Abstract
The purpose of this study was to evaluate the characteristics of the T-SPOT.TB test for the diagnosis of active tuberculosis (ATB) and to distinguish ATB from other diseases using a receiver operating characteristic (ROC) curve. A total of 535 patients with suspected active tuberculosis were enrolled in the study and divided into ATB and nonactive tuberculosis (NATB) groups, as well as pulmonary tuberculosis (PTB) and extrapulmonary tuberculosis (EPTB) subgroups. The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio of the T-SPOT.TB test for the diagnosis of ATB were 84.95%, 85.12%, 82.94%, 86.93%, 5.71, and 0.18, respectively. The median number of spot-forming cells (SFCs) in the ATB group was higher than that in the NATB group (71 versus 1; P < 0.0001). The sensitivities in the PTB and EPTB subgroups were 92.31% and 81.77%. The areas under the curve (AUC) for the diagnosis of ATB using the T-SPOT.TB, early secreted antigenic target 6 (ESAT-6), and culture filtrate protein 10 (CFP-10) were 0.906, 0.884, and 0.877, respectively. A cutoff of 42.5 SFCs for ATB yielded a positive predictive value of 100%. Our study shows that the T-SPOT.TB test is useful for the diagnosis of ATB. Utilizing an ROC curve to select an appropriate cutoff made it possible to discriminate ATB from NATB.
INTRODUCTION
Tuberculosis is a serious public health issue. Data from 202 countries and territories have shown that approximately 9 million people developed tuberculosis and 1.5 million people died from the disease in 2013 (1). China has the second heaviest burden of tuberculosis in the world, with 1 million new cases of active tuberculosis (ATB) each year and a reported Mycobacterium tuberculosis infection rate of 44.5% (2). The fifth national tuberculosis epidemiological survey in 2010 in China showed that the prevalence of active pulmonary tuberculosis (PTB) was 459/100,000 and the prevalence of smear-positive PTB was 66/100,000 in people over 15 years of age (3). At present, routine diagnostic methods for ATB in China include acid-fast bacillus smear, culture, pathology, erythrocyte sedimentation rate (ESR), and the tuberculin skin test (TST). However, as these methods have either low sensitivity or specificity or require a long time to provide a result, their clinical application has been limited.
After M. tuberculosis infection, effector T cells targeting M. tuberculosis are generated in peripheral blood mononuclear cells (PBMCs) or body fluid mononuclear cells (MCs). These effector T cells secrete interferon gamma (IFN-γ) when stimulated by specific RD1 antigens, such as early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) (4–8), but these RD1 antigens are not present in Mycobacterium bovis bacillus Calmette-Guérin or in most environmental mycobacteria. The T-SPOT.TB test uses ESAT-6 and CFP-10 to stimulate effector T cells in the sample, which then secrete IFN-γ. The cytokine is then captured by specific antibodies in microtiter plates and is visualized as spots on the bottom of each well; the spots are counted to determine whether the patient is infected with M. tuberculosis. T-SPOT.TB tests have been used to diagnose latent tuberculosis infection (LTBI), but with high M. tuberculosis infection rates in China, people are more concerned about the accuracy of the T-SPOT.TB test in diagnosing ATB rather than LTBI. This study aimed to determine whether the T-SPOT.TB test performs well for the diagnosis of ATB, whether the test can be used with all body fluid samples, whether the differences between ESAT-6 and CFP-10 responses have any clinical value, and whether the T-SPOT.TB test can be adjusted to differentiate ATB from nonactive tuberculosis (NATB).
MATERIALS AND METHODS
Study population.
The Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University gave its approval to not require informed consent because of the retrospective nature of the study. A total of 550 patients with suspected ATB from the First Affiliated Hospital of Xi'an Jiaotong University in China were enrolled from July 2013 to February 2014, and 15 patients with indeterminate T-SPOT.TB results (the negative control was greater than 10 in 10 cases, and the positive control was less than 20 in 5 cases) were excluded.
Patients were classified in the ATB group if they presented one of the following conditions: (i) granuloma or caseous necrosis changes on pathology (n = 78), (ii) detection of mycobacteria by acid-fast bacillus smear (n = 12), or (iii) clinical symptoms (such as long-term fever, night sweats, or weight loss) consistent with ATB without explicit evidence of microscopy or pathology but with effective antituberculosis treatment (n = 156). In the ATB group, subgroups were determined by the infected sites, with 65 patients in the PTB subgroup and 181 patients in the extrapulmonary tuberculosis (EPTB) subgroup. The remaining patients were classified in the NATB group based on (i) no explicit evidence on microscopy or pathology but a diagnosis of other diseases (such as acute tracheobronchitis, ulcerative colitis, and acute upper respiratory tract infection) and (ii) recovery without anti-TB treatment. Based on these criteria, 246 patients were placed in the ATB group and 289 patients were placed in the NATB group.
T-SPOT.TB test. (i) Sample collection.
Peripheral blood (5 ml) was collected in tubes coated with lithium-heparin; 10 ml cerebrospinal fluid (CSF) and 20 to 30 ml serous effusion were collected with 125 IU and 2,500 IU of heparin, respectively. T-SPOT.TB testing was initiated within 6 h after sampling.
(ii) PBMC separation.
The peripheral blood sample was added to a 15-ml conical centrifuge tube with an equal volume of RPMI 1640 (Gibco, Invitrogen) and 3 ml TBD lymphocyte separation medium (Hao Yang Biological Manufacture) and then centrifuged at 1,000 × g for 22 min at 18°C. The white, cloudy band of PBMCs was removed using a pipette and placed in a 15-ml conical centrifuge tube. Sterile cell culture medium (Gibco AIM-V; Invitrogen) was used to bring the volume up to 10 ml, and the sample was centrifuged at 600 × g for 7 min. The supernatant was removed, and the pellet was resuspended in 1 ml AIM-V; AIM-V was used to bring the volume up to 10 ml, and the sample was centrifuged at 350 × g for 7 min. The supernatant was removed, and the pellet was resuspended in 0.5 ml AIM-V. The cell concentration was adjusted to 2.5 × 106 PBMCs/ml using AIM-V to prepare 500 μl of the final cell suspension.
(iii) MC separation from body fluid sample.
The body fluid sample was poured into a 15-ml conical centrifuge tube and centrifuged at 700 × g for 7 min. The supernatant was removed; RPMI 1640 (Gibco, Invitrogen) was used to bring the volume up to 10 ml, and the sample was centrifuged at 700 × g for 7 min. The supernatant was removed, and AIM-V was used to bring the volume up to 5 ml. The treated sample was then processed similarly to peripheral blood, with MCs being separated according to the above-described PBMC separation method. For CSF, the adjusted cell concentration was 0.125 × 106 MCs/ml.
(iv) Incubation and spot-forming cell (SFC) count.
For each sample, 4 wells (precoated with anti-interferon gamma antibody) were utilized. RPMI (50 μl) was added to the first well as a negative control, 50 μl phytohemagglutinin (PHA) was added to the fourth well as a positive control, 50 μl panel A (CFP-10 peptides) was added to the second well, and 50 μl panel B (ESAT-6 peptides) was added to the third well. Then, 100 μl PBMCs/BFMCs (body fluid mononuclear cells) or 200 μl CSF MCs (cerebrospinal fluid mononuclear cells) (2.5 × 105 cells) was added to each of the 4 wells. The plates were incubated for 18 h at 37°C and 5% CO2, and the spots were counted under a microscope.
The T-SPOT.TB test manual in China defines negative results as ≤5 SFCs (both ESAT-6 and CFP-10) and positive results as ≥6 SFCs (either ESAT-6 or CFP-10, or both). When the number of spots in the negative-control well is greater than 10 or that in the positive-control well is less than 20, the test is classified as indeterminate. The maximum value of ESAT-6 and CFP-10 was selected as the SFC value for T-SPOT.TB in the study.
Statistical analysis.
Using SPSS13.0 (serial number 5026743; SPSS Inc., Chicago, Illinois, USA), the mean, median, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were calculated. Based on a binomial distribution, a 95% confidence interval (95% CI) was estimated. Nonparametric tests were used for the comparison of the medians. The chi-square test was used for the comparison of rates. A P value of <0.05 was considered to be statistically significant. A receiver operating characteristic (ROC) curve was drawn using sensitivity and “1 − specificity.” The selection of the SFC cutoff was based on the following criteria: (i) the cutoff was determined to be when the sum of the sensitivity and the specificity was at a maximum, and (ii) the cutoff to diagnose active tuberculosis was determined to be when the PPV reached a maximum.
RESULTS
Analysis of T-SPOT.TB test results.
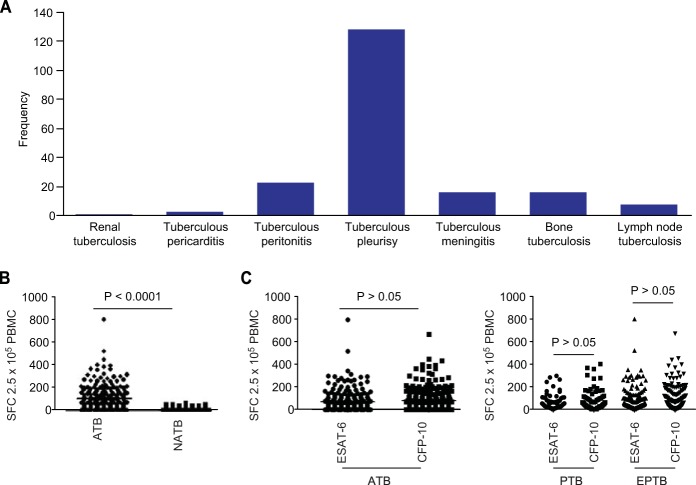
Of the 246 ATB patients, the proportions of PTB and EPTB patients were 26.42% and 73.58%, respectively; extrapulmonary sites included the central nervous system, pleura, bone, gastrointestinal tract, and lymphatic system. The sensitivity and specificity of the T-SPOT.TB test for the diagnosis of ATB were 84.95% and 85.12%, respectively. The comparisons between the groups were as follows: in the ATB group, the positive rates obtained for the T-SPOT.TB test, ESAT-6, and CFP-10 were 84.95%, 82.11%, and 73.58%, respectively, which were significantly higher than those for the NATB group (17.48%, 15.14%, and 11.58%, respectively; P < 0.05), and the median numbers of SFCs in the ATB group were 71, 43, and 38 for T-SPOT.TB, ESAT-6, and CFP-10, which were also significantly higher than those for the NATB group (1, 0, and 0, respectively; P < 0.0001). The sensitivity of the T-SPOT.TB test for each subgroup was assessed, and that for the PTB subgroup (92.31%) was higher than that for the EPTB subgroup (81.77%; P = 0.044). The comparison of the two antigens in the ATB group and the PTB and EPTB subgroups showed that the differences in sensitivity, specificity, and median numbers of SFCs between ESAT-6 and CFP-10 were not statistically significant (P > 0.05). The results are shown in Table 1 and Fig. 1.
TABLE 1.
Clinical data for included patients
| Parameter | Value for (sub)group: |
|||
|---|---|---|---|---|
| ATB | NATB | PTB | EPTB | |
| Total (n) | 246 | 289 | 65 | 181 |
| Age (yr) ± SD | 45.81 ± 19.69 | 54.04 ± 17.43 | 45.15 ± 18.26 | 45.94 ± 21.62 |
| Male [n (%)] | 139 (56.5) | 163 (56.4) | 39 (60) | 100 (55.25) |
| Female [n (%)] | 107 (43.5) | 126 (43.6) | 26 (40) | 81 (44.75) |
| Blood samples (n) | 208 | 280 | 65 | 143 |
| Body fluids (n) | 38 | 9 | 0 | 47 |
| CSF (n) | 11 | 3 | 0 | 11 |
| Pleural effusion (n) | 17 | 4 | 0 | 17 |
| Ascites (n) | 10 | 2 | 0 | 10 |
| T-SPOT.TB | ||||
| Median no. of SFCs | 71 | 1 | 83 | 67.5 |
| Positive/negative (n) | 209/37 | 43/246 | 60/5 | 148/33 |
| Sensitivity (%) (95% CI) | 84.95 (80.5–89.0) | NAa | 92.31(83.77–97.13) | 81.77(75.64–86.89) |
| Specificity (%) (95% CI) | NA | 85.12 (79.9–89.2) | NA | NA |
| ESAT-6 | ||||
| Median no. of SFCs | 43 | 0 | 52 | 43 |
| Positive/negative (n) | 202/44 | 38/251 | 56/9 | 147/34 |
| Sensitivity (%) (95% CI) | 82.11 (76.95–86.5) | NA | 86.15 (76.11–93.03) | 81.22 (75.03–86.41) |
| Specificity (%) (95% CI) | NA | 86.85 (82.58–90.4) | NA | NA |
| CFP-10 | ||||
| Median no. of SFCs | 38 | 0 | 58 | 42 |
| Positive/negative (n) | 181/65 | 30/259 | 50/15 | 136/45 |
| Sensitivity (%) (95% CI) | 73.58 (67.8–78.8) | NA | 76.92 (65.55–85.95) | 75.14 (68.45–81.02) |
| Specificity (%) (95% CI) | NA | 89.62 (85.69–92.8) | NA | NA |
NA, not applicable.
FIG 1.
Distribution of EPTB and results of the T-SPOT.TB test. (A) Distribution sites infected with M. tuberculosis in EPTB. (B) Spot-forming cells with the T-SPOT.TB test in the ATB and NATB groups. (C) Spot-forming cells with the two antigens in the ATB group and the PTB and EPTB subgroups.
Comparison of different tests for suspected active tuberculosis.
Of the 535 included patients, the numbers with available results for the T-SPOT.TB test, pathology, microscopy, and ESR were 535, 176, 179, and 437, respectively. The analysis of the results showed that the sensitivity and NPV of the T-SPOT.TB test (84.95% and 86.93%, respectively) were significantly higher than those for pathology (57.78% and 41.84%, respectively), microscopy (7.95% and 16.77%, respectively), and ESR (73.44% and 62.77%, respectively; P < 0.05). For the specificity and PPV, pathology and microscopy yielded the highest values (both 100%), while the T-SPOT.TB test showed 85.12% and 82.94%, respectively, and ESR, at 35.09% and 47%, was significantly lower than the others (P < 0.05). The PLR and NLR for the T-SPOT.TB test were the highest (5.71) and lowest (0.18) among the four methods. In patients in whom acid-fast bacillus smears or pathology with granuloma/caseous necrosis changes was detected, the positive rates of the T-SPOT.TB test were 91.67% and 87.18%, respectively. The results are shown in Table 2 and Fig. 2.
TABLE 2.
Comparison of T-SPOT.TB to other test methods
| Parameter | Value |
|||
|---|---|---|---|---|
| T-SPOT.TB (n = 535) | Pathologya (n = 176) | Smear (n = 179) | ESR (n = 437) | |
| ATB (n) | Positive, 209; negative, 37 | Yes, 78; no, 57 | Detected, 12; undetected, 139 | ≥20 mm/h, 141; <20 mm/h, 51 |
| NATB (n) | Positive, 43; negative, 246 | Yes, 0; no, 41 | Detected, 0; undetected, 28 | ≥20 mm/h, 159; <20 mm/h, 86 |
| Sensitivity (%) (95% CI) | 84.95 (80.5–89.0) | 57.78 (49.0–66.2) | 7.95 (4.2–13.5) | 73.44 (66.6–79.5) |
| Specificity (%) (95% CI) | 85.12 (79.9–89.2) | 100 (98.5–100) | 100 (87.7–100) | 35.09 (29.1–41.4) |
| PPV (%) (95% CI) | 82.94 (77.7–87.4) | 100 (95.4–100) | 100 (73.5–100) | 47 (41.2–52.8) |
| NPV (%) (95% CI) | 86.93 (82.4–90.6) | 41.84 (95.4–100) | 16.77 (73.5–100) | 62.77 (54.1–70.9) |
| PLR (95% CI) | 5.71 (4.19–7.65) | NAb | NA | 0.88 (0.78–1.00) |
| NLR (95% CI) | 0.18 (0.13–0.23) | 0.42 (1.94–2.89) | 0.92 (1.04–1.14) | 1.32 (0.99–1.77) |
Granuloma or caseous necrosis.
NA, not applicable.
FIG 2.
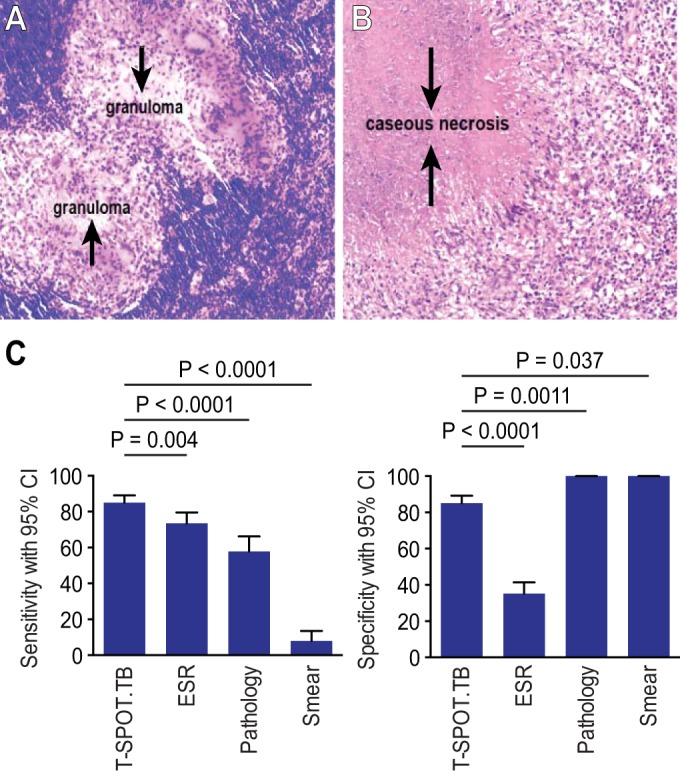
(A and B) Positive changes in pathology in ATB. (A) Pathology with a granuloma change. (B) Pathology with a caseous necrosis change. (C) Comparison of different tests for suspected ATB.
T-SPOT.TB test results from body fluid samples.
Body fluids from 47 patients were collected and analyzed using the T-SPOT.TB test. Fourteen samples were CSF, 21 were pleural effusion samples, and 12 were ascites samples. Thirty-eight patients were classified as ATB, and 9 patients had other diseases (NATB). The sensitivity of the T-SPOT.TB test in ATB patients ranged from 0 to 87.5% (the mean sensitivity was 57.89%); the sensitivities in pleural effusions and ascites were 82.35% and 80%, respectively, with a specificity of 75% for pleural effusions and 100% for ascites. However, for CSF, the tests were all negative in 11 patients with tuberculous meningitis. The results are shown in Table 3 and Fig. 3.
TABLE 3.
T-SPOT.TB test results from body fluid samples
| Sample | ATB (n) | NATB (n) | T-SPOT.TB (n) |
Sensitivity (%) | Specificity (%) | WBC count (median no. of cells) |
||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ATB | NATB | |||||
| CSF | 11 | 3 | 0 | 14 | 0 | 100 | 195 × 106/liter | 68 × 106/liter |
| Ascites | 10 | 2 | 8 | 4 | 80 | 100 | 3,120 × 106/liter | 3,590 × 106/liter |
| Pleural effusion | 17 | 4 | 15 | 6 | 82.35 | 75 | 2,980 × 106/liter | 376 × 106/liter |
FIG 3.
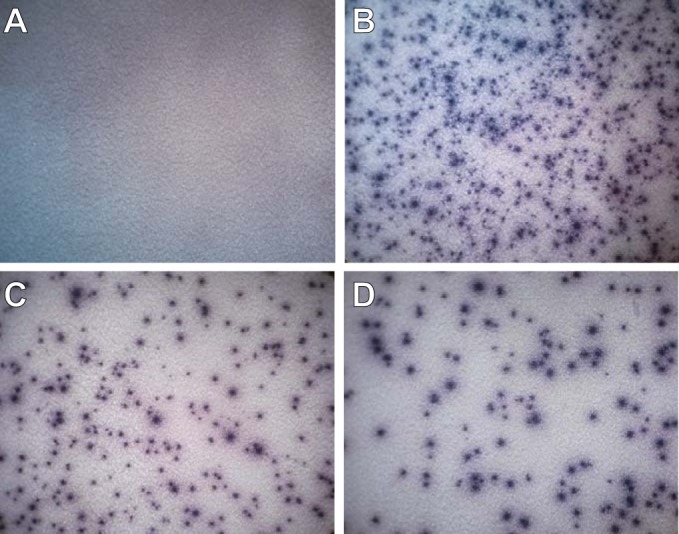
T-SPOT.TB test performance results on pleural effusions. (A) Nil control. (B) Positive control. (C) Positive result for ESAT-6 antigen. (D) Positive result for CFP-10 antigen.
ROC curve analysis of the T-SPOT.TB test for the diagnosis of active tuberculosis.
The parameters “1 minus specificity” and sensitivity were used to draw ROC curves for the T-SPOT.TB test, ESAT-6, and CFP-10. The areas under the curve (AUC) for the three tests were 0.906, 0.884, and 0.887, respectively. The sum of the sensitivity and specificity for the diagnosis of ATB was maximal when 14.5, 6.5, and 16.5 SFCs were used as cutoffs for the T-SPOT.TB test, ESAT-6, and CFP-10, respectively. A different SFC cutoff for the T-SPOT.TB test was selected to rule in ATB, and 42.5 SFCs provided the greatest discrimination, with the positive predictive value reaching the maximum (100%). The results are shown in Table 4 and Fig. 4.
FIG 4.
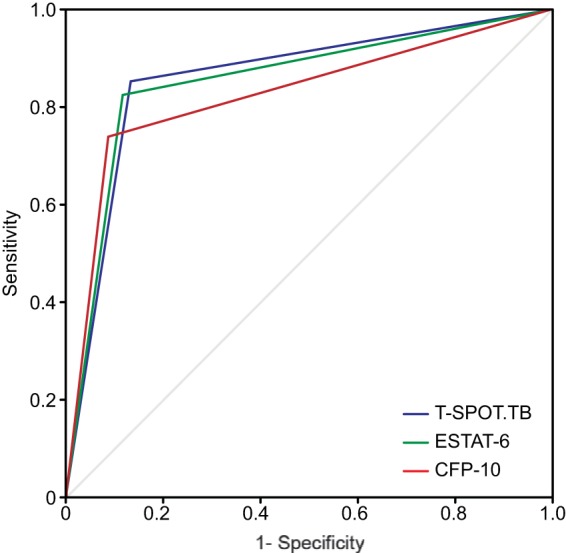
ROC curve analysis of T-SPOT.TB in ATB diagnosis.
TABLE 4.
ROC curve results of T-SPOT.TB test for diagnosing ATB
| Test | Analysis results of ROC curves |
Diagnosis of active tuberculosis at different cutoffs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Cutoff (no. of SFCs) | Sensitivity (%) | Specificity (%) | Youden indexa | Cutoff (no. of SFCs) | Sensitivity (%) | Specificity (%) | PPV | |
| T-SPOT.TB | 0.906 (0.878–0.935) | 14.5 | 80.9 | 91.3 | 0.72 | 32.5 | 70.3 | 97.9 | 97.1 |
| ESAT-6 | 0.884 (0.852–0.916) | 6.5 | 81.3 | 88.9 | 0.70 | 37.5 | 67.5 | 99.7 | 99.56 |
| CFP-10 | 0.877 (0.845–0.908) | 16.5 | 65.9 | 94.1 | 0.6 | 42.5 | 64.6 | 100 | 100 |
Youden index = 1 − (sensitivity + specificity).
DISCUSSION
The value of interferon gamma release assays (IGRAs) for the identification of LTBI has been recognized, but their use for the diagnosis of ATB remains controversial. It is thought that there is an association between the activity of mycobacteria and the level of interferon gamma secretion and that the interferon gamma level in ATB is significantly higher than that in LTBI. Some authors have suggested that IGRAs have a role in the diagnosis of ATB (4, 5, 9), whereas others object to their use in ATB due to the low specificity (6, 10, 11). Currently, there are two commercially available IGRAs: QFT-G-IT and T-SPOT.TB. Studies show that the sensitivity of the T-SPOT.TB test is higher than that of QFT-G-IT and that the indeterminate rate of T-SPOT.TB is lower than that of QFT-G-IT (12–14).
In this study, the T-SPOT.TB test had an indeterminate rate of 2.73% (15/550), similar to the results reported by Beffa et al. (15). The sensitivity, specificity, PPV, and NPV of the T-SPOT.TB test for the diagnosis of ATB are 84.95%, 85.12%, 82.94%, and 86.93%, respectively, according to recent research (4, 8, 12, 15, 16). When comparing the SFCs of the ATB and NATB groups, the median number of SFCs in the ATB group was significantly higher than that in the NATB group (P < 0.0001); in ATB patients, EPTB patients made up a significantly higher proportion than PTB patients (73.58% versus 26.42%; P < 0.05). These results are consistent with the findings of Feng et al. (16), who suggested that whether ATB in China was mainly comprised of EPTB required further study. The T-SPOT.TB test in PTB subjects had high sensitivity (92.31%), consistent with the results reported in the literature (8, 16), and its sensitivity was higher than that observed in EPTB patients (92.31% versus 81.77%; P = 0.04). When comparing the tests for the identification of ATB, the sensitivity of the T-SPOT.TB test was higher than that of ESR, pathology, or acid-fast bacillus smear (P < 0.05). In patients with an acid-fast bacillus smear or in whom pathology with granuloma or caseous necrosis was detected, the T-SPOT.TB test had high positive rates (91.67% and 87.18%, respectively).The sensitivity, specificity, and median numbers of SFCs for ESAT-6 and CFP-10 in the ATB group and the PTB and EPTB subgroups were not significantly different, which suggests that ESAT-6 and CFP-10 are equally valuable for the diagnosis of ATB.
Extrapulmonary tuberculosis often presents with nonspecific clinical manifestations, making it difficult to diagnose and leading to a delay in treatment and increases in mortality. Smears or cultures using body fluids from infected sites, such as serous effusions and joint fluid, have high specificity, but the sensitivity is limited because few mycobacteria are present in these body fluids. Using mouse models, Souza et al. found that when infected with M. tuberculosis, sensitized T cells moved from the blood to the sites of infection (17) and that mononuclear cells from body fluids at the site of infection release more interferon gamma than those from peripheral blood. Therefore, the T-SPOT.TB test may have a higher clinical value when performed on body fluids. This finding is supported by the literature (7, 18, 19). In our study, the T-SPOT.TB results from 47 patients with suspected active tuberculosis showed that when used with pleural effusion and ascites, the test had high sensitivity (82.35% and 80%, respectively) and specificity (75% and 100%, respectively), indicating that the T-SPOT.TB test is a fast and relatively accurate method for the diagnosis of tuberculous pleurisy and peritonitis.
Due to the blood brain barrier, the migration of effector T cells from the blood to the CSF is reduced compared to that observed with serous effusions. In our study, the T-SPOT.TB tests were all negative when used with the CSF from the 11 tuberculous meningitis patients. This differs from the results reported by Kim et al., in which the sensitivity of the T-SPOT.TB test was 59% (20). In our study, the time from CSF collection to the initiation of the test was approximately 6 h compared to 30 min for the Kim study. This delay resulted in the lysis of white blood cells (WBCs) in the CSF, which may be the cause of the low sensitivity of the T-SPOT.TB test in our study. Rajesh et al. reported that the CSF WBC count declined significantly from baseline (30 min) to 4 h (P < 0.001), with a decline rate of 33.89% (21). However, pleural effusion and ascites samples were treated in the same way as CSF samples but were not much influenced by the delay. This might suggest that the T-SPOT.TB test using CSF is not suitable for the diagnosis of tuberculous meningitis unless the CSF can be tested within 30 min.
We used ROC curves to evaluate the T-SPOT.TB test, and they showed that the AUC for the T-SPOT.TB test, ESAT-6, and CFP-10 were 0.93, 0.91, and 0.9, respectively, which was higher than those described by Janssens et al. and Ling et al. (6, 10). Our results suggest that the T-SPOT.TB test is a fast and accurate method for the diagnosis of ATB in China. SFC cutoffs of 14.5, 6.5, and 16.5 for the T-SPOT.TB test, ESAT-6, and CFP-10, respectively, provided the highest accuracy for the diagnosis of ATB in our study, with Youden indices of 0.72, 0.7, and 0.6, respectively. Using 42.5 SFCs as the cutoff to rule in ATB, the positive predictive value reached 100%.
This study shows that the T-SPOT.TB test has high sensitivity and specificity and may currently be the most effective method for diagnosing ATB in China. The use of ROC curves and the choice of appropriate cutoffs may improve the utility of the test.
ACKNOWLEDGMENTS
There was no financial support for the study.
We do not have commercial or other associations that might pose a conflict of interest.
REFERENCES
- 1.World Health Organization. 2014. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.National Technic Steering Group of the Epidemiological Sampling Survey for Tuberculosis, Duanmu H. 2002. Report on fourth national epidemiological sampling survey of tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi 25:3–7. http://www.ncbi.nlm.nih.gov/pubmed/11953089/. [PubMed] [Google Scholar]
- 3.Wang Y. 2012. The fifth national tuberculosis epidemiological survey in 2010. Chin J Antituberculosis 34:485–508. http://www.zgflzz.cn/CN/Y2012/V34/I8/485. [Google Scholar]
- 4.Zhang S, Shao L, Mo L, Chen J, Wang F, Meng C, Zhong M, Qiu L, Wu M, Weng X, Zhang W. 2010. Evaluation of gamma interferon release assays using Mycobacterium tuberculosis antigens for diagnosis of latent and active tuberculosis in Mycobacterium bovis BCG-vaccinated populations. Clin Vaccine Immunol 17:1985–1990. doi: 10.1128/CVI.00294-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian F, Wang W, Qiu Z, Shen Y, He J, Li D, Zhong J, Dai L. 2013. Evaluation of a new tuberculosis-related interferon gamma release assay for tuberculosis infection diagnosis in Huzhou, eastern China. Indian J Pathol Microbiol 56:125–128. doi: 10.4103/0377-4929.118694. [DOI] [PubMed] [Google Scholar]
- 6.Janssens J-P, Roux-Lombard P, Perneger T, Metzger M, Vivien R, Rochat T. 2007. Quantitative scoring of an interferon-gamma assay for differentiating active from latent tuberculosis. Eur Respir J 30:722–728. doi: 10.1183/09031936.00028507. [DOI] [PubMed] [Google Scholar]
- 7.Liao M, Yang Q, Zhang J, Zhang M, Deng Q, Liu H, Graner MW, Kornfeld H, Zhou B, Chen X. 2014. Gamma interferon immunospot assay of pleural effusion mononuclear cells for diagnosis of tuberculous pleurisy. Clin Vaccine Immunol 21:347–353. doi: 10.1128/CVI.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chee CB, KhinMar KW, Gan SH, Barkham TM, Koh CK, Shen L, Wang YT. 2010. Tuberculosis treatment effect on T-cell interferon-gamma responses to Mycobacterium tuberculosis-specific antigens. Eur Respir J 36:355–361. doi: 10.1183/09031936.00151309. [DOI] [PubMed] [Google Scholar]
- 9.Pai M, Menzies D. 2007. Interferon-gamma release assays: what is their role in the diagnosis of active tuberculosis? Clin Infect Dis 44:74–77. doi: 10.1086/509927. [DOI] [PubMed] [Google Scholar]
- 10.Ling DI, Pai M, Davids V, Brunet L, Lenders L, Meldau R, Calligaro G, Allwood B, Zyl-Smit R, van Peter J, Bateman E, Dawson R, Dheda K. 2011. Are interferon-gamma release assays useful for diagnosing active tuberculosis in a high-burden setting? Eur Respir J 38:649–656. doi: 10.1183/09031936.00181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd AE, Ashcroft A, Lipman M, Bothamley GH. 2011. Limited added value of T-SPOT.TB blood test in diagnosing active TB: a prospective Bayesian analysis. J Infect 62:456–461. doi: 10.1016/j.jinf.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai C-C, Tan C-K, Lin S-H, Liao C-H, Huang Y-T, Hsueh P-R. 2011. Diagnostic performance of whole-blood interferon-gamma assay and enzyme-linked immunospot assay for active tuberculosis. Diagn Microbiol Infect Dis 71:139–143. doi: 10.1016/j.diagmicrobio.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara G, Losi M, D'Amico R, Roversi P, Piro R, Meacci M, Meccugni B, Marchetti I, Alessandro D, Barbara A, Bergamini M, Mussini C, Rumpianesi F, Fabbri LM, Richeldi L. 2006. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 367:1328–1334. doi: 10.1016/S0140-6736(06)68579-6. [DOI] [PubMed] [Google Scholar]
- 14.Chee CB, Gan SH, KhinMar KW, Barkham TM, Koh CK, Liang S, Wang YT. 2008. Comparison of sensitivities of two commercial gamma interferon release assays for pulmonary tuberculosis. J Clin Microbiol 46:1935–1940. doi: 10.1128/JCM.02403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beffa P, Zellweger A, Janssens J-P, Wrighton-Smith P, Zellweger J-P. 2008. Indeterminate test results of T-SPOT.TB performed under routine field conditions. Eur Respir J 31:842–846. doi: 10.1183/09031936.00117207. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Diao N, Shao L, Wu J, Zhang S, Jin J, Wang F, Weng X, Zhang Y, Zhang W. 2012. Interferon-gamma release assay performance in pulmonary and extrapulmonary tuberculosis. PLoS One 7:e32652. doi: 10.1371/journal.pone.0032652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza MC, Penido C, Costa MF, Henriques MG. 2008. Mechanisms of T-lymphocyte accumulation during experimental pleural infection induced by Mycobacterium bovis BCG. Infect Immun 76:5686–5693. doi: 10.1128/IAI.00133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JY, Rhee CK, Kang NH, Kim JS, Yoon HK, Song JS. 2012. Clinical utility of two interferon-gamma release assays on pleural fluid for the diagnosis of tuberculous pleurisy. Tuberc Respir Dis (Seoul) 73:143–150. doi: 10.4046/trd.2012.73.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang Y, Shi X, Zhang Y, Deng G, Lalvani A, Liu X. 2014. Utility of T-cell interferon-gamma release assays for diagnosing tuberculous serositis: a prospective study in Beijing, China. PLoS One 9:e85030. doi: 10.1371/journal.pone.0085030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Cho OH, Park SJ, Lee EM, Kim MN, Lee SO, Choi SH, Kim YS, Woo JH, Lee SA, Kang JK. 2010. Rapid diagnosis of tuberculous meningitis by T cell-based assays on peripheral blood and cerebrospinal fluid mononuclear cells. Clin Infect Dis 50:1349–1358. doi: 10.1086/652142. [DOI] [PubMed] [Google Scholar]
- 21.Rajesh NT, Dutta S, Prasad R, Narang A. 2010. Effect of delay in analysis on neonatal cerebrospinal fluid parameters. Arch Dis Child Fetal Neonatal Ed 95:F25–F29. doi: 10.1136/adc.2008.150292. [DOI] [PubMed] [Google Scholar]