Abstract
Opportunistic subcutaneous fungal infections are increasing in present times due to increasing incidence of many medical conditions causing immunosupression like diabetes, AIDS, organ transplant recipients and anticancer therapy. Pyrenochaeta romeroi, a fungus from the dematiaceae group, first described by Borelli in 1959, is saprophyte to soil and plants.We present a rare case of subcutaneous phaeohyphomycotic cyst in a diabetic female caused by P. romeroi.
Keywords: Cyst, Phaeohypomycosis, Pyrenochaeta, Subcutaneous
1. Introduction
Opportunistic subcutaneous fungal infections are increasing in present times due to increasing incidence of many medical conditions causing immunosupression like diabetes, AIDS, organ transplant recipients and anticancer therapy. Phaeohyphomycosis is an heterogenous group of superficial or deep fungus with black filaments often causing opportunistic infections [1–3]. The member of the genus Pyrenochaeta are widely distributed in the environment and is saprophyte to soil, wood and plants. Pyrenochaeta romeroi, a fungus from dematiaceae group was first isolated by Borelli in 1959 [3]. It is generally inoculated through direct trauma and remains localized to cutaneous and subcutaneous tissue. P. romeroi is often isolated from mycetoma,[4–6] but recently it has been found as a causative agent of phaeohyphomycotic cyst in few cases [7–9].
We present a rare case of subcutaneous phaeohyphomycotic cyst in a diabetic female caused by P. romeroi. To the best of our knowledge, this is the second case reported from North India.
2. Case
A 50 year old female presented with progressively increasing painless swelling over left foot since 4 years to the surgical out patient department on day 0. There was no history of trauma, fever, cough, weight loss, anorexia or any other swelling on the body. She was a known case of diabetes mellitus for five years. But the compliance to the treatment was poor. There was no history of tuberculosis, hypertension or any kind of surgery.
Local examination revealed a soft to firm mass at the left foot measuring 3×2 cm2. Swelling was slightly mobile, non tender with overlying skin normal (Fig. 1). However, on deep pressure the patient exhibited discomfort.
Fig. 1.
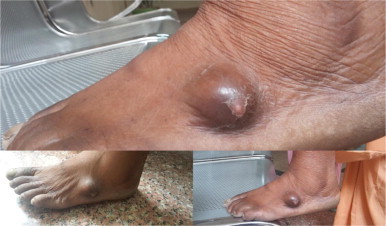
Photograph of the patient showing characteristic swelling on left foot.
On day +1, laboratory investigations revealed hemoglobin 10.9 g%, total leukocyte count 10,200 cells/cumm, differential count- neutrophils 68%, lymphocytes 25%, monocytes 4%, eosinophils 3%, basophil 0%, platelets 1.9 L/cumm and Erythrocyte Sedimentation Rate (ESR) 48 mm in 1st hour by Westergrens method. Biochemical tests revealed random blood sugar 413 g/dl. Liver function and renal function test were within normal limits. Urine examination revealed glucose +++ and traces of albumin. Serology for HIV and Hepatitis B antigen was non reactive.
Fine needle aspiration (FNA) of the swelling was performed using a 23-gauge needle and 10 ml syringe on day +1. FNA yielded 2 ml of dirty white purulent pus mixed with blood but free from any discrete granules. Smears were stained with Romanosky and papanicolaou stain. Examination of the smears revealed degenerated and intact inflammatory cells including neutrophils, few lymphocytes, macrophages and red blood cells in a background of necrotic material. Few septate fungal hyphae surrounded by inflammatory infiltrate were also observed (Fig. 2). Periodic Acid Schiff (PAS) stained smears show fungal hyphae which were PAS positive.
Fig. 2.
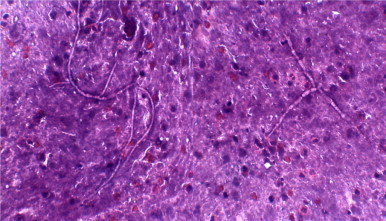
Haemotoxylin and eosin stained smear of the aspirate showing septate, tortuous hyphae.
The specimen was cultured by standard techniques onto the blood agar, MacConkey agar and Sabaraud’s dextrose agar (SDA) with chloramphenicol. Bacteriological culture did not show any growth. The SDA slants were incubated at 25 °C. After 10 days of incubation on SDA, white, velvetty growth was observed, which gradually turned in phaeoid form in about 3–4 weeks (Fig. 3). Lactophenol cotton blue (LCB) mount revealed brown colored, tortous hyphae. No conidia were observed on hyphae. On the basis of microscopic and macroscopic examination, the isolate was not identified. Therefore, it was subjected to identification on the basis of molecular methods and the isolate was then sent to National Culture Collection for Pathogenic Fungi (NCCPF), Post Graduate Institute of Medical Research and Education (PGIMER), Chandigarh for molecular analysis. PCR followed by sequencing was done on the culture grown on SDA slant. PCR amplification was done to amplify ITS1 region of 18S rRNA gene and was subsequently sequenced. A homology for the obtained sequences was carried out using BLAST (NCBI, Washington, DC). The clinical isolate was identified as Pyrenochaeta species with closest assocication with P. romeroi (ACCESION no. FN826905.2). The BLAST results revealed the 99% homology and 98% query coverage to the GenBank ACCESSION no. FN826905.2.
Fig. 3.
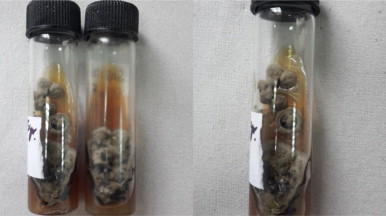
Saboraud Dextrose Agar slant showing phaeoid growth of the isolate.
On day +6, the swelling was incised and drained. The patient was started on itraconazole 200 mg/day for 15 days on the basis of preliminary FNA report. Routine follow up of the patient was done on day +30 and day +90. The patient showed excellent response and the swelling resolved completely.
3. Discussion
In 1974, Ajello introduced the term “Phaeohyphomycosis” to describe a heterogenous group of cutaneous, subcutaneous and systemic fungal infections caused by fungi with melanized hyphae or yeast cells in tissue [1]. Most of the phaeohyphomycotic infections have been reported in patients with underlying immunosuppressive or debilitating conditions. Khan et al. [9] have reported a case of phaeohyphomycotic cyst in a female with acute lymphoblastic leukemia. The patient is a known case of diabetes in the present study. Similarly, Girard et al. [8] also described a case of phaeohyphomycotic cyst in a patient with leprosy. However, in contrast, Badali et al. [7] have reported a case in healthy Indian female. Most species of Pyrenochaeta are true phytopathogens and are significantly different from clinical taxa [6]. So far, four species, namely Pyrenochaeta romeroi, Pyrenochaeta mackinnonii, Pyrenochaeta unguis-hominis and Pyrenochaeta keratinophila have been implicated in human infections [10–12]. Furthermore, only three cases of subcutaneous cyst by P romeroi have been documented since the first case reported in 1959 [7–9].
P. romeroi, a fungus from the dematiaceae group first described by Borelli in 1959, is saprophyte to soil and plants, seen particularly in association with plants or decaying matter or debris, so mainly agricultural workers are at increased risk of acquiring infection [9]. Although, in the present case, the patient is a housewife, but she used to go outside in fields for harvestation. In subcutaneous tissues, minor trauma, even when unrecognized by the patient as observed in our case, is supposed to be the inciting factor [1]. It is generally inoculated through direct traumatism by a plant or a soiled object and is usually encountered in tropical and subtropical areas [7,9]. The lesion usually starts as a cystic nodule which becomes chronic, as seen in the present case. The lesion may eventually undergo necrosis as observed by Khan et al. [9] in their patient. The identification of Pyrenochaeta species is difficult because of the inability of some of the strains to readily produce characteristic diagnostic structures in culture and also due to limited expertize available in most of the diagnostic laboratories [7,9]. Thus, molecular identification and characterization at a reference center remains mainstay for an accurate identification of the fungus and its antifungal susceptibility testing.
The literature shows that from a treatment point of view, surgical treatment has been successfully applied in a number of cases because no standardized therapy is available [8–13]. The literature also suggests that antifungal susceptibility of Pyrenochaeta species is scanty due to low availability and the low number of clinical isolates. No standard therapy is available for phaeohyphomycosis and also little is known about the relation between MIC and clinical outcome. Various authors have reported a higher MIC for amphotericin B, ketoconazole and fluconazole. However, newer azoles like voriconazole and posaconazole have shown good results [7–9]. Our patient showed signs of improvement on drainage and itraconazole therapy.
In conclusion, subcutaneous mycoses can be caused by both the common and rare species, therefore it is suggested that a clinician should keep a fungal etiology in mind especially in patients with underlying immunosuppressive conditions.
Conflict of interest
None to be declared.
Acknowledgements
We thank Dr. Arunaloke Chakraborty, Professor, Department of Medical Microbiology, and National Culture Collection for Pathogenic Fungi (NCCPF), Post Graduate Institute of Medical Research and Education (PGIMER), Chandigarh for molecular identification of the species.
References
- 1.L. Ajello, in: Proceedings of the Third International Conference on Mycosis. Scientific publication no. 304, Pan American Health Organization Washington DC, 1975. pp.126–133.
- 2.Rinaldi M.G. Phaeohyphomycosis. Dermatol. Clin. 1996;14:147–153. doi: 10.1016/s0733-8635(05)70335-1. [DOI] [PubMed] [Google Scholar]
- 3.Borelli D. Opportunistic fungi as producers of gray colonies and mycetomata. Dermatologica. 1979;159(Suppl 1):168–174. [PubMed] [Google Scholar]
- 4.Andre M., Brumpt V., Destombes P., Segretain G. Fungal mycetoma with black grains due to Pyrenochaeta romeroi in Cambodia. Bull. Soc. Pathol. Exot. Fil. 1968;61:108–112. [PubMed] [Google Scholar]
- 5.Thammayya A., Sanyal M., Basu N. Pyrenochaeta romeroi causing mycetoma pedis in India. J. Indian Med. Assoc. 1979;73:66–67. [PubMed] [Google Scholar]
- 6.Thiyagarajan U.M., Bagul A., Nicholson M.L. A nodulo-cystic eumycetoma caused by Pyrenochaeta romeroi in a renal transplant recipient: a case report. J. Med. Case Rep. 2011;5:460–461. doi: 10.1186/1752-1947-5-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badali H., Chander J., Gulati N., Attri A., Chopra R., Najafzadeh M.J. Subcutaneous phaeohyphomycotic cyst caused by Pyrenochaeta romeroi. Med. Mycol. 2010;48:763–768. doi: 10.3109/13693780903440383. [DOI] [PubMed] [Google Scholar]
- 8.Girard C., Dereure O., Rispail P., Duran L., Guilhou J.J. Subcutaneous phaeohyphomycosis due to Pyrenochaeta romeroi in a patient with leprosy. Acta Derm. Venereol. 2004;84:154–155. doi: 10.1080/00015550310006824. [DOI] [PubMed] [Google Scholar]
- 9.Khan Z., Ahmad S., Kapila K., Ramaswamy N.V., Alath P., Joseph L., Chandy R. Pyrenochaeta romeroi: a causative agent of phaeohyphomycotic cyst. J. Med. Microbiol. 2011;60:842–846. doi: 10.1099/jmm.0.029520-0. [DOI] [PubMed] [Google Scholar]
- 10.De Hoog G.S., Guarro J., Gene J., Figueras M.J. second ed. Utrech/Reus: Centraalbureau voor Schimmelcultures/Universtat Rovira i Virgili; Netherlands: 2000. Atlas of Clinical Fungi. [Google Scholar]
- 11.Ferrer C., Perez-Santonja J.J., Rodriguez A.E., Colom M.F., Gene J., Alio J.L. New Pyrenochaeta species causing keratitis. J. Clin. Microbiol. 2009;47:1596–1598. doi: 10.1128/JCM.01912-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verkley G.J., Gene J., Guarro J., Perez-Santonja J.J., Rodriguez A.E., Colom M.F. Pyrenochaeta keratinophila sp. nov., isolated from an ocular infection in spain. Rev. Iberoam. Micol. 2010;27:22–24. doi: 10.1016/j.riam.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Badali H., Najafzadeh M.J., Van Esbroeck M., Van den Enden E., Tarazooie B., Meis J.F. The clinical spectrum of Exophiala jeanselmei, with a case report and antifungal susceptibility of the species. Med. Mycol. 2009;47:1–10. doi: 10.1080/13693780903148353. [DOI] [PubMed] [Google Scholar]


