was conducted using the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL; Oxford, United Kingdom; http://fsl.fmrib.ox.ac.uk/fsl/). Eddy-current induced distortions and head-motion displacements were corrected through affine registration of the 31 diffusion volumes to the first b0 volume using FSL’s Linear Registration Tool (FLIRT) (25). The b-vector table (i.e. gradient directions) for each participant was then adjusted according to the rotation parameters of this linear correction. Non-brain tissue was removed using FSL’s Brain Extraction Tool. Fractional anisotropy (FA), radial diffusivity (RD) and axial diffusivity (AD) (1, 2) were then calculated at each voxel of the brain by fitting a diffusion tensor model to the raw diffusion data using weighted least squares in FSL’s Diffusion Toolbox. FA was chosen as the primary measure for analysis, because it has been the most widely used measure in relevant studies and thereby provides optimal between-study comparability.
Tractography
The probable trajectories of two interhemispheric tracts (splenium and genu of CC), two projection tracts (CST and anterior thalamic radiation (ATR)), and five bilateral association tracts (IFOF, ILF, SLF, cingulum, and uncinate fasciculus) were traced as follows. Within-voxel probability density functions of the principal diffusion direction were estimated using Markov Chain Monte Carlo sampling in FSL’s BEDPOSTX tool (19). A spatial probability density function was then estimated across voxels based on these local probability density functions using FSL’s PROBTRACKX tool (19), in which 5000 samples were taken for each input voxel with a 0.2 curvature threshold, 0.5 mm step length, and 2000 steps per sample. The above procedure was conducted in 304 study eligible individuals, but failed in 8 of those individuals, yielding a total sample size of 296. For each tract, seed masks, way-points, termination and exclusion masks were defined on the MNI152 T1 1mm template (see supplementary Methods). Masks were normalized to each subjects’ diffusion space using FLIRT (25), applying the affine parameters obtained by co-registering the first b0 volume to the MNI152 1mm T1 brain. The resulting tracts were thresholded at a normalized probability value (see supplementary Methods and Figure 1), and visually inspected to confirm successful tracing in each individual. Mean FA, RD and AD of each tract were then extracted for analysis.
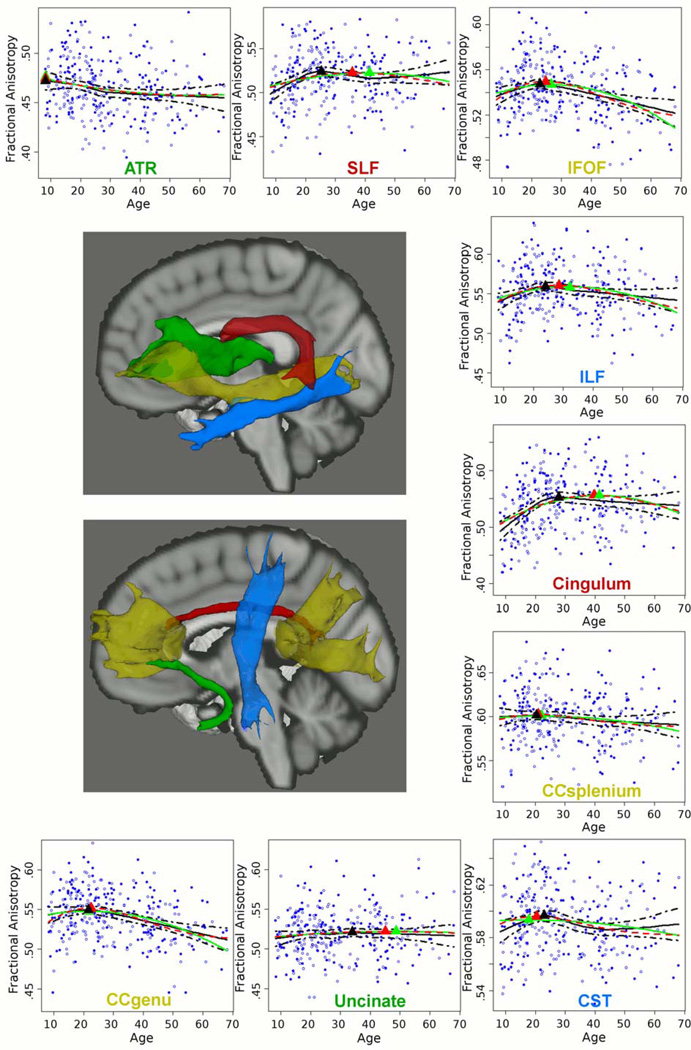
Figure 1. Age-Related Differences in Fractional Anisotropy of 9 White Matter Tracts.
x-axis: age; y-axis: fractional anisotropy. Filled dots = males, open dots = females. Red line = Poisson model; green line = quadratic model; black line = LOESS model. Ages at peak fractional anisotropy are marked with a triangle, color-coded by curve model.
ATR = anterior thalamic radiation; SLF = superior longitudinal fasciculus; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; CC = corpus callosum; CST = corticospinal tract.
Neurocognitive Assessments
A subgroup of participants (n=219–256) was administered the following battery of neurocognitive tests: Brief Assessment of Cognition in Schizophrenia, Trail Making Test, Continuous Performance Test - identical pairs, Wechsler Memory Scale 3rd ed., Controlled Oral Word Association Test, Animal Naming Test, UMd Letter-Number Span Task, Hopkins Verbal Learning Test rev., Brief Visuospatial Memory Test rev., Neuropsychological Assessment Battery, and Wisconsin Card Sorting Test (26, 27). For statistical analysis, we composed the following neurocognitive domains based on conceptual overlap between the tests and domain reliability as assessed with Cronbach’s alpha: speed of processing, attention, spatial working memory, verbal functioning, visual learning, and executive functioning (see supplementary Methods). Raw scores were converted to z-scores such that higher values were indicative of better performance across all tests. Neurocognitive data were only included when administered within 6 months of MRI scanning (thereby excluding twelve subjects).
Statistical Analysis
Outliers in tract FA values and neurocognitive z-scores, defined as exceeding 3 standard deviations (SD) from the mean, were substituted by the next lowest or highest value within 3 SD. This affected 0.8% of FA values and 0.7% of z-scores. Statistical tests were conducted in the Statistical Package for the Social Sciences, version 11.5.1 (IBM, USA; www.spss.com), and ‘R’, version 2.15.1 (http://www.r-project.org/).
Differences in demographic characteristics among age groups (see Table 1) were assessed using Pearson’s Chi-Squared Test and Fisher’s Exact Test with Monte Carlo sampling for counts lower than 5 (for categorical data) and univariate analysis of variance (for continuous data).
Age-FA curves were fitted with Poisson (c + a*Age*exp(−b*Age2)) and quadratic (c + a*Age + b*Age2) models using the ‘nls2’ package in ‘R’. Poisson models allow a different slope and curvature on either side of the peak, and in one study provided a better fit to lifespan WM differences than a quadratic model (3). Model fits were assessed with Akaike’s information criterion (AIC; 28). Poisson and quadratic models were plotted against the background of a nonparametric local regression (LOESS) model (29), to provide a hypothesis-free data-driven fit.
A mediation model was used to examine whether age-related differences in WM tract FA were associated with differences in neurocognitive test performance, (partially) independent of the effect of age (30). Due to nonlinear age-FA relationships, linear mediation models were implemented before and after the age peak of each tract. Age at peak FA derived from the LOESS model was chosen for the primary analysis, as this model provides maximum flexibility in finding local peaks without the influence of age-FA effects at further distance from the peak. Results obtained using the LOESS peaks were compared with results obtained using the peaks derived from the parametric models. Age-related differences in FA were considered to ‘mediate’ differences in test performance when: (1) age correlated significantly with both FA and test performance; (2) FA correlated significantly with test performance after adjusting for age; (3) FA partially explained an observed age effect, that is, the correlation between age and test performance was attenuated when FA was entered into the model. Criterion 1 of the mediation model was tested using linear regression. In case criterion 1 was confirmed, criteria 2 and 3 were tested using hierarchical linear regression with age first entered as the independent variable and test performance as the dependent variable, and then WM tract FA was added as a second independent variable. To limit the number of comparisons, analyses were first performed with a global cognition score averaged from the z-scores of all the individual tests (Cronbach’s alpha = 0.86). Tracts that correlated significantly with global cognition, independent of the effect of age, were included for follow-up mediation analyses of the six neurocognitive domains.
Results
Age-Related Differences in White Matter Tract Microstructure
There were no significant correlations between age and the asymmetry indices of the bilateral tracts (left−right / left+right) or significant age-by-sex interactions for the slope and curvature terms in the Poisson or quadratic models (level of significance set at p<0.0028, to correct for the two slope and curvature terms (‘a’ and ‘b’ terms) in each model and for the nine tracts, i.e. 0.050/(2*9)). Therefore, FA values were combined for males and females and collapsed across hemispheres. No significant interactions of age with socio-economic status (SES) or race were observed, with the exception of an age-by-SES interaction for the genu of CC in the Poisson model only (p<0.0028). Across the different models (Poisson, quadratic and LOESS), the ATR was the first to reach peak FA, followed by the genu and splenium of CC, CST and lastly association tracts (see Figure 1 and Table 2). For most tracts the Poisson model provided a better fit than the quadratic model as indicated by lower AIC values, but this did not reach statistical significance as tested using bootstrap resampling (p>0.050). Ages at peak FA of the association tracts were lowest in the LOESS model and highest in the quadratic model, and within a much smaller range in the LOESS model, approximately 8 years, compared to the Poisson and quadratic models reaching approximately 20 years (Table 2).
Table 2.
Age at Peak Fractional Anisotropy for 9 White Matter Tracts as Assessed with LOESS, Poisson and Quadratic Models.
| LOESS peak age (95% CI) |
Poisson peak age (95% CI) |
Quadratic peak age (95% CI) |
|
|---|---|---|---|
| ATR1 | 8.13 (8.12 – 8.14) | 8.13 (8.12 – 8.14) | 8.13 (8.12 – 8.14) |
| CC Splenium | 20.4 (16.7 – 24.0) | 20.9 (13.8 – 28.1) | 22.2 (11.7 – 32.7) |
| CC Genu | 21.6 (20.0 – 23.2) | 22.5 (21.0 – 24.0) | 21.7 (16.2 – 27.1) |
| CST | 22.8 (20.3 – 25.3) | 20.4 (17.5 – 24.4) | 17.7 (4.2 – 31.1) |
| IFOF | 22.8 (21.8 – 23.9) | 24.7 (23.0 – 26.3) | 26.6 (24.0 – 29.3) |
| ILF | 24.0 (23.0 – 25.1) | 28.5 (25.5 – – 31.6) | 32.3 (28.7 – 36.0) |
| SLF | 25.3 (23.5 – 27.1) | 35.8 (32.4 – 39.1) | 41.3 (38.6 – 44.1) |
| Cingulum | 27.7 (24.4 – 31.0) | 39.5 (36.5 – 42.5) | 41.5 (39.0 – 44.1) |
| Uncinate | 33.8 (21.1 – 46.5) | 45.0 (19.0 – 71.1) | 48.6 (21.9 – 75.3) |
ATR (anterior thalamic radiation) showed a negative correlation with age from childhood to late adulthood.
LOESS = local regression; CC = corpus callosum; CST = corticospinal tract; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; SLF = superior longitudinal fasciculus.
From childhood to age at peak FA, FA correlated positively and significantly with age in the splenium of CC (β=0.212, p=0.037), genu of CC (β=0.250, p=0.009), CST (β=0.263, p=0.004), IFOF (β=0.294, p=0.001), ILF (β=0.316, p<0.001), SLF (β=0.325, p<0.001) and cingulum (β=0.408, p<0.001). FA of the uncinate did not correlate significantly with age (p>0.050). From age at peak FA to late adulthood, FA correlated negatively and significantly with age in the genu of CC (β=−0.368, p<0.001), IFOF (β=−0.334, p<0.001) and ILF (β=−0.164, p=0.035), but not in the splenium of CC, CST, SLF, cingulum or uncinate (p>0.05). FA of the ATR correlated negatively and significantly with age from childhood to late adulthood (β=−0.174, p=0.003).
Age-Related Differences in Cognitive Performance
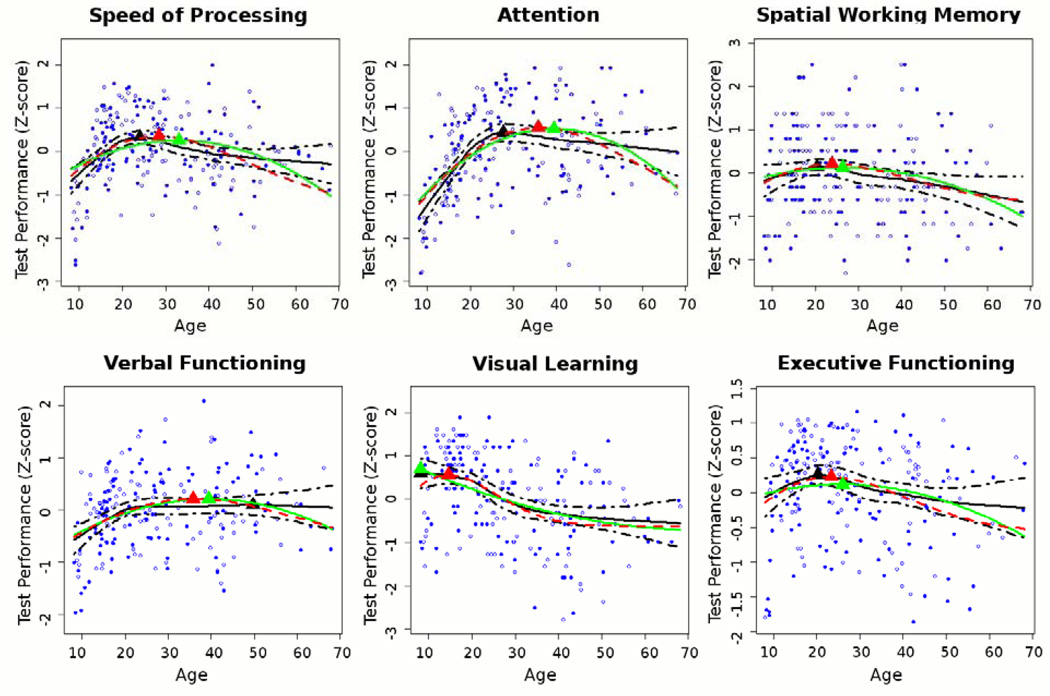
Age-related differences in performance on the six cognitive domains, as fitted with Poisson, quadratic and LOESS models, followed similar patterns as the age-related differences in WM tract FA (see Figure 2). The age peaks for each of the six domains are provided in Supplement: Table S1.
Figure 2. Age-Related Differences in Performance on 6 Cognitive Domains.
x-axis: age; y-axis: z-score of raw test score, such that higher values indicate better performance. Filled dots = males, open dots = females.
Mediation Models of Associations Between Age, White Matter Tract Microstructure and Cognitive Performance
From childhood to age at peak FA, FA of the cingulum and IFOF correlated positively and significantly with global cognitive performance, independent of the effect of age (see Table 3). FA of the splenium and genu of CC, CST, ILF and SLF did not correlate significantly with global cognitive performance, independent of the effect of age (p>0.050). Next, the cingulum and IFOF were each entered into separate mediation models of the six neurocognitive domains. FA of the cingulum correlated positively and significantly with executive functioning, independent of the effect of age (see Table 3), but not with the other cognitive domains independent of the age effect (p>0.050). FA of the IFOF correlated positively and significantly with speed of processing, attention, spatial working memory, verbal functioning, visual learning and executive functioning, independent of the effect of age (see Table 3). Most of these FA findings were driven by RD and not by AD (see Supplement: Table S2). Additionally to the cingulum and IFOF, we conducted mediation analyses of the other WM tracts and the six neurocognitive domains to explore potential mediation effects that were not identified due to the global cognition threshold. From childhood into early adulthood, FA of the ILF correlated positively and significantly with speed of processing and FA of the genu of CC correlated positively and significantly with attention and visual learning, independent of the effect of age (p<0.050; see Supplement: Table S3A).
Table 3.
Significant Mediation Models of Associations Between Age, White Matter Tract Fractional Anisotropy and Cognitive Performance from Childhood into Early Adulthood.1
| β | t-value | p-value | n | |
|---|---|---|---|---|
| Cingulum | ||||
| Global cognitive functioning:2 | 109 | |||
| Age 1a | 0.300 | 3.25 | 0.002 | |
| Age 2b | 0.229 | 2.38 | 0.019 | |
| FAc | 0.215 | 2.25 | 0.027 | |
| Executive functioning: | 115 | |||
| Age 1a | 0.229 | 2.50 | 0.014 | |
| Age 2b | 0.157 | 1.65 | 0.101 | |
| FAc | 0.217 | 2.28 | 0.025 | |
| IFOF | ||||
| Global cognitive functioning:2 | 81 | |||
| Age 1a | 0.500 | 5.13 | <0.001 | |
| Age 2b | 0.420 | 4.36 | <0.001 | |
| FAc | 0.293 | 3.04 | 0.003 | |
| Speed of processing: | 93 | |||
| Age 1a | 0.658 | 8.34 | <0.001 | |
| Age 2b | 0.599 | 7.35 | <0.001 | |
| FAc | 0.186 | 2.29 | 0.024 | |
| Attention: | 88 | |||
| Age 1a | 0.607 | 7.08 | <0.001 | |
| Age 2b | 0.547 | 6.19 | <0.001 | |
| FAc | 0.190 | 2.15 | 0.035 | |
| Spatial working memory: | 93 | |||
| Age 1a | 0.262 | 2.59 | 0.011 | |
| Age 2b | 0.194 | 1.85 | 0.068 | |
| FAc | 0.209 | 1.99 | <0.05 | |
| Verbal functioning: | 92 | |||
| Age 1a | 0.427 | 4.48 | <0.001 | |
| Age 2b | 0.356 | 3.60 | 0.001 | |
| FAc | 0.216 | 2.18 | 0.032 | |
| Visual learning: | 92 | |||
| Agea | 0.221 | 2.15 | 0.034 | |
| Ageb | 0.129 | 1.23 | 0.220 | |
| FAc | 0.287 | 2.73 | 0.008 | |
| Executive functioning: | 87 | |||
| Age 1a | 0.396 | 3.97 | <0.001 | |
| Age 2b | 0.335 | 3.33 | 0.001 | |
| FAc | 0.236 | 2.35 | 0.021 | |
Mediation analyses were performed using hierarchical linear regression (age first entered as independent variable and test performance as dependent variable, and then WM tract FA added as second independent variable) before and after the age peak of each tract, which was derived from a LOESS model.
The global cognitive score comprised the average z-score of all individual tests. Higher scores indicated better performance across all cognitive domains.
Not adjusted for FA;
adjusted for FA;
adjusted for age.
CI = confidence interval; FA = fractional anisotropy; IFOF = inferior fronto-occipital fasciculus.
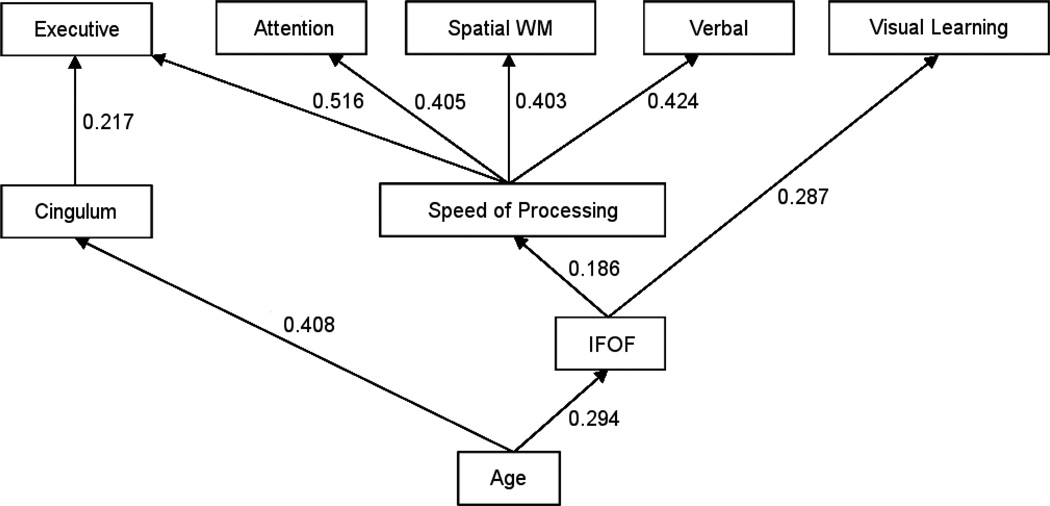
Since speed of processing substantially contributes to performance on most cognitive tests, we explored if speed of processing was driving these findings by repeating the hierarchical linear regressions for the five other cognitive domains while adding speed of processing as an independent variable. FA of the cingulum and IFOF remained significantly correlated with executive functioning and visual learning, respectively, independent of the effect of age (p<0.050), but not with the other cognitive domains (p>0.050) (Figure 3).
Figure 3. Significant Mediation Models of Associations Between Age, White Matter Tract Fractional Anisotropy and Cognitive Performance from Childhood into Early Adulthood.1.
1 Mediation analyses were performed using hierarchical linear regression, before and after the age peak of each tract as derived from a LOESS model. Standardized regression coefficients are provided for each association (p<0.050), where correlations between white matter tract fractional anisotropy and neurocognitive performance are adjusted for age, and correlations between speed of processing and other cognitive domains are adjusted for age as well as white matter tract fractional anisotropy (i.e. IFOF). WM = working memory; IFOF = inferior fronto-occipital fasciculus.
Since IQ (estimated using the WRAT-3 – reading subtest) correlated significantly with four of the six cognitive domains (see Supplement: Table S4), we assessed the effect of IQ on our findings by repeating the hierarchical linear regressions while adding IQ as an independent variable. FA of the cingulum and IFOF remained significantly correlated with executive functioning and visual learning, respectively, independent of the effect of age (p<0.050), but not with the other cognitive domains (p>0.050).
We compared the results obtained using the LOESS age peaks, with results obtained using the (later) Poisson age peaks. When using the Poisson peaks, the mediation model of the cingulum and global cognition was no longer significant, because age no longer correlated significantly with global cognition (p>0.050). FA of the IFOF remained significantly correlated with global cognition and four of the six cognitive domains, independent of the effect of age, while FA of the IFOF no longer correlated significantly with verbal functioning independent of the age effect, and visual learning no longer correlated significantly with age (p>0.050). FA of the SLF correlated positively and significantly with global cognition, speed of processing and verbal functioning, and FA of the ILF correlated positively and significantly with global cognition and speed of processing, independent of the effect of age (p<0.050).
From age at peak FA to late adulthood, the tracts that showed significant negative correlations with age (i.e. genu of CC, IFOF, ILF or ATR) did not correlate significantly with global cognition independent of the age effect (p>0.050; exploratory mediation analyses of these WM tracts and the six cognitive domains were also not significant; please see Supplement: Table S3B).
Discussion
In accordance with our hypothesis, and prior cross-sectional studies (3–5), significant FA differences were observed between childhood and early adulthood in a projection tract, two interhemispheric tracts and most association tracts. The uncinate fasciculus displayed little age-related FA difference, which is corroborated to some degree by a longitudinal study of healthy individuals 5–32 years of age (31). FA of the ATR showed a negative linear correlation with age from childhood to late adulthood, which was unexpected given prior observations of an inverted U-curve across the lifespan (4). The ATR, CC and CST showed the lowest ages at peak FA in our sample, followed by association tracts, and the cingulum showed the highest age at peak FA, which is strikingly consistent with prior studies (3–5). After age at peak FA, WM tract FA values showed small or moderate age-related differences across adulthood, in line with previous findings (3, 4).
Overall, a Poisson model provided a slightly better fit to these curves than a quadratic model, which was expected given the different slopes before and after the age peaks (3). Ages at peak FA varied by statistical model, with the age peaks generally being lowest using LOESS (20s), intermediate using Poisson (early 20s to late 30s) and highest using quadratic (early 20s to early 40s) models, which is in line with previous findings (3–5). In addition, the LOESS age peaks were within a much smaller range than the other two models. This likely reflects the constraints of Poisson and quadratic models, which produce smoother curves incorporating age effects across the complete data set, while LOESS allows ‘sharper’ local peaks. Thus, the Poisson and quadratic models may provide peaks many years after most age-related FA differences are observed. Therefore, age peaks derived from the LOESS model were used for our primary mediation analyses, and compared to results obtained using the Poisson age peaks.
Our hypothesis that age-related differences in WM tract FA are associated with neurocognitive performance from childhood to adulthood was confirmed for two major WM tracts: the cingulum and IFOF. Follow-up analyses indicated that most FA effects were driven by RD and not by AD. This is in accordance with prior observations from childhood into early adulthood (31), and the notion that white matter maturation during this period may be due to protracted myelination (32).
To our knowledge, this is the first study to report that age-related differences in cingulum FA are associated with executive functioning from childhood to early adulthood, independent of the effects of age and speed of processing, which complements recent findings implicating the cingulum in development of cognitive control across adolescence (33). In line with our findings, several lines of evidence have confirmed involvement of the cingulum in executive functions in healthy adult individuals (e.g. 34–37). For example, the anterior cingulate cortex has been associated with prediction of reward and evaluation of cost of future actions, which are both important for guiding decision making (34). Similarly, reduced dorsal anterior cingulate activity in adolescents, compared to adults, has been related to greater risk-taking performance (35). Furthermore, the posterior cingulate cortex, in communication with the dorsal anterior cingulate cortex, has been associated with error processing that is important for error recognition and response adjustment (36). The cingulum in our data included mainly the posterior and dorsal anterior divisions, while in only a few subjects the cingulum tracing continued into the ‘affective‘ rostral divisions (37). Post-hoc analyses indicated that mediation models were not significant when using the cingulum’s Poisson age peak in the late 30s, potentially reflecting that both cingulum FA and executive functioning had reached mature levels before age 30 (see Figures 1 and 2).
Age-related differences in FA of the IFOF between childhood and early adulthood were associated with performance on all cognitive domains, independent of the effect of age. There is sparse data on the IFOF’s role in neurocognitive functioning, and to our knowledge this is the first study to report on its association with healthy age-related differences in cognitive performance between childhood and early adulthood. Nevertheless, the IFOF’s association with all cognitive domains was unexpected. Post-hoc analyses indicated that the association between the IFOF and speed of processing explained the IFOF’s association with the other cognitive domains with the exception of visual learning (see Figure 3). The association with visual learning has anatomical validity considering the IFOF’s frontal connections to the occipital as well as temporal visual association cortices (38) and is in line with prior findings in healthy aging (14). Exploratory analyses suggested that the genu of CC may also be involved in maturation of visual learning, possibly through higher-level integration of visual information relayed to the frontal lobes by the IFOF from both hemispheres. When using the IFOF’s older age peak derived from the Poisson model, the IFOF was no longer associated with visual learning, independent of the age effect, reflecting the relatively early age peak that was observed for this cognitive domain (see Figure 2 and Supplement: Table S1). The IFOF remained however associated with the other cognitive domains, independent of the effect of age, which likely reflects the later age peaks of these domains, together with the proximity of the IFOF’s Poisson and LOESS age peaks (see Figure 2 and Supplement: Table S1).
The lack of WM tract-cognition associations from early adulthood to late adulthood is to some extent contradictory to other studies that observed associations between WM tract microstructure and cognitive performance across this age period, independent of the effect of age (14–18). However, these studies included subjects older than the subjects in our study, i.e. beyond age 70 (16, 17) or 80 (14, 15) years. WM tract-cognition associations may be more evident in very old age, in line with the sharp declines in WM FA observed from approximately age 60 years onward (4). In addition, over half of our participants were younger than 28 years of age, which decreased our power to detect WM tract-cognition associations from early adulthood into late adulthood.
There are some additional limitations to our study. First, although linear mediation models were fit on either side of the FA peak, the age-FA trends, particularly during childhood and adolescence, are still nonlinear. This limitation may be of particular relevance for analyses using the later age peaks derived from the Poisson model. For example, SLF FA was associated with age-related differences in verbal functioning when using its Poisson age peak, which is difficult to interpret, because the nonlinear curves do not indicate significant differences in SLF FA between the LOESS and Poisson age peaks (see Figure 1). Second, higher reliability estimates of the neurocognitive domains may have improved our ability to detect significant WM tract-cognition associations, although we note that speed of processing was significant in several mediation models. We chose to create domains, where possible, within the constraints of conceptual overlap between tests and domain reliability, rather than analyzing individual tests, to reduce the risk for spurious findings.
To our knowledge, this is the first DTI study to report neurocognitive correlates of age-related WM tract differences from childhood to adulthood. Importantly, including a broad age range allowed us to assess each tract’s neurocognitive correlates in relation to its unique aging pattern. Our findings suggest that protracted maturation of the cingulum and IFOF may play a critical role in neurocognitive maturation in the transition from adolescence to adulthood.
Supplementary Material
Acknowledgements
The authors thank Katie Mahon, Ph.D., for her contribution to defining the masks used for tractography, and Kimberly Cameron for her role in recruitment and assessment of the participants aged 8–18 years.
This work was supported in part by grants from the National Institutes of Health to Dr. Szeszko (R01 MH076995), the NSLIJ Research Institute General Clinical Research Center (M01 RR018535), an Advanced Center for Intervention and Services Research (P30 MH090590) and a Center for Intervention Development and Applied Research (P50 MH080173 to Dr. Malhotra). Dr Malhotra has received compensation from Eli Lilly, Schering-Plough/Merck, Sunovion Pharmaceuticals, Genomind, Shire, and Abbott.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Peters, Dr. Ikuta, Dr. DeRosse, Dr. John, Dr. Burdick, Dr. Gruner, Mr. Prendergast, and Dr. Szeszko reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 2.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 3.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 4.Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, et al. Life-span changes of the human brain white matter Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- 5.Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, Derosse P, et al. White matter development in adolescence Diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamnes CK, Fjell AM, Westlye LT, Ostby Y, Walhovd KB. Becoming consistent Developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci. 2012;32:972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds A diffusion tensor imaging study. J Cogn Neurosci. 2009;21:1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- 10.Østby Y, Tamnes CK, Fjell AM, Walhovd KB. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49:3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Muetzel RL, Collins PF, Mueller BA, M Schissel A, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. NeuroImage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 13.Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. Structural correlates of cognitive domains in normal aging with diffusion tensor imaging. Brain Struct Funct. 2012;217:503–515. doi: 10.1007/s00429-011-0344-7. [DOI] [PubMed] [Google Scholar]
- 14.Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, et al. Age-related decline in white matter tract integrity and cognitive performance A DTI tractography and structural equation modeling study. Neurobiol Aging. 2012;33:21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, et al. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval Contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry ME, McDonald CR, Hagler DJ, Jr, Gharapetian L, Kuperman JM, Koyama AK, et al. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47:2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, et al. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiol Aging. 2012;33:1699–1715. doi: 10.1016/j.neurobiolaging.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL) Initial reliability and validity data. J Am Acad of Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. (SCID-I/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 22.Wilkinson GS. The Wide Range Achievement Test (WRAT-3) Administration Manual. Wilmington, DE: Wide Range Inc.; 1993. [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Hollingshead AB. Mimeo-graphed. New Haven, CT: Yale University; 1957. Two Factor Index of Social Position. [Google Scholar]
- 25.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 26.Spreen O, Strauss E, editors. A Compendium of Neuropsychological Tests Administration, Norms, and Commentary. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 27.Nuechterlein KH, Green MF. MATRICS Consensus Battery Manual. Los Angeles, CA: MATRICS Assessment Inc.; 2006. [Google Scholar]
- 28.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 29.Cleveland WS, Devlin SJ. Locally-weighted regression An approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 30.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;5:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 31.Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ., Jr Multimodal imaging of the self-regulating developing brain. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1208243109. [published online ahead of print November 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- 35.Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agam Y, Hamalainen MS, Lee AK, Dyckman KA, Friedman JS, Isom M, et al. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proc Natl Acad of Sci U S A. 2011;108:17556–17561. doi: 10.1073/pnas.1103475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 38.Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.