Abstract
The correlate of protection for the licensure of meningococcal vaccines is serum bactericidal activity. However, evidence indicates that a complex situation and other mechanisms, such as antibody-mediated, complement-dependent opsonophagocytosis (OP), may play a role in protection and should be investigated in order to understand immunity to this disease. In this study, a high-throughput flow cytometric opsonophagocytic assay (OPA) was optimized. The assay measures the presence of killed fluorescently labeled Neisseria meningitidis within human granulocytes (differentiated HL60 cells) by flow cytometry, using IgG-depleted pooled human plasma as an exogenous source of complement. This method was found to be reliable and correlated with the results of an opsonophagocytic killing assay. The OPA was used to measure OP activity in 1,878 serum samples from individuals ranging from 0 to 99 years of age against N. meningitidis strain NZ98/254 (B:4:P1.7-2,4). The levels of OP activity in individual serum samples varied greatly. OP activity showed an initial peak in the 6- to 12-month age group corresponding to a peak in disease incidence. The OP activity dropped in childhood until the late teenage years, although there was still a higher percentage of individuals with OP activity than with protective bactericidal antibody titers. OP activity reached a peak in the 30- to 39-year age group and then declined. This later peak in OP activity did not coincide with the young adults in whom peak serum bactericidal activity and disease incidence occurred. The demonstration of OP activity when disease incidence is low and when protective bactericidal antibody titers are not detected may indicate a role for OP in protection from meningococcal disease in these age groups.
INTRODUCTION
The correlate of protection for the licensure of meningococcal vaccines is serum bactericidal activity, as measured by the serum bactericidal assay (SBA). This was established by direct evidence obtained in studies carried out in the 1960s by Goldschneider (1), who observed an epidemic of serogroup C meningococcal disease in military recruits. In this study, while 82% of the military recruits had SBA titers of ≥1:4 at the start of their basic training, 51 out of 53 recruits who developed meningococcal disease had SBA titers of <1:4 against the epidemic strain. Thus, it was proposed that a titer of ≥1:4 conferred protection against disease (1). In a concurrent paper by Goldschneider (2), it was also observed that SBA titers of ≥1:4 were rarely observed in children between 6 and 12 months of age, which is the age group with the highest incidence of meningococcal disease.
However, there is evidence to suggest that protection from meningococcal disease is possible even in the absence of an SBA titer of ≥1:4 (reviewed by Granoff [3]). Even in the initial reports by Goldschneider (1, 2), it was noted that there were many recruits who were likely to have been exposed to the epidemic strain and had SBA titers of <1:4 but did not develop meningococcal disease (1). Therefore, while titers of ≥1:4 conferred protection, titers of <1:4 did not necessarily indicate susceptibility. Seroprevalence studies in the United Kingdom (4–7) confirmed a decline in meningococcal disease without a corresponding increase in the proportion of individuals with higher SBA titers. This indicates that alternative mechanisms of protection must be responsible and may include the age-related maturation of the alternative complement pathway (8, 9), the presence of bactericidal activity in blood as observed using whole-blood killing assays (10, 11), opsonic activity (12), or a combination of these activities. Immunization with a serogroup B outer membrane vesicle (OMV) vaccine, alone or in combination with recombinant antigens, has also been shown to elicit antibodies that have opsonophagocytic killing activity despite SBA titers being <1:4 (12). In addition, efficacy studies of OMV vaccines in a number of countries have shown that while SBA titers of ≥1:4 are protective, they are not necessarily required for protection, as the efficacy of the vaccine appears to be greater and longer lasting than would be predicted by SBA titers alone (3). Evidence for opsonophagocytosis as a mechanism of protection against meningococcal disease is also seen in individuals with late-complement component deficiencies, who are more susceptible to recurrent episodes of meningococcal disease, as they are unable to form the membrane-attack complex and induce bacteriolysis (13–15). Following immunization with polysaccharide vaccines, these individuals elicited antibodies that promoted opsonophagocytic killing activity (13, 15, 16).
Opsonophagocytosis (OP) is important for protection provided by Streptococcus pneumoniae vaccines (17, 18). Opsonophagocytic assays (OPA) used for S. pneumoniae currently use the human leukocytic cell line HL60 as phagocytic effector cells and measure the killing of bacteria by viable count determination (19, 20). The meningococcal OPAs developed to date have used a variety of methods (12, 21–25). Predominantly, peripheral blood mononuclear cells have been used as effector cells (12, 21–24), as well as the HL60 cell line (25), with assays using either viable counts as their endpoint (12, 24) or flow cytometry to measure OP activity (21–23). Flow cytometric analysis can measure either the presence of fluorescently labeled bacteria within phagocytes (25) or the oxidative burst within the effector cells to indicate the presence of internalized bacteria (21, 23, 26).
In this study, we developed a reproducible high-throughput OPA using flow cytometry and characterized the age-specific seroprevalence of OP activity against serogroup B Neisseria meningitidis isolate NZ98/254 (B:4:P1.7-2,4) using sera collected in England from 2000 to 2001. This isolate has been used to measure the seroprevalence of bactericidal activity in a similar panel of serum samples (7) and is representative of clonal complex sequence type 41/44 (ST-41/44), which was responsible for approximately one-fifth of all laboratory-confirmed cases of invasive serogroup B meningococcal disease in England at that time (27). The prevalence of OP activity was also compared with the incidence of laboratory-confirmed cases of invasive serogroup B meningococcal disease in the same time period.
MATERIALS AND METHODS
Serum samples.
Sera were obtained from the Public Health England seroepidemiology unit, which maintains a depository of anonymized residual sera from routine diagnostic testing at the participating laboratories. There is no record of the indications for blood testing, but samples from immunocompromised individuals are not included. The 1,878 samples selected for this study were collected in 2000 or 2001 from male and female individuals ranging in age from 0 to 99 years. Donors from all regions of England were included. The serum samples were grouped by age of the patients, as follows: 0 to 0.5 years, 0.5 to 1 years, 1 to 2 years, 3 to 6 years, 7 to 13 years, 14 to 19 years, 20 to 29 years, 30 to 39 years, 40 to 49 years, 50 to 59 years, and ≥60 years. Pooled mouse serum raised against OMVs prepared from N. meningitidis strain NZ98/254 was also used as a positive control in all assays.
Complement source.
IgG-depleted pooled human plasma was prepared as described by Brookes et al. (28).
Bacteria.
N. meningitidis NZ98/254 (B:4:P1.7-2,4; ST-42, complex ST-41/44) was used throughout this study (29). Bacteria were grown in 10 ml of Frantz medium for 4 h at 37°C in 5% CO2. For use in the flow cytometric OPA, the bacteria were then washed in phosphate-buffered saline (PBS) before being stained internally with 10 μg ml−1 2′,7′-bis-(2-carboxyethyl)-5-(and-6) carboxyfluorescein acetoxymethyl ester (BCECF-AM) (Life Technologies, United Kingdom) in PBS. The bacteria were then killed by resuspension in PBS containing 0.2% (wt/vol) sodium azide and 17 μg ml−1 phenylmethylsulfonyl fluoride for 48 h at 37°C with 5% CO2. Killing was confirmed by plating overnight on Columbia agar containing 5% horse blood (bioMérieux). Once killing was confirmed, the bacteria were washed once with PBS and then resuspended in 1 ml of PBS. This suspension at an optical density at 600 nm (OD600) of 1.0 corresponded to 2 × 109 bacteria ml−1.
Cell line growth and differentiation.
HL60 cells (human promyelocytic leukemia cells, CCL240; American Type Culture Collection, Manassas, VA, USA) were maintained in RPMI 1640 medium (without phenol red; Gibco) supplemented with 20% fetal bovine serum (LabTech) and 1% l-glutamine (Gibco). The cells were subcultured daily to maintain the cell density between 1 × 105 and 1 × 106 cells ml−1. The cells were differentiated into granulocytes in the same medium containing 0.8% (vol/vol) N,N-dimethylformamide (DMF; Sigma) for 5 days.
Flow cytometric OPA.
The OPA was adapted from the method described previously by Findlow et al. (25). Heat-inactivated serum samples were tested in duplicate as follows, using Dulbecco's PBS with 0.5% bovine serum albumin, 5 mM glucose, 0.9 mM CaCl2·H2O, and 0.5 mM MgSO4·H2O (DPBS-GACM) throughout. Initially, test serum (5 μl) was added to wells in a 96-well microtiter plate; 20 μl of a BCECF-AM-stained N. meningitidis suspension (2.5 × 109 bacteria ml−1) was then added to each well, and the final volume was made up to 40 μl with DPBS-GACM before incubation at 37°C for 30 min with shaking at 900 rpm in an iEMS shaking incubator (Thermo Scientific). Next, 10 μl of a 1:10 dilution of IgG-depleted human plasma as the complement source was added and incubated at 37°C for 15 min with shaking at 900 rpm. Finally, differentiated HL60 cells were harvested by centrifugation at 400 × g for 5 min and resuspended in DPBS-GACM at a cell concentration of 2.5 × 107 cells ml−1. HL60 cells (40 μl) were added to each well and incubated for 30 min at 37°C with shaking at 900 rpm. Following this incubation, opsonophagocytosis was immediately stopped by placing the plate on ice and then adding 80 μl of ice-cold DPBS containing 0.2% (wt/vol) EDTA. Immediately prior to analysis on the flow cytometer, 50 μl of 0.4% trypan blue solution (Sigma) was added to quench the fluorescence of bacteria remaining on the surfaces of the HL60 cells.
For each 96-well microtiter plate, various controls were included: a single well containing HL60 cells only; a single well containing HL60 cells plus bacteria; duplicate wells containing HL60 cells, bacteria, and complement (complement-only control); duplicate wells of a positive control, which included 2 μl of mouse antiserum raised against OMV isolated from N. meningitidis NZ98/254; and a human standard serum control with a low level of OP activity. Also included on all plates was an OP control in duplicate containing 10 μl of Phagotest (a preparation of preopsonized fluorescein isothiocyanate [FITC]-labeled Escherichia coli; Glycotope, Germany) plus complement and differentiated HL60 cells.
Flow cytometric analysis.
Samples were analyzed using a Beckman Coulter CyAn flow cytometer with an automated Cytek 96-well plate sampler. A minimum of 10,000 gated HL60 cells were analyzed per sample and the fluorescence measured in the fluorescein channel. A horizontal gate was set above the autofluorescence seen in the cells-only control. The percentage of HL60 cells showing BCECF fluorescence (percent gated) was multiplied by the mean fluorescence of the gated population (X-mean) to calculate a fluorescence index (FI) for each sample; a mean FI for each sample was calculated, and the mean FI for the complement-only control was subtracted to give an FI-C value. The coefficient of variation (CV) between the duplicates was calculated for each sample; if this was >35%, the sample was repeated. The Phagotest samples were analyzed as described above, except that the horizontal gate was set above the autofluorescence (first peak) in the Phagotest samples, and were expressed as FI values, as there is no equivalent complement-only control for this sample.
Opsonophagocytic killing assay.
The opsonophagocytic killing assay was adapted from that described previously (12), with several variations. Bacteria were harvested from a 4-h culture in Frantz medium, resuspended at 5 × 104 CFU ml−1 in opsonophagocytic killing assay (OPKA) buffer (1% bovine serum albumin [BSA] in Hanks' balanced salts solution [HBSS] containing 0.9 mM CaCl2·H2O and 0.5 mM MgSO4·H2O), and 10 μl was added to 8 μl of test serum in duplicate. To this, 20 μl of IgG-depleted human plasma diluted 1:12.5 (final complement concentration, 2%) was added, which had been incubated with a 1:125 dilution of anti-human C7 monoclonal antibody (Quidel, San Diego, CA) for 20 min at room temperature to remove bactericidal activity. After a 30-min incubation at 37°C with shaking at 900 rpm, 20 μl of differentiated HL60 cells at 1 × 106 cells ml−1 in OPKA buffer was added to all wells (to give a 40:1 cell-to-bacterium ratio) and incubated for 30 min with shaking at 900 rpm. Samples (10 μl) from each well were then plated out using the tilt method onto Columbia agar containing 5% horse blood and incubated for 24 h at 37°C with 5% CO2. The results were then expressed as the mean percentage of killing between the duplicates in comparison to that of the complement-only no-test serum control.
Statistics.
The OPA results from each day were normalized using the Phagotest value for each plate, and the mean FI value for Phagotest samples across all plates was used to calculate a multiplication factor for each sample. For all serum samples, the coefficient of variation (CV) was calculated as the percent ratio of the standard deviation and the mean of the duplicates.
Reverse cumulative distribution curves were constructed as described by Reed, Meade, and Steinhoff (30). The samples with OP activity below that of complement-only background (FI-C, <0) were assigned a value of 1. The differences between the curves were then determined by a Kolmogorov-Smirnov test using the GraphPad Prism software (version 6.04).
To analyze the correlation between the flow cytometric OPA and the OPKA, the Pearson product moment correlation coefficient was calculated using GraphPad Prism to determine significance (P = 0.01).
RESULTS
Development and optimization of a flow cytometric OPA.
A previously described flow cytometric assay using chemically killed BCECF-AM-stained N. meningitidis and dimethylformamide (DMF)-differentiated HL60 cells (25) was further optimized to improve reproducibility. The previous assay used HBSS with 0.9 mM magnesium and 0.5 mM calcium, with 2% skimmed milk powder as the blocking agent. It was found that results were more consistent using DPBS-GACM, in which bovine serum albumin is the blocking agent.
The bacterium-to-cell ratio was also investigated, and in the presence of serum, a ratio of 50:1 was found to give a consistently increased OP level above that of the complement alone compared to the previously described ratio of 4:1 (25). Increasing the bacterium-to-cell ratio did, however, lead to an increase in the background OP activity seen in the complement-only control (without test antiserum). To reduce this, the final complement concentration in the assay was lowered from 25% to 2%. Various incubation times were assessed, and increased levels of antibody-mediated OP activity were seen when a 30-min incubation was performed to allow opsonization with specific antibody in the test sera prior to the addition of complement. The complement was then added and incubated for 15 min before the HL60 cells were added, and phagocytosis was allowed to occur for 30 min.
Performance of the flow cytometric OPA.
The OPA was then assessed for reproducibility by measuring the values obtained in repeat experiments. A panel of 20 human serum samples with a range of OP activities was tested in duplicate concurrently on three separate plates by one operator (intra-assay variability), by one operator, on three separate plates, on three separate occasions (interassay variability), and by three operators on three separate plates concurrently (interoperator variability). The results for all assays were collated, and the mean FI for each sample across different assays, as described above, was used to calculate the CV values for each sample. The mean CV values for all samples were then obtained and are shown in Table 1. They are all <35%, which indicates low variability for a functional immunological assay. The dilutional linearity of the flow cytometric OPA was determined with two human serum samples. The mean correlation between the observed and expected values obtained was 0.88.
TABLE 1.
Reproducibility of flow cytometric OPA as measured by CV of repeat samples performed on different days by different operatorsa
| Reproducibility measure (n) | No. of plates/operators/days | Mean CV (SEM) |
|---|---|---|
| Intra-assay variability (60) | 3/1/1 | 13.72 (2.42) |
| Interassay variability (180) | 3/1/3 | 30.07 (2.19) |
| Interoperator variability (180) | 3/3/1 | 25.85 (1.60) |
| Total precision (600) | All assays | 29.03 (1.40) |
CV, coefficient of variation; SEM, standard error of the mean. Twenty serum samples were assessed that had a range of FI values of 6,372 to 58,665, with a mean of 24,201.
Correlation of the flow cytometric OPA with OPKA.
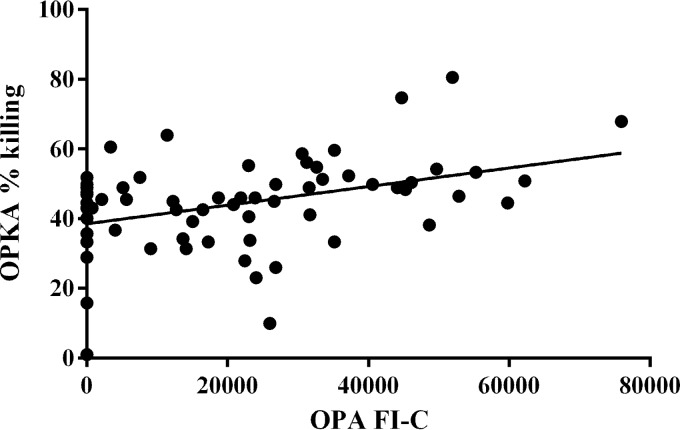
A panel of 65 human serum samples with various OP activities was analyzed using both the flow cytometric and the live meningococcal OPKA. For both assays, each sample was tested at a dilution of 1:10 only and used the same final complement concentration of 2%. The same differentiated HL60 cells were used in both assays, although the cell-to-bacterium ratio and incubation times differed. When the OP activity measured by flow cytometry, expressed as the FI-C, and the OP killing, expressed as percent killing, were compared for these 65 samples, a positive correlation of r = 0.39 (P < 0.01) was found (Fig. 1).
FIG 1.
Correlation of flow cytometric OPA with OPKA performed using 65 human serum samples with a range of OP activities, r = 0.39 (P < 0.01).
Seroprevalence of OP activity.
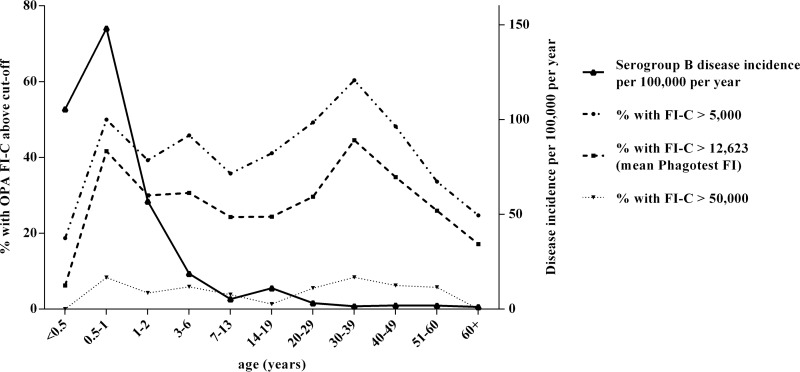
The OP activity was assessed against that of the serogroup B N. meningitidis strain NZ98/254 using a panel of 1,878 serum samples taken from individuals in England between 2000 and 2001. These sera were stratified by patient age, and the percentages of serum showing OP activity greater than those with various cutoff values are shown in Fig. 2. In the youngest age group (<6 months of age), <10% of the children had an OP activity above the mean FI value of the Phagotest sample (623) (12). This increased to >40% of the individuals having an OP activity above this level in children between 6 months and 1 year of age.
FIG 2.
Age-specific incidence of serogroup B disease compared to the percentage of individuals with OP activity at various cutoffs for sera collected in England in 2000 to 2001.
Between 20 and 30% of the individuals showed OP activity above the mean Phagotest FI throughout childhood, dropping to <15% in teenagers (Fig. 2). The percentage of individuals with OP activity greater than the mean Phagotest FI then increased to a peak in the 30- to 39-year age group, in which 45% of the individuals had OP activity above this level. The percentage of individuals showing OP activity then dropped with age, and <20% of the individuals >60 years of age showed a cutoff greater than this. When the cutoff is raised to an FI-C of >20,000 (Fig. 2), the percentage of individuals above this level broadly follows a similar trend as that seen with the mean Phagotest FI cutoff. Few individuals in any age group had OP values of >50,000.
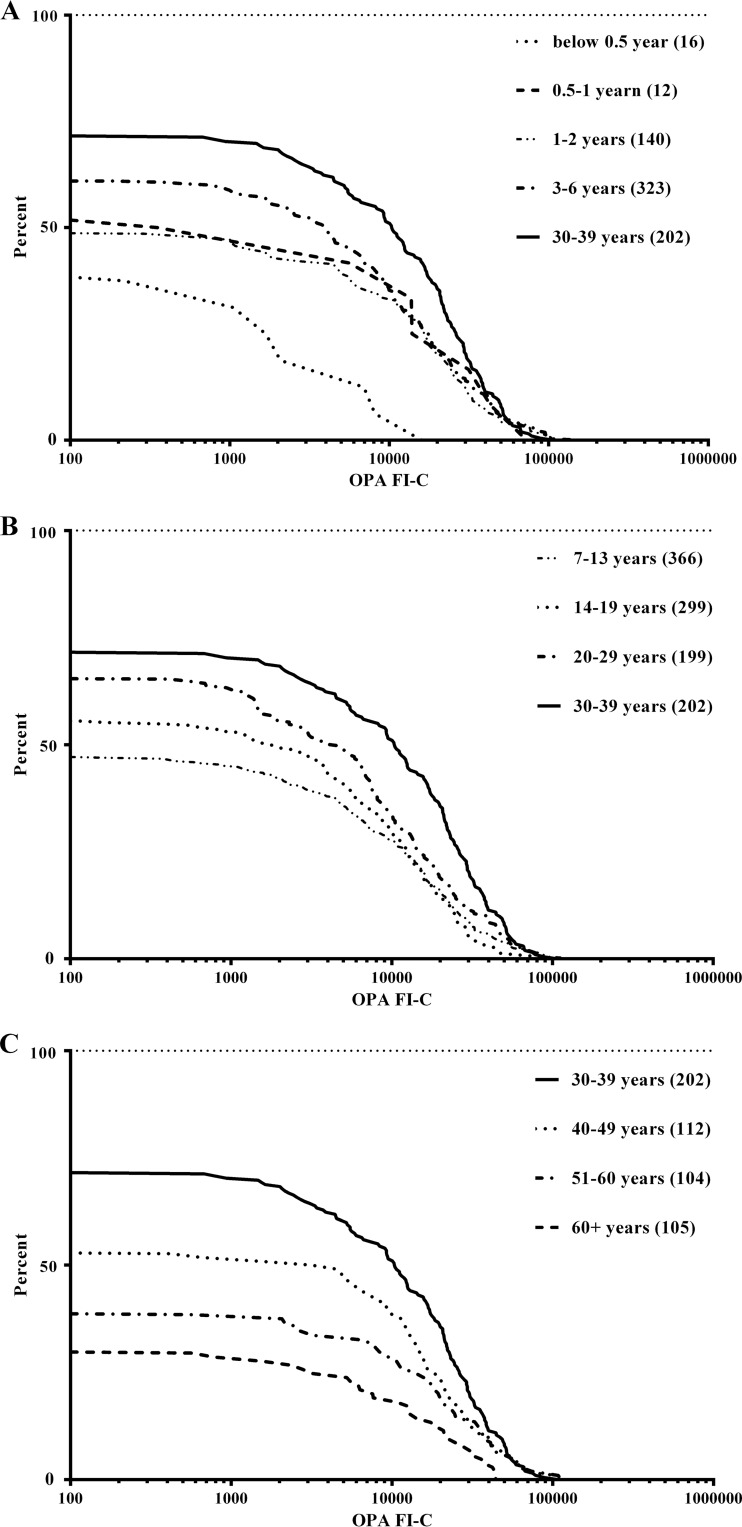
Within each age group, there are wide ranges in the levels of OP activity seen, with FI-C values ranging from below the limit of detection, i.e., below complement-only background phagocytosis, up to FI-C values of >100,000. The reverse cumulative distribution (RCD) curves of OP activity in each age group are shown in Fig. 3 to allow a comparison of the distributions of the determined activities. The lowest OP activity is seen for the samples from individuals <6 months (Fig. 3A) and >60 years (Fig. 3C) of age. The highest OP activity is seen in the 30- to 39-year age group. The steep gradient of this RCD curve also indicates smaller variation within the OP activities of individuals from this age group. The OP activity distributions between the age groups were compared using the Kolmogorov-Smirnov t test (which assumes nonnormal distribution), and significant differences (P < 0.05) are shown in Table 2. The ≥60-year age group had significantly lower OP activity than all age groups except the group aged <0.5 years. It is interesting to note that the OP activity of the 3- to 6-year age group is significantly higher than that of the age groups of <6 months, 7 to 13 years, and >60 years.
FIG 3.
Reverse cumulative distributions for OP activity against serogroup B meningococci for various age groups. The different age ranges are identified in the legends, with the number of samples per group in parentheses; all panels contain the 30- to 39-year age group (solid line) as a reference.
TABLE 2.
Significant differences between relative cumulative distribution curves for OP activities in different age groupsa
| Age group (yr) | Age group(s) (yr), compared to column 1, in which distribution is significantly: |
|
|---|---|---|
| Lower | Higher | |
| <0.5 | 3–6, 20–29, 30–39 | |
| 0.5–1 | ||
| 1–2 | ≥60 | 20–29, 30–39 |
| 3–6 | <0.5, 7–13, ≥60 | 30–39 |
| 7–13 | ≥60 | 3–6, 30–39 |
| 14–19 | 50–59, ≥60 | 30–39 |
| 20–29 | <0.5, 1–2, 7–13, 50–59, ≥60 | 30–39 |
| 30–39 | All except 0.5–1 | |
| 40–49 | ≥60 | 30–39 |
| 50–59 | 14–19, 20–29, 30–39 | |
| ≥60 | All except <0.5, 0.5–1, 50–59 | |
Significance at a P value of <0.05, by the Kolmogorov-Smirnov test.
Relationship between OP activity seroprevalence and disease incidence.
Using population data from the Office of National Statistics as the denominator and the number of laboratory-confirmed cases of invasive serogroup B meningococcal disease for England in 2001 collected by the Public Health England (PHE) meningococcal reference unit as the numerator, disease incidence was calculated and compared to the OP activity seroprevalence data (Fig. 2). The highest disease incidence was seen in children between 0.5 and 1 year of age, and this coincided with high levels of OP activity. Disease incidence then dropped throughout childhood, up to and including the 7- to 13-year age group. OP activity, however, drops after the second year of life but then remains at a steady level throughout childhood. There is a second increase in the rate of meningococcal serogroup B disease seen in teenagers (14- to 19-year age group), and this corresponds with the lowest percentage of individuals with OP activities of >20,000 and >50,000. There is an increase in the percentage of individuals with higher OP activity in adulthood, corresponding to low levels of disease. However, there was no increase in disease incidence in the age group of >60 years, despite the low levels of OP activity seen in this age group.
DISCUSSION
We have further developed an opsonophagocytosis assay for N. meningitidis using HL60 cells, with the uptake of fluorescent bacteria determined by flow cytometry, and used it to describe the age-specific prevalence of antibody-mediated, complement-dependent OP activity to serogroup B meningococci. Following optimization of the OPA protocol using a single batch of bacteria, pooled IgG-depleted human plasma as the complement source, and HL60 cells, the assay was found to be reproducible. The flow cytometric OPA used killed meningococci, thus avoiding the complication of serum bactericidal activity interfering with the result, and it has the added advantage that the daily handling of live meningococci is not required. Additionally, unlike some assays, we used a single point dilution, thus increasing the number of sera that can be assessed on a single assay plate. To date, several protocols for flow cytometric OPAs have been developed. Initial studies used antigen-coated fluorescent beads in place of bacteria, coating the beads in OMVs of the meningococcal strain of interest (31), specific meningococcal proteins (32), or lipopolysaccharide (LPS) epitopes of interest (33). By direct staining of the bacteria with the internal stain BCECF-AM, as was done in this study, the bacterial surface is available for specific-antibody binding and complement binding. One concern when measuring the presence of fluorescent bacteria by flow cytometry is that adherent but not internalized meningococci might contribute to the fluorescence signal. However, trypan blue was added to the assay to quench the fluorescence from surface-bound bacteria. An alternative approach would be to measure the oxidative burst within the phagocytes following the engulfment of bacteria (26).
As mentioned above, the standardization of the assay was aided by the use of pooled IgG-depleted human plasma as the source of complement (28). SBAs for serogroup B meningococci are generally performed using human complement, but finding human donors with sufficiently low anti-meningococcal serum antibodies is a considerable problem (34) that is solved by the IgG-depleted complement source used here. As the assay used killed meningococci, it was important to determine how the results compare to opsonophagocytic killing of live meningococci. A weak positive correlation was found between the flow cytometry OPA and the OPKA, which used viable counts as the endpoint. It should be noted that the strength of the correlation is influenced by the difference in the dynamic ranges for the readouts of these two assays. Killing is expressed as a percentage, whereas the FI-C results range from below detection to >100,000. It may also be that the two assays assess different antibody specificities.
This is the first time that anti-meningococcal OP seroprevalence has been investigated across the population and age ranges. The seroprevalence of OP activity above our chosen cutoff was compared to the incidence of serogroup B disease, and two peaks in the percentages of individuals with OP activity were seen. The initial peak occurs in the age group of 6 months to 1 year, the age group with the highest incidence of meningococcal disease, indicating that OP is unlikely to play a role in protection against meningococcal disease in this age group. In contrast, many more children age 3 to 13 years possess OP activity than possess protective SBA titers, and this corresponds with low rates of meningococcal disease. Thus, it is conceivable that OP may play a role in maintaining low rates of disease in this age group, although there is no direct evidence that this is responsible. The maximum percentage of individuals with OP activity above the cutoff is seen in the 30- to 39-year-olds, which is >10 years later than the small increase in disease incidence that occurs in teenagers (14- to 19-year age group; Fig. 2), which may indicate that OP activity develops following repeated episodes of meningococcal carriage and is a longer-lasting immune response than is bactericidal antibody. There are also notable differences seen in the profile of OP activity across the age ranges compared with the profile of SBA titers of ≥1:4 against strain NZ98/254 (7). The maximum peak in SBA titers seen in teenagers to young adults (18 to 24 years) is not replicated in OP activity, with the peak of this immunity occurring in the 30- to 39-year age group. This study and that of Trotter et al. (7) used the same serogroup B meningococcal isolate for the assays, and so the SBA titer and OP activity detected may not reflect immunity to other serogroup B isolates. It is understood that the serum bactericidal activity is likely to be PorA specific, at least to some extent, with small changes in that antigen having a large effect on the level of killing detected (35). The antigens that elicit antibodies with OP activity are less well understood, but PorA may be less immunodominant. It would be expected that anti-meningococcal antibodies in teenagers and adults are the result of multiple carriage episodes with different strains. Thus, it is perhaps surprising that the peak OP activity seen in 30- to 39-year-olds is not also seen for bactericidal activity. This may be because the bactericidal anti-PorA and other antigen antibodies are shorter lived than are the possibly broader range of antigens responsible for OP activity. Broader cross-strain OP activity than serum bactericidal activity was seen following the immunization of adult volunteers with an OMV vaccine based on Neisseria lactamica (36). Thus, it may be detrimental that vaccine candidates such as Neisseria antigen 2123 (37) and transferrin binding protein A (32, 38) have not been pursued as vaccine candidates, as they do not elicit serum bactericidal activity despite their ability to mediate OP. This situation is likely to continue, as while an SBA titer of ≥1:4 is sufficient for protection against meningococcal disease, the level of OP activity needed to confer protection in the absence of detectable bactericidal activity is not known.
We have described an OPA that requires a low serum volume for each test and overcomes many of the shortcomings of previous methods. We used this to characterize the seroprevalence of OP activity in the English population to a serogroup B N. meningitidis isolate and showed peak activity in young children and in 30- to 39-year-olds. We propose that OPA should be used in addition to SBA to help understand immunity to serogroup B meningococci.
ACKNOWLEDGMENTS
We thank Richard Pebody and the PHE Meningococcal Reference Unit for providing the sera for this study. We also thank Stephen Gray and Helen Campbell for providing the disease incidence data and advice.
REFERENCES
- 1.Goldschneider I. 1969. Human immunity to the meningococcus: I. The role of humoral antibodies. J Exp Med 129:1307–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldschneider I. 1969. Human immunity to the meningococcus: II. Development of natural immunity. J Exp Med 129:1327–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granoff DM. 2009. Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine 27(Suppl 2):B117–B125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trotter CL, Borrow R, Findlow J, Holland A, Frankland S, Andrews NJ, Miller E. 2008. Seroprevalence of antibodies against serogroup C meningococci in England in the postvaccination era. Clin Vaccine Immunol 15:1694–1698. doi: 10.1128/CVI.00279-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trotter CL, Yaro S, Njanpop-Lafourcade B-M, Drabo A, Kroman SS, Idohou RS, Sanou O, Bowen L, Findlow H, Diagbouga S, Gessner BD, Borrow R, Mueller JE. 2013. Seroprevalence of bactericidal, specific IgG antibodies and incidence of meningitis due to group A Neisseria meningitidis by age in Burkina Faso 2008. PLoS One 8:e55486. doi: 10.1371/journal.pone.0055486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trotter CL, Findlow H, Borrow R. 2012. Seroprevalence of serum bactericidal antibodies against group W135 and Y meningococci in England in 2009. Clin Vaccine Immunol 19:219–222. doi: 10.1128/CVI.05515-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trotter C, Findlow J, Balmer P, Holland A, Barchha R, Hamer N, Andrews N, Miller E, Borrow R. 2007. Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England. Clin Vaccine Immunol 14:863–868. doi: 10.1128/CVI.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferriani VP, Barbosa JE, de Carvalho IF. 2007. Complement haemolytic activity (classical and alternative pathways), C3, C4 and factor B titres in healthy children. Acta Paediatr 88:1062–1066. doi: 10.1111/j.1651-2227.1999.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 9.de Paula PF, Barbosa JE, Junior PR, Ferriani VPL, Latorre MRDO, Nudelman V, Isaac L. 2003. Ontogeny of complement regulatory proteins—concentrations of factor H, factor I, C4b-binding protein, properdin and vitronectin in healthy children of different ages and in adults. Scand J Immunol 58:572–577. doi: 10.1046/j.1365-3083.2003.01326.x. [DOI] [PubMed] [Google Scholar]
- 10.Ison CA, Heyderman RS, Klein NJ, Peakman M, Levin M. 1995. Whole blood model of meningococcal bacteraemia—a method for exploring host-bacterial interactions. Microb Pathog 18:97–107. doi: 10.1016/S0882-4010(95)90093-4. [DOI] [PubMed] [Google Scholar]
- 11.Ison CA, Anwar N, Cole MJ, Galassini R, Heyderman RS, Klein NJ, West J, Pollard AJ, Morley S, Levin and the Meningococcal Research Group. 1999. Assessment of immune response to meningococcal disease: comparison of a whole-blood assay and the serum bactericidal assay. Microb Pathog 27:207–214. doi: 10.1006/mpat.1999.0296. [DOI] [PubMed] [Google Scholar]
- 12.Plested JS, Granoff DM. 2008. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol 15:799–804. doi: 10.1128/CVI.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platonov AE, Kuijper EJ, Vershinina IV, Shipulin GA, Westerdaal N, Fijen CAP, Van De Winkel JGJ. 1998. Meningococcal disease and polymorphism of FcγRIIa (CD32) in late complement component-deficient individuals. Clin Exp Immunol 111:97–101. doi: 10.1046/j.1365-2249.1998.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platonov AE, Vershinina IV, Kuijper EJ, Borrow R, Käyhty H. 2003. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine 21:4437–4447. doi: 10.1016/S0264-410X(03)00440-7. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa J, Andreoni J, Densen P. 1993. Complement deficiency states and meningococcal disease. Immunol Res 12:295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- 16.Andreoni J, Käyhty H, Densen P. 1993. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J Infect Dis 168:227–231. doi: 10.1093/infdis/168.1.227. [DOI] [PubMed] [Google Scholar]
- 17.Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. 2007. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 25:2518–2527. doi: 10.1016/j.vaccine.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Hu BT, Yu X, Jones TR, Kirch C, Harris S, Hildreth SW, Madore DV, Quataert SA. 2005. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae. Clin Diagn Lab Immunol 12:287–295. doi: 10.1128/CDLI.12.2.287-295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jódar L, Butler J, Carlone G, Dagan R, Goldblatt D, Käyhty H, Klugman K, Plikaytis B, Siber G, Kohberger R, Chang I, Cherian T. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265–3272. doi: 10.1016/S0264-410X(03)00230-5. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol 13:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassøe CF, Smith I, Sørnes S, Halstensen A, Lehmann AK. 2000. Concurrent measurement of antigen- and antibody-dependent oxidative burst and phagocytosis in monocytes and neutrophils. Methods 21:203–220. doi: 10.1006/meth.2000.1001. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann AK, Sørnes S, Halstensen A. 2000. Phagocytosis: measurement by flow cytometry. J Immunol Methods 243:229–242. doi: 10.1016/S0022-1759(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 23.Aase A, Bjune G, Høiby EA, Rosenqvist E, Pedersen AK, Michaelsen TE. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect Immun 63:3531–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welsch JA, Granoff D. 2007. Immunity to Neisseria meningitidis group B in adults despite lack of serum bactericidal antibody. Clin Vaccine Immunol 14:1596–1602. doi: 10.1128/CVI.00341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Findlow J, Taylor S, Aase A, Horton R, Heyderman R, Southern J, Andrews N, Barchha R, Harrison E, Lowe A, Boxer E, Heaton C, Balmer P, Kaczmarski E, Oster P, Gorringe A, Borrow R, Miller E. 2006. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect Immun 74:4557–4565. doi: 10.1128/IAI.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann AK, Halstensen A, Bassøe CF. 1998. Flow cytometric quantitation of human opsonin-dependent phagocytosis and oxidative burst responses to meningococcal antigens. Cytometry 33:406–413. doi:. [DOI] [PubMed] [Google Scholar]
- 27.Meningococcal Reference Unit, Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, Borrow R, Mallard RH, Kaczmarski EB. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol 55:887–896. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 28.Brookes C, Kuisma E, Alexander F, Allen L, Tipton T, Ram S, Gorringe A, Taylor S. 2013. Development of a large scale human complement source for use in bacterial immunoassays. J Immunol Methods 391:39–49. doi: 10.1016/j.jim.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23:2191–2196. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 30.Reed GF, Meade BD, Steinhoff MC. 1995. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics 96:600–603. [PubMed] [Google Scholar]
- 31.Lehmann AK, Halstensen A, Holst J, Bassøe CF. 1997. Functional assays for evaluation of serogroup B meningococcal structures as mediators of human opsonophagocytosis. J Immunol Methods 200:55–68. doi: 10.1016/S0022-1759(96)00185-8. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann AK, Gorringe AR, Reddin KM, West K, Smith I, Halstensen A. 1999. Human opsonins induced during meningococcal disease recognize transferrin binding protein complexes. Infect Immun 67:6526–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plested JS, Ferry BL, Coull PA, Makepeace K, Lehmann AK, MacKinnon FG, Griffiths HG, Herbert MA, Richards JC, Moxon ER. 2001. Functional opsonic activity of human serum antibodies to inner core lipopolysaccharide (galE) of serogroup B meningococci measured by flow cytometry. Infect Immun 69:3203–3213. doi: 10.1128/IAI.69.5.3203-3213.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos GF, Deck RR, Donnelly J, Blackwelder W, Granoff DM. 2001. Importance of complement source in measuring meningococcal bactericidal titers. Clin Diagn Lab Immunol 8:616–623. doi: 10.1128/CDLI.8.3.616-623.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin SL, Borrow R, van der Ley P, Dawson M, Fox AJ, Cartwright KA. 2000. Effect of sequence variation in meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine. Vaccine 18:2476–2481. doi: 10.1016/S0264-410X(00)00047-5. [DOI] [PubMed] [Google Scholar]
- 36.Gorringe AR, Taylor S, Brookes C, Matheson M, Finney M, Kerr M, Hudson M, Findlow J, Borrow R, Andrews N, Kafatos G, Evans CM, Read RC. 2009. Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Clin Vaccine Immunol 16:1113–1120. doi: 10.1128/CVI.00118-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. 2003. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis 188:1730–1740. doi: 10.1086/379375. [DOI] [PubMed] [Google Scholar]
- 38.West D, Reddin K, Matheson M, Heath R, Funnell S, Hudson M, Robinson A, Gorringe A. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect Immun 69:1561–1567. doi: 10.1128/IAI.69.3.1561-1567.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]