Abstract
The exoproteome of Staphylococcus aureus contains enzymes and virulence factors that are important for host adaptation. We investigated the exoprotein profiles and cytokine/chemokine responses obtained in three different S. aureus-host interaction scenarios by using two-dimensional gel electrophoresis (2-DGE) and two-dimensional immunoblotting (2D-IB) combined with tandem mass spectrometry (MS/MS) and cytometric bead array techniques. The scenarios included S. aureus bacteremia, skin and soft tissue infections (SSTIs), and healthy carriage. By the 2-DGE approach, 12 exoproteins (the chaperone protein DnaK, a phosphoglycerate kinase [Pgk], the chaperone GroEL, a multisensor hybrid histidine kinase, a 3-methyl-2-oxobutanoate hydroxymethyltransferase [PanB], cysteine synthase A, an N-acetyltransferase, four isoforms of elongation factor Tu [EF-Tu], and one signature protein spot that could not be reliably identified by MS/MS) were found to be consistently present in more than 50% of the bacteremia isolates, while none of the SSTI or healthy-carrier isolates showed any of these proteins. By the 2D-IB approach, we also identified five antigens (methionine aminopeptidase [MetAPs], exotoxin 15 [Set15], a peptidoglycan hydrolase [LytM], an alkyl hydroperoxide reductase [AhpC], and a haptoglobin-binding heme uptake protein [HarA]) specific for SSTI cases. Cytokine and chemokine production varied during the course of different infection types and carriage. Monokine induced by gamma interferon (MIG) was more highly stimulated in bacteremia patients than in SSTI patients and healthy carriers, especially during the acute phase of infection. MIG could therefore be further explored as a potential biomarker of bacteremia. In conclusion, 12 exoproteins from bacteremia isolates, MIG production, and five antigenic proteins identified during SSTIs should be further investigated for potential use as diagnostic markers.
INTRODUCTION
Staphylococcus aureus is capable of causing a wide range of infections, including skin and soft tissue infections (SSTIs), bacteremia, osteomyelitis, and more. However, certain sequence types (STs) of S. aureus may better colonize and infect patients. For instance, necrotizing pneumonia or sepsis is commonly associated with ST1 of clonal cluster 1 (CC1) (1). In addition, the burden of multidrug-resistant (MDR) S. aureus renders infection control challenging in hospital settings. Furthermore, non-MDR strains may also cause severe staphylococcal infections (2–4).
In S. aureus, exoproteins play a major role in virulence, particularly during invasion and host tissue damage. S. aureus produces exogenous phenol-soluble modulins that exhibit strong cytolytic activity against human neutrophils, erythrocytes, and monocytes (5). The exoprotein LukGH was recently reported to exhibit synergistic effects with Panton-Valentine leukocidin on human neutrophil lysis (6). Similarly, the exoprotein SasX facilitates intercellular aggregation and promotes biofilm formation (7). A continuous search for new S. aureus virulence factors is ongoing, and comparative exoproteomics of strains isolated from different infection types may help in the identification of additional virulence factors.
Several studies have reported heterogeneous virulence gene expression in strains from different infection types and different clones (8, 9). These studies also reported exoproteome heterogeneity likely due to genetic regulation, posttranslational modification, or targeted protein degradation or stabilization. Such heterogeneity complicates the identification of potential biomarkers or vaccine candidates for S. aureus.
It is well known that some exoproteins are antigenic. This antigenicity has been shown in both S. aureus-infected patients and healthy carriers (10, 11). The exoproteins Hla, IsdB, IsaA, and ClfA elicit significantly higher levels of antibodies in patients than in healthy carriers. Such immunogens play key roles in host protection by enhancing opsonic activity and inducing cytokine and chemokine production to promote immune cell recruitment (12, 13). Unfortunately, the efficacy of these protective actions has not been transformed into a licensed vaccine, underscoring the need for a better understanding of host responses to the different types of infection caused by S. aureus. A comprehensive analysis of exoproteome variations and immunoproteomics may allow correlations between different infection types and host immune profiling to be discovered. In turn, this knowledge may help to identify new disease-specific protein markers.
Here, we described the first pilot study of differentially expressed S. aureus exoproteins from different strains and detected during different infections. Until now, most staphylococcal immunoproteomic studies have focused mainly on proteins in the pI range of 6 to 11, as this range is known to cover the majority of well-known virulence factors (11, 14, 15). We investigated exoproteins at lower pI values of 4 to 7 in order to get a clearer picture of all of the proteins involved. In order to investigate the S. aureus-induced patterns of cytokines or chemokines, we analyzed their concentrations during SSTIs, bacteremia, and healthy colonization events by using the cytometric bead array (CBA) and the BD LSRFortessa flow cytometer. Ultimately, our study aimed to determine the protein signatures corresponding to various infection types and to identify biomarkers for the clinical detection of S. aureus infections.
MATERIALS AND METHODS
S. aureus strains.
This study was conducted with the approval of the Faculty of Medicine and Health Sciences of the Universiti Putra Malaysia, the Clinical Research Centre of Hospital Serdang, and the Ministry of Health Malaysia Medical Research Ethics Committee. It was conducted at the Universiti Putra Malaysia, and the samples were obtained from patients at Hospital Serdang. Six isolates each were collected from patients with bacteremia and SSTIs and from healthy carriers. SSTIs included superficial skin infections (such as impetigo, folliculitis/furunculosis, and mastitis) that can progress to more complicated skin infections (such as cellulitis, surgical wound infections, subcutaneous abscesses, and necrosis). All isolates were confirmed as being S. aureus by standard methods, which included Gram staining (Gram-positive cocci in clusters), mannitol fermentation, and coagulase and DNase production. All S. aureus isolates were stored in Luria-Bertani broth containing 20% (vol/vol) sterile glycerol at −70°C.
S. aureus strain characterization.
All 18 isolates were subjected to mecA PCR for the detection of methicillin resistance. Staphylococcal cassette chromosome mec (SCCmec) typing was performed for the mecA-positive isolates according to a protocol optimized by Ghaznavi-Rad et al. (16).
Multilocus sequence typing (MLST) and staphylococcal protein A (spa) typing were performed as previously described (17, 18). Pulsed-field gel electrophoresis (PFGE) was performed to investigate the genetic relationships among the 18 isolates (19). The SmaI restriction fragment patterns were analyzed with BioNumerics software (Applied Maths, Ghent, Belgium), and the percent similarities were determined by the unweighted-pair group method using average linkages based on Dice coefficients.
Each isolate was screened by PCR for 15 virulence factors involved in attachment, tissue invasion, evasion of host defense, and possible toxin production. The panel of genes included cna (20), fnbA (21), icaA, icaD (22), pvl (23), arginine catabolic mobile element-associated arcA (24), seg, seh, sei (25), sea, seb, sec, eta, etb, and tssT (26).
Sera.
Patients admitted to the hospital were randomly chosen for this study. For the bacteremia study, the patients were monitored daily on the basis of their symptoms, which included persistent high fever, chills, low blood pressure, and a high total white blood cell count. Only patients who had no symptoms of bacteremia during their first day in the hospital were selected. Blood was drawn from the patients once they were suspected to have bloodstream infections. Generally, serum samples were collected from two groups (those with SSTIs and those with bacteremia) at day 1 and at day14, after the infection was considered cured. Serum was collected once from healthy carriers upon their identification as carriers. Samples were collected only from those participants who had provided signed informed consent.
The criteria for inclusion in this study were an age of >18 years, consent to be included in the study, and willingness to participate in regular clinical follow-ups. Immunocompromised subjects and patients with renal insufficiency were not included in this study. Additionally, patients who died during the study or were diagnosed with bacteremia, diabetes mellitus, eczema, or polymicrobial infection at the time of admission were excluded.
Exoprotein extraction.
An overnight broth culture of the S. aureus strains collected during the clinical study was pipetted into 500 ml of tryptic soy broth supplemented with 0.001 M 2,2′-dipyridyl. The optical density at 600 nm of the culture was adjusted to 0.03 to 0.04, and the culture was grown at 37°C with constant agitation at 150 rpm. Once the culture reached the postexponential phase, the exoproteins from 500 ml of culture were precipitated by the addition of ice-cold ethanol-trichloroacetic acid. The precipitated exoproteins were dried at room temperature and solubilized in rehydration buffer containing 8 M urea, 2 M thiourea, 2.0% (wt/vol) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 0.2% (vol/vol) Bio-Lyte 3/10 ampholytes, and 50 mM dithiothreitol (DTT) to a final volume of 150 μl. The exoprotein solution was then centrifuged at 21,000 × g at room temperature for 10 min to remove insoluble proteins. The concentration of the exoprotein was determined with the RC-DC (reducing agent- and detergent-compatible) Protein Assay (Bio-Rad).
2-DGE.
Analytical two-dimensional (2D) gel electrophoresis (2-DGE) was performed as described previously (27). A total of 6 μg of exoproteins solubilized in 125 μl of rehydration buffer (8 M urea, 2 M thiourea, 2.0% [wt/vol] CHAPS, 0.2% [vol/vol] Bio-Lyte 3/10 ampholytes, 50 mM DTT) was loaded onto 7-cm immobilized pH gradient (IPG) strips (linear, pI 4 to 7; Bio-Rad) and passively rehydrated for 14 h. Subsequent isoelectric focusing, followed by second-dimension electrophoresis and silver staining, was carried out as previously described (27). Briefly, the absorbed protein inside the IPG strips was isoelectrically focused for a total of 14,000 Vh in a PROTEAN isoelectric focusing cell (Bio-Rad) and processed to equilibration steps with equilibration buffer I (6 M urea, 2% [wt/vol] SDS, 20% [vol/vol] glycerol, 2% [wt/vol] DTT, 50 mM Tris-HCl, pH 8.8) and equilibration buffer II (buffer I with 2.5% [wt/vol] iodoacetamide instead of 2% DTT). For second-dimension separation, equilibrated IPG strips were embedded in a 1-mm-thick SDS-PAGE gel (12.5% T) with overlay agarose (Bio-Rad) and subjected to electrophoresis in a Mini Dodeca-Cell (Bio-Rad) for 45 min at a constant 200 V in Laemmli SDS-PAGE running buffer. The gels were subjected to silver staining with the ProteoSilver Silver Stain kit (Sigma-Aldrich), scanned with a calibrated densitometer GS-800 (Bio-Rad), and analyzed with the PDQuest Advanced 8.0.1 2D Gel Analysis software. Technical triplicates were performed with each sample.
2D immunoblotting (2D-IB).
A total of 12 μg of pooled exoproteins for each strain in the different groups was separated by 12.5% 2-DGE as described above. The separated exoproteins and methanol-pretreated polyvinylidene difluoride (PVDF) membranes were incubated in Towbin buffer (25 mM Tris, 190 mM glycine, 20% [vol/vol] methanol [pH 8.3]) for 10 min. Trans-Blot membranes were also rinsed with Towbin buffer. The exoproteins on the 2D electrophoresis gels were then blotted onto PVDF membranes for 30 min at a constant 25 V with a Trans-Blot SD semidry blotting device according to the manufacturer's instructions (Bio-Rad). A prestained protein ladder was used to confirm transfer efficiency. The PVDF membranes with the blotted exoproteins were then blocked overnight in a solution of 5% nonfat dry milk in TBST (Tris-buffered saline–Tween containing 20 mM Tris-HCl, 137 mM NaCl, and 0.1% [vol/vol] Tween 20 [pH 7.6]) at 4°C. This blocking step was followed by five washes with TBST buffer prior to overnight incubation of the PVDF membranes with various human sera at a 1:10,000 dilution in 5% nonfat dry milk-TBST buffer at 4°C in the dark. Five washes with TBST buffer were performed, and the bound human IgG was detected by incubation with peroxidase-conjugated goat anti-human IgG (H+L) diluted 1:50,000 in 5% nonfat dry milk–TBST buffer for 1 h at room temperature. Five washes with TBST buffer were then performed. The membranes were then incubated with SuperSignal West Pico substrate for 5 min. Visualization was performed with an AlphaImager with a 5-min recording time. Three independent experiments were performed for each patient, and acute- and convalescent-phase serum samples were always analyzed in the same experiment. The acquired images were imported into PDQuest for quantitative comparison.
Pooled human serum samples from each clinical group were used to determine the total immune proteome. The 2D-IB image was matched with silver-stained 2-DGE images of the corresponding pooled exoproteins. We aimed to ensure that the immunoreactive protein spots of interest were accurately excised and subjected to tandem mass spectrometry (MS/MS) analysis.
Protein identification.
For identification of proteins by liquid chromatography (LC)-electrospray ionization (ESI) MS/MS, the silver-stained protein spots were manually excised from 2D electrophoresis gels. The proteins were trypsin digested, and the peptides were extracted according to standard techniques (28). ESI MS analysis of the peptides was performed with an Ultimate 3000 Nano high-performance liquid chromatography system (Dionex) coupled to a 4000 Q TRAP mass spectrometer (Applied Biosystems, Foster City, CA). Briefly, peptides were eluted with an increasing gradient of acetonitrile with the mobile phase containing 0.1% formic acid at 300 nl/min. The mass spectrometer was set to perform information-dependent acquisition to acquire fragmentation data on the three most intense ions with the following criteria: a mass range of 400 to 2,800, a charge 1 to 3, an intensity of >5e4 counts, and exclusion of former target ions for 300 s. The spectra were analyzed to identify proteins of interest with the Mascot sequence matching software (Matrix Science, London, United Kingdom) and the Ludwig NR database. An individual ion score of >60 was considered significant to identify a protein spot with a P value of ≤0.05.
Cytokine and chemokine assays.
To investigate cytokine and chemokine production patterns, patient serum was analyzed with the BD CBA human Th1/Th2/Th17 cytokine kit and the BD CBA Human Chemokine kit as recommended by the manufacturer. The cytokine and chemokine molecules detected by this kit include gamma interferon (IFN-γ), interleukin-10 (IL-10), IL-2, IL-4, IL-6, IL-17A, IL-8, tumor necrosis factor (TNF), IFN-γ-inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), monokine induced by IFN-γ (MIG), and regulated upon activation of normal T cell expressed and secreted protein (RANTES). Before the CBA was started, the cytometer setup beads provided by the manufacturer were used for the photomultiplier tube voltage and compensation settings in the BD LSRFortessa flow cytometer. The singlet bead population was obtained by adjustment of the forward and side light scatter voltages. The individual beads were then separated by using the optimal allophycocyanin and fluorescein isothiocyanate voltages, approximately 70,000 and 75 V, respectively. After the setting, a mixture of the recombinant standards (standards included all of the recombinant cytokines or chemokines tested) was reconstituted and serially diluted in assay diluent to obtain a standard curve (either 20 to 5,000 pg/ml of the cytokine standards or 10 to 2,500 pg/ml of the chemokine standards). For the cytokine assay, 50 μl of the diluted recombinant cytokine standards or 50 μl of individual human sera (diluted 1:8 or undiluted) was incubated with 50 μl of serum-enhanced pretreated cytokine-mixed capture beads and human Th1/Th2/Th17 phycoerythrin (PE) detection reagent (containing PE-conjugated detection antibodies) for 3 h at room temperature and protected from light. Following incubation, washing, and acquisition of fluorescence data (through FACSDiva software), the results were analyzed with the FCAP Array software. In order to facilitate the analysis of samples with the FCAP Array software, recombinant cytokine standards were acquired from the lowest to the highest concentration, followed by the most concentrated test sample. The level of each cytokine was measured on the basis of PE and 300 events and was recorded per analyte by using the singlet population as the storage and stopping gates. The standard curve was plotted by using a four-parameter logistic model (FCAP Array software). During analysis, the mean fluorescence intensity (MFI) of each population of cytokine capture beads was recorded. The intensity measurement with PE is proportional to the cytokine concentration. The cytokine concentrations were then quantified by comparison of the MFIs of samples to a standard curve. Most of the steps in the chemokine assay were similar to those used in the cytokine assay; however, serum enhancement for capture beads was omitted and signal detection was dependent on the human chemokine PE detection reagent instead of the human Th1/Th2/Th17 PE detection reagent.
Statistics.
Statistical analyses were conducted with SPSS Statistics (version 17.0). For each protein spot, antigenic protein signal, or cytokine concentration, the mean value of technical replicates was calculated from three independent experiments. The spot intensities or cytokine concentrations of the different study groups were then assessed with Student's t test. The Mann-Whitney U test or Wilcoxon signed-rank test for paired samples was performed if the data were not normally distributed. Fisher's exact test was also used for categorical data. A P value (two tailed) of ≤0.05 was considered statistically significant.
RESULTS
Eighteen subjects of mixed ethnicity (Chinese, Indian, and Malay) participated in this study. Twelve participants developed S. aureus infections, while six were only nasally colonized without any clinical symptoms (see Table S1 in the supplemental material).
Molecular characterization of S. aureus isolates.
According to the DNA sequence-based typing and PFGE results, the isolates were diverse. Eighteen isolates comprised 10 STs, eight clonal lineages (Table 1), and 11 pulsotypes (A to K). Of these 18 isolates, 3 were resistant to methicillin and harbored the mecA gene; 2 of these 3 were hospital associated (2W and 5W; ST239-t037-SCCmec III hospital-associated methicillin-resistant S. aureus [MRSA]) and genetically indistinguishable, while the other isolate was community-associated MRSA (4B; ST80-t4152-SCCmec IVc).
TABLE 1.
Characterization of S. aureus isolates
| Isolatea | MLSTc result | CCd | spa typee | Allelic profile | spa repeat succession | Kreiswirth IDf | Virulence gene profile |
|---|---|---|---|---|---|---|---|
| 1B | ST1899 | 101 | t7760 | 3-1-14-15-11-212-3 | 04-13-12-17-20-17-12-17-17 | ZEGMDMGMM | fnbA, icaA, icaD |
| 2B | NAb | NA | t127 | NA | 07-23-21-16-34-33-13 | UJFKBPE | fnbA, icaA, icaD |
| 3B | ST1179 | 97 | t359 | 144-1-1-1-1-5-3 | 07-23-12-21-17-34-34-33-34 | UJGFMBBPB | fnbA, icaA, icaD |
| 4B | ST80 | 80 | t4152 | 1-3-1-14-11-51-10 | 07-23-12-02-12-34-34-33-34 | UJGAGBBPB | fnbA, icaA, icaD, pvl, mecA |
| 5B | ST1 | 1 | t127 | 1-1-1-1-1-1-1 | 07-23-21-16-34-33-13 | UJFKBPE | sea, seh, cna, fnbA, icaA, icaD, pvl |
| 6B | ST1 | 1 | t127 | 1-1-1-1-1-1-1 | 07-23-21-16-34-33-13 | UJFKBPE | sea, seb, seg, seh, sei, cna, fnbA, icaA, icaD |
| 1W | ST30 | 30 | t021 | 2-2-2-2-6-3-2 | 15-12-16-02-16-02-25-17-24 | WGKAKAOMQ | sei, cna, fnbA, icaA, icaD, pvl |
| 2W | ST239 | 239 | t037 | 2-3-1-1-4-4-3 | 15-12-16-02-25-17-24 | WGKAOMQ | sea, cna, fnbA, icaA, icaD, mecA |
| 3W | ST1290 | Singletons | t131 | 1-4-1-1-11-1-3 | 07-23-12-34-33-34 | UJGBPB | sea, fnbA, icaA, icaD, pvl |
| 4W | ST1 | 1 | t127 | 1-1-1-1-1-1-1 | 07-23-21-16-34-33-13 | UJFKBPE | sea, seh, cna, fnbA, icaA, icaD, pvl |
| 5W | ST239 | 239 | t037 | 2-3-1-1-4-4-3 | 15-12-16-02-25-17-24 | WGKAOMQ | sea, fnbA, icaA, icaD, mecA |
| 6W | ST1 | 1 | t127 | 1-1-1-1-1-1-1 | 07-23-21-16-34-33-13 | UJFKBPE | sea, sec, seh, cna, fnbA, icaA, icaD |
| 1H | ST1963 | 101 | t714 | 69-1-14-1-11-19-3 | 04-13-21-12-17 | ZEFGM | seb, sec, seh, fnbA, icaA, icaD |
| 2H | ST8 | 8 | t7758 | 3-3-1-1-4-4-3 | 11-19-21-17-21-34-23-25 | YHFMFBJO | sea, seh, fnbA, icaA, icaD, tssT |
| 3H | ST1964 | 121 | t159 | 3-5-6-2-7-14-5 | 14-44-13-12-17-17-23-18-17 | I2Z2EGMMJH2 M | seb, seg, sei, cna, fnbA, icaA, icaD |
| 4H | ST8 | 8 | t7759 | 3-3-1-1-4-4-3 | 11-10-34-33-25 | YC2BPO | seb, seh, fnbA, icaA, icaD |
| 5H | NAb | NA | t7463 | NA | 259-25-17-17-16-16-16-17-16-16-16-16 | OMMKKKMKKKK | sea, seg, seh, sei, fnbA, icaA, icaD |
| 6H | ST30 | 30 | t318 | 2-2-2-2-6-3-2 | 15-12-16-16-02-16-02-25-17-24 | WGKKAKAOMQ | sea, seg, seh, sei, cna, fnbA, icaA, icaD |
Cultured S. aureus isolates from 1B-6B patients were characterized as bacteremia strains, S. aureus isolates from 1W-6W patients were grouped as SSTI strains, and S. aureus isolates harvested from healthy 1H-6H participants were recognized as colonizer strains.
NA, not available. Seven sequences of internal fragments of housekeeping genes in the 2B S. aureus isolate were totally different from the alleles in the database; the aroE gene of the 5H isolate was not detected in this study.
MLST is a nucleotide sequence-based characterization of isolates that uses the sequences of internal fragments of seven housekeeping genes that are translated into a specific ST for each isolate.
CCs are defined as clusters of closely related STs with up to a single difference in the allelic profile.
spa type, Staphylococcus protein A gene typing is defined by polymorphic direct repeat regions in the spa gene. The repeats are assigned a numerical code, and the order of specific repeats is translated into a specific spa type for each isolate by the Ridom system (17).
Kreiswirth ID (18) is another nomenclature-based method of spa typing that assigns letters (A to Z, A2, B2, and more) to each repeat sequence rather than a numerical code. Subsequently, the combination of all of the letters is translated into a spa type motif.
The combinations of virulence genes harbored by the isolates tested are listed in Table 1 (see also Table S2 in the supplemental material). Most of the isolates, even those with similar genetic backgrounds, harbored clearly different combinations of virulence genes (Table 1). Only a few isolates that shared the same genotype (methicillin-susceptible S. aureus ST1-t127, isolates 5B and 4W) showed the same virulence gene pattern (sea seh can fnbA icaA icaD pvl).
Acidic and neutral exoproteome of S. aureus.
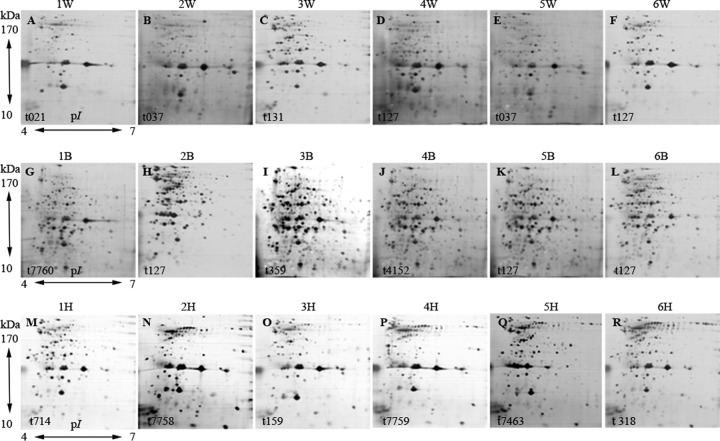
All 18 of the isolates studied showed a vast heterogeneity in their in vitro exoproteome (2-DGE). This exoprotein expression heterogeneity was more commonly observed among isolates from different infection types, even when the isolates belonged to the same clonal complex, such as CC1 (Fig. 1). In this study, we observed that at least half of the bacteremia isolates contained a large number of extracellular proteins with complex spot patterns and high-intensity signals when expressed in vitro, in contrast to SSTIs and carrier isolates (Fig. 1). Exoprotein spots specific to each infection group (either SSTI or bacteremia isolates) were very difficult to define. However, we still identified 12 exoprotein spots with moderate intensity that were expressed only by the bacteremia isolates. Eleven of these unique exoprotein spots were successfully identified by LC MS/MS (Fig. 2); these exoproteins included the chaperone protein DnaK, a phosphoglycerate kinase (SAO11_2521, also referred as Pgk), the molecular chaperone GroEL, a multisensor hybrid histidine kinase (Anae109_2543), a 3-methyl-2-oxobutanoate hydroxymethyltransferase (PanB), cysteine synthase A (SATG_02657), an N-acetyltransferase (SA21209_0471), and elongation factor Tu (EF-Tu).
FIG 1.
Characterization of in vitro expression of S. aureus exoproteins by 2-DGE. Isolates from healthy carriers expressed a pattern of acidic exoproteins (1H to 6H) completely different from the patterns of exoproteins secreted by SSTI isolates (1W to 6W) and bacteremia isolates (1B to 6B). Shown is one of three independent replicates analyzed.
FIG 2.
The proteome signatures of bacteremia isolates detected by 2-DGE.
Humoral response to S. aureus exoproteins.
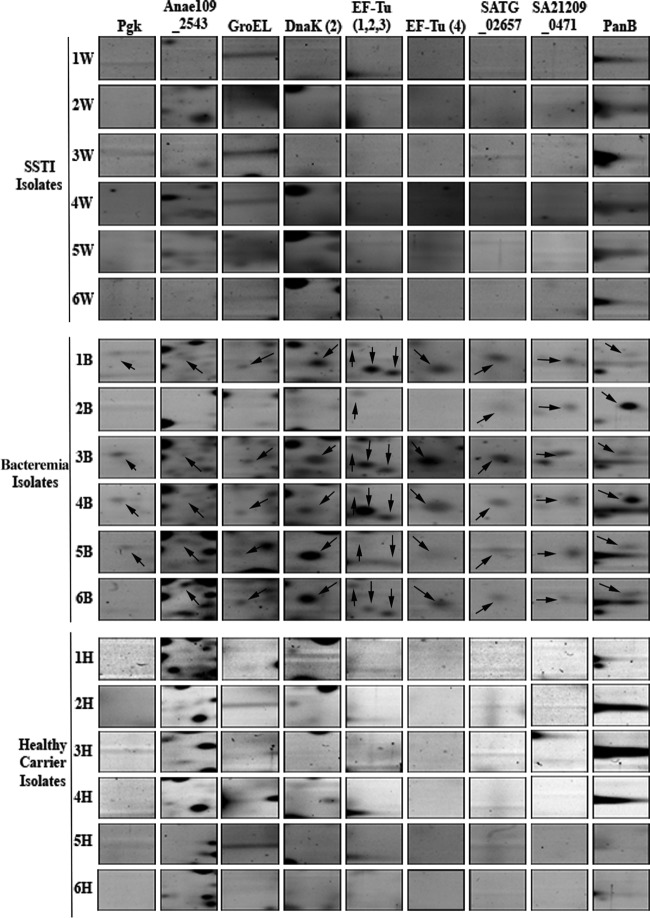
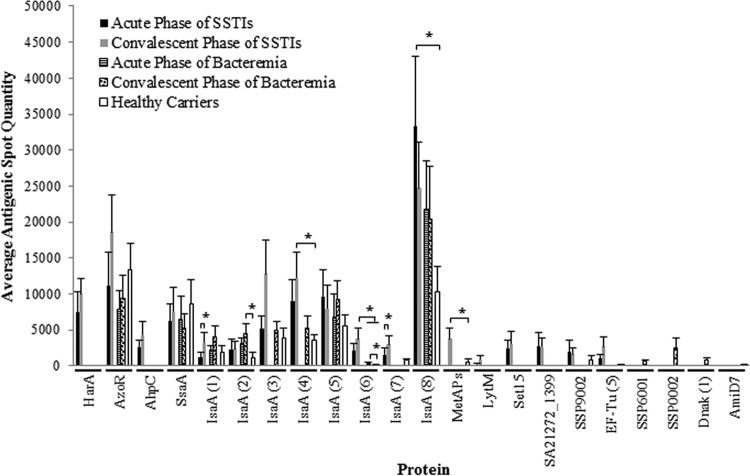
The antibodies from more than half of the participants in each group frequently reacted with nine exoprotein spots (see Fig. S1 and Tables S3 and S4 in the supplemental material). These exoproteins included staphylococcal secretory antigen (SsaA), flavin mononucleotide-dependent NADH-azoreductase (AzoR), and several isoforms of the immunodominant antigen A (IsaA). We found highly significant (P ≤ 0.05) signals for the majority of the IsaA isoforms in patients, in contrast to healthy carriers (Fig. 3; see Fig. S1 in the supplemental material). Although very few antigenic protein spots were observed in bacteremia patients, in contrast to SSTI patients and healthy carriers, bacteremia strains generated comparatively more signals during the convalescent phase (see Fig. S1 in the supplemental material). While the newly developed spots were not very intense, they correlated with various proteins, such as IsaA isoforms 3, 4, and 6 and DnaK isoform 1, as well as two unidentified proteins (SSP0002 and SSP6001). Superimposition of 2-DGE and 2D-IB images successfully identified the immunoproteome signatures of distinct infection types (see Fig. S2 in the supplemental material). For example, serum IgG against methionine aminopeptidase (MetAps), peptidoglycan hydrolase (LytM), haptoglobin-binding heme uptake protein (HarA), alkyl hydroperoxide reductase (AhpC), and exotoxin 15 (Set15) were specifically observed in SSTI patients. Healthy-carrier and SSTI isolates commonly showed antigenic proteins for EF-Tu isoform 5 and IsaA isoform 7. Two antigenic protein spots (DnaK and SSP0002) were found to be selectively present in the bacteremia group. None of the bacteremia isolates expressed Isd system proteins in vivo (Fig. 4), but these proteome signatures were found to be produced in vitro (from cultures) by both carrier and infection isolates, showing that the coding potential was there.
FIG 3.
Variability in serum IgG responses to S. aureus exoproteins. New antigenic proteins with weak signals are observed between the acute and convalescent phases of infection. *, P ≤ 0.05.
FIG 4.

Presence of a HarA (IsdH) protein spot and binding of antibodies to the HarA protein in 2-DGE and 2D-IB images. Most of the S. aureus isolates expressed the HarA protein during in vitro culture, as shown on the 2-DGE gel images. In the 2D-IB images, the HarA protein was not produced in vivo by any of the bacteremia or healthy-carrier isolates. An arrow indicates the presence of HarA, while an open circle indicates the absence of HarA.
Serum cytokine levels in patients and healthy carriers.
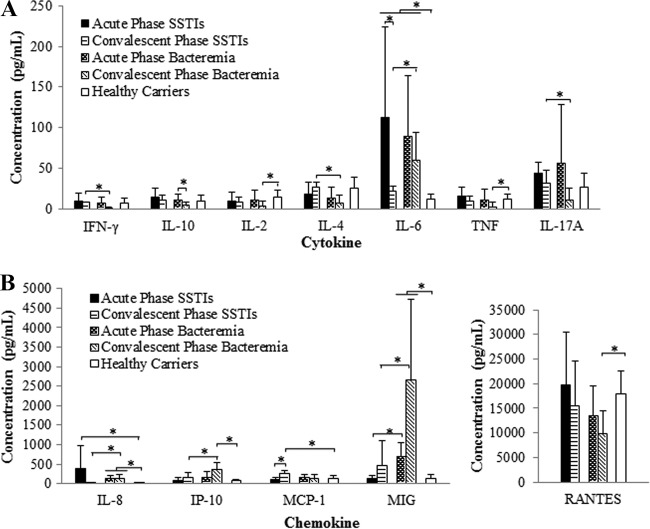
Cytokine and chemokine expression patterns were investigated to see if there were any biomarkers specific to staphylococcal infections in addition to the antigenic protein signatures corresponding to the defined infection types. As shown in Fig. 5, IL-6 expression levels were significantly higher in most of the S. aureus-infected patients than in healthy carriers (P < 0.035). Most of the patients showed reduced IL-6 levels during the convalescent phase of infection. However, bacteremia patients still displayed higher serum IL-6 levels (59.53 ± 34.48 pg/ml) than SSTI patients in the convalescent phase (21.45 ± 7.02 pg/ml, P = 0.044). We found that the majority of the S. aureus-infected patients exhibited higher concentrations of IL-17A during the acute phase of infection (Fig. 5A). In the convalescent phase, the IL-17A production in bacteremia patients was reduced 4.9-fold and was significantly different from that observed in SSTI patients (P = 0.043).
FIG 5.
Profiling of cytokines and chemokines. (A) Concentrations of the cytokines IFN-γ, IL-10, IL-2, IL-4, IL-6, TNF, and IL-17A in various groups. (B) Concentrations of the chemokines IL-8/CXCL8, IP-10/CXCL10, MCP-1/CCL2, MIG/CXCL9, and RANTES/CCL5 in various groups. *, P value (two tailed) of ≤0.05, as determined by a Mann-Whitney or Wilcoxon signed-rank test.
Serum chemokine levels in patients and healthy carriers.
Significantly higher IL-8 levels were observed in the patient groups than in healthy carriers (P = 0.016). In SSTI patients, a rise in the chemokine MCP-1 concentration (254.7 ± 91.21 pg/ml) was detected during the convalescent phase that was significantly different from the values of healthy carriers (143.59 ± 55.66 pg/ml). Conversely, elevation of MIG and IP-10 levels during the convalescent phase was observed in the bacteremia patients, and these increased levels were significantly different from the levels observed in both the SSTI group and the healthy carriers (Fig. 5B). The MIG levels in the bacteremia patients were significantly higher than those observed in SSTI patients in both the acute and convalescent phases (P = 0.036 and 0.028, respectively).
DISCUSSION
To date, several proteomic studies have been reported and this improved our understanding of staphylococcal cell physiology, virulence, and associated host responses (9, 13, 29, 30). However, most of these studies were carried out with a single strain or infection type with limited follow-up using data on patient serum antibodies. In addition, most of these studies used IPG strips with pHs ranging from 3 to 10 to cover a wide range of proteins or pH 6 to 11, as most of the immunoreactive proteins are resolved in this range (9, 15). 2-DGE in a pH range of 3 to 10 displayed a crowded appearance and low resolution of protein spots, especially for pI values between 4 and 7 (31). Thus, in this study, we compared different infection types and healthy carriers with 7-cm IPG strips at pHs of 4 to 7 by using a modified 2-DGE silver staining method (27) to get better resolution and to identify the acidic and neutral proteins for which information is still limited. For example, in this study, we identified HarA, MetAPs, Set15, AhpC, DnaK, EF-Tu, LytM, PanB, AzoR, and SsaA, which have not been identified in earlier studies that focused on the pH range of 6 to 11. In previous studies, the changes in the human serum cytokine or chemokine profiles over the disease course or between different infection types have also been relatively underexplored. It is important to target the cytokine or chemokine patterns, as these might generate novel diagnostic information to implement early and appropriate treatment. Therefore, the present study addressed SSTIs, bloodstream infections, and subclinical colonization, albeit in a small sample. Hence, our observations are exploratory and generate hypotheses that need to be validated in a larger patient cohort.
First, we performed a molecular characterization of isolates. In the limited number of isolates studied, we were not able to define any specific correlation between clones and disease types. Although two studies have reported that no single staphylococcal genotype is specifically associated with a defined infection type (32, 33), two other studies have shown CC5 and CC30 to be associated with infectious endocarditis and osteomyelitis (34, 35).
Second, the heterogeneous exoprotein expression patterns of S. aureus strains made it difficult to identify exoproteins specific to strains or particular infection types. Here we successfully identified only 12 protein spots that were specifically found in bacteremia isolates. Surprisingly, most of these proteins (EF-Tu, DnaK, PanB, Pgk, GroEL, SATG_02657, SA21209_0471, and Anae109_2543) were found to be located in the cytoplasm and play roles in protein synthesis and degradation, metabolism, stress response, amino acid biosynthesis, transcription, and replication. Possibly, this is caused by cellular autolysis in patient blood or these proteins may be released through nonclassical secretion pathways (36–38). In addition, these proteins may be involved in other functions. For example, EF-Tu is also known to be a plasminogen or fibronectin binding protein (39, 40) and might be involved in reducing the phagocytosis activity of immune cells (41). Plasminogen is a glycoprotein that circulates in the blood. In this study, EF-Tu, which binds host plasminogen on its surface, was found in strains that cause bacteremia. It was speculated that this activity could help S. aureus to disseminate in the blood and cause invasive infections. On the other hand, DnaK and GroEL are very important in ensuring the survival of S. aureus under various stress conditions such as heat, oxidative, and antibiotic stress (42, 43). For the individual bacteremia patient, the heat shock protein of S. aureus (DnaK), which mediates the solubilization of denatured protein, might have been induced in response to the elevated temperature during fever. More recently, DnaK has been shown to be involved in biofilm formation on medical devices and this contributes to intravascular-catheter-related bacteremia in patients (44). Unfortunately, there is not much information about the relevance of PanB in S. aureus. PanB has been proven to be an essential virulence factor in Mycobacterium tuberculosis pathogenesis (45). It is interesting that these data indicate that the secreted cytoplasmic proteins may also be involved in staphylococcal virulence.
Unfortunately, none of the 12 protein spots unique to the bacteremia isolates appeared to be antigenic. One of the explanations for this could be denaturation of epitopes during 2D-IB because of the reducing agent DTT. Moreover, there is no native 2D-IB approach with high resolution that could be performed. However, some proteins (such as IsaA isoforms 1 to 8) that were commonly present in both infection types and healthy carriers showed antigenicity (2D-IB) but with various intensities. This result is in agreement with data from other studies (11, 46), where serum from patients showed antistaphylococcal antibody titers higher than those of serum from healthy carriers. Therefore, it is likely that healthy carriers do not depend solely on the humoral immune responses for protection against (auto)infection.
In the present study, anti-IsaA antibody was detected in almost all individual human sera as this protein is expressed by the majority of S. aureus strains (11, 47, 48). Some of the antibodies against IsaA isoforms appeared only in the convalescent phase after bacteremia. This clearly shows that all isoforms of a protein should be taken into account during the design of vaccines or therapeutic agents. IsaA is one of the major antigens of S. aureus that are located either in the cell wall or secreted extracellularly. IsaA has a putative hydrophilic lytic domain and may have specific peptidoglycan hydrolysis activity when close to the cell wall, which indirectly assists in cell wall turnover, staphylococcal growth, and hence microbial survival (49). Therefore, IsaA is produced by most S. aureus strains during colonization or infection. Consequently, high titers of antibody to IsaA protein in patients have been reported in several earlier studies (50, 51). In addition, SsaA- and AzoR-specific IgGs were also found to be common in our study subjects. The high levels of anti-SsaA antibody observed in the 2D-IB assay were in accordance with the results of a previous study (11). The role of anti-SsaA antibody in pathogenesis is not clearly understood; however, SsaA has been postulated to be involved in biofilm-associated infections (11, 52). AzoR was recently proven to play a role in providing resistance to thiol-specific stress (53).
Here, we report for the first time the antigenicity of MetAPs and Set15 in immunoblot assays of SSTI isolates. In addition, antigenicity for LytM, AhpC, and HarA (known as IsdH or SasI) was also specifically found in SSTI isolates. The presence of antigenicity indicates that certain exoproteins are expressed in vivo and may be prerequisites for infection. For example, MetAPs play a main role in protein maturation by catalyzing N-terminal methionine excision from newly synthesized proteins. Inhibitors of MetAPs have been shown to significantly limit the growth of MRSA (54). Additionally, MetAPs were also reported to be involved in the recycling pathway of methionine and production of polyamines which are important for SSTIs (55, 56). Although very few data on Set15 are available, it has been shown to be immunoreactive and important in the survival of S. aureus. set15 mutants isolated from murine kidney abscesses were found to be low in number compared with the wild type (57, 58). It was recently demonstrated that Set proteins act as inhibitors of polymorphonuclear leukocyte adherence. Consequently, this might prevent the recruitment of circulating neutrophils to the site of skin infection. Infected skin produces hydrogen peroxide (H2O2), which is an important factor in wound healing (59). Therefore, S. aureus might produce AhpC to protect itself against H2O2 (60). In the present study, antibody against LytM was detected in patients with SSTIs, but only during the convalescent phase. A previous study has shown that higher levels of IgGs against LytM may prevent an S. aureus infection from becoming bacteremia (61), and none of our SSTI patients progressed to bacteremia. Interestingly, HarA was found to be produced only in vivo by SSTI isolates. HarA is a protein in the Isd system (including IsdABCDEFGI) that is upregulated under iron-restricted conditions. HarA has also been shown to protect S. aureus from opsonophagocytosis, and it inhibits neutrophil rolling on the endothelial surface of blood vessels close to the site of infection (62). Immunization with IsdH induced protection against S. aureus mastitis (63). However, Isd was not shown to be antigenic in all of the patients and controls in our study. This is in agreement with the previous findings of Kolata et al. demonstrating that Isd proteins such as IsdH, IsdA, and IsdB could not been identified in bacteremia patients (15). Taken together, these results may help to explain the failure of V710 (vaccine containing IsdB) in clinical trials (64). Vaccine preparations using Isd proteins should be reevaluated to determine their coverage against an array of staphylococcal infections.
Ultimately, the kinetics of cytokine and chemokine production indicated that a variety of human immune responses may contribute to the outcome of S. aureus-human interactions. The serum IL-6 levels were higher in patients than in healthy carriers. IL-6 plays a major role in inflammation and acute-phase reactions, affecting both B and T cells (65, 66). The IL-17A level was significantly higher in SSTI patients than in bacteremia patients, even after 2 weeks of infection. An increase in the IL-17A titer during cutaneous S. aureus infections has been previously described (67). Abnormal functioning of IL-17A may increase host susceptibility to S. aureus skin infection (68, 69). IL-17A stimulates the production of proinflammatory cytokines, chemokines, and adhesion molecules in keratinocytes to promote neutrophil maturation and to recruit additional neutrophils to infection sites (70). Overall, the production of cytokines may trigger the release of chemokines to recruit additional immune cells to the infection site.
We found that bacteremia patients possessed higher average MIG and IP-10 titers than SSTI patients and healthy carriers during the convalescent phase. This result is in agreement with previous reports showing that both of these specific chemokines are highly induced in S. aureus bacteremia, where they act as specific chemoattractants for Th1 cells (71–73). Luster et al. showed that elevated expression of IP-10 in the epidermis causes delayed wound healing (74).
Significantly elevated MIG titers in bacteremia patients during the acute phase suggest that MIG could be a candidate diagnostic marker for early detection of bacteremia. However, further investigation is warranted to confirm its significance, especially during early stages of bacteremia. Similarly, the convalescent-phase MCP-1 level in SSTI patients was significantly higher than that in healthy carriers. Our study represents the first demonstration of MCP-1 expression in SSTI patients. MCP-1 is known to be crucial for polarizing Th cells toward Th2 differentiation (73).
Our study confirmed that the in vitro and in vivo expression of S. aureus exoproteins is highly variable, irrespective of strain relatedness, and documents new common protein signatures that warrant further study. Most of the immunoproteome signatures were found to be produced in vitro by most of the isolates. The differential expression of Isd proteins in different infection types should be taken into consideration when designing future therapeutics. The diversity in the cytokine and chemokine response pattern will aid the understanding of staphylococcal disease types over the course of infection. Production of the chemokine MIG was found to be significantly high during bacteremia onset, which shows its potential for use as a marker for early diagnosis. However, whether MIG is specific for S. aureus or whether it could be used as a more generic marker of bacterial sepsis must be evaluated by using larger sample panels. In conclusion, we confirm here that the interactions between S. aureus and its human host are very complex. The fact that we did not find reliable prospective infection markers suggests that widening the scope of the present study will, in the end, result in the definition of highly individual infection and colonization scenarios that will be difficult to predict on the basis of both bacterial and host marker molecules. Although our current understanding of the importance of proteome signatures for bacteremia and SSTIs is incomplete, the present study has revealed the pattern of protein expression during the course of an infection. In light of the observations listed above, these protein signatures need to be validated in large populations comprising different infection types. Each protein signature should then be characterized by using in vivo models to determine its potential for use as a vaccine or a biomarker. Overall, this strategy has important clinical implications since targeting of proteome signatures shows promise as a preventive and therapeutic strategy to achieve complete bacterial clearance.
ACKNOWLEDGMENTS
Special thanks to Julia Kolata, University of Greifswald, Greifswald, Germany, for her excellent technical assistance in 2D-IB. We are grateful to Richard Goering from Creighton University Medical Center, Omaha, NE, for commenting on and proofreading the manuscript.
This work was supported by Universiti Putra Malaysia through the Research University Grant Scheme (04-02-12-1756RU).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00493-14.
REFERENCES
- 1.Gordon RJ, Lowy FD. 2008. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David MZ, Glikman D, Crawford SE, Peng J, King KJ, Hostetler MA, Boyle-Vavra S, Daum RS. 2008. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis 197:1235–1243. doi: 10.1086/533502. [DOI] [PubMed] [Google Scholar]
- 4.Mongkolrattanothai K, Aldag JC, Mankin P, Gray BM. 2009. Epidemiology of community-onset Staphylococcus aureus infections in pediatric patients: an experience at a Children's Hospital in central Illinois. BMC Infect Dis 9:112. doi: 10.1186/1471-2334-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung GYC, Duong AC, Otto M. 2012. Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microb Infect 14:380–386. doi: 10.1016/j.micinf.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. 2011. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol 79:814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Du X, Villaruz AE, Diep BA, Wang D, Song Y, Tian Y, Hu J, Yu F, Lu Y, Otto M. 2012. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med 18:816–819. doi: 10.1038/nm.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Q, Han L, Li B, Sun J, Ni Y. 2012. Virulence characteristic and MLST-agr genetic background of high-level mupirocin-resistant, MRSA isolates from Shanghai and Wenzhou, China. PLoS One 7:e37005. doi: 10.1371/journal.pone.0037005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziebandt A, Kusch H, Degner M, Jaglitz S, Sibbald MJ, Arends JP, Chlebowicz MA, Albrecht D, Pantucek R, Doskar J, Ziebuhr W, Bröker BM, Hecker M, van Dijl JM, Engelmann S. 2010. Proteomics uncovers extreme heterogeneity in the Staphylococcus aureus exoproteome due to genomic plasticity and variant gene regulation. Proteomics 10:1634–1644. doi: 10.1002/pmic.200900313. [DOI] [PubMed] [Google Scholar]
- 10.Prevaes SM, van Wamel WJ, de Vogel CP, Veenhoven RH, van Gils EJ, van Belkum A, Sanders EA, Bogaert D. 2012. Nasopharyngeal colonization elicits antibody responses to staphylococcal and pneumococcal proteins that are not associated with a reduced risk of subsequent carriage. Infect Immun 80:2186–2193. doi: 10.1128/IAI.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtfreter S, Nguyen TT, Wertheim H, Steil L, Kusch H, Truong QP, Engelmann S, Hecker M, Völker U, van Belkum A, Bröker BM. 2009. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin Vaccine Immunol 16:1607–1614. doi: 10.1128/CVI.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, DeLeo FR. 2012. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis 206:1185–1193. doi: 10.1093/infdis/jis495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. 2012. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS One 7:e46648. doi: 10.1371/journal.pone.0046648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtfreter S, Jursa-Kulesza J, Masiuk H, Verkaik NJ, de Vogel C, Kolata J, Nowosiad M, Steil L, van Wamel W, van Belkum A, Völker U, Giedrys-Kalemba S, Bröker BM. 2011. Antibody responses in furunculosis patients vaccinated with autologous formalin-killed Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 30:707–717. doi: 10.1007/s10096-010-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolata J, Bode LG, Holtfreter S, Steil L, Kusch H, Holtfreter B, Albrecht D, Hecker M, Engelmann S, van Belkum A, Völker U, Bröker BM. 2011. Distinctive patterns in the human antibody response to Staphylococcus aureus bacteremia in carriers and non-carriers. Proteomics 11:3914–3927. doi: 10.1002/pmic.201000760. [DOI] [PubMed] [Google Scholar]
- 16.Ghaznavi-Rad E, Nor Shamsudin M, Sekawi Z, van Belkum A, Neela V. 2010. A simplified multiplex PCR assay for fast and easy discrimination of globally distributed staphylococcal cassette chromosome mec types in meticillin-resistant Staphylococcus aureus. J Med Microbiol 59:1135–1139. doi: 10.1099/jmm.0.021956-0. [DOI] [PubMed] [Google Scholar]
- 17.Enright MC, Day NPJ, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S, Kreiswirth BN. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol 37:3556–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goering RV. 2010. Pulsed field gel electrophoresis: a review of application and interpretation in the molecular epidemiology of infectious disease. Infect Genet Evol 10:866–875. doi: 10.1016/j.meegid.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Montanaro L, Arciola CR, Baldassarri L, Borsetti E. 1999. Presence and expression of collagen adhesin gene (cna) and slime production in Staphylococcus aureus strains from orthopaedic prosthesis infections. Biomaterials 20:1945–1949. doi: 10.1016/S0142-9612(99)00099-X. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Xiong ZY, Li HP, Zheng YL, Jiang YQ. 2006. An immunogenicity study of a newly fusion protein Cna-FnBP vaccinated against Staphylococcus aureus infections in a mice model. Vaccine 24:4830–4837. doi: 10.1016/j.vaccine.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Arciola CR, Baldassarri L, Montanaro L. 2001. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol 39:2151–2156. doi: 10.1128/JCM.39.6.2151-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 24.Ellington MJ, Yearwood L, Ganner M, East C, Kearns AM. 2008. Distribution of the ACME-arcA gene among methicillin-resistant Staphylococcus aureus from England and Wales. J Antimicrob Chemother 61:73–77. doi: 10.1093/jac/dkm422. [DOI] [PubMed] [Google Scholar]
- 25.Jarraud S, Cozon G, Vandenesch F, Bes M, Etienne J, Lina G. 1999. Involvement of enterotoxins G and I in staphylococcal toxic shock syndrome and staphylococcal scarlet fever. J Clin Microbiol 37:2446–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson WM, Tyler SD, Ewan EP, Ashton FE, Pollard DR, Rozee KR. 1991. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol 29:426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liew YK, Neela V, Hamat RA, Nordin SA, Chong PP. 2013. Modified silver staining in 2DE improves protein detection even at extremely low sample concentration. Electrophoresis 34:397–400. doi: 10.1002/elps.201200380. [DOI] [PubMed] [Google Scholar]
- 28.Shevchenko A, Wilm M, Vorm O, Mann M. 1996. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 29.Kohler C, von Eiff C, Peters G, Proctor RA, Hecker M, Engelmann S. 2003. Physiological characterization of a heme-deficient mutant of Staphylococcus aureus by a proteomic approach. J Bacteriol 185:6928–6937. doi: 10.1128/JB.185.23.6928-6937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziebandt A, Weber H, Rudolph J, Schmid R, Höper D, Engelmann S, Hecker M. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and sigma B. Proteomics 1:480–493. doi:. [DOI] [PubMed] [Google Scholar]
- 31.Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. 2006. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun 74:3415–3426. doi: 10.1128/IAI.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melles DC, Gorkink RF, Boelens HA, Snijders SV, Peeters JK, Moorhouse MJ, van der Spek PJ, van Leeuwen WB, Simons G, Verbrugh HA, van Belkum A. 2004. Natural population dynamics and expansion of pathogenic clones of Staphylococcus aureus. J Clin Invest 114:1732–1740. doi: 10.1172/JCI200423083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim KT, Yeo CC, Suhaili Z, Thong KL. 2012. Comparison of methicillin-resistant and methicillin-sensitive Staphylococcus aureus strains isolated from a tertiary hospital in Terengganu, Malaysia. Jpn J Infect Dis 65:502–509. doi: 10.7883/yoken.65.502. [DOI] [PubMed] [Google Scholar]
- 34.Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, Barriere S, Woods CW, Chu VH, Marín M, Bukovski S, Garcia P, Corey GR, Korman T, Doco-Lecompte T, Murdoch DR, Reller LB, Fowler VG. 2011. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis 204:704–713. doi: 10.1093/infdis/jir389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fowler VG, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, Federspiel J, Naidich S, Remortel B, Rude T, Brown P, Reller LB, Corey GR, Gill SR. 2007. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis 196:738–747. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 36.Enany S, Yoshida Y, Magdeldin S, Zhang Y, Bo X, Yamamoto T. 2012. Extensive proteomic profiling of the secretome of European community acquired methicillin resistant Staphylococcus aureus clone. Peptides 37:128–137. doi: 10.1016/j.peptides.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Yang C, Ewis HE, Zhang X, Lu C, Hu H, Pan Y, Abdelal AT, Tai PC. 2011. Nonclassical protein secretion by Bacillus subtilis in the stationary phase is not due to cell lysis. J Bacteriol 193:5607–5615. doi: 10.1128/JB.05897-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieves W, Heang J, Asakrah S, Höner zu Bentrup K, Roy CJ, Morici LA. 2010. Immunospecific responses to bacterial elongation factor Tu during Burkholderia infection and immunization. PLoS One 5:e14361. doi: 10.1371/journal.pone.0014361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dallo SF, Kannan TR, Blaylock MW, Baseman JB. 2002. Elongation factor Tu and E1 β subunit of pyruvate dehydrogenase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Mol Microbiol 46:1041–1051. doi: 10.1046/j.1365-2958.2002.03207.x. [DOI] [PubMed] [Google Scholar]
- 40.Schaumburg J, Diekmann O, Hagendorff P, Bergmann S, Rohde M, Hammerschmidt S, Jänsch L, Wehland J, Kärst U. 2004. The cell wall subproteome of Listeria monocytogenes. Proteomics 4:2991–3006. doi: 10.1002/pmic.200400928. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi M, Terao Y, Mori Y, Hamada S, Kawabata S. 2008. PfbA, a novel plasmin- and fibronectin-binding protein of Streptococcus pneumoniae, contributes to fibronectin-dependent adhesion and antiphagocytosis. J Biol Chem 283:36272–36279. doi: 10.1074/jbc.M807087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh VK, Jayaswal RK, Wilkinson BJ. 2001. Cell wall-active antibiotic induced proteins of Staphylococcus aureus identified using a proteomic approach. FEMS Microbiol Lett 199:79–84. doi: 10.1111/j.1574-6968.2001.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 43.Kubota H, Hynes G, Willison K. 1995. The chaperonin containing t-complex polypeptide 1 (TCP-1). Eur J Biochem 230:3–16. doi: 10.1111/j.1432-1033.1995.tb20527.x. [DOI] [PubMed] [Google Scholar]
- 44.Singh VK, Syring M, Singh A, Singhal K, Dalecki A, Johansson T. 2012. An insight into the significance of the DnaK heat shock system in Staphylococcus aureus. Int J Med Microbiol 302:242–252. doi: 10.1016/j.ijmm.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Pearce MJ, Arora P, Festa RA, Butler-Wu SM, Gokhale RS, Darwin KH. 2006. Identification of substrates of the Mycobacterium tuberculosis proteasome. EMBO J 25:5423–5432. doi: 10.1038/sj.emboj.7601405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, Hooijkaas H, Foster TJ, Verbrugh HA, van Belkum A, van Wamel WJ. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis 199:625–632. doi: 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 47.Liew YK, Neela V. 2012. Comparative exoproteome profile of MRSA-ST9 isolated from pigs and pig handler. Indian J Microbiol 52:507–509. doi: 10.1007/s12088-012-0262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorenz U, Ohlsen K, Karch H, Thiede A, Hacker J. 2000. Immunodominant proteins in human sepsis caused by methicillin resistant Staphylococcus aureus. Adv Exp Med Biol 485:273–278. doi: 10.1007/0-306-46840-9_36. [DOI] [PubMed] [Google Scholar]
- 49.Stapleton MR, Horsburgh MJ, Hayhurst EJ, Wright L, Jonsson I, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. 2007. Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J Bacteriol 189:7316–7325. doi: 10.1128/JB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke SR, Brummell KJ, Horsburgh MJ, McDowell PW, Mohamad SA, Stapleton MR, Acevedo J, Read RC, Day NP, Peacock SJ, Mond JJ, Kokai-Kun JF, Foster SJ. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis 193:1098–1108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 51.Vytvytska O, Nagy E, Blüggel M, Meyer HE, Kurzbauer R, Huber LA, Klade CS. 2002. Identification of vaccine candidate antigens of Staphylococcus aureus by serological proteome analysis. Proteomics 2:580–590. doi:. [DOI] [PubMed] [Google Scholar]
- 52.Lang S, Livesley MA, Lambert PA, Littler WA, Elliott TS. 2000. Identification of a novel antigen from Staphylococcus epidermidis. FEMS Immunol Med Microbiol 29:213–220. doi: 10.1111/j.1574-695X.2000.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu G, Zhou J, Fu QS, Wang J. 2009. The Escherichia coli azoreductase AzoR is involved in resistance to thiol-specific stress caused by electrophilic quinones. J Bacteriol 191:6394–6400. doi: 10.1128/JB.00552-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chai SC, Wang W, Ding D, Ye Q. 2011. Growth inhibition of Escherichia coli and methicillin-resistant Staphylococcus aureus by targeting cellular methionine aminopeptidase. Eur J Med Chem 46:3537–3540. doi: 10.1016/j.ejmech.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kao RY, Yuen KY, Che CM, Siu FM. 2011. Methionine aminopeptidase as a novel target for antibiotic therapy against Staphylococcus aureus: a proteomic approach. Hong Kong Med J 17(Suppl 2):S29–S31. [PubMed] [Google Scholar]
- 56.Bao Y, Li Y, Jiang Q, Zhao L, Xue T, Hu B, Sun B. 2013. Methylthioadenosine/S-adenosylhomocysteine nucleosidase (Pfs) of Staphylococcus aureus is essential for the virulence independent of LuxS/AI-2 system. Int J Med Microbiol 303:190–200. doi: 10.1016/j.ijmm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Langley R, Arcus V, Fraser J. 2003. Virulence factors from Staphylococcus aureus: tools to study innate and adaptive immunity. Aust Biochemist 34:11–18. [Google Scholar]
- 58.Schneider WP, Ho SK, Christine J, Yao M, Marra A, Hromockyj AE. 2002. Virulence gene identification by differential fluorescence induction analysis of Staphylococcus aureus gene expression during infection-simulating culture. Infect Immun 70:1326–1333. doi: 10.1128/IAI.70.3.1326-1333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loo AE, Ho R, Halliwell B. 2011. Mechanism of hydrogen peroxide-induced keratinocyte migration in a scratch-wound model. Free Radic Biol Med 51:884–892. doi: 10.1016/j.freeradbiomed.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Cosgrove K, Coutts G, Jonsson I, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. 2007. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol 189:1025–1035. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szweda P, Schielmann M, Kotlowski R, Gorczyca G, Zalewska M, Milewski S. 2012. Peptidoglycan hydrolases—potential weapons against Staphylococcus aureus. Appl Microbiol Biotechnol 96:1157–1174. doi: 10.1007/s00253-012-4484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Visai L, Yanagisawa N, Josefsson E, Tarkowski A, Pezzali I, Rooijakkers SH, Foster TJ, Speziale P. 2009. Immune evasion by Staphylococcus aureus conferred by iron-regulated surface determinant protein IsdH. Microbiology 155:667–679. doi: 10.1099/mic.0.025684-0. [DOI] [PubMed] [Google Scholar]
- 63.Ster C, Beaudoin F, Diarra MS, Jacques M, Malouin F, Lacasse P. 2010. Evaluation of some Staphylococcus aureus iron-regulated proteins as vaccine targets. Vet Immunol Immunopathol 136:311–318. doi: 10.1016/j.vetimm.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A. 2013. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309:1368–1378. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 65.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. 1987. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A 84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu G, Xia JH, Zhou H, Yu CZ, Zhang Y, Zuo KJ, Shi JB, Li HB. 2009. Interleukin-6 is essential for staphylococcal exotoxin B-induced T regulatory cell insufficiency in nasal polyps. Clin Exp Allergy 39:829–837. doi: 10.1111/j.1365-2222.2009.03218.x. [DOI] [PubMed] [Google Scholar]
- 67.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schäffer AA, Puck JM, Grimbacher B. 2007. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 69.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y. 2012. Compartmentalized control of skin immunity by resident commensals. Science 337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 cells. Annu Rev Immunol 27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 71.Lauw FN, Simpson AJH, Prins JM, van Deventer SJH, Chaowagul W, White NJ, van der Poll T. 2000. The CXC chemokines gamma interferon (IFN-γ)-inducible protein 10 and monokine induced by IFN-γ are released during severe melioidosis. Infect Immun 68:3888–3893. doi: 10.1128/IAI.68.7.3888-3893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng PC, Li K, Chui KM, Leung TF, Wong RPO, Chu WC, Wong E, Fok TF. 2007. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res 61:93–98. doi: 10.1203/01.pdr.0000250207.95723.96. [DOI] [PubMed] [Google Scholar]
- 73.Esche C, Stellato C, Beck LA. 2005. Chemokines: key players in innate and adaptive immunity. J Investig Dermatol 125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- 74.Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. 1998. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Physicians 110:183–196. [PubMed] [Google Scholar]