Abstract
Whooping cough remains a problem despite vaccination, and worldwide resurgence of pertussis is evident. Since cellular immunity plays a role in long-term protection against pertussis, we studied pertussis-specific T-cell responses. Around the time of the preschool acellular pertussis (aP) booster dose at 4 years of age, T-cell memory responses were compared in children who were primed during infancy with either a whole-cell pertussis (wP) or an aP vaccine. Peripheral blood mononuclear cells (PBMCs) were isolated and stimulated with pertussis vaccine antigens for 5 days. T cells were characterized by flow-based analysis of carboxyfluorescein succinimidyl ester (CFSE) dilution and CD4, CD3, CD45RA, CCR7, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) expression. Before the aP preschool booster vaccination, both the proliferated pertussis toxin (PT)-specific CD4+ and CD8+ T-cell fractions (CFSEdim) were higher in aP- than in wP-primed children. Post-booster vaccination, more pertussis-specific CD4+ effector memory cells (CD45RA− CCR7−) were induced in aP-primed children than in those primed with wP. The booster vaccination did not appear to significantly affect the T-cell memory subsets and functionality in aP-primed or wP-primed children. Although the percentages of Th1 cytokine-producing cells were alike in aP- and wP-primed children pre-booster vaccination, aP-primed children produced more Th1 cytokines due to higher numbers of proliferated pertussis-specific effector memory cells. At present, infant vaccinations with four aP vaccines in the first year of life result in pertussis-specific CD4+ and CD8+ effector memory T-cell responses that persist in children until 4 years of age and are higher than those in wP-primed children. The booster at 4 years of age is therefore questionable; this may be postponed to 6 years of age.
INTRODUCTION
Whooping cough remains a worldwide problem in high-income countries despite high pertussis vaccination coverage. Already since the 1990s, acellular pertussis (aP) vaccines have been implemented in the immunization programs to replace whole-cell pertussis (wP) vaccines in many countries. In the past decade, several studies have shown that the immunity to pertussis will wane within several years after primary wP or aP vaccinations but also after the subsequent aP booster vaccinations at preschool age (1–3). In The Netherlands, three yearly peaks in the incidence of whooping cough have been observed since 1996 (4, 5). Since 2001, preschool children in The Netherlands are boosted with an aP vaccine at 4 years of age. In the beginning of 2005, the Dutch wP vaccine administered at infant age was replaced by an aP vaccine. Nowadays, Dutch infants are immunized at 2, 3, 4, and 11 months and boosted at 4 years of age with a high-dose aP vaccine.
Remarkably, in 2012, an enormous rise in pertussis disease was observed starting at 8 years of age and in teenagers and young adults. This unexpected rise in pertussis was not restricted to The Netherlands but also was observed in many other countries worldwide (1, 6).
It is known that antibodies to the different pertussis vaccine components wane within 2 years both after wP and aP infant vaccinations (3, 7–11). We have found that the priming vaccination history in infancy also influences the pertussis-specific memory response, resulting in higher memory B-cell responses in aP-primed children than in wP-primed children (12). This suggests a different effect of aP and wP vaccines on B-cell memory immunity.
Besides the memory B-cell response, T-cell immune responses play an important role in the maintenance of immunological memory and may be relevant for clinical protection to pertussis (13, 14). We have demonstrated that aP-immunized children still show high pertussis-specific T-cell responses at 4 years of age just before the preschool booster. Surprisingly, these responses did not increase after booster vaccination despite a further rise in antibody levels. However, in wP-primed children, the booster vaccination induced a rise in T-cell memory responses (15).
We now have further characterized these memory T cells phenotypically and functionally. Different subsets of T cells have been identified based on expression patterns of CD45RA and the chemokine receptor CCR7 (16, 17) starting with the CD45RA+ CCR7+ naive T cells. The CD45RA− CCR7+ cells were described as central memory T cells (TCM) which have the capacity to proliferate and differentiate to CD45RA− CCR7− effector memory T cells (TEM) in response to antigenic stimulation. The CD45RA+ CCR7− terminally differentiated T cells (TTD) were defined as the most differentiated T cells, still capable of producing cytokines (16–18).
The aim of the present study was to improve the insight in the immunological memory T-cell expression patterns of proliferated CD4+ and CD8+ T cells generated by pertussis vaccine antigens to show potential differences between aP- and wP-primed children. T cells were characterized by cell staining for CD3, CD4, proliferation (carboxyfluorescein succinimidyl ester [CFSE]), the memory markers CD45RA and CCR7, and the intracellular Th1-type cytokines gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). In this way, the induction of T-cell memory immunity was studied just before and 10 days after the aP preschool booster vaccination in children 4 years of age primed with either aP or wP in their first year of life.
MATERIALS AND METHODS
Study population.
In this study, T cells of children 4 years of age were analyzed. The children were a subset of a cross-sectional observational study in The Netherlands (ISRCTN65428640) which aimed to investigate pertussis-specific immunity in children 3 to 9 years of age. The cohorts of 4-year-old children were enrolled in 2007 and 2008, and children were either wP- or aP-primed in infancy. The pertussis vaccine-specific IgG antibody and the T-cell cytokine responses in these 4-year-old children have been published previously (7, 15). Now, we evaluated T-cell expression patterns in a randomly selected subset of these children (n = 27). As previously described (7), we divided the children in 4 different groups, according to the vaccination history in infancy (aP or wP priming in the first year of life) and time of blood sampling, i.e., before and 10 days after booster vaccination (Fig. 1). This study was conducted according to the Declaration of Helsinki and good clinical practice guidelines, with the approval of the relevant ethics review committee. Written informed consent was obtained from both parents or from legal representatives.
FIG 1.

Four different groups of children were used in this study. Children primed with either aP or wP vaccine at 2, 3, 4, and 11 months of age received an aP booster vaccine at 4 years of age. The aP-primed group received a high-dose vaccine and the wP-primed group received a low-dose vaccine. The groups of children were studied pre-booster vaccination and at 10 days post-booster vaccination. Numbers of individuals used varied in the different stimulations, as indicated.
Vaccines.
All aP-primed children had received diphtheria–tetanus–acellular-pertussis vaccine (DTaP)-inactivate polio vaccine (IPV)-Haemophilus influenzae type b (Hib) (Infanrix-IPV-Hib; GlaxoSmithKline Biologicals S.A., Rixensart, Belgium), containing 25 μg pertussis toxin (PT), 25 μg filamentous hemagglutinin (FHA), and 8 μg pertactin (Prn) (high-dose vaccine), at 2, 3, 4, and 11 months of age according to the Dutch National Immunization Programme. All wP-primed children had received DTwP-IPV-Hib (NVI, Bilthoven, The Netherlands) at the same age. At 4 years of age, the aP-primed children received a high-dose preschool booster vaccine, Infanrix, and the wP-primed children received a low-dose preschool booster vaccine, Triaxis (Sanofi Pasteur, Lille, France), containing 2.5 μg PT, 5 μg FHA, 3 μg Prn, and 5 μg fimbriae types 2 and 3 (Fig. 1). During the inclusion period of this study, children received randomly either a low-dose or high-dose preschool booster vaccine. Due to a temporarily shortage in supply of the high-dose booster vaccine, we had to include wP-primed children who had received a low-dose vaccine, Triaxis.
Eight-color FACS analysis.
Peripheral blood mononuclear cells (PBMCs) were isolated from blood as described earlier and frozen (19). After thawing, 1 × 106 PBMCs were stained with 5 μM carboxylfluorescein succinimidyl ester (CFSE) (Sigma Chemicals, St. Louis, MO) for 10 min in the dark at 4°C to measure proliferation of cells. After the PBMCs were washed, cells were cultured in AIMV medium (Gibco Invitrogen, Grand Island, NY) containing 5% human AB serum (Harlan Laboratories, Leicestershire, United Kingdom). The cells were stimulated with 5 μg/ml PT or 10 μg/ml FHA (Novartis, Siena, Italy) or 4 μg/ml recombinant Prn (20) at 37°C and 5% CO2 in 24-well culture plates (Greiner, Invitrogen, Breda, The Netherlands) for 5 days. PT and FHA were heat inactivated at 95°C for 15 min to avoid any mitogenicity (21). Nonstimulated cells (NS) and cells stimulated with 1 μg/ml pokeweed mitogen (PWM) (Sigma) served as negative and positive controls, respectively. Cells were stimulated subsequently with PWM, NS, PT, FHA, and Prn if enough cells were available. Within an international collaboration, the optimal antigen concentrations for fluorescence-activated cell sorting (FACS) analysis had been tested in preliminary experiments (21, 22). At day 5, GolgiPlug (BD Biosciences, San José, CA) was added to block intracellular transport processes 4 h before further intracellular cytokine staining. Cells were collected, washed, and stained for dead cell discrimination by Aqua amine-reactive dye (Invitrogen, Paisley, Scotland, United Kingdom) and for the cell surface markers allophycocyanin (APC)-H7-labeled CD4 (BD), phycoerythrin (PE)-Cy7-labeled CD45RA (BD), and PE-labeled CCR7 (R&D Systems, Minneapolis, MN). Subsequently, cells were resuspended in a Cytofix/Cytoperm Plus kit (BD) and stained for V450-labeled CD3 (BD), PerCP-Cy5.5-labeled TNF-α (BioLegend, San Diego, CA), and APC-labeled IFN-γ (BD). After being washed, the cells were analyzed using the FACSCanto cytometer (BD) in combination with Diva software (version 5.2 BD) and FlowJo software (Mac version 9.3.2; Tree Star, Ashland, OR). Proliferated (CFSEdim) viable CD3+ CD4+ and CD3+ CD4− T-cell populations were further divided in naive (CCR7+ CD45RA+), TCM (CCR7+ CD45RA−), TEM (CCR7− CD45RA−), and TTD (CCR7− CD45RA+) subsets and analyzed for intracellular Th1 cytokine production as described recently (21, 22). The CD3+ CD4+ and CD3+ CD4− T-cell populations are further defined as CD4+ and CD8+ T cells.
Flow cytometric data analysis.
Flow cytometry standard format 3.0 files were exported, and data were evaluated using FlowJo software. The gating strategy is shown in Fig. 2. Dead cells were excluded if stained with Aqua amine-reactive dye as showed in Fig. 2A. Singlets were selected using forward-scatter area (FSC-A) and forward-scatter height (FSC-H) of viable cells, and lymphocytes were gated based on FSC-A/side-scatter area (SSC-A) parameters (Fig. 2A). Both CD4+ and CD8+ total cells were gated within the viable lymphocyte singlet gate (Fig. 2A). Total CD4+ (Fig. 2B) and CD8+ (Fig. 2D) gated cells as well as proliferated cells (CD4+ CFSEdim and CD8+ CFSEdim) (Fig. 2C and E, respectively) were further analyzed for memory subset populations (CD45RA, CCR7) and intracellular cytokine-producing IFN-γ+ TNF-α−/IFN-γ+ TNF-α+/IFN-γ− TNF-α+ (Th1+ cells) T cells. Using the FSC-A/SSC-A morphological parameters, we identified the blast region of the proliferated CD4+ cells (Fig. 3A). Only samples showing both more than 1 × 104 lymphocytes and proliferation after positive control (PWM) stimulation were included in the data analysis. Because of the limited numbers of PBMCs available per sample, not all antigens could be tested in each sample. Pertussis antigen-specific stimulation is the mean from stimulation data from the three pertussis antigens (PT, FHA, and Prn) or even 2 antigens if numbers of PBMCs were limited.
FIG 2.
Example of the 8-color FACS analysis of T cells stimulated with pertussis antigen. (A) Viable cells were gated, doublet cells were excluded, and lymphocytes were selected using forward scatter (FSC-A) and side scatter (SSC-A), and subsequently CD4+ and CD8+ cells were gated. Total CD4+ (B) and CD8+ (D) gated cells as well as proliferated (CFSEdim) CD4+ (C) and proliferated (CFSEdim) CD8+ (E) gated cells were further characterized by CCR7 and CD45RA surface markers and analyzed for intracellular Th1 cytokine production of IFN-γ and TNF-α.
FIG 3.
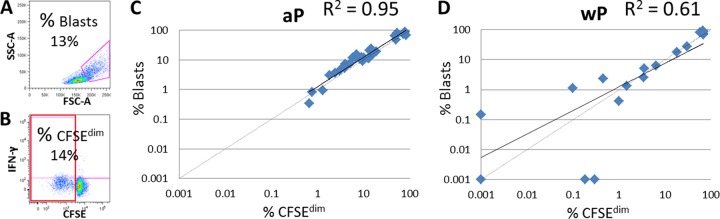
(A and B) Representative example of pertussis-specific T-cell proliferation determined by FACS analysis of the blast region of T cells using the morphological parameters FSC-A/SSC-A (A) or the CFSEdim frequencies of T cells (B). (C and D) Correlation of proliferated T cells identified by the blast region with proliferated T cells determined by the CFSEdim frequencies of aP (C)- and wP (D)-primed children 4 years of age before booster vaccination. Per sample, data from nonstimulated proliferated cells (NS) were subtracted from those from the pertussis-specific stimulated cells.
Statistical methods.
Results were expressed in medians (25th to 75th percentile) or means ± standard deviations of the mean (SD). The Mann-Whitney U test was used to determine differences between groups. P values of <0.05 were considered significantly different. Correlations were compared by linear regression and by calculating the correlation coefficients (R2 values).
RESULTS
Proliferation of pertussis antigen-specific T cells.
In general, the frequencies of proliferated CD4+ and CD8+ T cells after stimulation with the 3 pertussis-specific antigens separately were higher in aP-primed children than in wP-primed children, and the frequency was significantly elevated for PT pre-booster vaccination (Table 1). The total frequencies of PT-specific proliferating CD4+ T cells of aP-primed children were significantly decreased post-booster vaccination compared to pre-booster vaccination. The FHA and Prn stimulation of T cells of aP-primed children resulted in comparable frequencies of proliferated CD4+ and CD8+ T cells before and after booster vaccination. In wP-primed children who were boosted with a low-dose aP vaccine at 4 years, all PT and FHA stimulations resulted in similar T-cell proliferation pre- and post-booster vaccination. The Prn-specific T cells showed a tendency to increase post-booster vaccination. The frequencies of proliferated CD8+ T cells were highly comparable to those of CD4+ T cells after pertussis stimulation at all time points in both groups (Table 1). Within a European collaboration, the T-cell proliferation has been determined by either identifying the blast region using the morphological parameters FSC-A/SSC-A or by staining for CFSE (21) (Fig. 3A and B). Pre-booster vaccination, a good correlation (R2 = 0.95) between the blasts and the CFSEdim CD4+ T cells of the aP-primed children was found (Fig. 3C), and a lower correlation was found with the T cells of the wP-primed children (R2 = 0.61) (Fig. 3D). This was caused by the lower cell proliferation of some samples. The post-booster vaccination correlation of these two parameters was similar (R2 = 0.75) for aP- and wP-primed children (data not shown).
TABLE 1.
Proliferation of CD4+ and CD8+ T cells determined by CFSE staininga
| Antigen by cell type | Pre-booster vaccination |
10 days post-booster vaccination |
||||||
|---|---|---|---|---|---|---|---|---|
| aP primed |
wP primed |
aP primed (Infanrix) |
wP primed (Triaxis) |
|||||
| % of T cells (median [25th to 75th percentile]) | No. of children | % of T cells (median [25th to 75th percentile]) | No. of children | % of T cells (median [25th to 75th percentile]) | No. of children | % of T cells (median [25th to 75th percentile]) | No. of children | |
| CD4+ | ||||||||
| PT | 14.5 (5.2–18.5)*, # | 7 | 1.3 (0.4–10.2) | 6 | 3.9 (1.5–7.9) | 6 | 0.72 (0.06–3.5) | 4 |
| FHA | 3.0 (1.0–9.2) | 9 | 0.30 (0.001–5.0) | 5 | 4.7 (1.7–8.8) | 7 | 1.7 (0.2–4.2) | 5 |
| Prn | 8.9 (3.9–12.6) | 7 | 0.09 (0.001–0.19) | 2 | 5.8 (2.5–12.5) | 6 | 1.5 (1.3–3.0) | 4 |
| CD8+ | ||||||||
| PT | 15.6 (8.0–27.1)* | 7 | 1.6 (0.3–7.4) | 6 | 5.1 (3.3–12.0) | 6 | 1.6 (0.3–3.9) | 4 |
| FHA | 2.0 (0.8–7.8) | 9 | 0.20 (0.05–5.3) | 5 | 2.6 (0.7–7.6) | 7 | 0.60 (0.001–3.0) | 5 |
| Prn | 8.1 (2.2–14.1) | 7 | 0.08 (0.001–0.16) | 2 | 3.9 (1.6–7.5) | 6 | 1.7 (1.0–2.3) | 4 |
Children 4 years of age were vaccinated with either Infanrix (high-dose) or Triaxis (low-dose) aP booster vaccine. PBMCs were stimulated for 5 days with PT, FHA, or Prn. Per sample, the nonstimulated T-cell proliferation data were subtracted. *, significant difference between aP- and wP-primed children; #, significant difference between pre- and post-booster vaccination T cells of aP-primed children.
Memory subset characterization of pertussis antigen-specific T cells.
We determined the distribution of the memory subsets of proliferated CD4+ CFSEdim T cells both pre-booster vaccination and at 10 days post-booster vaccination. The results of three representative cross-sectional sampled subjects 4 years of age primed with either aP or wP are illustrated in Fig. 4. Overall, the nonstimulated samples in all four different groups consisted of large amounts of naive T cells. Upon pertussis-specific stimulation, T cells of aP-primed children displayed higher proportions of TCM and especially TEM and TTD than the control nonstimulated cells (Fig. 4A and B). In contrast, T-cell subsets of wP-primed children before the booster did not really change upon stimulation (Fig. 4A). The distributions of the T-cell memory subsets of either aP- or wP-primed children also remain similar upon booster vaccination (Fig. 4B). Overall, stimulation with the three different pertussis antigens resulted in a similar distribution of the subsets. The ratio of the proportion of TEM after stimulation with pertussis antigen related to nonstimulated cells per individual is presented in Fig. 4C. In aP-primed children, the proportion of TEM before the booster showed an increase up to13-fold, whereas post-booster vaccination, a 2- to 6-fold increase with less variation was observed. In wP-primed children, the proportion of TEM before the booster at 4 years increased just up to 3-fold and was lower than that in aP-primed children. Post-booster vaccination, the proportion of TEM of only one wP-primed child showed an increase up to 7-fold (Fig. 4C).
FIG 4.

Memory subsets of proliferated (CFSEdim) CD4+ T cells of aP- and wP-primed children cross-sectionally sampled before (A) and 10 days after (B) booster vaccination are presented. The proportions of naive T cells (CCR7+ CD45RA+; purple), central memory T cells (TCM) (CCR7+ CD45RA−; magenta), effector memory T cells (TEM) (CCR7− CD45RA−; yellow), and terminally differentiated T cells (TTD) (CCR7− CD45RA+; black) specific for PT, FHA, and Prn antigens are shown. (C) The ratio of the fraction of proliferated (CFSEdim) TEM (yellow in panels A and B) in antigen-stimulated cultures to individual nonstimulated cultures is shown.
In aP-primed children, several differences between nonstimulated cells and cells with pertussis stimulation were detected. Pre-booster vaccination, the proportions of pertussis-specific naive T cells of these children were lower and the proportions of TEM and TTD were significantly higher than those in nonstimulated T cells (Fig. 5A). After booster vaccination in aP-primed children, the proportions of both TCM and TEM were significantly higher and the proportion of naive cells was still lower than those in the nonstimulated T cells (Fig. 5B). Within the aP-primed children, there were no significant differences in memory subsets between the pre- and post-booster vaccination samples.
FIG 5.
Memory subsets of proliferated (CFSEdim) CD4+ T cells of aP-primed (left) and wP-primed (right) children, before (A) and 10 days after (B) booster vaccination at 4 years of age. Data are presented as proportions of naive (CCR7+ CD45RA+), central memory (TCM) (CCR7+ CD45RA−), effector memory (TEM) (CCR7− CD45RA−), and terminally differentiated (TTD) (CCR7− CD45RA+) T-cell subsets. Bars represent means and SD for nonstimulated cells (NS) (white bars) and pertussis-stimulated T cells (hatched bars). Pertussis stimulation is shown as the mean from the stimulation data for three pertussis antigens (PT, FHA, and Prn), aP-primed pre-booster vaccination (n = 23), aP-primed post-booster vaccination (n = 19), wP-primed pre-booster vaccination (n = 13), and wP-primed post-booster vaccination (n = 13). *, significant difference between nonstimulated and pertussis-stimulated cells; #, significant difference between aP- and wP-primed children; §, significant difference between pre- and post-booster vaccination T cells of wP-primed children.
In the pre-booster vaccination samples of wP-primed children, just the pertussis-specific TTD levels were lower than those of nonstimulated T cells (Fig. 5A), although these proportions were very small. Post-booster vaccination, no significant differences were found between the T-cell distributions of these children and nonstimulated T cell distribution (Fig. 5B). Between wP-primed children, there was a significant rise in pertussis-specific TTD post-booster compared to pre-booster vaccination, although the proportions are very low.
Comparing aP-primed children to wP-primed children pre-booster vaccination, the proportion of pertussis-specific naive T cells was lower in aP-primed children, whereas that of TTD was higher (Fig. 5A). Post-booster vaccination, the proportion of the pertussis-specific naive T cells was lower and that of TEM was significantly higher in aP-primed children than in wP-primed children (Fig. 5B).
Overall, in both aP- and wP-primed children, the pertussis-specific CD8+ CFSEdim T cells and the frequencies of the different memory subsets were comparable with that found in the CD4+ CFSEdim T cells. Notably, all pertussis-specific CD8+ CFSEdim T cells had elevated numbers of TTD (about 20%) compared to CD4+ CFSEdim T cells, and there was a lower proportion of the TCM memory subset (about 5%) in both aP- and wP-primed children (data not shown).
Th1 intracellular cytokine production of pertussis antigen-specific CD4+ CFSEdim and CD8+ CFSEdim T cells.
The T cells producing intracellular IFN-γ and TNF-α have been identified in aP- and wP-primed children before and 10 days after booster vaccination both in the proliferated (CFSEdim) CD4+ and CD8+ T cells (Fig. 6). In both aP- and wP-primed children, the same proportions of CD4+ CFSEdim IFN-γ+ TNF-α−/IFN-γ+ TNF-α+/IFN-γ− TNF-α+ (Th1+ cells) T cells were found before the booster. Pre-booster vaccination, only a few aP-primed children had high proportions of both CD4+ CFSEdim and CD8+ CFSEdim Th1 cytokine-producing cells. Post-booster vaccination, a significantly higher proportion of CD4+ CFSEdim Th1+ cells was detected in aP-primed than in wP-primed children. Moreover, the proportion of Th1 cytokine-producing CD4+ CFSEdim cells of aP-primed children post-booster vaccination displayed less variation than those of wP-primed children. In general, in the same individual, higher proportions of Th1 cytokine-producing CD4+ CFSEdim cells than CD8+ CFSEdim cells were found after pertussis antigen stimulation.
FIG 6.
Percentages of IFN-γ+ TNF-α−/IFN-γ+ TNF-α+/IFN-γ− TNF-α+-producing CD4+ CFSEdim (A) and CD8+ CFSEdim (B) proliferated T cells of aP-primed (open symbols) and wP-primed (filled symbols) children, before (circles) and 10 days after (squares) booster vaccination at 4 years of age. Per sample, the nonstimulated data of cytokine-producing T cells were subtracted. Percentage of IFN-γ+ TNF-α−/IFN-γ+ TNF-α+/IFN-γ− TNF-α+-producing proliferated T cells is shown as the mean from the stimulation data for three pertussis antigens (PT, FHA, and Prn), aP-primed pre-booster vaccination (n = 23), aP-primed post-booster vaccination (n = 19), wP-primed pre-booster vaccination (n = 13), and wP-primed post-booster vaccination (n = 13). Horizontal lines indicate the median per group. *, P ≤ 0.05.
Notably, there were no significant differences in the proportions of CD4+ or CD8+ CFSEdim memory subsets producing Th1 cytokines between pre- and post-booster vaccination samples of both aP- and wP-primed children. The proliferated T cells that produce Th1 cytokines in aP-primed children were mainly CD4+ TCM (15% ± 14%), CD4+ TEM (17% ± 13%), CD8+ TCM (9% ± 15%), and CD8+ TEM (11% ± 12%). The memory subset proportions in wP-primed children are lower than those found in aP-primed children.
DISCUSSION
We investigated possible differences in memory T-cell subsets of proliferated CD4+ and CD8+ T cells specific for pertussis vaccine antigens in pre- and post-booster vaccination samples from children 4 years of age who were primed with either aP or wP in their first year of life.
We demonstrated that both the CD4+ and the CD8+ pertussis-specific T cells of aP-primed children proliferated more than those of wP-primed children before the preschool booster. The T cells of aP-primed children contained more effector memory T cells and terminally differentiated T cells than those of wP-primed children. Moreover, priming with four acellular infant vaccine doses in the first year of life resulted in a higher proportion of effector memory T cells already before the preschool booster than in children primed with wP at 4 years of age. Conversely, the proliferated T cells of wP-primed children compared to aP-primed children still had a significantly larger amount of naive T cells in pertussis-stimulated cultures, both pre- and post-booster vaccination.
Although the percentages of Th1 cytokine-excreting cells were similar in both groups of children, the total amount of the Th1 cytokine-positive cells was higher in aP-primed children than in wP-primed children already before the booster, because of the higher total numbers of proliferated T cells producing these cytokines. The preschool booster vaccination at 4 years of age appeared not to significantly affect the memory T-cell subsets and functionality of pertussis-specific T cells in either aP-primed or wP-primed children.
In this study, we focused on proliferated T cells, since these cells are involved mainly in the development of TCM, TEM, and TTD, as agreed upon within a European collaboration network, Child-Innovac. Our results, showing a clear proliferation of CD4+ and CD8+ T cells of aP-primed children upon pertussis stimulation, are in agreement with studies that showed that CD4+ and CD8+ T cells are involved in the immune response against pertussis (13, 21, 23). Moreover, the observations that pertussis-specific TCM were more prevalent in CD4+ than in CD8+ T cells and that the relative proportions of TCM and TEM do not change after a booster immunization are in line with other studies on memory T-cell subsets (16, 24). In general, we confirmed the presence of higher proportions of TTD in nonstimulated CD8+ T cells than in CD4+ T cells both in wP- and aP-primed children (16, 24).
Pertussis-specific memory subsets have been studied in relation to the T-cell activation status after vaccination in other age groups (13, 14). Sharma and Pichichero found that pertussis-specific T-cell responses of infants who have received 3 doses of DTaP vaccine were restricted to CD4+ TCM and that adults had more fully differentiated pertussis-specific CD4+ T cells than infants due to multiple vaccinations (14). We earlier have demonstrated that wP-primed children 9 years of age showed higher numbers of pertussis-specific TEM than wP-primed children 4 years of age due to the preschool booster vaccination in combination with the high circulation of pertussis (25). So, specific T cells are able to differentiate further upon pertussis vaccination and infection.
Other groups have also reported higher cell proliferation responses in aP- than in wP-vaccinated children, although they studied children of different ages and at other time points after immunization (8, 26). Since the optimum response of antigen-specific human CD4+ T cells following reimmunization lies between 5 and 15 days after vaccination (27), the 10-day post-booster vaccination T-cell responses in the vaccinated groups of children were analyzed in this study.
Both CD4+ and CD8+ T cells of aP- and wP-primed children contain pertussis-specific Th1+ cells before and 10 days after the preschool booster vaccination at 4 years of age. Some studies indicate that cytokine production is dependent on the kind of T-cell subset involved and that naive T cells can also produce the IFN-γ and TNF-α cytokines but at a lower level than TCM and TEM (16–18, 28). In this study, Th1 cytokines in the CD4+ or CD8+ CFSEdim T cells were produced mainly by the TCM and TEM memory subsets. Because of the limited numbers of cells available, we could not determine just the IFN-γ or TNF-α cytokine-producing T cells.
We have already shown by measuring cytokine profiles in the supernatants of T-cell cultures (15) that most of the pre-booster vaccination Th1 cytokine responses specific for all three pertussis proteins were significantly higher in aP- than in wP-primed children. The high cytokine levels in aP-primed children remained elevated post-booster vaccination and were enhanced in wP-primed children following the booster vaccination. This is in agreement with the high proliferation of effector memory T cells of aP-primed children producing Th1 cytokines upon pertussis-specific stimulation already before the booster, as shown in the present study. All together, the higher numbers of pertussis-specific TEM producing Th1 cytokines in aP-primed children than in wP-primed children leads to a higher total production of these cytokines.
Several limitations of this study need to be discussed. The aP- and wP-primed children were boosted with different aP (high-dose versus low-dose) vaccines. However, we did not find any difference in the induction of effector memory cells producing cytokines at 10 days post-booster vaccination compared to pre-booster vaccination. In addition, we previously showed that cytokine responses at 10 days were similar to those at 28 days post-booster vaccination. We found that even the low-dose booster vaccine in wP-primed children did induce a T-cell memory response by increasing the T-cell cytokine responses, whereas a high-dose booster in aP-primed children did not (15). This indicates that a difference in the booster vaccine dose at 4 years of age did not appear to influence the T-cell responses.
Differences in T-cell responses seem to have been induced already by the different priming vaccinations in infancy. The Dutch wP vaccine contained very small amounts of inactivated PT (29), which is in line with the weak antibody response to PT (7). Interestingly, however, the various recent peaks in pertussis disease in aP-vaccinated populations of Europe, the United States, and Australia indicate that priming with an aP vaccine in the first year of life induces a shorter immune protection later in childhood than priming with a wP vaccine (30–33). This recent knowledge stresses the impact of the pertussis vaccine used for neonates and substantiates the need for a better understanding of the immunogenicity induced by the widely used safe and efficacious aP vaccines and their duration of protection. In this study, we showed that effector memory responses to pertussis are present in aP-vaccinated children 4 years of age at just a few years after the former vaccination at 11 months of age. However, we speculate that this immune response is rather short-lived, and long-term memory might be induced better after wP vaccination. Now we are investigating immune responses in aP-primed children 9 years of age, at 5 years after the 5th aP booster vaccination at preschool age. These results will add to our knowledge on the more long-term immunogenicity of the aP vaccines.
Another limitation is that the method we used for identifying the memory subsets of pertussis-specific proliferating T cells and their Th1 cytokine production is rather complex, and the amount of cells was limited. The CFSE staining of T cells resulted in a high loss of cells. Carollo et al. showed that the identification of pertussis-specific proliferation of T-cell blasts was comparable to that found with CFSE staining (21). We also observed a good correlation between the numbers of proliferated cells identified by blasts and by CFSE staining. This indicates that the enumeration of blasts instead of CFSEdim cells is a better proliferation marker for pertussis-specific T cells.
The FACS analysis provides the benefit of possible identification of a specific cytokine profile per T-cell phenotype. In this study, we were able to measure pertussis-specific Th1 cytokine production by intracellular FACS analysis. However, for the measurement of Th1 cytokine responses at cellular levels in population studies, the IFN-γ enzyme-linked immunosorbent spot assay (ELISpot) method showed a higher sensitivity (34, 35) and is easier to handle than FACS analysis. Because of the limited cell numbers, we were unable to assess detailed intracellular cytokines per T-cell phenotype or to determine Th2 intracellular cytokines. However, in the same groups of vaccinated children, we have previously already shown specific Th1 as well as Th2 and Th17 responses by measuring cytokines in the supernatant of pertussis-stimulated T cells, all of which were higher at pre-booster vaccination assessment in aP-primed than in wP-primed children (15).
To summarize, we demonstrated that both effector memory CD4+ and CD8+ T cells are induced by acellular and whole-cell priming vaccinations, but the aP priming led to higher T-cell immunity already before the boosting. The 5th aP vaccination at age 4 years had no effect on the phenotype and functionality of these T cells. In the present national immunization program, infants are primed with four high-dose aP vaccines in the first year of life. Therefore, it is tempting to speculate that the highly differentiated pertussis-specific T cells in these aP-primed children cannot be boosted in an efficient way, although antibody levels and B-cell memory responses are enhanced after the aP booster (7, 12). Although the implementation of an aP booster at 4 years of age for wP-primed children in The Netherlands has been successful in the protection of schoolchildren from pertussis, the fifth consecutive aP vaccination at 4 years of age is probably too early for aP-primed children and could be postponed to a later time point, contributing to a longer protection (7, 15). In addition, the dosage may have an impact, although we were not able to investigate this. Other countries have implemented an aP booster at 6 years of age for aP-primed children without an increase in clinical pertussis cases before that age, indicating that the postponement could be a good possibility (8, 36). Moreover, better memory immunity might be induced when children are vaccinated at a later age, when the immune system is more mature (37). In the recent epidemic of 2012, however, it became clear that pertussis-specific immunity induced by a booster vaccination at 6 years of age is only short-lived in many children (1), and the role or rather the lack of T-cell immunity needs to be carefully investigated in these cases. Priming, dosage, vaccine type (wP/aP), and timing of vaccinations all need to be taken into account in new studies aiming to improve the memory immune responses to pertussis.
ACKNOWLEDGMENTS
We thank all children who participated in this study.
This study was performed within a European research network, Child-Innovac (201502), supported by the European Commission under the Health Cooperation Work Programme of the 7th Framework.
REFERENCES
- 1.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. 2012. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 367:1012–1019. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 2.Witt MA, Katz PH, Witt DJ. 2012. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis 54:1730–1735. doi: 10.1093/cid/cis287. [DOI] [PubMed] [Google Scholar]
- 3.Schure RM, Hendrikx LH, de Rond LG, Ozturk K, Sanders EA, Berbers GA, Buisman AM. 2013. Differential T- and B-cell responses to pertussis in acellular vaccine-primed versus whole-cell vaccine-primed children 2 years after preschool acellular booster vaccination. Clin Vaccine Immunol 20:1388–1395. doi: 10.1128/CVI.00270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Melker HE, Schellekens JF, Neppelenbroek SE, Mooi FR, Rumke HC, Conyn-van Spaendonck MA. 2000. Reemergence of pertussis in the highly vaccinated population of the Netherlands: observations on surveillance data. Emerg Infect Dis 6:348–357. doi: 10.3201/eid0604.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Melker HE, Conyn-van Spaendonck MA, Rumke HC, van Wijngaarden JK, Mooi FR, Schellekens JF. 1997. Pertussis in The Netherlands: an outbreak despite high levels of immunization with whole-cell vaccine. Emerg Infect Dis 3:175–178. doi: 10.3201/eid0302.970211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Maas NA, Mooi FR, de Greeff SC, Berbers GA, Spaendonck MA, de Melker HE. 2013. Pertussis in the Netherlands, is the current vaccination strategy sufficient to reduce disease burden in young infants? Vaccine 31:4541–4547. doi: 10.1016/j.vaccine.2013.07.060. [DOI] [PubMed] [Google Scholar]
- 7.Hendrikx LH, Berbers GA, Veenhoven RH, Sanders EA, Buisman AM. 2009. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: effect of vaccine antigen content. Vaccine 27:6530–6536. doi: 10.1016/j.vaccine.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 8.Guiso N, Njamkepo E, Vie le Sage F, Zepp F, Meyer CU, Abitbol V, Clyti N, Chevallier S. 2007. Long-term humoral and cell-mediated immunity after acellular pertussis vaccination compares favourably with whole-cell vaccines 6 years after booster vaccination in the second year of life. Vaccine 25:1390–1397. doi: 10.1016/j.vaccine.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 9.Hallander HO, Gustafsson L, Ljungman M, Storsaeter J. 2005. Pertussis antitoxin decay after vaccination with DTPa. Response to a first booster dose 3 1/2-6 1/2 years after the third vaccine dose. Vaccine 23:5359–5364. doi: 10.1016/j.vaccine.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Edelman K, He Q, Makinen J, Sahlberg A, Haanpera M, Schuerman L, Wolter J, Mertsola J. 2007. Immunity to pertussis 5 years after booster immunization during adolescence. Clin Infect Dis 44:1271–1277. doi: 10.1086/514338. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak SG. 1998. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J Pediatr 132:983–988. doi: 10.1016/S0022-3476(98)70395-6. [DOI] [PubMed] [Google Scholar]
- 12.Hendrikx LH, de Rond LG, Ozturk K, Veenhoven RH, Sanders EA, Berbers GA, Buisman AM. 2011. Impact of infant and preschool pertussis vaccinations on memory B-cell responses in children at 4 years of age. Vaccine 29:5725–5730. doi: 10.1016/j.vaccine.2011.05.094. [DOI] [PubMed] [Google Scholar]
- 13.Rieber N, Graf A, Hartl D, Urschel S, Belohradsky BH, Liese J. 2011. Acellular pertussis booster in adolescents induces Th1 and memory CD8+ T cell immune response. PLoS One 6:e17271. doi: 10.1371/journal.pone.0017271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma SK, Pichichero ME. 2012. Functional deficits of pertussis-specific CD4+ T cells in infants compared to adults following DTaP vaccination. Clin Exp Immunol 169:281–291. doi: 10.1111/j.1365-2249.2012.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schure RM, Hendrikx LH, de Rond LG, Ozturk K, Sanders EA, Berbers GA, Buisman AM. 2012. T-cell responses before and after the fifth consecutive acellular pertussis vaccination in 4-year-old Dutch children. Clin Vaccine Immunol 19:1879–1886. doi: 10.1128/CVI.00277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 17.Appay V, van Lier RA, Sallusto F, Roederer M. 2008. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 18.Harari A, Vallelian F, Pantaleo G. 2004. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol 34:3525–3533. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buisman AM, de Rond CG, Ozturk K, Ten Hulscher HI, van Binnendijk RS. 2009. Long-term presence of memory B-cells specific for different vaccine components. Vaccine 28:179–186. doi: 10.1016/j.vaccine.2009.09.102. [DOI] [PubMed] [Google Scholar]
- 20.Loosmore SM, Yacoob RK, Zealey GR, Jackson GE, Yang YP, Chong PS, Shortreed JM, Coleman DC, Cunningham JD, Gisonni L, Klein MH. 1995. Hybrid genes overexpress pertactin from Bordetella pertussis. Vaccine 13:571–580. doi: 10.1016/0264-410X(94)00015-F. [DOI] [PubMed] [Google Scholar]
- 21.Carollo M, Palazzo R, Bianco M, Smits K, Mascart F, Ausiello CM. 2012. Antigen-specific responses assessment for the evaluation of Bordetella pertussis T cell immunity in humans. Vaccine 30:1667–1674. doi: 10.1016/j.vaccine.2011.12.104. [DOI] [PubMed] [Google Scholar]
- 22.Smits K, Pottier G, Smet J, Dirix V, Vermeulen F, De Schutter I, Carollo M, Locht C, Ausiello CM, Mascart F. 2013. Different T cell memory in preadolescents after whole-cell or acellular pertussis vaccination. Vaccine 32:111–118. doi: 10.1016/j.vaccine.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 23.Dirix V, Verscheure V, Vermeulen F, De Schutter I, Goetghebuer T, Locht C, Mascart F. 2012. Both CD4(+) and CD8(+) lymphocytes participate in the IFN-gamma response to filamentous hemagglutinin from Bordetella pertussis in infants, children, and adults. Clin Dev Immunol 2012:795958. doi: 10.1155/2012/795958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. 2006. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev 127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Schure RM, de Rond L, Ozturk K, Hendrikx L, Sanders E, Berbers G, Buisman AM. 2012. Pertussis circulation has increased T-cell immunity during childhood more than a second acellular booster vaccination in Dutch children 9 years of age. PLoS One 7:e41928. doi: 10.1371/journal.pone.0041928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. 1997. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun 65:2168–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cellerai C, Harari A, Vallelian F, Boyman O, Pantaleo G. 2007. Functional and phenotypic characterization of tetanus toxoid-specific human CD4+ T cells following re-immunization. Eur J Immunol 37:1129–1138. doi: 10.1002/eji.200636885. [DOI] [PubMed] [Google Scholar]
- 28.Khamesipour A, Nateghi Rostami M, Tasbihi M, Miramin Mohammadi A, Shahrestani T, Sarrafnejad A, Sohrabi Y, Eskandari SE, Keshavarz Valian H. 2012. Phenotyping of circulating CD8(+) T cell subsets in human cutaneous leishmaniasis. Microbes Infect 14:702–711. doi: 10.1016/j.micinf.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Thalen M, van der Ark A, van den Ijssel J, van Straaten I, Jansen D, Beuvery C, Martens D, Tramper J. 2008. Improving the cellular pertussis vaccine: increased potency and consistency. Vaccine 26:653–663. doi: 10.1016/j.vaccine.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 30.Sheridan SL, Ware RS, Grimwood K, Lambert SB. 2012. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 308:454–456. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 31.Liko J, Robison SG, Cieslak PR. 2013. Priming with whole-cell versus acellular pertussis vaccine. N Engl J Med 368:581–582. doi: 10.1056/NEJMc1212006. [DOI] [PubMed] [Google Scholar]
- 32.Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. 2013. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 56:1248–1254. doi: 10.1093/cid/cit046. [DOI] [PubMed] [Google Scholar]
- 33.Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. 2013. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 131:e1716–e1722. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson AC, Martin JN, Younger SR, Bredt BM, Epling L, Ronquillo R, Varma A, Deeks SG, McCune JM, Nixon DF, Sinclair E. 2003. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J Immunol Methods 283:141–153. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Tassignon J, Burny W, Dahmani S, Zhou L, Stordeur P, Byl B, De Groote D. 2005. Monitoring of cellular responses after vaccination against tetanus toxoid: comparison of the measurement of IFN-gamma production by ELISA, ELISPOT, flow cytometry and real-time PCR. J Immunol Methods 305:188–198. doi: 10.1016/j.jim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Hallander HO, Andersson M, Gustafsson L, Ljungman M, Netterlid E. 2009. Seroprevalence of pertussis antitoxin (anti-PT) in Sweden before and 10 years after the introduction of a universal childhood pertussis vaccination program. APMIS 117:912–922. doi: 10.1111/j.1600-0463.2009.02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegrist CA, Aspinall R. 2009. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]





