Abstract
Urgently needed species-specific enzyme-linked immunosorbent assays (ELISAs) for the detection of antibodies against Chlamydia spp. have been elusive due to high cross-reactivity of chlamydial antigens. To identify Chlamydia species-specific B cell epitopes for such assays, we ranked the potential epitopes of immunodominant chlamydial proteins that are polymorphic among all Chlamydia species. High-scoring peptides were synthesized with N-terminal biotin, followed by a serine-glycine-serine-glycine spacer, immobilized onto streptavidin-coated microtiter plates, and tested with mono-specific mouse hyperimmune sera against each Chlamydia species in chemiluminescent ELISAs. For each of nine Chlamydia species, three to nine dominant polymorphic B cell epitope regions were identified on OmpA, CT618, PmpD, IncA, CT529, CT442, IncG, Omp2, TarP, and IncE proteins. Peptides corresponding to 16- to 40-amino-acid species-specific sequences of these epitopes reacted highly and with absolute specificity with homologous, but not heterologous, Chlamydia monospecies-specific sera. Host-independent reactivity of such epitopes was confirmed by testing of six C. pecorum-specific peptides from five proteins with C. pecorum-reactive sera from cattle, the natural host of C. pecorum. The probability of cross-reactivity of peptide antigens from closely related chlamydial species or strains correlated with percent sequence identity and declined to zero at <50% sequence identity. Thus, phylograms of B cell epitope regions predict the specificity of peptide antigens for rational use in the genus-, species-, or serovar-specific molecular serology of Chlamydia spp. We anticipate that these peptide antigens will improve chlamydial serology by providing easily accessible assays to nonspecialist laboratories. Our approach also lends itself to the identification of relevant epitopes of other microbial pathogens.
INTRODUCTION
Chlamydiae are obligate intracellular bacteria that replicate in eukaryotic cells within membrane-bound vacuoles (1). Infectious, but nonreplicating elementary bodies (EBs) and noninfectious, but metabolically active reticulate bodies are two unique physiological forms for chlamydiae (1). Chlamydia spp. cause a variety of diseases in humans, other mammals, and birds (1). Until very recently, nine species were recognized, including Chlamydia abortus, C. psittaci, C. caviae, C. felis, C. pecorum, C. pneumoniae, C. muridarum, C. suis, and C. trachomatis (1). In 2014, new species, C. avium and C. gallinacea, were published (2), which are not part of the present study. In 1999, Everett et al. proposed to subdivide Chlamydiaceae into two genera, Chlamydia (consisting of C. muridarum, C. suis, and C. trachomatis) and Chlamydophila (consisting of the remaining six species) (3). This subdivision has now been formally reversed to a single Chlamydia genus consisting of 11 Chlamydia spp. (4).
Numerous serovars of C. suis (5), C. pecorum (6, 7), C. trachomatis (8, 9), and C. psittaci (10–12) have been reported. Different serovars of a species cause multiple diseases in a single host, e.g., serovars of C. trachomatis cause trachoma, infections of reproductive organs, or lymphogranuloma venereum in humans (1). Single hosts can also be infected by multiple Chlamydia spp., e.g., humans may be infected by both respiratory transmitted C. pneumoniae and sexually transmitted C. trachomatis (1) or by C. trachomatis and C. psittaci in the case of trachoma patients (13). Antibodies produced against one species strongly cross-react with other species, making interpretation of serological assays difficult (14). For instance, anti-C. pneumoniae antibodies may interfere with the diagnosis of sexually transmitted diseases caused by C. trachomatis due to cross-reactivity of chlamydial antigens in serological assays (15–18).
Acute, chronic, or asymptomatic infections with C. trachomatis and C. pneumoniae have a significant impact on human health (19). Infections with all Chlamydia spp. may occur with epidemic to endemic prevalence, with sporadic, subclinical, and occasional clinical manifestations in a wide range of animal hosts, resulting in a significant economic impact on animal production (20, 21). The occasional transmission of Chlamydia spp. from animals to humans has been reported (1). Specific serological assays to detect anti-Chlamydia antibodies are important to providing differential diagnoses of chlamydial infections for patient care and to understanding chlamydial diseases and epidemiology.
The microimmunofluorescence (MIF) test is the standard serological assay for species-specific detection of antibodies against chlamydiae (22). Detection of specific antichlamydial antibodies for nine species and their serovars using the MIF test requires cumbersome production of antigens by growing these Chlamydia spp. and their numerous strains in cell culture or developing chicken embryos. Standardization of the MIF test also requires technical expertise in microscopy and antigen preparation that is available only in reference laboratories. Nevertheless, poor sensitivity and cross-reactivity of the MIF test have been reported (15–18, 23, 24). Simple and high-throughput methods are typically unsuitable for species- or serovar-specific antichlamydial antibody detection because of high cross-reactivity of standard chlamydial antigens in serological assays, such as whole Chlamydia EBs, lysed EBs, immunodominant proteins, or lipopolysaccharide (23–25).
Chlamydia genus-, species-, subspecies-, and serotype-specific B cell epitopes have been mapped before to the four variable domains of the outer membrane protein A (OmpA) by use of monoclonal antibodies (26, 27), recombinant protein fragments (28–31), and synthetic peptides (32–36). Based on these epitope mapping studies, synthetic OmpA peptides were tested for Chlamydia species-specific serology (23–25, 37, 38). However, these studies used peptides as short as 6 to 10 amino acids in length and did not use spacers between solid support and peptide to minimize steric hindrance of antibody binding (32–36). Recent computational studies of antigen-antibody complex 3D structures showed that 15- to 25-amino-acid (aa) residues of an epitope are structurally involved in antibody binding (39–42). Short 6- to 10-aa peptides tend to capture only antibodies binding to linear epitopes composed of adjacent functional residues that comprise <10% of all epitopes (42). In contrast, longer peptides capture antibodies binding to conformational epitopes with functionally binding residues spaced apart over 16- to 30-aa sequences that comprise 55 to 80% of all epitopes (39). The presence of all functional residues of an epitope also contributes to high-affinity antibody binding (42). Thus, the probability of high-affinity binding is proportional to the length of a peptide antigen. These recent data suggest that previous studies failed to achieve high sensitivity (23–25, 43, 44), which is most likely due to weak antibody binding to the short peptides used (32–36) and to steric hindrance of antibody binding to these peptides (45).
The long-term objectives in the present study were to identify unique B cell epitopes among the complete proteomes of nine Chlamydia species and to use these peptides in specific and sensitive Chlamydia species-specific enzyme-linked immunosorbent assays (ELISAs). This goal is now facilitated by the availability of complete genome sequences of all chlamydial species and of many serovars of some species (46–57), by proteome-wide mapping of immunodominant proteins in several chlamydial species (58–66), and by advanced computational tools for B cell epitope discovery (39–42). Using a murine respiratory infection model, we report here peptide-based molecular serology for nine Chlamydia spp. in a robust and high-throughput ELISA format by identifying immunodominant B cell epitopes and determining their specific reactivities with mouse hyperimmune sera against these nine Chlamydia species. With sera from cattle naturally infected with C. pecorum we also confirmed the suitability of these assays for chlamydial seroepidemiology.
MATERIALS AND METHODS
Chlamydia strains.
Of the nine Chlamydia spp. (1), the strains C. abortus B577 (ATCC VR 656, DSMZ DSM 27654), C. pecorum E58 (ATCC VR 628), C. pneumoniae CDC/CWL-029 (ATCC VR-1310), and C. trachomatis D UW-3/CX (ATCC VR 885, DSMZ DSM 19411) were grown on buffalo green monkey kidney (BGMK) cells, and elementary bodies (EBs) were purified and stored at −80°C (67). Strains of the remaining five Chlamydia spp., i.e., C. psittaci 02DC15 (DSMZ DSM 27008), C. caviae GPIC (ATCC VR 813, DSMZ DSM 19441), C. felis 02DC26 (Collection FLI, Jena, Germany), C. muridarum MoPn/Nigg (ATCC VR 123, DSMZ DSM 28544), and C. suis 99DC3 (collection FLI), were propagated in developing chicken embryos, and infected yolk sacs were homogenized in sucrose phosphate glutamate (SPG) buffer with disposable tissue grinders and stored at −80°C (68). Chlamydial genome copy numbers in stocks were quantified by Chlamydia spp. 23S rRNA PCR (69, 70).
Chlamydia species monospecific mouse hyperimmune sera.
A/J and BALB/c mice were used to generate sera against each of the nine chlamydial species. Mice were inoculated intranasally under light isoflurane anesthesia with 20-μl chlamydial stocks (71). High doses of SPG-diluted chlamydiae (103 to 108 genomes/mouse) were inoculated three times at 4- to 6-week intervals to produce high-titer, high-affinity IgG antibodies against chlamydial antigens encountered during natural Chlamydia infection. To minimize the losses of mice, particularly for highly virulent strains, doses at the low end of the used spectrum were chosen for the first inoculation (1.0 × 107, 4.9 × 104, 1.3 × 105, 1.4 × 105, 3.6 × 108, 1.3 × 106, 9.7 × 102, 2.6 × 104, and 5.0 × 107 genomes of C. abortus, C. psittaci, C. caviae, C. felis, C. pecorum, C. pneumoniae, C. muridarum, C. suis, and C. trachomatis, respectively). To maximize the antigenic stimulus, these doses were increased by 2- to 10-fold in the second inoculation (2.0 × 107, 4.9 × 105, 1.3 × 106, 5.6 × 105, 3.6 × 108, 5.0 × 106, 3.9 × 103, 1.3 × 105, and 1.0 × 108 genomes, respectively) and third inoculation (4.0 × 107, 4.9 × 105, 1.3 × 106, 2.8 × 106, 3.6 × 108, 5.0 × 106, 9.7 × 103, 6.5 × 105, and 2.0 × 108 genomes, respectively). Mice were exsanguinated 3 weeks after the last inoculation by axillary cut-down under anesthesia. Heparin plasma samples (termed sera from here forth) were collected in microtainer tubes with a gel band (Becton Dickinson, Franklin Lakes, NJ) by centrifugation at 3,000 rpm and stored at −80°C. In the final species-specific serum pools, totals of 47 C. abortus-, 12 C. psittaci-, 9 C. caviae-, 48 C. felis-, 50 C. pecorum-, 39 C. pneumoniae-, 28 C. muridarum-, 28 C. suis-, and 47 C. trachomatis-specific sera were combined. Of these, 18 sera for C. abortus, 8 for C. psittaci, 48 for C. felis, 10 for C. muridarum, and 30 for C. trachomatis were from obtained BALB/c mice, and the remaining sera were obtained from A/J mice. All animal experimental protocols were approved by the Institutional Animal Care and Use Committees at Auburn University and Friedrich-Loeffler-Institut.
Bovine anti-C. pecorum sera.
Sera were collected from cows that had experienced multiple episodes of natural infection with multiple C. pecorum strains (72), while calves were sampled between 11 and 15 weeks of age, after serum colostrum antibodies had disappeared and the calves had experienced a first episode of C. pecorum infection (20). The C. pecorum infection in all calves and cows had been confirmed by the detection of chlamydial DNA by real-time PCR for the Chlamydia 23S rRNA gene and by genotyping of infecting C. pecorum strains by Chlamydia OmpA PCR and DNA sequencing (20, 72).
Chlamydial peptide sequences for epitope discovery.
Matching type strains and genome sequences were available and used for raising hyperimmune sera for five chlamydial species: C. trachomatis (strain D/UW-3/CX) (46), C. pneumoniae (strain CWL029) (47), C. muridarum (strain Nigg) (48), C. pecorum (strain E58) (49), C. caviae (strain GPIC) (50), and peptides were designed from the type strain proteomes. The genome of the non-type C. psittaci strain 02DC15 used in this investigation was available and virtually identical to type strain 6BC (51–53), and peptides were designed from the 02DC15 proteome. For C. abortus type strain B577 and C. felis non-type strain 02DC26 used for raising antisera, the genome sequences were not available. Instead, the genomes of C. abortus strain S26/3 (54) and of C. felis strain Fe/C-56 (55) were used to design peptide antigens. Compared to these strains with known genomes, the strains used to raise antisera had an identical OmpA sequence (73) or an OmpA with a single polymorphism (C. felis Fe/C-56; GenBank accession number KP165540), and these species are also known to show minimal strain diversity based on available genomes and multilocus sequence typing (NCBI GenBank database). As the last one of the nine chlamydial species, the first complete genome of C. suis non-type strain MD56 has only recently become available (56). Initial sequences used in the alignment were deduced from the incomplete genome of type strain S45 (G. Myers, unpublished data). For raising hyperimmune sera, C. suis strain 99DC3 was used. Because of the high diversity of C. suis strains (5), peptides of antibody-reactive regions of C. suis strain 99DC3 were confirmed by DNA sequencing. The sequences of the gene fragments of ompA, omp2, pmpD, incA, and incG and of C. suis 99DC3 homologs to CT529 and CT618 are available at GenBank (accession numbers KP165534 to KP165539 and KP165542).
Bioinformatic analyses of chlamydial genomes, immunodominant proteins, and B cell epitopes.
To identify species-specific B cell epitopes, we first identified and ranked 72 immunodominant proteins among all chlamydial proteomes (46–57), based on published data (58–66, 74–82). These protein sequences of all nine chlamydial species, and of serovars among species, were first aligned in the freeware Jalview (83) by use of the MUSCLE algorithm and weights for amino acid substitutions based on the Blosum62 AA substitution matrix (83–85). Alignments were optimized by varying alignment parameters, in particular by increasing gap opening and extension penalties or by manual editing. Polymorphic regions suitable for identification of species-specific epitope candidates were further subjected to in silico B cell epitope analyses. Optimal predictive algorithms based on recent knowledge of B cell epitope structure and length (39–42) were used to define peptides for testing. These algorithms determined protein intrinsically unstructured/disordered tendency (86), relative solvent accessibility/surface exposed tendency (87), and hydrophilicity (88). A linear combination of these scores was used to rank and select peptides from polymorphic regions for species-specific reactivity, as well as from selected conserved regions for genus-specific reactivity.
For display of aligned peptide sequences, phylogenetic trees were constructed by the unweighted pair-group method (UPGMA [83]). Within-tree sequence distances were calculated by determining the percent identity of the amino acids of the antibody-reactive regions (83).
Determination of peptide antigen seroreactivity by chemiluminescent ELISA.
Peptides were chemically synthesized with an N-terminal biotin, followed by a serine-glycine-serine-glycine (SGSG) spacer (89, 90), the specific sequence, and a carboxyl C terminus (AAPPTec, Louisville, KY; ProImmune, Oxford, United Kingdom; and GenScript, Piscataway, NJ). White flat-bottom microtiter plates coated with covalently linked streptavidin were used for binding of biotinylated peptides (Nunc; Fisher Scientific, Pittsburgh, PA). Peptides were initially dissolved at 2.5 μmol/ml in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). For coating microtiter plates, each peptide was diluted first to 25 nmol/ml in DMSO and then to 0.25 nmol/ml in assay diluent (0.15 M NaCl, 20 mM Tris-HCl [pH 7.5] containing 2% rabbit serum, 0.2% bovine serum albumin, 0.2% casein, 0.2% polyethylene glycol, 0.05% Tween 20, 0.004% benzalkonium chloride; Sigma-Aldrich).
Plates were incubated for 15 min at room temperature with 400 μl of wash buffer (0.25 M NaCl, 20 mM Tris-HCl [pH 7.5], containing 0.1% Tween 20, and 0.001% benzalkonium chloride)/well and then rinsed twice with 300 μl of wash buffer. Subsequently, 100 μl of peptide solution in assay diluent (0.25 nmol/ml) was added per well (25 pmol of peptide), followed by incubation at room temperature for 30 min with agitation. Peptide solutions were aspirated, and the plates were washed five times with 300 μl of wash buffer, followed by 30 min of incubation with 300 μl of blocking buffer per well (0.15 M NaCl, 20 mM Tris-HCl [pH 7.5] containing 10% rabbit serum, 1% bovine serum albumin, 1% casein, 1% polyethylene glycol, 0.004% benzalkonium chloride). To capture low-affinity antibodies, lower concentrations of NaCl were used in wash buffer (0.15 M), assay diluent (0.1 M), and blocking buffer (0.1 M). Sera were diluted 1:50 or 1:100 in assay diluent, and 100 μl of diluted sera was added per well, followed by incubation for 1 h with agitation at room temperature. After five washes with wash buffer, 100 μl of secondary antibody diluted 1:1,000 in assay diluent was added [horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG-F(ab′)2 fragment; Bethyl Laboratories, Inc., Montgomery, TX], and microtiter plates were incubated for 30 min. After five washes, 100 μl of freshly prepared HRP-chemiluminescent substrate (Roche Diagnostics Corp., Indianapolis, IN) was added, and the luminescence was determined after 2 min on a Spectrafluor Plus reader at a 500-ms integration time at 100% gain and maximum dynamic range. The signals were indicated as relative light units (RLU) per second and, for ease of display, are divided by 1,000 (RLU/s × 10−3).
For bovine sera, modified assay conditions were used. The assay diluent was composed of 0.2 M NaCl, 20 mM Tris-HCl (pH 7.5) containing 10% chicken serum, 0.5% polyethylene glycol, 0.1% Tween 20, and 0.004% benzalkonium chloride. The blocking buffer was composed of 0.2 M NaCl, 20 mM Tris-HCl (pH 7.5), 10% chicken serum, 1% polyethylene glycol, and 0.004% benzalkonium chloride, and the wash buffer was composed of 0.3 M NaCl, 20 mM Tris-HCl (pH 7.5), containing 0.1% Tween 20, and 0.001% benzalkonium chloride. Goat anti-bovine IgG heavy and light chain–HRP conjugate (Bethyl Laboratories) was diluted 1:500.
All sera were analyzed in wells coated with specific peptides and in an uncoated well, and for the final background-corrected results, 150% of the background signal (mean plus two standard deviations) in the uncoated well of each serum was subtracted from its specific peptide signals. For all peptides, we stringently defined species-specific reactivity as any reactivity above this background with the homologous mouse serum pool, but no signal above the background with any of the eight heterologous serum pools. Any peptide reactive with a heterologous mouse serum pool was considered cross-reactive. To avoid false-positive results in the quantitative evaluation of the reactivity of any peptide with individual mouse sera and with bovine sera from naturally infected cattle, we used a more stringent cutoff of 10,000 RLU/s.
Statistical analyses.
All statistical analyses were performed using the Statistica 7.1 software package (Statsoft, Tulsa, OK), and P values of ≤0.05 were considered significant. Differences between means of peptide reactivities and background were analyzed by paired one-tailed Student t test. The probability of cross-reactivity among peptides of a clade was estimated by logistic regression against the percent sequence identity. Linear regression analysis was used to calculate the signal intensity of cross-reactive peptides as a function of percent sequence identity. For analysis of categorical data, a one- or two-tailed Fisher exact test was used as described below.
RESULTS
Identification of antibody-reactive regions of chlamydial proteins.
To identify protein regions containing strong B cell epitopes, a total of 72 chlamydial proteins, described in the literature as immunodominant, were catalogued and ranked based on seroreactivity in the published literature, the number of studies describing the reactivity, and the evolutionary polymorphism among homologs of the nine Chlamydia spp. All available sequences of each immunodominant protein were aligned, and polymorphic peptide regions in each protein alignment were ranked based on B cell epitope scores (polymorphism, disorder, and surface-exposed tendencies). Conserved peptide regions were ranked based on predicted scores for disorder, surface-exposed tendencies, and hydrophilicity.
High-scoring peptides were synthesized from sequences of C. trachomatis (42 proteins), C. pneumoniae (24 proteins), C. abortus (15 proteins), C. pecorum (12 proteins), and C. muridarum (11 proteins). Peptides were synthesized chemically with N-terminal biotin, followed by serine-glycine-serine-glycine (SGSG) as a spacer/linker and the chlamydial sequence, and attached to streptavidin-coated white microtiter plates. Peptides were tested with homologous or heterologous Chlamydia monospecific hyperimmune sera pooled from 9 to 50 mice for IgG antibodies in a chemiluminescent ELISA format. For the remaining Chlamydia spp., additional peptides that were homologous to the reactive peptides were tested.
In total, 812 peptides were tested, and 23 antibody-reactive regions (ARRs) in 10 proteins were identified. These proteins, in order of ARR dominance and numbers, are OmpA/MOMP, PmpD, CT618, IncA, CT529, CT442, IncG, OmcB/Omp2, TarP, and IncE (Table 1). A total of 593 peptides were synthesized from the 23 ARRs of these 10 proteins for all nine Chlamydia spp. The remaining 219 peptides were synthesized for initial screening of all ranked proteins and did not show reactivity with mouse sera (see footnote c in Table 1). Collectively, we defined the reactivity of 134 genus-, species-, and serovar-specific peptides of B cell epitopes of these proteins from nine chlamydial species.
TABLE 1.
Chlamydia proteins with antibody-reactive regions suitable for molecular serology
| Protein | Locus taga | Sequence identity range (%)b | No. of peptides evaluatedc | No. of ARRs identifiedd | Referencese |
|---|---|---|---|---|---|
| OmpA/MOMP (outer membrane protein A) | CT_681 | 65–86 | 126 | 6 | 44, 58, 60–62, 64, 66, 74–77 |
| PmpD (polymorphic outer membrane protein D) | CT_812 | 33–87 | 129 | 5 | 58, 60, 62, 66, 77, 79 |
| CT618 (inclusion membrane protein CT618) | CT_618 | 23–72 | 41 | 3 | 59–61 |
| IncA (inclusion membrane protein A) | CT_119 | 12–80 | 182 | 1 | 59–65, 81 |
| CT529 (inclusion membrane protein CT529) | CT_529 | 31–88 | 33 | 1 | 59–62, 64 |
| CrpA/Srp (cysteine-rich outer membrane protein A) | CT_442 | 20–88* | 16 | 2 | 59–64 |
| IncG (inclusion membrane protein G) | CT_118 | 41–45† | 17 | 1 | 59–60 |
| OmcB/Omp2 (outer membrane cysteine-rich protein B) | CT_443 | 71–99 | 24 | 2 | 44, 58, 60–62, 64, 77–78 |
| TarP (translocated actin-recruiting phosphoprotein) | CT_456 | 24–92 | 20 | 1 | 60, 62–63, 66, 82 |
| IncE (inclusion membrane protein E) | CT_116 | 53–59† | 5 | 1 | 59–62, 64 |
That is, the gene locus tag in the genome of C. trachomatis strain D/UW-3/CX.
Range of pairwise sequence identities among the nine Chlamydia spp. *, homolog not found in C. felis; †, homologs to the C. trachomatis protein found only in C. muridarum and C. suis.
Peptides were tested with mouse hyperimmune sera. Peptides from the following proteins did not react with mouse sera: CtrCpaf, CtrFtsH, CtrIncB, CtrIncC, CtrIncD, CtrLcrE, CtrIncF, CtrPmpB, CtrPmpC, CtrPkn5, CtrCT058, CtrCT089, CtrCT143, CtrCT147, CtrCT223, CtrCT226, CtrCT228, CtrCT228, CtrCT241, CtrCT381, CtrCT484, CtrCT541, CtrCT561, CtrCT579, CtrCT603, CtrCT619, CtrCT694, CtrCT741, CtrCT795, CtrCT813, CtrCT823, CtrCT875, Cpn0808, CpnPmp2, CpnPmp6, CpnPmp10, CpnPdhC, CpnPorB, CpnRecA, CpnRpsA, CpnRpsB, CpnYscC, CpnYscL, CpnYwbM, CpnCT858, Cpn0525, Cab063, CabCT058, CabCT541, CabIncAf, CabPmp15G, CpeCT143, CpeORF663, and CmuCT228.
Antibody-reactive regions (ARRs) that were identified with mouse sera.
Key references are cited.
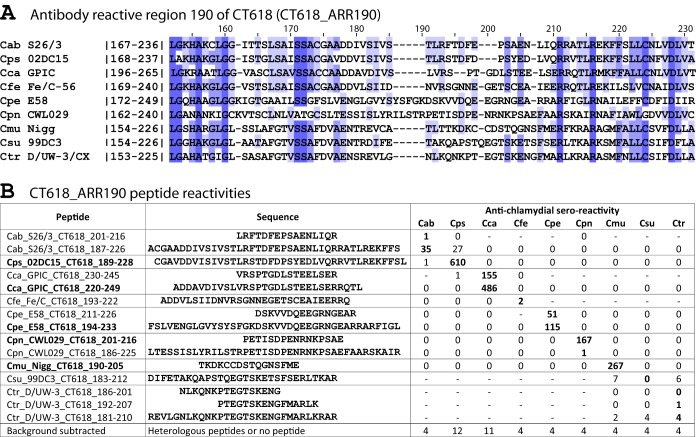
Figure 1 is an example of an ARR, showing the alignment of the sequences of nine chlamydial species of a polymorphic region of chlamydial protein CT618 (Fig. 1A) and the reactivity of peptides from this region with a battery of nine corresponding species-specific antichlamydial sera (Fig. 1B). Cross-reactivity with heterologous sera was only found for C. abortus and C. psittaci peptides. All remaining peptides reacted significantly (P <10−3) only with homologous sera, thus providing species-specific reactivity (Fig. 1B). Of the total of 23 ARRs identified, 8 additional ARRs are shown in Fig. S1 to S8 in the supplemental material. Information regarding the remaining 14 ARRs can be obtained from the authors upon request.
FIG 1.
CT618 antibody-reactive region 190 (CT618_ARR190). (A) Complete ARR190 alignment of the putative inclusion membrane protein CT618 (locus tag CT_618 of C. trachomatis strain D/UW-3/CX) with the homologs of the remaining eight Chlamydia species. The alignment includes the antibody-reactive polymorphic region bracketed by regions conserved among the nine chlamydial species. Numbers within vertical lines correspond to amino acid residue numbers of each species. Numbers on top of the alignment indicate approximate residue numbers of the Ctr_CT618 protein. The designation antibody-reactive region 190 (ARR190) is derived from the approximate central residue of the species-specific antibody determining region of the Ctr_CT618. (B) Antibody reactivity of CT618_ARR190 peptides with Chlamydia monospecies-specific antisera pooled from 9 to 50 mice. Peptides are named for Chlamydia species specificity (Cab, Cps, etc.), followed by strain and protein designation, and amino acid positions of the peptide in the respective proteins. Average reactivity of three repeats of each peptide from a high-stringency ELISA (interassay coefficient of variation [CV] = 11.2%, intra-assay CV = 8.5%). Boldface peptide signals indicate the specific reactivity with homologous sera. All signals of positive serum pools are significantly above background (P < 10−3; one-tailed Student t test). Boldface peptides show strong species-specific reactivity with homologous sera. Cross-reactivity with Cps-specific sera is evident for the strongly reactive Cab peptide Cab_S26/3_CT618_187-226.
Sequence divergence and cross-reactivity.
To derive the relationship between sequence divergence and cross-reactivity, 93 peptides that had strongly reacted with their respective cognate antiserum were identified. Species/strain variant peptides of these 93 peptides were tested with sera raised by immunization with different chlamydial species (heterologous sera), resulting in 700 tests of peptides with heterologous sera (Fig. 2A). We defined peptide cross-reactivity as the ability of a peptide to bind antibodies elicited by a different chlamydial species or strain with at least one amino acid variation in the peptide sequence (42).
FIG 2.
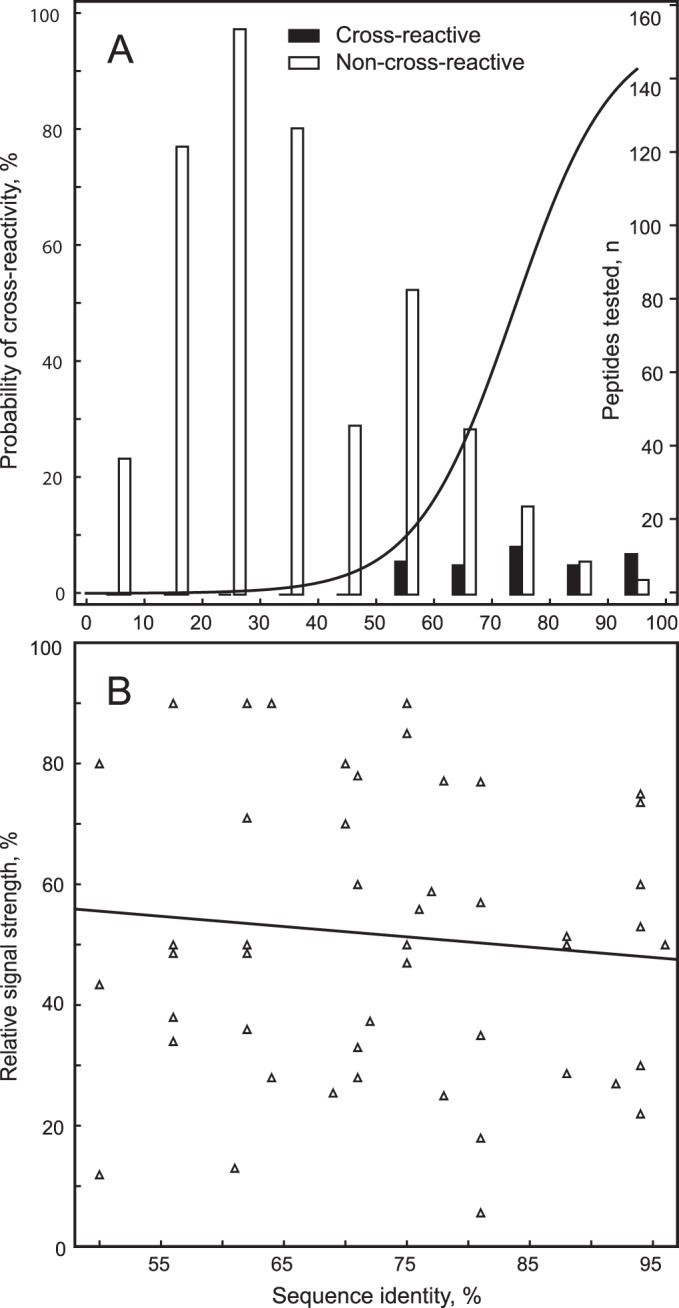
Cross-reactivity of peptides with heterologous sera. (A) The probability of reactivity of a peptide with heterologous sera raised against the other chlamydial species/strains is shown in dependence on the percent sequence identity of the peptide to the respective heterologous chlamydial peptide. Peptides that produced with the heterologous sera >5% of the signal of homologous sera were designated as cross-reactive. The probability of cross-reactivity analyzed by logistic regression (n = 700, P < 10−6) is shown on the left ordinate; the number of peptides analyzed for each 10% bracket, visualized by bars, is shown on the right ordinate. Cross-reactive peptides are found exclusively at a 50% or higher sequence identity. (B) Signal strength of cross-reactive peptides relative to the signal of the homologous peptides in dependence of sequence identity. In linear regression analysis, the signal strength is independent of the degree of sequence identity (n = 49, r2 = 0.01, P = 0.5).
Only peptides with 50 to 96% amino acid sequence identity to the respective heterologous chlamydial peptide showed cross-reactivity, while those with less than 50% identity did not. As expected, the frequency of cross-reactivity strongly increased with sequence identity, with 11% of peptides with 50 to 60% identity, but 70% of peptides with 90 to 96% identity, showing cross-reactivity. Logistic regression analysis showed that the probabilities of cross-reactivity at 50, 65, and 85% sequence identity were 4, 20, and 80%, respectively (Fig. 2A). In linear regression analysis, the signal strength of these cross-reactive peptides relative to the signal of their homologous peptides did not correlate with percent sequence identity (Fig. 2B) and cross-reactive variant peptides produced, on average, 50%, and always less than 100%, of the signal generated by the homologous peptides. The results show that increasing sequence identity of heterologous peptides increases the probability, but not intensity, of cross-reactivity.
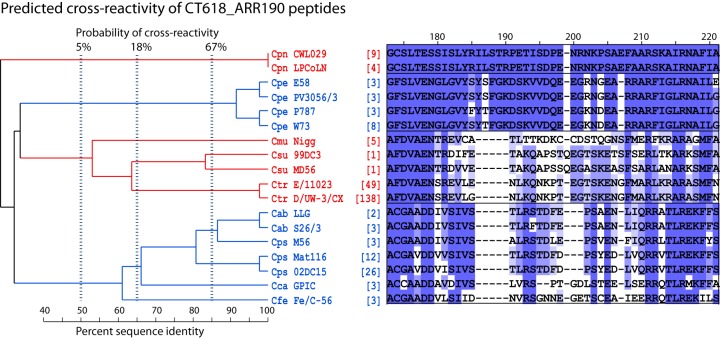
Phylogenetic reconstruction of reactive peptides predicts antigenic specificity.
Figure 3 is an example of an ARR subregion alignment of reactive peptides and their strain variations within species (serovars). The phylogram of these CT618_ARR190 peptides enables calculation of the probability of peptide cross-reactivity using the logistic regression between phylogenetic distances and cross-reactivity, as shown in Fig. 2A. The 80% sequence identity between C. abortus and C. psittaci peptides in Fig. 3, resulting in ∼60% probability of cross-reactivity (Fig. 2A), translates into actual cross-reactivity, as shown in Fig. 1B. In contrast, none of the well-separated peptides shown in Fig. 3 is cross-reactive (Fig. 1B).
FIG 3.
Evolutionary relationship and predicted cross-reactivity among CT618_ARR190 sequences. Numbers at the top of the subregion alignment indicate approximate residue numbers of the Ctr_CT618 protein in the alignment of the overall region (Fig. 1). Corresponding residues in mismatched sequences are not always identical to the overall ARR alignment because of additional sequences, different sequence lengths, gap insertions, and higher gap insertion penalty in the subregion alignments. Numbers in brackets indicate the frequency of the strain-specific sequence available in the NCBI protein database. For clarity, deep clades (sequences of typically <50% amino acid identity) are indicated in alternating color, with the sequences separated into boxes. Vertical dashed lines indicate the calculated probabilities of antibody cross-reactivity among peptides with a single common ancestor at these thresholds. This ARR shows high evolutionary divergence, allowing for robust differentiation of a chlamydial species (Cpn or Cpe) or a group of closely related chlamydial species (clade Cmu, Csu, and Ctr and clade Cab, Cps, Cca, and Cfe). Cps species/strain-specific peptides are evolutionarily closest to Cab, followed by the Cca and Cfe species/strain. Therefore, Cps peptides have the highest probability of cross-reactivity with Cab. Cpn or Cpe peptide sequences are evolutionarily well separated, and these peptides have a very low probability of serological cross-reactivity with the remaining eight chlamydial species.
Identification of monospecies- and multispecies-specific reactive peptide antigens.
To determine the specificity of reactive peptides identified in the screening phase, they were tested with the nine monospecies-specific serum pools. Initial phylogenetic trees were constructed for each reactive peptide from its alignment with the homologous sequences from the remaining eight strains of the Chlamydia species used to raise the sera, followed by subtrees constructed from all serovars of all nine species (Fig. 1 and 3; see also Fig. S1 to S8 in the supplemental material). The probability of cross-reactivity among peptides of a clade was estimated by logistic regression against the percent sequence identity (Fig. 2A). Highly reactive peptides were grouped into Chlamydia single species-specific (Table 2) or multispecies-specific (Table 3) peptides based on their empirical and probabilistic cross-reactivity with heterologous sera. All peptide antigens in Table 2 reacted strongly with homologous sera but not with the remaining eight heterologous sera. These peptides are also evolutionarily well separated from the remaining Chlamydia spp.
TABLE 2.
Highly reactive Chlamydia species-specific peptides
| Peptide | Sequence (5′–3′) | ELISA signal ([RLU/s] × 10−3)a | No. of sequence variants/total sequencesb | Peptides/species detectionc | ARR cross-reactivityd |
|---|---|---|---|---|---|
| Cab_S26/3_OmpA_153-176 | NLVGLIGVKGSSIAADQLPNVGIT | 365 | 2/39 | 1 | I |
| Cab_S26/3_PmpD_1060-1089 | KIESPTSNVYYSAHESVKQPENKTLADINS | 185 | 2/7 | 1 | II |
| Cab_S26/3_IncA_324-353 | STAVTEHADIPRDPNRDPRGGRGGQSSPSV | 109 | 2/5 | 1 | |
| Cps_02DC15_OmpA_158-181 | LVGLIGFSAASSISTDLPTQLPNV | 106 | 30/288 | 8* | III |
| Cps_05DC15_OmpA_250-265 | ASSNFPLPITAGTTEA | 78 | 17/204 | 4* | IV |
| Cps_02DC15_OmpA_333-348 | STTALPNNSGKDVLSD | 299 | 20/209 | 5* | III |
| Cps_02DC15_IncA_321-360 | SLTSTTETADQGDLRDPSGDRYGGWGAQSSYRLSPSVTMS | 328 | 7/77 | 4* | V |
| Cps_02DC15_CT618_105-134 | YEVDSATGSFKIVTKNIQKPNGEVEIVSSR | 249 | 3/41 | 2 | |
| Cps_02DC15_CT618_189-228 | CGAVDDVISIVSTLRSTDFDPSYEDLVQRRVTLREKFFSL | 343 | 3/41 | 2 | |
| Cca_GPIC_OmpA_159-168 | VTGTDLQGQY | 117 | 1/5 | 1 | |
| Cca_GPIC_IncA_316-355 | LIGVMVQDGAESSTVEEASQDDSAQPQDENQSDAGEHKDS | 321 | 1/4 | 1 | |
| Cca_GPIC_CT618_134-163 | EVDAQTGNFVLQTKTVQLEDGTQRVVPSRV | 383 | 1/3 | 1 | |
| Cca_GPIC_CT618_220-249 | ADDAVDIVSLVRSPTGDLSTEELSERRQTL | 180 | 1/3 | 1 | |
| Cfe_Fe/C_OmpA_160-175 | IGLAGTDFANQRPNVE | 46 | 1/10 | 1 | |
| Cfe_Fe/C_PmpD_1055-1084 | PNVKSVEKIESPSAKSYYSNYEIEKNPIEK | 52 | 1/6 | 1 | |
| Cfe_Fe/C_CT618_108-137 | DSASGNFKIGVKSVKNENGETVLVPCRILK | 528 | 1/3 | 1 | |
| Cpe_E58_OmpA_090-105 | TSPNNAADSSTTAERA | 48 | 24/203 | 8* | |
| Cpe_E58_OmpA_161-176 | ISGSSLEGKYPNANIS | 63 | 27/209 | 10* | |
| Cpe_E58_OmpA_323-338 | LGQATTVDGTNKFADS | 198 | 27/215 | 11* | |
| Cpe_E58_IncA_281-300 | AAPAAPAAPAAPAAPA | 110 | 14/126 | 9* | |
| Cpe_E58_IncA_311-326 | PAPENNDNNNDDNAAS | 513 | 8/89 | 5 | |
| Cpe_E58_CT529_209-248 | IIRERRAYQRCLERLNQKEVGQEESGSAQEVQAMRSSYVK | 154 | 2/15 | 1 | |
| Cpe_E58_CT442_151-190 | DGSNQIFVDSNRDIRRPGSGGSGGVSASGALEQVANIVMN | 94 | 3/19 | 3 | |
| Cpn_CWL029_PmpD_0147-0187 | EKISSDTKENRKDLETEDPSKKSGLKEVSSDLPKSPETAV | 80 | 1/16 | 1 | |
| Cpn_CWL029_PmpD_1131-1170 | NKEETLVSAGVQINMSSPTPNKDKAVDTPVLADIISITVD | 116 | 1/16 | 1 | |
| Cpn_CWL029_IncA_331-370 | QKAESEFIACVRDRTFGRRETPPPTTPVVEGDESQEEDEG | 340 | 2/13 | 2 | |
| Cpn_CWL029_CT618_201-216 | PETISDPENRNKPSAE | 215 | 1/13 | 1 | |
| Cmu_Nigg_PmpD_724-739 | KVETADINSDKQEAEE | 20 | 1/6 | 1 | |
| Cmu_Nigg_PmpD_1038-1053 | EIGDLEDSVNSEKTPS | 50 | 1/6 | 1 | |
| Cmu_Nigg_CT618_190-205 | TKDKCCDSTQGNSFME | 41 | 1/5 | 1 | |
| Csu_99DC3_OmpA_166-181 | FGLTTTSVAAQDLPNV | 6 | 25/55 | 22* | |
| Csu_99DC3_OmpA_317-332 | TISGKGQDAQTLQDTM | 20 | 20/40 | 12* | |
| Csu_99DC3_IncA_259-293 | LANIKEALIKPSRPPLPKNGFPRTMPPCPPRQTPP | 76 | 1/1 | 1 | |
| Csu_99DC3_CT529_207-236 | EERCNRILCGQADEVLGINNTMCEQFVRQR | 13 | 2/2 | 2 | |
| Ctr_D/UW-3_OmpA_082-105 | FQMGAKPTTDTGNSAAPSTLTARE | 166 | 28/601 | 11* | |
| Ctr_D/UW-3_OmpA_159-174 | FGDNENQKTVKAESVP | 20 | 22/741 | 10* | |
| Ctr_D/UW-3_OmpA_313-328 | IFDTTTLNPTIAGAGD | 535 | 11/738 | 3 | VI |
| Ctr_D/UW-3_OmpA_324-339 | AGAGDVKTGAEGQLGD | 73 | 18/725 | 6* | |
| Ctr_D/UW-3_OmpA_306-345 | QPKSATAIFDTTTLNPTIAGAGDVKTGAEGQLGDTMQIVS | 587 | 18/725 | 8 | VI |
| Ctr_D/UW-3_PmpD_536-565 | ARAPQALPTQEEFPLFSKKEGRPLSSGYSG | 3 | 3/201 | 1 | |
| Ctr_D/UW-3_PmpD_1036-1065 | SGTPVQQGHAISKPEAEIESSSEPEGAHSL | 6 | 5/202 | 3 | |
| Ctr_D/UW-3_CT529_200-239 | SAERADCEARCARIAREESLLEVPGEENACEKKVAGEKAK | 27 | 4/182 | 2 | |
| Ctr_D/UW-3_IncG_108-147 | RPSDQQESGGRLSEESASPQASPTSSTFGLESALRSIGDS | 40 | 4/183 | 2 |
Background-corrected average signals of six repeats with homologous sera at high stringency are shown. The signal for all peptides was highly significantly above background (P < 10−5; one-tailed Student t test). The method has an interassay CV of 11.2% and an intra-assay CV of 8.5%. All peptides were nonreactive above background with nonhomologous mouse sera against the eight remaining chlamydial species.
Sequence variants among all GenBank sequence accession numbers for the respective Chlamydia peptide sequences.
That is, the number of variant peptides within the respective Chlamydia spp. required to provide Chlamydia species-specific antibody binding to ≥95% of all GenBank sequence accession numbers at a ≤2 amino acid mismatch tolerance. *, peptides from this ARR show serovar-specific reactivity.
Potential for cross-reactivity of the antibody reactive peptide region (ARR) with other Chlamydia species/strains: I, Cps strains VS225 and GR9; II, Cps GR9 and possibly other Cps strains; III, Cab strains with Cps strains VS225 and GR9; IV, Cab strains with Cps strain VS225; V, ARR absent in Cps strain VS225, no reactivity; VI, central residues shared among all chlamydial species. Homologs from other species show unpredictable patterns of cross-reactivity.
TABLE 3.
Peptides with cross-reactivity among Chlamydia species
| Peptide | Sequence (5′–3′) | ELISA signal with Chlamydia monospecific antisera ([RLU/s] × 10−3)a |
No. of sequence variants/total sequences of reactive speciesb | No. of peptides/species detectionc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cab | Cps | Cca | Cfe | Cpe | Cpn | Cmu | Csu | Ctr | ||||
| Cab_S26/3_OmpA_089-104 | PTGTAAANYKTPTDRP | 23 | 0 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | Cab (2/49), Cpn (2/36) | 1, 1 |
| Cab_S26/3_OmpA_223-262 | NVVSSPAQFVVHKPRGYKGTAFPLPLTAGTDQATDTKSAT | 0 | 0 | 0 | 0 | 0 | 39 | 1 | 0 | 1 | Cpn (3/37)* | 1 |
| Cab_S26/3_OmpA_309-324 | AVLNLTTWNPTLLGEA | 5 | 74 | 6 | 0 | 6 | 61 | 0 | 0 | 113 | Cab (1/38), Cps (14/132), Cpn (2/39) | 1, 4, 1, 3 |
| Cab_S26/3_OmpA_302-341 | AQPKLAAAVLNLTTWNPTLLGEATALDTSNKFADFLQIAS | 17 | 89 | 15 | 0 | 6 | 117 | 0 | 0 | 89 | Cab (2/38), Cps (20/209), Cca (1/5), Cpn (2/39) | 1, 7, 1, 1, 2 |
| Cab_S26/3_OmpA_366-389 | KWSITGEARLINERAAHMNAQFRF | 0 | 321 | 0 | 0 | 0 | 0 | 35 | 74 | 0 | Cps (4/287), Csu (1/33), Cmu (1/13) | 1 |
| Cab_S26/3_PmpD_1074-1113 | ESVKQPENKTLADINSIGIDLASFVSSDDETPVPPQIIVP | 136 | 269 | 40 | 134 | 0 | 0 | 0 | 0 | 0 | Cab (1/8), Cps (3/76), Cca (1/3), Cfe (1/6) | 1, 1, 1, 1 |
| Cca_GPIC_PmpD_571-610 | ITFSYNKGTILPFPKVAASSEGESAPEAPKESSPVDLGVR | 0 | 3 | 191 | 0 | 1 | 1 | 0 | 0 | 0 | Cca (1/3)* | 1 |
| Cfe_Fe/C_PmpD_569-598 | FSYNSGKFLPLPMPSAEVSEENSSQNAPVE | 0 | 0 | 182 | 0 | 0 | 0 | 0 | 0 | 0 | Cca (1/3)* | 1 |
| Cfe_Fe/C_PmpD_1083-1112 | EKTLADISSIGVDLASFVTNDDGSSPLPPQ | 116 | 214 | 151 | 260 | 0 | 1 | 1 | 0 | 0 | Cab (1/8), Cps (3/76), Cca (1/3), Cfe (1/6) | 1, 1, 1, 1 |
| Cpn_CWL029_PmpD_654-693 | EKSLNACSHGDHYPPKTVEEEVPPSLLEEHPVVSSTDIRG | 0 | 0 | 0 | 2 | 0 | 115 | 0 | 0 | 0 | Cpn (1/16)* | 1 |
| Cpe_E58_OmpA_313-328 | PIFNLTTWNPTLLGQA | 4 | 11 | 4 | 0 | 5 | 40 | 0 | 0 | 87 | Cpn (2/39), Ctr (11/738) | 4 |
| Csu_99DC3_OmpA_345-384 | RKSCGLAVGTTIVDADKYAVTVETRLIDERAAHVNAQFRF | 0 | 267 | 0 | 0 | 0 | 0 | 36 | 87 | 0 | Cps (1/287), Csu (1/33), Cmu (1/13) | 2, 1, 1 |
| Csu_99DC3_PmpD_535-564 | NARAPQAVPTRDPEEVFSLSAESLNGCSGG | 0 | 0 | 6 | 0 | 8 | 0 | 0 | 16 | 0 | Csu (2/2)* | 2 |
| Ctr_D/UW-3_OmpA_041-056 | EGFGGDPCDPCATWCD | 0 | 27 | 0 | 16 | 1 | 0 | 6 | 0 | 0 | Cps (4/284), Cfe (1/13) | 1, 1 |
| Ctr_D/UW-3_OmpA_104-119 | RENPAYGRHMQDAEMF | 0 | 0 | 0 | 0 | 0 | 119 | 2 | 0 | 197 | Cpn (1/36), Ctr (4/741) | 1, 2 |
| Ctr_D/UW-3_OmpA_080-119 | KEFQMGAKPTTDTGNSAAPSTLTARENPAYGRHMQDAEMF | 0 | 0 | 0 | 0 | 0 | 294 | 9 | 0 | 376 | Cpn (2/36), Ctr (24/763) | 1, 2, 16, 9 |
| Ctr_D/UW-3_OmpA_152-181 | SFNLVGLFGDNENQKTVKAESVPNMSFDQS | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 97 | Ctr (28/601) | 12 |
| Ctr_D/UW-3_OmpA_233-248 | EFTINKPKGYVGKEFP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 100 | Csu (25/98), Ctr (8/750) | 10, 3 |
| Ctr_D/UW-3_OmpA_245-260 | KEFPLDLTAGTDAATG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 105 | Ctr (9/750)* | 4 |
| Ctr_D/UW-3_OmpA_226-265 | NVLCNAAEFTINKPKGYVGKEFPLDLTAGTDAATGTKDAS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 295 | Csu (28/98), Ctr (12/750) | 12, 4 |
That is, the average reactivity of three repeats of each peptide in a high-stringency ELISA with background subtracted (P < 10−3; one-tailed Student t test).
Sequence variants among all GenBank peptide sequence accession numbers for the reactive chlamydial species. *, despite experimental reactivity with only a single species, potential for cross-reactivity between species exists because of extensive shared peptide sequences.
Number of variant peptides within the respective Chlamydia spp. required to provide Chlamydia species-specific antibody binding to ≥95% of all GenBank sequence accession numbers at ≤2-amino-acid mismatch tolerance.
In addition to polymorphisms at the Chlamydia genus level, certain ARRs show polymorphism at the Chlamydia species level. For instance, all ARRs of the C. suis OmpA protein are highly divergent, followed by C. pecorum, C. trachomatis, and C. psittaci. The C. suis OmpA peptide of aa 166 to 181 (Csu_99DC3_OmpA_166-181) shows 25 sequence variants among just 55 available sequences, including 22 major variants with a >2-aa difference (Table 2). In contrast, 22 sequence variants of this peptide are present among 741 C. trachomatis sequences, including 10 major variants. Compared to OmpA ARRs, PmpD, CT442, CT529, CT618, IncG, or IncA ARRs showed low divergence within a species but high genus divergence. Thus, non-OmpA ARRs require only a single peptide or a few peptides to safely allow the serodetection of all strains of each Chlamydia species (Table 2, footnote c).
The multispecies peptides shown in Table 3 were cross-reactive with heterologous sera or have extensive shared amino acids so that cross-reactivity in original hosts is likely, even if they did not react with the heterologous mouse serum pools. For instance, the Ctr_D/UW-3_OmpA_104-119 peptide reacted strongly with homologous C. trachomatis-specific sera, as well as heterologous C. pneumoniae-specific sera (Table 3). In addition, Ctr_D/UW-3_OmpA_245-260 reacted only with homologous sera, and yet this peptide cannot be used for C. trachomatis-specific seroassays because this region extensively shares amino acids with C. suis (≤88% sequence identity). Therefore, the reliable detection of antibodies, e.g., against C. trachomatis, with a single peptide variant or a few peptide variants (Table 3, footnote c) will be a promising application of such peptides, even at the risk of some loss of species specificity.
Stochastic antibody responses of individual mouse sera.
Unlike major histocompatibility complex-restricted T cell immunity, the presence or absence of a B cell response to a given epitope is stochastic due to immunoglobulin gene recombination that determines complementarity of the epitope binding regions (91). Once an immunoglobulin has evolved that binds a specific B cell epitope, affinity maturation under antigenic reexposure drives the dominance of this antibody. To account for this stochasticity of individual seroreactivity with single peptide epitopes, we used serum pools from 9 to 50 mice to identify highly reactive peptide antigens in the screening process. To evaluate antibody reactivity of individual animals, we selected six C. pecorum E58 peptides of low to high reactivity with the homologous C. pecorum serum pool and tested them with the pool constituent individual mouse sera. The results in Fig. 4A attest to the stochastic reactivity to these six peptides. They also show that the percentage of animals producing antibodies had a 2.23-fold greater influence on the averaged reactivity of all mice tested (pool reactivity) than the antibody level of the individual reactive sera (Fig. 4A).
FIG 4.
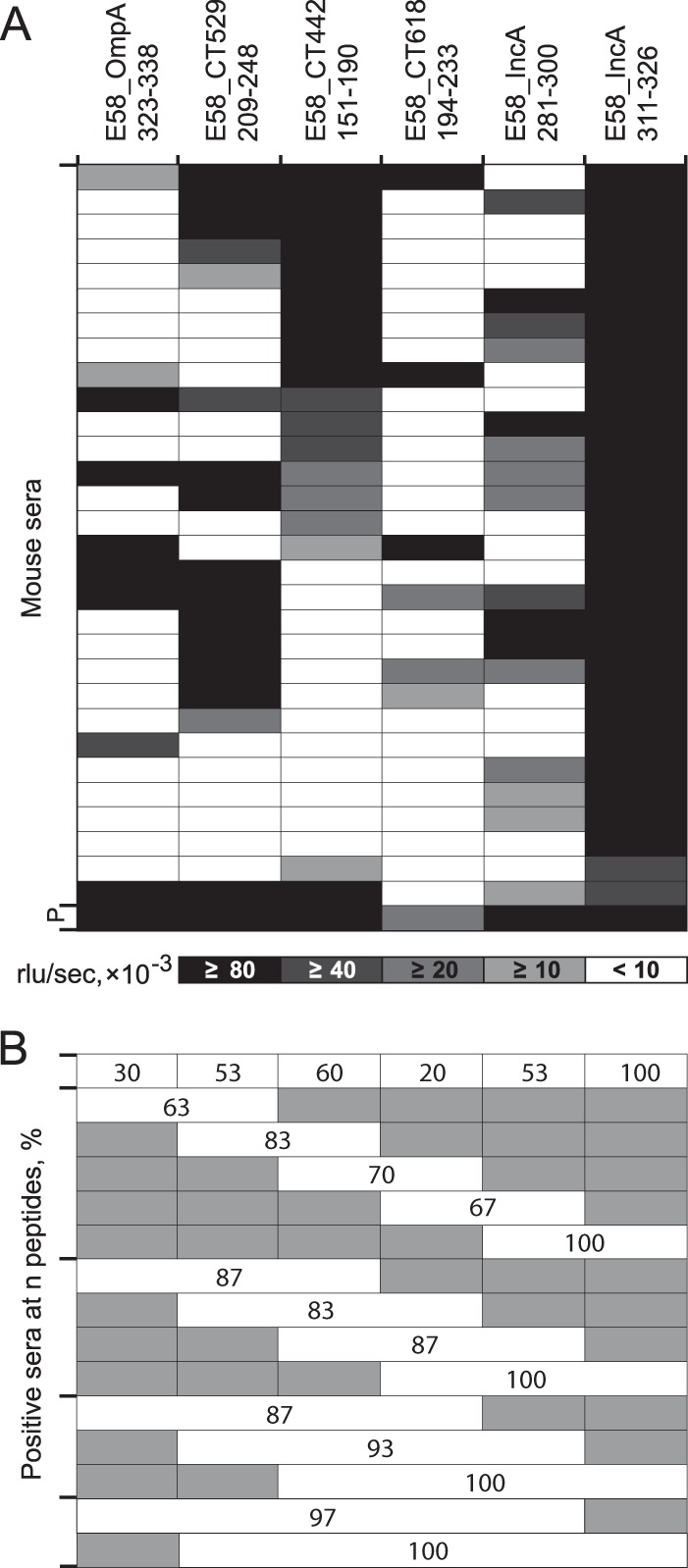
Reactivity of C. pecorum-specific individual mouse sera with C. pecorum peptides. (A) Heat map of reactivity of 30 mouse sera with six peptides designed from five proteins (OmpA, CT529, CT442, CT618, and IncA) of C. pecorum strain E58. Shading intensity is proportional to peptide reactivity with each serum (mean of two experiments, interassay CV = 8.5%), with a cutoff of 10,000 RLU/s. P indicates the pool of all mouse sera. The mean of all 15 ratios of the six peptide ELISA reactivities of all 30 sera (positive and negative) versus that of only positive sera is 3.57 versus 1.60 (P = 0.046; Student t test), indicating that the percentage of positive sera has a 2.23-fold-higher influence on the level of reactivity of the pool of all 30 sera than the amplitude of reactivity of individual positive sera. In other words, the number of positive sera among all sera of the pool has a 2.23-fold-higher influence on the signal amplitude of the serum pool than the signal amplitude of the individual positive sera of the pool. (B) Combined percent seropositivity of these single and multiple C. pecorum E58 peptides. Shaded areas indicate peptides not included in the combined reactivity of the evaluated peptides.
The results in Fig. 4B show that the first five peptides (excluding immunodominant peptide IncA_311-326 that reacted strongly with 100% of the sera) reacted with only 30, 53, 60, 20, and 53% of the individual sera, respectively. In contrast, combining results of 2, 3, 4, or all 5 of the less immunodominant peptides rendered 83, 87, 93, and 97% of the individual sera positive (P ≤ 0.05; one-tailed Fisher exact test). Therefore, the use of multiple, even weakly reactive, peptides reduces the stochastic variation of antibody responses to peptide epitopes and allows the detection of peptide-specific antibodies with higher sensitivity.
Validity of chlamydial B cell epitope identification in the heterologous murine host confirmed in the homologous bovine host.
In previous experiments, B cell epitopes had been identified by use of sera raised in the heterologous murine host by three high-dose intranasal inoculations of viable chlamydiae. To evaluate the antigenic potential of these same epitopes in the natural host, we analyzed seroreactivity to the six C. pecorum peptide antigens tested in Fig. 4A with sera of cattle naturally exposed to high levels of endemic C. pecorum infection. All peptides identified in the murine assays exhibited immunodominant reactivity with these bovine sera, albeit in different patterns (Fig. 5A). Cows that had been exposed to multiple episodes of natural infection showed a higher percentage of positive sera against the six peptides than calves that had experienced only a single episode (59.8% versus 34.2%; P = 0.0002; two-tailed Fisher exact test). Interestingly, peptide epitope IncA281-300 dominated in calves over IncA311-326, while the order was reversed in cows, suggesting that IncA311-326 outcompetes IncA281-300 during affinity maturation. Similar to mouse sera, combinations of two to three strongly reactive peptides produced 100% sensitivity, and the combination of less immunodominant peptides also increased the robustness of detection of antichlamydial antibodies in bovine sera (Fig. 5B).
FIG 5.
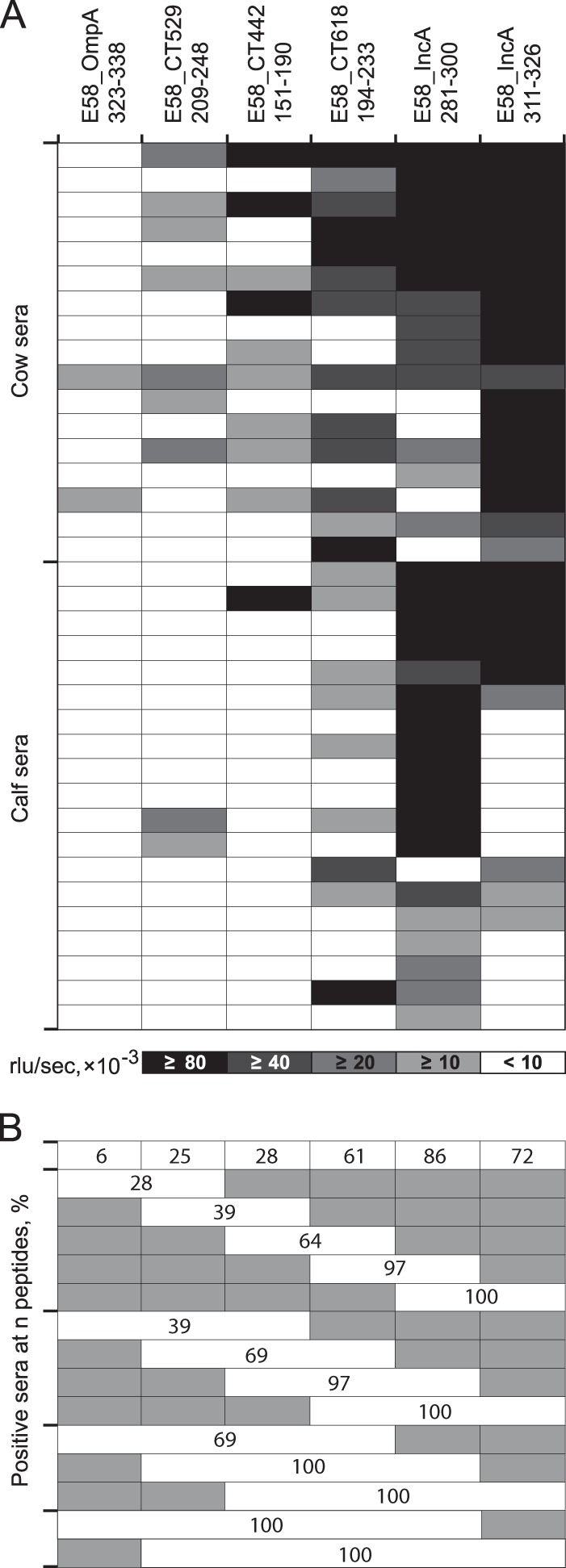
Reactivity of C. pecorum-specific individual cow and calf sera with C. pecorum peptides. (A) Heat map of reactivity of 17 cow and 19 calf sera with six C. pecorum E58 peptides. These bovine sera were obtained from animals in two herds with endemic C. pecorum infections. The cows had experienced multiple episodes of natural infection with multiple C. pecorum strains (72), while calves were sampled between 11 and 15 weeks of age, after serum colostrum antibodies had disappeared and calves had experienced a first episode of C. pecorum infection (20). Shading intensity is proportional to peptide reactivity with each serum (mean of two experiments, interassay CV = 7.5%), with a cutoff of 10,000 RLU/s. (B) Combined percent seropositivity of these single and multiple C. pecorum E58 peptides. Shaded areas indicate peptides not included in the combined reactivity of the evaluated peptides.
DISCUSSION
For each of the nine Chlamydia species, we identified three to nine immunodominant B cell epitopes in 23 regions of 10 immunodominant proteins. Peptides corresponding to these epitopes provided highly reactive and specific antigens in robust ELISAs for chlamydial serology, thus achieving the original objective of the present study. Immunodominance of the protein was a requirement for identifying strong B cell epitopes, directing our search to highly polymorphic regions of the immunodominant proteins that were otherwise conserved throughout all chlamydial species. The need for polymorphism within highly conserved proteins to obtain species specificity reduced the number of candidates very substantially to ∼72 known immunodominant chlamydial proteins, information that had been accessible from classical (74–82) as well as several recent proteome-wide comprehensive studies (58–66). We tested peptides from a total of 64 proteins, mainly at polymorphic regions for species or strain specificity, but also at conserved regions for multispecies or genus specificity (Table 1).
Inadvertently, the search for polymorphic regions directed the investigation to sequences that are enriched for B cell epitopes (41). As positive results emerged, these data in combination with negative data allowed iterative improvements of B cell epitope predictive algorithms, as well as peptide design for maximum reactivity. Highly reactive peptides strongly accumulated in regions with highest sequence polymorphism, which typically also had high predicted scores for protein disorder tendency, surface-exposed tendency, and hydrophilicity (data not shown).
Improvement of peptide reactivity was achieved by use of long, 16- to 40-aa peptides rather than short 8- to 11-aa peptides (Fig. 1B; see also Fig. S1 to S8, panels C, in the supplemental material), a finding consistent with the results of several recent computational studies on B-cell epitopes (39–42). We found that 16-aa peptides produced, on average, a 20-fold-higher signal than 8- to 11-aa peptides, and 20 to 40 aa-long peptides produced another average 3-fold increase compared to 16-aa peptides (unpublished data).
An important component in maximizing the reactivity of the peptide antigens was their accessibility to cognate antibodies. This was maximized by (i) capturing biotinylated peptides on streptavidin that was covalently attached to the solid surface rather than by hydrophobic binding of unmodified peptides or peptide carrier proteins; and by (ii) using the highly flexible hydrophilic SGSG amino acid linker/spacer (89, 90) as an N-terminal portion of the peptide, followed by the specific chlamydial amino acid sequence. Other commonly used linkers, such as the hydrophobic aminohexanoic acid (45), provided inconsistent results for some peptides with reactivity equal to SGSG but lower or absent reactivity in others (data not shown). Overall, the methodology used in this investigation resulted in a robust ELISA platform with an interassay coefficient of variation (CV) of 11.2% and an intra-assay CV of 8.5%.
The large data set that was created by testing many species/strain variant peptides with eight heterologous sera (n = 700) allowed a probabilistic estimation of peptide antigen cross-reactivity (Fig. 2). This allows a rational choice of peptide antigens depending on assay objectives (genus, species, or serovar specificity) in chlamydial serology. Comprehensive phylograms of B cell epitope regions among all Chlamydia spp., including the strain variants, provide critical information on the probability of cross-reactivity (Fig. 3; see also Fig. S1 to S8, panels C, in the supplemental material). In addition, the more than 1,600 currently available OmpA sequences of Chlamydia spp. allow the construction of serovar phylograms, thus pinpointing epitopes for serovar-specific molecular serology of Chlamydia spp. (see Fig. S3B, S4B, and S8B in the supplemental material).
Peptide-based chlamydial serology has been approached before (23–25, 37, 38). However, the reactivity of these peptides had not been tested with a comprehensive battery of monospecific antisera against all Chlamydia species. We report here chlamydial proteome-wide and genus-wide discovery of dominant B cell epitopes by comprehensive testing with improved methodology. Previous studies focused mainly on OmpA peptides and, to avoid cross-reactivity, typically used very short peptides and applied them directly to solid surfaces (30–34). Our data show that both short peptides and suboptimal linkers profoundly reduce peptide reactivity in ELISAs. In addition, the high OmpA polymorphism necessitates large numbers of peptide antigens for species-specific detection of antichlamydial antibodies. Our investigation has identified dominant B cell epitopes from other proteins that are highly conserved within chlamydial species and yet well separated among species (PmpD, IncA, CT618, CT529, and CT442), thus facilitating Chlamydia species-specific assays by use of a few, but highly reactive, peptide antigens. In contrast, OmpA as the main serovar determinant of Chlamydia provides optimal epitopes for determination of serovar-specific antibodies, as we demonstrated for one species, C. pecorum (see Fig. S3, S4, and S8 in the supplemental material).
The strength of the multipeptide approach for serological determination of the chlamydial exposure status is also that the probability of false-positive results is vastly reduced if reactivity not with a single species but with multiple species-specific peptides is used as a stringent requirement for establishing exposure status to a chlamydial species. Importantly, the simultaneous use of multiple peptides of each chlamydial species in serological assays also reduces the stochastic variation of antibody levels against individual peptides and thereby increases the sensitivity of the assays, as shown in Fig. 4B and 5B.
Antibody responses against many chlamydial proteins, such as inclusion membrane proteins (92), are produced only in the context of the replication of chlamydiae during infection. Therefore, infectious, rather than inactivated and adjuvanted, chlamydiae were used to reproduce immune responses after natural infection. These inoculations were repeated twice to create high-titer, affinity-matured antibody responses. Thus, these hyperimmune antisera represent the natural antibody response to chlamydial infections as closely as experimentally achievable, with the notable, and possibly consequential, exception that the murine host does not represent the natural host for most chlamydial species. However, in contrast to laboratory mice, for most natural host species it is very difficult to obtain animals that have never been exposed to chlamydial infection. Thus, the murine host offers the certainty of nonexposure which is essential for creation of monospecific antichlamydial sera.
Antibody production against many chlamydial proteins is detectable only in the original host and not in heterologous hosts such as laboratory rodents (60). This host dependency may be the result of differential protein expression and immune accessibility in the original versus the heterologous host. However, immunodominance of some chlamydial proteins is independent of the host in which the antichlamydial antibodies were raised (60). In this investigation, the vast majority of the identified species-specific epitopes are located on host-independent proteins such as OmpA, PmpD, IncA, Omp2, CT442, CT529, and CT618 (Table 1, footnote e). This indicates that epitopes identified in our murine heterologous model approach (Tables 2 and 3) will also be immunodominant in natural infections of the homologous host. We have confirmed this homologous immunodominance, i.e., the host-independent nature of these reactive peptides, with bovine sera for six C. pecorum peptides from five proteins (Fig. 4 and 5) and for the peptides shown in Tables 2 and 3 of C. abortus, C. pneumoniae, and C. suis (data not shown).
Among the 54 chlamydial proteins that have been reported as immunodominant, we could not identify any B cell epitopes (Table 1, footnote c). Although this may be explained by the fact that we tested only a few peptides, it may also have reflect the poor immunogenicity in the heterologous host. This suggests that additional host-dependent B cell epitopes may be identified by screening with homologous sera after the chlamydial exposure status has been ascertained by use of the current set of species-specific peptides. In fact, a preliminary screen of nonreactive peptides with bovine sera has demonstrated high and specific reactivity of a number of these peptides (data not shown).
In summary, the peptide antigens found in this investigation produce high and absolutely species-specific signals in a robust ELISA format. In addition to Chlamydia species-specific peptides, type-specific peptides for strains of C. suis, C. pecorum, C. trachomatis, and C. psittaci and genus-specific peptides were identified. Because of the simplicity and robustness of these peptide ELISAs, molecular serology of chlamydial infections may now become accessible for nonspecialist laboratories. Such serological assays would also have the added advantage of retrospectively capturing the history of chlamydial infection, rather than stochastically sample a single point in time as PCR detection does. These peptide antigens may also be used in multiplexed assays such as microarrays (89, 90) or fluorescent bead assays (91, 92). We anticipate that these peptide ELISAs have the potential to vastly improve chlamydial serology, in particular Chlamydia species-specific serological diagnosis. By allowing serological dissection of multispecies chlamydial infections, they will further the understanding of chlamydial diseases in retrospective epidemiological investigations of human and animal chlamydial infections. In a wider context, the present methodological approach of epitope identification has great potential for being useful in both immunological research and the laboratory diagnosis of other microbial infections.
ACKNOWLEDGMENTS
We thank Dongya Gao for help with DNA sequencing. We also thank Garry Myers for kindly providing draft genome sequences of C. suis strain S45.
This investigation was supported by funding from the Molecular Diagnostics Laboratory at the Department of Pathobiology, College of Veterinary Medicine, at Auburn University. Part of the study was supported by funding from the Seventh Framework Programme of the European Union (FP7/2007-2013) under grant agreement 222633 (WildTech).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00102-15.
REFERENCES
- 1.Kuo CC, Stephens RS, Bavoil PM, Kaltenboeck B. 2011. Genus I. Chlamydia Jones, Rake, and Stearns 1945, 55AL, p 846–865. In Krieg NR, Parte A, Ludwig W, Whitman W, Hedlund P, Paster BJ, Staley JT, Ward N, Brown D (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 4 Springer, New York, NY. [Google Scholar]
- 2.Sachse K, Laroucau K, Riege K, Wehner S, Dilcher M, Creasy HH, Weidmann M, Myers G, Vorimore F, Vicari N, Magnino S, Liebler-Tenorio E, Ruettger A, Bavoil PM, Hufert FT, Rosselló-Móra R, Marz M. 2014. Evidence for the existence of two new members of the family Chlamydiaceae and proposal of Chlamydia avium sp. nov. and Chlamydia gallinacea sp. nov. Syst Appl Microbiol 37:79–88. doi: 10.1016/j.syapm.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Everett KD, Bush RM, Andersen AA. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol 49:415–440. doi: 10.1099/00207713-49-2-415. [DOI] [PubMed] [Google Scholar]
- 4.Sachse K, Bavoil PM, Kaltenboeck B, Stephens RS, Kuo C-C, Rosselló-Móra R, Horn M. 2015. Emendation of the family Chlamydiaceae: proposal of a single genus, Chlamydia, to include all currently recognized species. Syst Appl Microbiol http://dx.doi.org/10.1016/j.syapm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Kaltenboeck B, Schmeer N, Schneider R. 1997. Evidence for numerous omp1 alleles of porcine Chlamydia trachomatis and novel chlamydial species obtained by PCR. J Clin Microbiol 35:1835–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamad KY, Roche SM, Myers GS, Bavoil PM, Laroucau K, Magnino S, Laurent S, Rasschaert D, Rodolakis A. 2008. Preliminary phylogenetic identification of virulent Chlamydophila pecorum strains. Infect Genet Evol 8:764–771. doi: 10.1016/j.meegid.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Mohamad KY, Kaltenboeck B, Rahman KS, Magnino S, Sachse K, Rodolakis A. 2014. Host adaptation of Chlamydia pecorum toward low virulence evident in co-evolution of the ompA, incA, and ORF663 loci. PLoS One 9:e103615. doi: 10.1371/journal.pone.0103615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SP, Grayston JT. 1974. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis 130:388–397. doi: 10.1093/infdis/130.4.388. [DOI] [PubMed] [Google Scholar]
- 9.Nunes A, Borrego MJ, Nunes B, Florindo C, Gomes JP. 2009. Evolutionary dynamics of ompA, the gene encoding the Chlamydia trachomatis key antigen. J Bacteriol 191:7182–7192. doi: 10.1128/JB.00895-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanrompay D, Butaye P, Sayada C, Ducatelle R, Haesebrouck F. 1997. Characterization of avian Chlamydia psittaci strains using omp1 restriction mapping and serovar-specific monoclonal antibodies. Res Microbiol 148:327–333. doi: 10.1016/S0923-2508(97)81588-4. [DOI] [PubMed] [Google Scholar]
- 11.Andersen AA. 2005. Serotyping of US isolates of Chlamydophila psittaci from domestic and wild birds. J Vet Diagn Invest 17:479–482. doi: 10.1177/104063870501700514. [DOI] [PubMed] [Google Scholar]
- 12.Sachse K, Laroucau K, Vorimore F, Magnino S, Feige J, Müller W, Kube S, Hotzel H, Schubert E, Slickers P, Ehricht R. 2009. DNA microarray-based genotyping of Chlamydophila psittaci strains from culture and clinical samples. Vet Microbiol 135:22–30. doi: 10.1016/j.vetmic.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Dean D, Rothschild J, Ruettger A, Kandel RP, Sachse K. 2013. Zoonotic Chlamydiaceae species associated with trachoma, Nepal. Emerg Infect Dis 19:1948. doi: 10.3201/eid1912.130656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachse K, Vretou E, Livingstone M, Borel N, Pospischil A, Longbottom D. 2009. Recent developments in the laboratory diagnosis of chlamydial infections. Vet Microbiol 135:2–21. doi: 10.1016/j.vetmic.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Ozanne G, Lefebvre J. 1992. Specificity of the microimmunofluorescence assay for the serodiagnosis of Chlamydia pneumoniae infections. Can J Microbiol 38:1185–1189. doi: 10.1139/m92-194. [DOI] [PubMed] [Google Scholar]
- 16.Kern DG, Neill MA, Schachter J. 1993. A seroepidemiologic study of Chlamydia pneumoniae in Rhode Island: evidence of serologic cross-reactivity. Chest J 104:208–213. [DOI] [PubMed] [Google Scholar]
- 17.Wagenvoort JHT, Koumans D, Van de Cruijs M. 1999. How useful is the Chlamydia micro-immunofluorescence (MIF) test for the gynaecologist? Eur J Obstet Gynecol Reprod Biol 84:13–15. doi: 10.1016/S0301-2115(98)00303-0. [DOI] [PubMed] [Google Scholar]
- 18.Wong YK, Sueur JM, Fall CH, Orfila J, Ward ME. 1999. The species specificity of the microimmunofluorescence antibody test and comparisons with a time resolved fluoroscopic immunoassay for measuring IgG antibodies against Chlamydia pneumoniae. J Clin Pathol 52:99–102. doi: 10.1136/jcp.52.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard CA, Borel N. 2014. Chronic chlamydial diseases: from atherosclerosis to urogenital infections. Curr Clin Microbiol Rep 1:1–12. doi: 10.1007/s40588-014-0002-y. [DOI] [Google Scholar]
- 20.Poudel A, Elsasser TH, Rahman KS, Chowdhury EU, Kaltenboeck B. 2012. Asymptomatic endemic Chlamydia pecorum infections reduce growth rates in calves by up to 48%. PLoS One 7:e44961. doi: 10.1371/journal.pone.0044961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhold P, Sachse K, Kaltenboeck B. 2011. Chlamydiaceae in cattle: commensals, trigger organisms, or pathogens? Vet J 189:257–267. doi: 10.1016/j.tvjl.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Wang SP, Grayston JT. 1970. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol 70:367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]
- 23.Bas S, Muzzin P, Ninet B, Bornand JE, Scieux C, Vischer TL. 2001. Chlamydial serology: comparative diagnostic value of immunoblotting, microimmunofluorescence test, and immunoassays using different recombinant proteins as antigens. J Clin Microbiol 39:1368–1377. doi: 10.1128/JCM.39.4.1368-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bas S, Genevay S, Schenkel MC, Vischer TL. 2002. Importance of species-specific antigens in the serodiagnosis of Chlamydia trachomatis reactive arthritis. Rheumatology 41:1017–1020. doi: 10.1093/rheumatology/41.9.1017. [DOI] [PubMed] [Google Scholar]
- 25.Bas S, Muzzin P, Vischer TL. 2001. Chlamydia trachomatis serology: diagnostic value of outer membrane protein 2 compared with that of other antigens. J Clin Microbiol 39:4082–4085. doi: 10.1128/JCM.39.11.4082-4085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens RS, Tam MR, Kuo CC, Nowinski RC. 1982. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol 128:1083–1089. [PubMed] [Google Scholar]
- 27.Batteiger BE, Newhall WJ, Terho P, Wilde CE, Jones RB. 1986. Antigenic analysis of the major outer membrane protein of Chlamydia trachomatis with murine monoclonal antibodies. Infect Immun 53:530–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baehr W, Zhang YX, Joseph T, Su HUA, Nano FE, Everett KD, Caldwell HD. 1988. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A 85:4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mygind P, Christiansen G, Persson K, Birkelund S. 2000. Detection of Chlamydia trachomatis-specific antibodies in human sera by recombinant major outer-membrane protein polyantigens. J Med Microbiol 49:457–465. [DOI] [PubMed] [Google Scholar]
- 30.Klein M, Kötz A, Bernardo K, Krönke M. 2003. Detection of Chlamydia pneumoniae-specific antibodies binding to the VD2 and VD3 regions of the major outer membrane protein. J Clin Microbiol 41:1957–1962. doi: 10.1128/JCM.41.5.1957-1962.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livingstone M, Entrican G, Wattegedera S, Buxton D, McKendrick IJ, Longbottom D. 2005. Antibody responses to recombinant protein fragments of the major outer membrane protein and polymorphic outer membrane protein POMP90 in Chlamydophila abortus-infected pregnant sheep. Clin Diagn Lab Immunol 12:770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong GM, Reid RE, Brunham RC. 1990. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect Immun 58:1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conlan JW, Clarke IN, Ward ME. 1988. Epitope mapping with solid-phase peptides: identification of type-, subspecies-, species-, and genus-reactive antibody binding domains on the major outer membrane protein of Chlamydia trachomatis. Mol Microbiol 2:673–679. doi: 10.1111/j.1365-2958.1988.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 34.Pal S, Cheng X, Peterson EM, de la Maza LM. 1993. Mapping of a surface-exposed B cell epitope to the variable sequent 3 of the major outer-membrane protein of Chlamydia trachomatis. J Gen Microbiol 139:1565–1570. doi: 10.1099/00221287-139-7-1565. [DOI] [PubMed] [Google Scholar]
- 35.Batteiger BE. 1996. The major outer membrane protein of a single Chlamydia trachomatis serovar can possess more than one serovar-specific epitope. Infect Immun 64:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villeneuve A, Brossay L, Paradis G, Hébert J. 1994. Determination of neutralizing epitopes in variable domains I and IV of the major outer-membrane protein from Chlamydia trachomatis serovar K. Microbiology 140:2481–2487. [DOI] [PubMed] [Google Scholar]
- 37.Närvänen A, Puolakkainen M, Hao W, Kino K, Suni J. 1997. Detection of antibodies to Chlamydia trachomatis with peptide-based species-specific enzyme immunoassay. Infect Dis Obstet Gynecol 5:349–354. doi: 10.1155/S1064744997000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baud D, Regan L, Greub G. 2010. Comparison of five commercial serological tests for the detection of anti-Chlamydia trachomatis antibodies. Eur J Clin Microbiol Infect Dis 29:669–675. doi: 10.1007/s10096-010-0912-4. [DOI] [PubMed] [Google Scholar]
- 39.Sivalingam GN, Shepherd AJ. 2012. An analysis of B cell epitope discontinuity. Mol Immunol 51:304–309. doi: 10.1016/j.molimm.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kringelum JV, Nielsen M, Padkjær SB, Lund O. 2013. Structural analysis of B cell epitopes in antibody: protein complexes. Mol Immunol 53:24–34. doi: 10.1016/j.molimm.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubinstein ND, Mayrose I, Halperin D, Yekutieli D, Gershoni JM, Pupko T. 2008. Computational characterization of B cell epitopes. Mol Immunol 45:3477–3489. doi: 10.1016/j.molimm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Van Regenmortel MHV. 2009. What is a B cell epitope? Methods Mol Biol 524:3–20. doi: 10.1007/978-1-59745-450-6_1. [DOI] [PubMed] [Google Scholar]
- 43.Wills GS, Horner PJ, Reynolds R, Johnson AM, Muir DA, Brown DW, Winston A, Broadbent AJ, Parker D, McClure MO. 2009. Pgp3 antibody enzyme-linked immunosorbent assay, a sensitive and specific assay for seroepidemiological analysis of Chlamydia trachomatis infection. Clin Vaccine Immunol 16:835–843. doi: 10.1128/CVI.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham AF, Ward ME. 2003. Characterization of human humoral responses to the major outer membrane protein and OMP2 of Chlamydophila pneumoniae. FEMS Microbiol Lett 227:73–79. doi: 10.1016/S0378-1097(03)00650-5. [DOI] [PubMed] [Google Scholar]
- 45.Andresen H, Grötzinger C, Zarse K, Kreuzer OJ, Ehrentreich-Förster E, Bier FF. 2006. Functional peptide microarrays for specific and sensitive antibody diagnostics. Proteomics 6:1376–1384. doi: 10.1002/pmic.200500343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 47.Kalman S, Mitchell W, Marathe R, Lammel C, Fan J, Hyman RW, Olinger L, Grimwood J, Davis RW, Stephens R. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat Genet 21:385–389. doi: 10.1038/7716. [DOI] [PubMed] [Google Scholar]
- 48.Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, Hickey EK, Peterson J, Utterback T, Berry K, Bass S, Linher K, Weidman J, Khouri H, Craven B, Bowman C, Dodson R, Gwinn M, Nelson W, DeBoy R, Kolonay J, McClarty G, Salzberg SL, Eisen J, Fraser CM. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res 28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mojica S, Creasy HH, Daugherty S, Read TD, Kim T, Kaltenboeck B, Bavoil P, Myers GS. 2011. Genome sequence of the obligate intracellular animal pathogen Chlamydia pecorum E58. J Bacteriol 193:3690. doi: 10.1128/JB.00454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Read TD, Myers GS, Brunham RC, Nelson WC, Paulsen IT, Heidelberg J, Holtzapple E, Khouri H, Federova NB, Carty HA, Umayam LA, Haft DH, Peterson J, Beanan MJ, White O, Salzberg SL, Hsia RC, McClarty G, Rank RG, Bavoil PM, Fraser CM. 2003. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res 31:2134–2147. doi: 10.1093/nar/gkg321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schöfl G, Voigt A, Litsche K, Sachse K, Saluz HP. 2011. Complete genome sequences of four mammalian isolates of Chlamydophila psittaci. J Bacteriol 193:4258. doi: 10.1128/JB.05382-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voigt A, Schöfl G, Heidrich A, Sachse K, Saluz HP. 2011. Full-length de novo sequence of the Chlamydophila psittaci type strain, 6BC. J Bacteriol 193:2662–2663. doi: 10.1128/JB.00236-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Read TD, Joseph SJ, Didelot X, Liang B, Patel L, Dean D. 2013. Comparative analysis of Chlamydia psittaci genomes reveals the recent emergence of a pathogenic lineage with a broad host range. mBio 4:e00604-12. doi: 10.1128/mBio.00604-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomson NR, Yeats C, Bell K, Holden MT, Bentley SD, Livingstone M, Cerdeño-Tárraga AM, Harris B, Doggett J, Ormond D, Mungall K, Clarke K, Feltwell T, Hance Z, Sanders M, Quail MA, Price C, Barrell BG, Parkhill J, Longbottom D. 2005. The Chlamydophila abortus genome sequence reveals an array of variable proteins that contribute to interspecies variation. Genome Res 15:629–640. doi: 10.1101/gr.3684805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azuma Y, Hirakawa H, Yamashita A, Cai Y, Rahman MA, Suzuki H, Mitaku S, Toh H, Goto S, Murakami T, Sugi K, Hayashi H, Fukushi H, Hattori M, Kuhara S, Shirai M. 2006. Genome sequence of the cat pathogen, Chlamydophila felis. DNA Res 13:15–23. doi: 10.1093/dnares/dsi027. [DOI] [PubMed] [Google Scholar]
- 56.Donati M, Huot-Creasy H, Humphrys M, Di Paolo M, Di Francesco A, Myers GS. 2014. Genome sequence of Chlamydia suis MD56, isolated from the conjunctiva of a weaned piglet. Genome Announc 2:e00425-14. doi: 10.1128/genomeA.00425-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Lent S, Piet JR, Beeckman D, van der Ende A, van Nieuwerburgh F, Bavoil P, Myers G, Vanrompay D, Pannekoek Y. 2012. Full genome sequences of all nine Chlamydia psittaci genotype reference strains. J Bacteriol 194:6930–6931. doi: 10.1128/JB.01828-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bunk S, Susnea I, Rupp J, Summersgill JT, Maass M, Stegmann W, Schrattenholz A, Wendel A, Przybylski M, Hermann C. 2008. Immunoproteomic identification and serological responses to novel Chlamydia pneumoniae antigens that are associated with persistent C. pneumoniae infections. J Immunol 180:5490–5498. doi: 10.4049/jimmunol.180.8.5490. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Chen C, Chen D, Wu Y, Zhong Y, Zhong G. 2008. Characterization of fifty putative inclusion membrane proteins encoded in the Chlamydia trachomatis genome. Infect Immun 76:2746–2757. doi: 10.1128/IAI.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Zhang Y, Lu C, Lei L, Yu P, Zhong G. 2010. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J Immunol 185:1670–1680. doi: 10.4049/jimmunol.1001240. [DOI] [PubMed] [Google Scholar]
- 61.Cruz-Fisher MI, Cheng C, Sun G, Pal S, Teng A, Molina DM, Kayala MA, Vigil A, Baldi P, Felgner PL, Liang X, de la Maza LM. 2011. Identification of immunodominant antigens by probing a whole Chlamydia trachomatis open reading frame proteome microarray using sera from immunized mice. Infect Immun 79:246–257. doi: 10.1128/IAI.00626-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu C, Holland MJ, Gong S, Peng B, Bailey RL, Mabey DW, Wu Y, Zhong G. 2012. Genome-wide identification of Chlamydia trachomatis antigens associated with trachomatous trichiasis. Invest Ophthalmol Vis Sci 53:2551–2559. doi: 10.1167/iovs.11-9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teng A, Cruz-Fisher MI, Cheng C, Pal S, Sun G, Ralli-Jain P, Molina DM, Felgner PL, Liang X, de la Maza LM. 2012. Proteomic identification of immunodominant chlamydial antigens in a mouse model. J Proteomics 77:176–186. doi: 10.1016/j.jprot.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasui Y, Yanatori I, Kawai Y, Miura K, Suminami Y, Hirota T, Tamari M, Ouchi K, Kishi F. 2012. Genomic screening for Chlamydophila pneumoniae-specific antigens using serum samples from patients with primary infection. FEMS Microbiol Lett 329:168–176. doi: 10.1111/j.1574-6968.2012.02520.x. [DOI] [PubMed] [Google Scholar]
- 66.Forsbach-Birk V, Foddis C, Simnacher U, Wilkat M, Longbottom D, Walder G, Benesch C, Ganter M, Sachse K, Essig A. 2013. Profiling antibody responses to infections by Chlamydia abortus enables identification of potential virulence factors and candidates for serodiagnosis. PLoS One 8:e80310. doi: 10.1371/journal.pone.0080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li D, Vaglenov A, Kim T, Wang C, Gao D, Kaltenboeck B. 2005. High-yield culture and purification of Chlamydiaceae bacteria. J Microbiol Methods 61:17–24. doi: 10.1016/j.mimet.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 68.Storz J, Shupe JL, Smart RA, Thornley RW. 1966. Polyarthritis of calves: experimental induction by a psittacosis agent. Am J Vet Res 27:987. [PubMed] [Google Scholar]
- 69.DeGraves FJ, Gao D, Hehnen HR, Schlapp T, Kaltenboeck B. 2003. Quantitative detection of Chlamydia psittaci and C. pecorum by high-sensitivity real-time PCR reveals high prevalence of vaginal infection in cattle. J Clin Microbiol 41:1726–1729. doi: 10.1128/JCM.41.4.1726-1729.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehricht R, Slickers P, Goellner S, Hotzel H, Sachse K. 2006. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol Cell Probes 20:60–63. doi: 10.1016/j.mcp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Wang C, van Ginkel FW, Kim T, Li D, Li Y, Dennis JC, Kaltenboeck B. 2008. Temporal delay of peak T-cell immunity determines Chlamydia pneumoniae pulmonary disease in mice. Infect Immun 76:4913–4923. doi: 10.1128/IAI.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poudel A, Reid E, Newport-Nielsen E, Chowdhury EU, Rahman KS, Sartin J, Kaltenboeck B. 2014. Endemic asymptomatic Chlamydia pecorum genital tract infection reduces fertility in dairy cows, p 258–260. Proceedings of the 13th International Symposium on Human Chlamydial Infections, Pacific Grove, CA. [Google Scholar]
- 73.Kaltenboeck B, Kousoulas KG, Storz J. 1993. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J Bacteriol 175:487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caldwell HD, Stewart S, Johnson S, Taylor H. 1987. Tear and serum antibody response to Chlamydia trachomatis antigens during acute chlamydial conjunctivitis in monkeys as determined by immunoblotting. Infect Immun 55:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stephens RS, Wagar EA, Schoolnik GK. 1988. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med 167:817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell LA, Kuo C, Wang S, Grayston JT. 1990. Serological response to Chlamydia pneumoniae infection. J Clin Microbiol 28:1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Essig A, Simnacher U, Susa M, Marre R. 1999. Analysis of the humoral immune response to Chlamydia pneumoniae by immunoblotting and immunoprecipitation. Clin Diagn Lab Immunol 6:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Portig I, Goodall JC, Bailey RL, Gaston JSH. 2003. Characterization of the humoral immune response to Chlamydia outer membrane protein 2 in chlamydial infection. Clin Diagn Lab Immunol 10:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crane DD, Carlson JH, Fischer ER, Bavoil P, Hsia R, Tan C, Kuo C, Caldwell HD. 2006. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc Natl Acad Sci U S A 103:1894–1899. doi: 10.1073/pnas.0508983103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hongliang C, Zhou Z, Zhan H, Yanhua Z, Zhongyu L, Yingbiao L, Guozhi D, Yimou W. 2010. Serodiagnosis of Chlamydia pneumoniae infection using three inclusion membrane proteins. J Clin Lab Anal 24:55–61. doi: 10.1002/jcla.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohamad YK, Rekiki A, Berri M, Rodolakis A. 2010. Recombinant 35-kDa inclusion membrane protein IncA as a candidate antigen for serodiagnosis of Chlamydophila pecorum. Vet Microbiol 143:424–428. doi: 10.1016/j.vetmic.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 82.Wang J, Zhang Y, Yu P, Zhong G. 2010. Immunodominant regions of a Chlamydia trachomatis type III secretion effector protein, Tarp. Clin Vaccine Immunol 17:1371–1376. doi: 10.1128/CVI.00218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview version 2: a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henikoff S, Henikoff JG. 1992. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A 89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dosztányi Z, Csizmok V, Tompa P, Simon I. 2005. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 21:3433–3434. doi: 10.1093/bioinformatics/bti541. [DOI] [PubMed] [Google Scholar]
- 87.Mirabello C, Pollastri G. 2013. Porter, PaleAle 4.0: high-accuracy prediction of protein secondary structure and relative solvent accessibility. Bioinformatics 29:2056–2058. doi: 10.1093/bioinformatics/btt344. [DOI] [PubMed] [Google Scholar]
- 88.Parker JMR, Guo D, Hodges RS. 1986. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry 25:5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- 89.Geysen HM, Rodda SJ, Mason TJ, Tribbick G, Schoofs PG. 1987. Strategies for epitope analysis using peptide synthesis. J Immunol Methods 102:259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- 90.Kaltenboeck B, Heard D, DeGraves FJ, Schmeer N. 1997. Use of synthetic antigens improves detection by enzyme-linked immunosorbent assay of antibodies against abortigenic Chlamydia psittaci in ruminants. J Clin Microbiol 35:2293–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brack C, Hirama M, Schuller RL, Tonegawa S. 1978. A complete immunoglobulin gene is created by somatic recombination. Cell 15:1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- 92.Rockey DD, Rosquist JL. 1994. Protein antigens of Chlamydia psittaci present in infected cells but not detected in the infectious elementary body. Infect Immun 62:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andresen H, Zarse K, Grötzinger C, Hollidt JM, Ehrentreich-Förster E, Bier FF, Kreuzer OJ. 2006. Development of peptide microarrays for epitope mapping of antibodies against the human TSH receptor. J Immunol Methods 315:11–18. doi: 10.1016/j.jim.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 94.Cosandey V, Debrot FN, Kaeser J, Marti R, Passeraub P, Petremand J, Prim D, Pfeifer ME. 2012. Construction of a peptide microarray for auto-antibody detection. Chimia Int J Chem 66:803–806. doi: 10.2533/chimia.2012.803. [DOI] [PubMed] [Google Scholar]
- 95.Elshal MF, McCoy JP. 2006. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods 38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chowdhury F, Williams A, Johnson P. 2009. Validation and comparison of two multiplex technologies, Luminex® and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods 340:55–64. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]