Abstract
The effects of Saccharomyces cerevisiae supplementation (6.6×108 cfu) and anhydrous ammonia treatment (3%) of wheat straw (WS) were investigated on in-situ dry matter (DM) degradability, and on rumen fermentation and growth performance of lambs. Rumen-fistulated Menemen sheep fed a diet with and without live yeast were used to assess the DM degradability characteristics of WS and ammonia-treated wheat straw (WSNH3). Twenty-six yearling Menemen male lambs were fed in four groups. Lambs of control group (WS) received untreated WS without supplemental yeast, whereas other three groups were fed WS treated with anhydrous ammonia (WSNH3 group), untreated WS and yeast (WS+YEAST group) or WS treated with anhydrous ammonia and yeast (WSNH3+YEAST group). Supplemented live yeast (4 g/d) was added in the diet. Lambs were offered untreated or ammonia treated WS ad-libitum and concentrate was fed at 1% of live body weight. The degradability of the water-insoluble (fraction B) was significantly increased by all of the treatment groups. Potential degradability (A+B), effective DM degradability’s (pe2, pe5, and pe8) and average daily weight gain increased only in WSNH3+YEAST group (p<0.05). Voluntary DM intake was not increased by the treatments (p>0.05), but voluntary metabolizable energy and crude protein intake were increased by WSNH3 and by WSNH3+YEAST (p<0.05). Average daily rumen pH was not affected by any of the treatments, but average daily NH3-N was significantly higher in the WSNH3 and WSNH3+YEAST groups, and total volatile fatty acids were significantly higher in the WS+YEAST and WSNH3+YEAST groups. In conclusion, the improvement of feed value of WS was better by the combination of ammonia-treatment and yeast supplementation compared to either treatment alone.
Keywords: Wheat Straw, Ammonia-treatment, Live Yeast, In-situ Degradability, Growth Performance
INTRODUCTION
Large quantities of cereal straws are used in ruminant nutrition in Turkey because of the lack of quality forage in this region (Şehu et al., 1996). Cereal straws are difficult to digest because of their high cellulose content and lignification (Horton and Steacy, 1979). Among various cereal straws in use, wheat straw (WS) is the most common and has the highest feed value, and various studies have been conducted to improve apparent digestibility and voluntary intake of WS (Kılıç et al., 1990). Wheat straw is treated with sodium hydroxide, anhydrous ammonia, and other compounds to break the ester linkages between lignin and polysaccharides (e.g., cellulose and hemicelluloses) in the cell membrane. This process makes the polysaccharides more accessible to microbial enzymes that catalyze their hydrolysis (Tuah et al., 1986; Silva et al., 1989). Various chemical treatments show similar effects, however ammonia is used most commonly because ammonia increases the crude protein (CP) content (Özkul and Şayan, 1996) and decreases the insoluble lignin content of WS (Singh and Gupta, 1990; Habib et al., 1998).
Various studies have shown that ammonia-treatment increases in-situ rumen degradability and effective dry matter (DM) degradability at different rumen outflow rates (Ma et al., 1990; Habib et al., 1998). On the other hand, the studies of ammonia-treatment of straws on rumen fermentation and growth performance show inconsistency. Horton and Steacy (1979) claimed that voluntary intake of WS was significantly improved by 19% with ammonia-treatment, while Kılıç et al. (1990) found that ammonia-treatment of WS did not affect live weight, live weight gain, or DM intake. Bruchemi et al. (1993) reported that ammoniating of WS led to increased voluntary intake in sheep, but the increased intake did not affect the rumen fermentation. No differences were found between average daily pH and total volatile fatty acids (TVFA), but rumen ammonia-N (NH3-N) of ammonia-treated wheat straw (WSNH3) was significantly higher than that of non-treated WS.
In addition to chemical treatment of WS, various studies have investigated methods for increasing the digestibility of cereal straws with live yeast supplementation, because live yeast provides a natural approach for manipulating animal performance by altering the rumen microfauna (İnal et al., 2010). Researchers attribute this effect to the establishment of an environment conducive to the growth of anaerobic bacteria by depleting oxygen in the rumen, thus promoting the development of cellulosic bacteria (Newbold et al., 1996). However, there have been variable effects of yeast supplementation on growth performance and rumen fermentation in ruminants. İnal et al. (2010) reported increased in-situ DM degradation rate with yeast supplementation, whereas yeast supplementation did not improve feed DM intake in lambs (Haddad and Goussous, 2005; Khadem et al., 2007). However, improved growth performance (Tripathi and Karim, 2011) and feed conversion ratio (Haddad and Goussous, 2005) were reported in lambs. Influence of live yeast supplementation in ruminants has been demonstrated on rumen fermentation, which was dependent with the yeast strain, viable cell numbers, basal diet, and the forage type. Promoted rumen pH, TVFA, and NH3-N were reported in sheep (Roa et al., 1997; Khadem et al., 2007), whereas improved pH and no-effect on NH3-N was reported in cows (Guedes et al., 2008). A greater influence of live yeast was reported on digestibility of fiber with fiber rich diets even on low quality forages (Mazzia and Walker, 2008).
Few studies have examined the individual effects of live yeast supplementation and anhydrous ammonia-treatment on the DM degradation and, growth performance and rumen parameters of forage, but no studies have assessed the yeast effects with ammonia-treated or untreated WS. With the combined effect, it is expected that feed value of WS could be positively improved by the increasing crude protein and rumen cellulosic bacteria. Therefore, the present study aim was to investigate the effects of with live yeast (Saccharomyces cerevisiae) supplementation with ammonia-treated or untreated WS on in-situ DM degradability and, rumen fermentation and growth performance in yearling lambs.
MATERIALS AND METHODS
The experiment was carried out at the Biological Analyses Laboratory of Animal Science Department, Faculty of Agriculture, Ege University Izmir, Turkey. The study consisted of two experiments including in-situ study (nylon bag method) and animal experiment.
Wheat straw, ammonia-treatment, and live yeast
The Menemen-88 genotype WS which is commonly produced in Western Anatolia part of Turkey was used to evaluate the effects Saccharomyces cerevisiae supplementation and anhydrous ammonia-treatment of WS on in-situ DM degradability, rumen fermentation, and growth performance in yearling lambs. Anhydrous ammonia-treatment (3%) was carried out according to Kılıç et al. (1990). Eighty-five bales of WS were randomly placed in a single stack in a barn. The rectangular stack was covered with 0.20 mm polyethylene sheet and sealed at the base using sandbags. Anhydrous ammonia was delivered to the WS via a perforated 5 cm diameter iron pipe inserted three quarters of the way into the stack. The cover was removed after 35 days treatment. Another eighty-five bales of WS were stored at the different part of the barn at the time of ammoniating. A hay maker (manufactured in National Agricultural Supplying Foundation) was used to grind the straws into 16 mm at the start of animal experiment. The WS and WSNH3 were packed in the polyethylene bags after grinding. The straws were kept at the room temperature (approximately 19°C) during the experiment. The activity of live yeast (Alltech Inc., Saccharomyces cerevisiae, Yea-Sacc 1026, Nicholasville, AL, USA) was 6.6×108 cfu/g (Method: FDA/BAM-2001).
In-situ study
In-situ dry matter degradability were determined according to the nylon bag method of Bhargava and Orskov, (1987) using three rumen fistulated (40 mm diameter) yearling Menemen male yearling sheep (crossbred of 75% Ile de France and 25% Tahirova). The nylon bags of 9×14 cm in size with pore diameter of 40μm were used for in-situ study. The sheep (live weight about 50 kg) were kept individually (1.2 m×2 m) and had free access to fresh water. Before the study, the parasite and vaccination applications were performed based on veterinary advices. The sheep were distributed according to 3×3 Latin Square design and fed with 60% alfalfa hay (143.0 g/kg of CP and 2,003 kcal/kg of metabolizable energy [ME]) and 40% concentrate (146.0 g/kg of CP and 2,754 kcal/kg of ME) twice a day with the 1.25×of maintenance requirements. The vitamin and mineral composition of the concentrate consisted of vitamin A 7,000 U/kg, vitamin D3 700 U/kg, vitamin E 25 mg/kg, calcium 1.1%, phosphorus 0.4%, and sodium 0.25%.
The in-situ study consisted of 2 steps: First, in-situ dry matter degradability and degradability parameters (A, B, A+B and c) of WS and WSNH3 were determined with nylon bag method without live yeast diet. The WS were grinded using 2.5 mm sieve, weighed 3 g, and then incubated in the rumen for periods 8, 16, 24, 48, 72, and 96 h. After removal from the rumen, the bags were rinsed in cold tap water. The washing losses were determined by measuring one hour incubation in 39°C water. Then, all bags were washed for 10 min in a washing machine, dried at 55°C to 60°C for 48 h and weighed to determine dry matter degradability. The dry matter degradability parameters were A; easily soluble fraction or washing losses, (B = (a+b)-A); degradability of water insoluble fraction, c; degradable rate constant, A+B, potential degradability. The effective dry matter degradability (pe2, pe5, pe8) at different rumen outflow rates (k = 0.02, 0.05, and 0.08 h−1) were calculated using the mathematical model “a+(bxc/c+k)” according to Orskov and McDonald (1979). In the second step, each fistulated sheep was daily supplemented with 4 g live yeast in the diet for 15 days, thereafter attributes of the degradability of DM of WS+Yeast and WSNH3+Yeast were determined by nylon bag method. Three samples were incubated for each observation and time.
Animal experiment
Animal and feeding
Twenty-six Menemen yearling male lambs were used for the 70-day study with a fifteen days adaptation period to the barn and diets prior to the start of the experiment. The national obligatory vaccination programme were applied as declared on TURKVET/KKKS. The interparazitised and exterparazitised applications were done according to veterinary suggestions. The lambs were housed in individual pens (1 m×1.2 m) with 30 cm feed trough. They had free access to fresh water during the experiment. The lambs were fed in four groups. Lambs of control group (WS, n = 6) received untreated WS without supplemental yeast, whereas other three groups were fed WS treated with anhydrous ammonia (WSNH3 group, n = 7), untreated WS and yeast (WS+YEAST group, n = 6) or WS treated with anhydrous ammonia and yeast (WSNH3+YEAST group, n = 7).
Intake and performance
The WS and WSNH3 were fed ad-libitum whereas, the concentrate was offered 1% of live weight of lambs in two equal amounts twice a day at 8:00 AM and 16:00 PM. The live yeast culture was supplemented with concentrate (4 g/d: 2 g in the morning meal, 2 g in the afternoon meal). Records of daily intakes of straws and concentrate were maintained and daily intakes of ME (kcal) and CP (g) were calculated. The live weight gains (g/d) were calculated by weighing the animals on the first and last days of the experimental period and at 14-d intervals for 70 d. The feed conversion ratio was calculated on the basis of unit feed consumed to unit body weight gain for each groups separately (Kılıç et al., 1990).
Rumen fermentation
Rumen fluid samples were collected using stomach tube on day 35 at 0, 3, 6, and 9 hours after morning meal for determination of pH, NH3-N and TVFA. The pH measured with a digital pH meter (Hanna HI 8314 pH-meter) after the sampling and 50 mL rumen fluid samples were collected from four sheep of the each group were strained through five layers of cheesecloth. A 0.5 mL 1 M HCl was added to each rumen fluid samples and stored at −20°C for NH3-N and TVFA analysis (Demirel and Bolat, 1996). NH3-N was determined by using The Hanna C213 Multiparameter Ion specific Meter (Nessler Method). To determine TVFA, rumen fluid samples were centrifuged at 6,000×g for 10 min at 4°C with 15 mL tubes. Then 5 mL rumen fluid were taken to the new tubes and 1 mL 25% meta phosphoric acids was added in each 5 mL supernatant, after 30 min, the tubes were centrifuged again to collect the supernatants. The supernatants were sent to The Central Laboratory of Ege University Agriculture Faculty to determine TVFA by gas-liquid chromatography (Agilent Technologies 6890N Network GC System, Anaheim, CA, USA). The Supelco Analytical WSFA-2 (900 to 1,100 μg/mL in water) was used as a TVFA standard. Detector temperature: 260°C, Injection block temperature: 250°C, Owen temperature: gradually from 45°C to 250°C, helium as the carrier gas: 1 mL/min, Hydrogen gas passes ratio: 30 mL/min (Erwin et al., 1961).
Chemical analyses and calculations
The chemical compositions (dry matter, crude protein, ether extract, crude fiber) were determined according to AOAC (1995) (Table 2). The organic matter and nitrogen free extract were calculated from chemical compositions. The ME values were calculated by using Turkish Standard Institute equation (TSE9610, 2004) ME, kcal/kg organic matter = 3,260+(0.455×crude protein+3.517×ether extract) −4.037×crude fiber.
Table 2.
Chemical composition of feeds and live yeast
| Feed1 | DM % | OM | CP | EE | CF | NFE | ME kcal/kg |
|---|---|---|---|---|---|---|---|
| WS | 90.72 | 82.95 | 4.53 | 1.58 | 38.94 | 37.90 | 1165 |
| WSNH3 | 89.50 | 83.16 | 10.07 | 1.56 | 37.11 | 34.42 | 1252 |
| Concentrate* | 88.60 | 81.45 | 14.60 | 4.41 | 3.05 | 59.39 | 2754 |
| Live yeast | 92.88 | 79.58 | 19.38 | 3.94 | 7.93 | 48.33 | - |
DM, dry matter; OM, organic matter; CP, crude protein; EE, ether extract; CF, crude fiber; NFE, nitrogen-free extract (NFE = [OM−{CP+EE+CF}]); ME, metabolizable energy.
WS, wheat straw; WSNH3, ammonia-treated wheat straw.
Concentrate consisted of: Vitamin A, 7,000 U/kg; Vitamin D3, 700 U/kg; Vitamin E, 25 mg/kg; calcium, 1.1%; phosphorus, 0.4%; sodium, 0.25%.
Statistical analyses
All tests were carried out using the statistical package of SPSS (15.0). The data were analyzed using Genel Linear Model procedure with a model including anhydrous ammonia-treatment and live yeast (Saccharomyces cerevisiae) supplementation and, interaction between yeast supplementation and anhydrous ammonia-treatment. Duncan test was used to compare the means, when significant differences occurred.
RESULTS AND DISCUSSION
In- situ study: Dry matter degradation characteristics
In-situ DM degradability of WS, WSNH3, WS+YEAST, WSNH3+YEAST varied between 24.67% and 70.03% for 0 to 96 h of incubation times, and these values were used to estimate degradability characteristics of the treatments (Table 1). In agreement with Inal et al. (2010), fraction (A) did not vary significantly between the control (WS) and experimental treatments. Although the degradability of the water-insoluble fraction (B) was significantly increased by all of the experimental treatments, potential degradability (A+B) was significantly increased only by WSNH3+YEAST (16.73%). Similar to these findings, Ma et al. (1990) found that ammonia-treatment strongly influenced the degradation characteristic of B, while c was not markedly changed. These findings could be explained by the fact that yeast supplementation affected only the water-insoluble material in the rumen by promoting the development of rumen fauna. The effective DM degradability of WS was significantly increased by WSNH3+YEAST (Table 1). Habib et al. (1998) reported that effective DM degradability of WS genotypes at different rumen outflow rates was positively influenced (p<0.001) by ammoniation. However, we observed a significant increase in effective DM degradability of WS (Menemen-88) only with the combined effect. The effects of different wheat varieties could be explained depending on feed intake, because wheat varieties have different stem-to-leaf ratios (Özkul and Sayan, 1996) and the physical capacity of the rumen is limited (Givens and Moss, 1995).
Table 1.
In-situ DM degradability parameters of wheat straw and ammonia-treated wheat straw with and without dietary yeast supplementation
| Items | WS1 | WSNH31 | WS+YEAST1 | WSNH3+YEAST1 | SEM | p-value | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| NH3 | YEAST | NH3×YEAST | ||||||
| Parameter2 | ||||||||
| A | 24.67 | 27.96 | 24.67 | 27.96 | 0.67 | 0.042 | 0.666 | 0.111 |
| B | 35.71 c | 39.21 b | 43.92 ab | 49.14 a | 1.27 | 0.087 | 0.001 | 0.001 |
| A+B | 60.38 b | 67.17 b | 68.59 ab | 77.11 a | 1.75 | 0.026 | 0.007 | 0.004 |
| c (h−1) | 0.0323 | 0.0383 | 0.0240 | 0.0283 | 0.003 | 0.307 | 0.062 | 0.210 |
| RSD | 2.30 | 2.24 | 1.92 | 2.56 | 1.00 | 0.144 | 0.889 | 0.153 |
| Effective DM degradability2 | ||||||||
| pe2 | 44.24 b | 51.14 ab | 46.28 b | 54.02 a | 1.31 | 0.003 | 0.354 | 0.025 |
| pe5 | 35.99 b | 41.71 ab | 36.14 b | 43.02 a | 1.14 | 0.004 | 0.753 | 0.042 |
| pe8 | 32.27 b | 37.10 ab | 31.99 b | 38.33 a | 1.02 | 0.004 | 0.819 | 0.044 |
SEM, standard error of means; RSD, residual standard deviation.
WS, wheat straw; WSNH3, ammonia-treated wheat straw; WS+YEAST, with 4 g/d live yeast supplementation; WSNH3+YEAST, ammonia-treated wheat straw with 4 g/d live yeast supplementation.
A, easily soluble fraction; B, degradability of water-insoluble fraction; A+B, potential degradability; c, degradability rate constant.
pe2, pe5, and pe8, effective DM degradability at rumen outflow rate (k) = 0.02, 0.05, and 0.08 h−1, respectively. Different letters (a,b,c) within rows indicate significant differences (p<0.05).
Animal experiment: Growth performance study
The chemical composition of feeds and live yeast is presented in Table 2. The CP content of WS was doubled with 3% ammonia-treatment (Table 2), consistent with findings of Singh and Gupta (1990), Özkul and Şayan (1996).
Growth performance and voluntary intake parameters
Growth performance and voluntary intake parameters of yearling lambs are shown in Table 3; no significant differences were observed in changes in body weight between the control and experimental groups during the study. However, numerical differences in body weight between the control and experimental groups were observed after 14 d (Figure 1).
Table 3.
The growth performance and voluntary intake parameters of wheat straw and ammonia-treated wheat straw with and without yeast supplementation diets of one-yearling lambs between 0 to 70 days
| Parameter | WS1 | WSNH31 | WS+YEAST1 | WSNH3+ YEAST1 | SEM | p-value | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| NH3 | YEAST | NH3 ×YEAST | ||||||
| Initial weight (kg) | 48.50 | 48.43 | 48.33 | 48.07 | 0.98 | 0.517 | 0.644 | 0.999 |
| Final weight (kg) | 57.33 | 58.79 | 59.37 | 60.74 | 1.24 | 0.676 | 0.359 | 0.829 |
| Average daily gain (g) | 126 b | 148 ab | 157 ab | 181 a | 7.2 | 0.012 | 0.145 | 0.044 |
| Voluntary DM intake (g/d) | 896 | 946 | 919 | 1016 | 17.9 | 0.037 | 0.185 | 0.082 |
| Feed conversion ratio (kg feed/kg gain) | 6.90 | 5.74 | 5.44 | 5.21 | 0.25 | 0.166 | 0.048 | 0.074 |
| Voluntary ME intake (kcal) | 1,151 b | 1,324 a | 1,180 b | 1,422 a | 31.2 | 0.001 | 0.278 | 0.001 |
| Voluntary CP intake (g/d) | 44.77 c | 106.50 b | 45.89 c | 114.39 a | 41.3 | 0.001 | 0.726 | 0.001 |
SEM, standard error of the mean; DM, dry matter; ME, metabolizable energy; CP, crude protein.
WS, wheat straw; WSNH3, ammonia-treated wheat straw; WS+YEAST, with 4 g/d live yeast supplementation; WSNH3+YEAST, ammonia-treated wheat straw with 4 g/d live yeast supplementation.
Different letters (a,b,c) within rows indicate significant differences (p<0.05).
WS and WSNH3 were given ad-libitum; concentrate was limited to 1% of live weight (DM basis).
Voluntary intake of DM, ME, and CP were calculated in the absence of offered concentrate.
Figure 1.
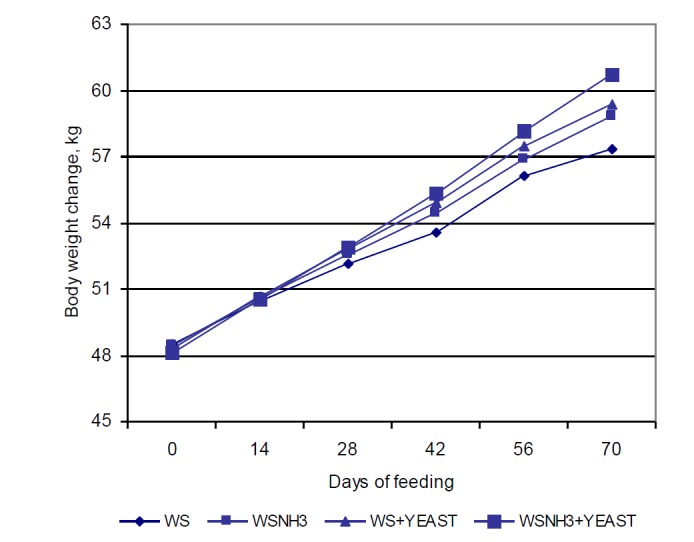
Changes in body weight of yearling lambs according to treatment during the experimental period. WS, wheat straw; WSNH3, ammonia-treated wheat straw; WS+YEAST, with 4 g/d live yeast supplementation; WSNH3+YEAST, ammonia-treated wheat straw with 4 g/d live yeast supplementation.
Average daily weight gain was significantly increased relative to the control in WSNH3+YEAST group, and tended to be higher in WSNH3 and WS+YEAST groups (Table 3). However, daily weight gain was not positively affected by ammonia-treatment (Kılıç et al., 1990) and there were no effect of yeast culture on growth performance (Rouzbehan et al., 1994). Similarly to our results, increased daily weight gain were reported by the addition of live yeast (Haddad and Goussous, 2005; Tripathi and Karim, 2011). The feed conversion ratios of our experimental groups tended to be lower than those of the control (Table 3). In agreement with our results, Rouzbehan et al. (1994) and Khadem et al. (2007) found no differences in feed conversion ratios, whereas significant differences among feed conversion ratios of live-yeast-supplemented groups were reported in the studies (Haddad and Goussous, 2005; Fadel Elseed and Abusamra, 2007). The differences between studies could be attributed to the use of commercial yeast strains that differed in viable cell numbers, and to differences in the dosage administered.
The differences in voluntary DM intake between the control and experimental groups were not significant (Table 3). Voluntary ME and CP intake were not significantly increased by yeast supplementation, but only ammonia-treatment and the combined treatment were associated with significantly higher voluntary ME intake compared to WS (p<0.05), and voluntary CP intake was significantly increased by only ammonia-treatment and the combined treatment. The differences in voluntary ME and CP intake could be a result of ammonia-treatment, but we found the highest voluntary intake for the combined treatment. Similar to our results, no significant effects were reported on voluntary DM intake in ruminants by yeast supplementation (Rouzbehan et al., 1994; Kawas et al., 2007; Titi et al., 2008; Tripathi and Karim, 2011; Issakowicz et al., 2013). However, in agreement with our findings, Tripathi and Karim (2011) reported that feed intake was numerically improved with yeast supplementation. The differences between the studies could be attributed to the basal diet, forage type and feeding strategy, because different contents of readily soluble carbohydrates in the diet affect DM intake in ruminants.
Rumen fermentation parameters
Differences among average daily post-feeding pH values of the control and experimental groups were not significant (Table 4). Yeast supplementation tended to produce higher pH values. Similar to our findings, Bruchemi et al. (1993) reported that ammoniation of WS and Tripathi and Karim (2011) reported that yeast supplementation did not produce differences in average daily pH values. On the other hand, Khadem et al. (2007) found higher rumen pH values with yeast supplementation and proposed that the use of live yeast in sheep rations resulted in more stable pH and probably more effective ruminal activity and yeast addition was associated with a significant increase in ruminal pH at 3 h post-feeding. We did not observe this effect, which could be observed in animals fed diets high in soluble carbohydrates. Differences between the results of these studies could be attributed to the yeast cultures, which may have different mechanisms of action.
Table 4.
Rumen fermentation parameters of wheat straw and ammonia-treated wheat straw with and without dietary yeast supplementation at 0, 3, 6, and 9 h post-feeding during the 35-d experiment
| Parameter | WS1 | WSNH31 | WS+YEAST1 | WSNH3+ YEAST1 | SEM | p-value | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| NH3 | YEAST | NH3 ×YEAST | ||||||
| pH | ||||||||
| 0 h | 7.03 c | 7.02 c | 7.38 a | 7.18 b | 0.05 | 0.288 | 0.001 | 0.001 |
| 3 h | 6.86 | 6.91 | 7.02 | 7.13 | 0.05 | 0.394 | 0.490 | 0.122 |
| 6 h | 6.68 b | 6.84 a | 6.92 a | 6.70 b | 0.03 | 0.688 | 0.025 | 0.003 |
| 9 h | 6.76 | 6.83 | 6.77 | 6.77 | 0.02 | 0.470 | 0.565 | 0.732 |
| Average daily | 6.83 | 6.90 | 7.02 | 6.94 | 0.03 | 0.944 | 0.045 | 0.136 |
| NH3-N (mg/100 mL) | ||||||||
| 0 h | 7.65 b | 11.20 a | 6.45 c | 9.88 ab | 0.62 | 0.002 | 0.329 | 0.011 |
| 3 h | 6.75 b | 15.75 a | 8.78 b | 15.55 a | 1.13 | 0.001 | 0.701 | 0.001 |
| 6 h | 10.15 b | 18.38 a | 10.20 b | 16.58 a | 1.04 | 0.001 | 0.689 | 0.001 |
| 9 h | 10.00 b | 15.95 a | 7.83 b | 13.90 a | 0.96 | 0.001 | 0.284 | 0.001 |
| Average daily | 8.64 b | 15.32 a | 8.31 b | 13.98 a | 0.52 | 0.001 | 0.426 | 0.001 |
| TVFA (mmol/100 mL) | ||||||||
| 0 h | 8.22 | 7.06 | 8.64 | 7.77 | 0.47 | 0.321 | 0.592 | 0.780 |
| 3 h | 6.88 c | 7.47 bc | 8.93 ab | 9.28 a | 0.41 | 0.604 | 0.003 | 0.042 |
| 6 h | 7.16 b | 7.43 b | 9.91 a | 9.79 a | 0.50 | 0.947 | 0.001 | 0.011 |
| 9 h | 8.32 | 8.12 | 8.06 | 9.10 | 0.31 | 0.534 | 0.601 | 0.716 |
| Average daily | 7.64 b | 7.52 b | 8.88 a | 8.99 a | 0.21 | 0.978 | 0.001 | 0.008 |
SEM; Standard error of the mean; TVFA, total volatile fatty acids.
WS, wheat straw; WSNH3, ammonia-treated wheat straw; WS+YEAST, with 4 g/d live yeast supplementation; WSNH3+YEAST, ammonia-treated wheat straw with 4 g/d live yeast supplementation.
Different letters (a,b,c) within rows indicate significant differences (p<0.05).
The average daily NH3-N values of WS were not significantly increased by yeast supplementation, except at 0 h post-feeding. However, only ammonia-treatment or combined treatment led to the highest average daily NH3-N values (p<0.05) (Table 4). Therefore, differences in rumen NH3-N could be a result of the ammonia-treatment. Consistent with our results, Bruchemi et al. (1993) found that NH3-N of WSNH3 was significantly higher than that of WS (175.2 and 42.6 mg/L respectively). Roa et al. (1997) and Khadem et al. (2007) observed that NH3-N was increased by yeast supplementation, whereas not positively improved NH3-N was reported by yeast supplementation (Guedes et al., 2008; Tripathi and Karim, 2011). In agreement with our findings, Khadem et al. (2007) reported that peak rumen ammonia concentration occurred after 4 h post-feeding. In our study, the declining concentration of ammonia in the rumen after 6 h post-feeding appeared to be a result of increased incorporation of ammonia into microbial proteins, possibly because of stimulation of microbial activity.
The average daily TVFA in WS were not significantly increased by ammonia-treatment. However, the combined treatment and the yeast-supplementation had significantly higher TVFA than the control (p<0.05) (Table 4). At 3 and 6 h post-feeding, TVFA of WS were similar to WSNH3 and significantly lower than WSNH3+YEAST and WS+YEAST. These differences in TVFA could be due to the yeast supplementation. Similarly, Bruchemi et al. (1993) found no effect of ammonia-treatment on daily TVFA values. Some studies reported that TVFA were increased by yeast supplementation (Roa et al., 1997; Khadem et al., 2007), however some others did not find this increases (Tripathi and Karim, 2011). Khadem et al. (2007) noted that TVFA content in rumen fluid was not affected by live yeast supplementation in rations containing low to moderate concentrations of grain.
In conclusion, the present study showed that ammonia-treatment increased the degradability of water-insoluble material (the B fraction), voluntary ME intake, voluntary CP intake, and rumen NH3-N of WS; yeast supplementation significantly increased the B fraction and rumen TVFA. Furthermore, the combination of ammonia-treatment and yeast supplementation significantly increased the B fraction, potential degradability (A+B), effective DM degradability values (p2, p5, and p8), average daily weight gain, voluntary ME intake, voluntary CP intake, rumen NH3-N, and rumen TVFA of WS. We conclude that the improvement of feed value of WS was better by the combination of ammonia-treatment and yeast supplementation compared to either treatment alone. These results reveal combined effect of yeast supplementation and ammonia-treatment of WS, leading to improve rumen fermentation efficiency and a possible productivity in ruminants.
ACKNOWLEDGMENTS
Authors appreciate the financial support from Ege University Scientific Research Project Department (2007-ZRF-020). Also, they are thankful to Erkoç Feed Factory for providing concentrate used for cannulated sheep and Alltech-Turkey for providing the live yeast.
REFERENCES
- AOAC. Official Methods of Analysis of AOAC International. 16th ed. Association Official Analytical Chemists; Washington, DC, USA: 1995. [Google Scholar]
- Bhargava PK, Orskov ER. Manual for the use of nylon bag technique in the evaluation of feedstuffs. The Rovett Research Institute; Bucksburn, Aberdeen, Scotland, UK: 1987. p. 21. [Google Scholar]
- Bruchemi JV, Oosting SJ, Lammers-Wienhoveni SCW, Leffering CP. Doctoral Thesis of Simon J. Oosting. Agricultural University Wageningen; Netherlands: 1993. Ammonia treatment of wheat straw. Chapter 3, 3.1. Voluntary intake, chewing behavior, rumen pool size and turnover and partition of digestion along the gastrointestinal tract of sheep. Wheat straw as ruminant feed effect of supplementation and ammonia treatment on voluntary intake and nutrient availability; p. 232. [Google Scholar]
- Erwin ES, Marco GJ, Emery EM. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J Dairy Sci. 1961;44:1768–1771. [Google Scholar]
- Demirel M, Bolat D. The effects of urea and niacin supplementation to the concentrate feed added dried sugar beet pulp on rumen fluid and blood parameters. Turkish J Vet Anim Sci. 1996;20:203–210. [Google Scholar]
- Fadel Elseed AMA, Abusamra MAR. Effect of supplemental yeast (Saccharomyces cerevisiae) culture on NDF digestibility and rumen fermentation of forage sorghum hay in Nubian goat’s kids. Res J Agric Biol Sci. 2007;3:133–137. [Google Scholar]
- Givens DI, Moss AR. The nutritional value of cereal straws. A review. Nutr Abstr Rev (B) 1995;65:793–808. [Google Scholar]
- Guedes CM, Goncalves D, Rodrigues MAM, Dias da Silva A. Effects of a Saccharomyces cerevisiae yeast on ruminal fermentation and fibre degradation of maize silages in cows. Anim Feed Sci Technol. 2008;145:27–40. [Google Scholar]
- Habib G, Hassan MF, Siddiqui MM. Degradation characteristics of straw from different wheat genotypes and their response to urea-ammoniation treatment. Anim Feed Sci Technol. 1998;72:373–386. [Google Scholar]
- Haddad SG, Goussous SN. Effect of yeast culture supplementation on nutrient intake, digestibility and growth performance of Awassi lambs. Anim Feed Sci Technol. 2005;118:343–348. [Google Scholar]
- Horton GMJ, Steacy GM. Effect of anhydrous ammonia treatment on the intake and digestibility of cereal straws by steers. J Anim Sci. 1979;48:1239–1249. [Google Scholar]
- İnal F, Gürbüz E, Coşkun B, Alataş MS, Çitil ÖB, Polat ES, Şeker E, Özcan C. The effects of live yeast culture (Saccharomyces cerevisiae) on rumen fermentation and nutrient degradability in yearling lambs. Kafkas Ünv Vet Fak Derg. 2010;16:799–804. [Google Scholar]
- Issakowicz J, Bueno MS, Sampaio ACK, Duarte KMR. Effect of concentrate level and live yeast (Saccharomyces cerevisiae) supplementation on Texel lamb performance and carcass characteristics. Livest Sci. 2013;155:44–52. [Google Scholar]
- Kawas JR, García-Castillo R, Garza-Cazares F, Fimbres-Durazo H, Olivares-Sáenz E, Hernández-Vidal G, Luc CD. Effects of sodium bicarbonate and yeast on productive performance and carcass characteristics of light-weight lambs fed finishing diets. Small Rumin Res. 2007;67:157–163. [Google Scholar]
- Khadem AA, Pahlavan M, Afzalzadeh A, Rezaeinan M. Effects of live yeast Saccharomyces cerevisiae on fermentation parameters and microbial populations of rumen, total tract digestibility of diet nutrients and on the in situ degradability of alfalfa hay in Iranian Chall sheep. Pak J Biol Sci. 2007;10:590–597. doi: 10.3923/pjbs.2007.590.597. [DOI] [PubMed] [Google Scholar]
- Kılıç A, Sevgican F, Şayan Y, Çapçı T. The feed value of untreated and ammonia-treated wheat straw and wheat straw stem, and a research of these straws on the using possibilities of fattening performance of lamps. Turkish J Vet Anim Sci. 1990;14:72–82. [Google Scholar]
- Ma TK, Gu CX, Dai BC, Orskov ER. Effect of ammonia treatment of wheat straw and level of concentrate on performance of Chinese yellow cattle. [Accessed March 12, 2014];Livest Res Rural Develop. 1990 2 Article # 25. http://www.lrrd.org/lrrd2/3/orskov1.htm. [Google Scholar]
- Mazzia M, Walker N. [Accessed March 10, 2014];Live yeast action on the rumen: unlocking fiber potential to optimize feed value. 2008 http://en.engormix.com/MA-dairy-cattle/health/articles/live-yeast-action-rumen-t1051/p0.htm.
- Newbold CJ, Wallace RJ, Mc Intosh FM. Mode of action of the yeast Saccharomyces cerevisae as a feed additive for ruminants. Br J Nutr. 1996;76:249–261. doi: 10.1079/bjn19960029. [DOI] [PubMed] [Google Scholar]
- Orskov ER, McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to ratio of passage. J Agric Sci. 1979;92:499–503. [Google Scholar]
- Özkul H, Şayan Y. The determination of feed value of some straw varieties by nylon bag technique. Ege Üniv Zir Fak Derg. 1996;33(2–3):151–157. [Google Scholar]
- Roa ML, Barcena-Gama JR, Gonzalez S, Mondeza MG, Ortega CME, Garcia BC. Effect of fiber source and yeast culture (Saccharomyces cerevisiae) on the digestion and the environment in the rumen of cattle. Anim Feed Sci Technol. 1997;64:327–336. [Google Scholar]
- Rouzbehan Y, Galbraith H, Rooke JA, Perrott JG. A note on the effects of dietary inclusion of a yeast culture on growth and ruminal metabolism of lambs given diets containing unground pelleted molassed dried sugar-beet pulp and barley in various proportions. Anim Sci. 1994;59:147–150. [Google Scholar]
- Silva AT, Greenhalgh JFD, Orskov ER. Influence of ammonia treatment and supplementation on the intake, digestibility and weight gain of sheep and cattle on barley straw diets. Anim Prod. 1989;48:99–108. [Google Scholar]
- Singh SP, Gupta BN. Effect of ammonia treatment of wheat straw on the chemical composition, in vitro digestion and fibre degradability in the rumen. Indian J Anim Sci. 1990;60:99–103. [Google Scholar]
- SPSS for Windows 2006. Released 15.0 version. SPPS Inc.; Chicago, IL, USA: [Google Scholar]
- Şehu A, Yalçın S, Önol AG. The in vivo digestibility coefficients and rumen degradability characteristics of some cereal straws. Ankara Univ Vet Fak Derg. 1996;43:469–477. [Google Scholar]
- Titi HH, Dmour RO, Abdullah AY. Growth performance and carcass characteristics of Awassi lambs and Shami goat kids fed yeast culture in their finishing diet. Anim Feed Sci Technol. 2008;142:33–43. [Google Scholar]
- Tripathi MK, Karim SA. Effect of yeast cultures supplementation on live weight change, rumen fermentation, ciliate protozoa population, microbial hydrolytic enzymes status and slaughtering performance of growing lamb. Livest Sci. 2011;135:17–25. [Google Scholar]
- Tuah AK, Lufadeju E, Ørskov ER, Blackett GA. Rumen degradation of straw 1. Untreated and ammonia-treated barley, oat and wheat straw varieties and triticale straw. Anim Prod. 1986;43:261–269. [Google Scholar]


