Abstract
Objectives
To evaluate data for the period 2004–2013 to identify changes in demographics, pathogens, and outcomes in a single, level IV neonatal intensive care unit (NICU).
Study design
Sepsis episodes were identified prospectively and additional information obtained retrospectively from infants with sepsis while in the NICU from 2004–2013. Demographics, hospital course, and outcome data were collected and analyzed. Sepsis was categorized as early (≤3 days of life) or late-onset (>3 days of life).
Results
Four hundred and fifty two organisms were identified from 410 episodes of sepsis in 340 infants. Ninety percent of cases were late-onset. Rates of early-onset sepsis remained relatively static throughout the study period (0.9 per 1000 live births). The majority (60%) of infants with early-onset sepsis were very low birth weight for the first time in decades, and E. coli (45%) replaced GBS (36%) as the most common organism associated with early-onset sepsis. Rates of late-onset sepsis, particularly due to coagulase-negative staphylococci (CoNS), decreased significantly after implementation of several infection prevention initiatives. CoNS was responsible for 31% of all cases from 2004–2009 but accounted for no cases of late-onset sepsis after 2011.
Conclusions
The epidemiology and microbiology of early- and late-onset sepsis continue to change, impacted by targeted infection prevention efforts. We believe the decrease in sepsis indicates that these interventions have been successful, but additional surveillance and strategies based on evolving trends are necessary.
Keywords: bloodstream infections, newborn, healthcare associated infections
Yale-New Haven Hospital (YNHH) has maintained a continuously-running, single-center database of neonatal blood stream infections (BSI), beginning with Ethel Dunham’s case series from 1928–1933.1 Since that time, documentation and analyses of evolving center-specific data have greatly assisted in the formulation of strategies to treat and prevent sepsis in the neonatal intensive care unit (NICU) population, and have allowed tracking of the emergence and disappearance of certain pathogenic organisms from the NICU landscape.2–6
One example is the rise2–4 and subsequent decline6 of group B Streptococcus (GBS)-related early-onset sepsis. The identification and tracking of high rates of GBS early-onset sepsis and its significant associated morbidity and mortality resulted in the implementation of maternal screening and intrapartum antibiotic prophylaxis in the 1990s.6,7 In our NICU6, in Connecticut8, and across the US7, the results of that effort exemplified how a targeted intervention could impact neonatal morbidity and mortality dramatically. Even though cases of early-onset sepsis declined, late-onset sepsis was increasing at an alarming rate.5,6 From 1989–2003, 27% of all very low birth weight (VLBW) infants in our NICU suffered at least one episode of sepsis and coagulase-negative staphylococci (CoNS) emerged as the predominant organisms responsible for late-onset sepsis.6,9 A similar trend was observed by Stoll et al on behalf of the Neonatal Research Network, who commented “strategies to reduce late infections…are urgently needed” and “successful interventions should improve survival, shorten mechanical ventilation and hospital stay, decrease antibiotic usage, and reduce the high cost of caring for VLBW infants.”9 Several infection prevention strategies were implemented in our NICU during the 2004–2013 study period with these goals in mind.
This report details changes in the epidemiology of early- and late-onset sepsis at YNHH from 2004–2013 and compares these findings with those observed at our institution from the previous 75 years. We also assess the impact of a series of interventions, including an effort to reduce central-line associated BSI (CLABSI)10, on the microbiology, rates, and outcomes associated with late-onset sepsis in a single, level IV NICU.
METHODS
The 54-bed level IV NICU at YNHH supports a high-risk obstetrical service and is a major referral center for fetuses and newborns with complex medical and surgical conditions. Positive blood cultures from our NICU were identified prospectively via frequent review of medical records and by direct communication with the microbiology lab and infection control providers. This study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Any positive blood culture yielding a traditional neonatal pathogen met arbitrary criteria for inclusion as a case of neonatal sepsis. Cultures that yielded commensal species (eg CoNS) were reviewed using criteria modified from the Centers for Disease Control and Prevention.6,11 Prior to 2008, the surveillance definition stated that, in addition to the presence of signs and symptoms of infection, CoNS had to be retrieved from at least 2 blood cultures or from 1 blood culture after which appropriate antimicrobial therapy was administered.11 As of January, 2008, the definition was made more stringent to specify that a minimum of 2 positive blood cultures are required to fulfill criteria for a CoNS-related BSI.12 In order to maintain consistency in reporting and to allow for comparisons with prior study periods, we chose to adhere to the prior definition. Blood cultures that did not fulfill these criteria or that yielded organisms believed to be contaminants, including Corynebacterium and non-speciated Gram-positive bacilli, were excluded. Multiple positive blood cultures from a single infant yielding the same species with identical antibiotic susceptibility patterns were considered a single episode of sepsis if the time between positive cultures was ≤7 days.
Cases of sepsis were classified according to the infant’s age at the time of the positive blood culture as: early-onset (≤ 3 days of life) and late-onset (>3 days) infection. Two modifications were made from prior cases series. The category late, late-onset sepsis (>30 days) was eliminated and the dividing line between early and late-onset was chosen at 3 instead of 4 days to coincide with current reporting.9,13
Additional data were collected retrospectively from the medical records of infants with a positive blood culture(s) obtained while inpatients in the YNHH NICU from January 1, 2004, through December 31, 2013. Demographics, information related to the hospital course, and outcomes were collected and reviewed.
The majority of variables were defined as previously described.6 Sepsis-related death was defined as death occurring within 7 days of a positive blood culture(s) or when clinical signs and symptoms of sepsis were believed to be, and documented as, the direct cause of death. Sepsis-related death was calculated with the numerator representing the number of episodes of sepsis resulting in death and the denominator as the total number of episodes of sepsis. In the case where an infant had multiple episodes of sepsis, only the last one was included in the calculation of mortality and previous episodes were recorded as sepsis with survival. Blood cultures were assessed using a fluorescent-detection system for the presence of carbon dioxide (Bactec II or 9240®, Becton Dickinson, USA).
Statistical analyses
Univariate comparisons were made using the independent samples student’s t test for continuous data and Chi-square or Fisher exact test, if any cell in the analysis contained <5, for dichotomous data. A p-value of < 0.05 based on two-sided tests was considered statistically significant (SPSS Inc., Chicago, IL).
Trends in infection rates were assessed from 1979 (the first year with complete data available on live births and NICU admissions at YNHH) through 2013 and independently from 2004 through 2013. The number of infections over a specified year-interval was assumed to be Poisson-distributed, therefore, the change in rates of infections over time was analyzed using Poisson Regression with the number of live births (for early-onset sepsis ) or total NICU admissions (for late-onset sepsis) in a given year used as an offset variable (SAS 9.3, Cary, NC). The time effect was not assumed to be linear, and polynomial time effects such as quadratic, cubic, and quartic were tested in the regression models. To supplement the parametric modeling of the infection rates using Poisson regression, the primary goal of which was to investigate the effect of time, a non-parametric method was used to estimate infection rates in a more dynamic manner using smoothing spline Poisson regression, with approximate Bayesian CIs for the smoothed rates.14 Statistical significance was established with α = 0.05.
RESULTS
Early-onset sepsis
Forty-two episodes of early-onset sepsis yielding 44 organisms were identified in 42 infants over the 10-year study period. Escherichia coli was the most common organism isolated from blood culture (45%), followed by GBS (36%), Haemophilus influenzae (7%), and Staphylococcus aureus (7%) (Table I). In infants with E. coli early-onset sepsis, 13 of 19 (68%) were exposed to intrapartum antibiotics, but in only 55% of cases was the E. coli strain susceptible to the antimicrobial regimen administered to the mother. Ampicillin resistance was observed in 63% of available E. coli isolates. Fifteen infants had GBS early-onset sepsis. In 11 of 15 cases (73%), the maternal GBS status was either unknown (8 of 15; 53%) or negative (3 of 15; 20%) at the time of delivery. In cases where the maternal GBS status was unknown, all 8 infants were <35 weeks’ GA.
Table I.
The Microbiology of Neonatal Sepsis at Yale-New Haven Hospital, 2004–2013
| Early-Onset Sepsis* (42 episodes) | Late-Onset Sepsis* (368 episodes) | All Sepsis* (410 episodes) | |
|---|---|---|---|
| Gram-positive aerobic and facultative bacteria | |||
| Staphylococcus aureusa | 3 (7) | 64 (17) | 67 (16) |
| Coagulase-negative Staphylococcus speciesa | 2 (5) | 115 (31) | 117 (29) |
| Beta-hemolytic streptococci | |||
| Group B | 15 (36) | 17 (5) | 32 (8) |
| Group D | |||
| Enterococcus faecalis | 0 | 49 (13) | 49 (12) |
| Enterococcus faecium | 0 | 3 (0.8) | 3 (0.7) |
| Enterococcus spp | 0 | 9 (2) | 9 (2) |
| Viridans streptococcius speciesa,b | 1 (2) | 9 (2) | 10 (2) |
| Bacillus sppa | 0 | 2 (0.5) | 2 (0.5) |
| Listeria monocytogenes | 0 | 0 | 0 |
| Gram-negative aerobic and facultative bacteria | |||
| Escherichia coli | 19 (45) | 39 (11) | 58 (14) |
| Klebsiella pneumoniae | 0 | 23 (6) | 23 (6) |
| Klebsiella oxytoca | 0 | 9 (2) | 9 (2) |
| Haemophilus influenzae | 3 (7) | 0 | 3 (0.7) |
| Pseudomonas aeruginosa | 0 | 16 (4) | 16 (4) |
| Enterobacter cloacae | 0 | 13 (4) | 13 (3) |
| Enterobacter spp | 0 | 5 (1) | 5 (1) |
| Serratia marcescens | 0 | 4 (1) | 4 (1) |
| Acinetobacter baumannii | 0 | 3 (0.8) | 3 (0.7) |
| Citrobacter spp | 0 | 1 (0.3) | 1 (0.2) |
| Leclercia adenocarboxylata | 0 | 1 (0.3) | 1 (0.2) |
| Morganella morganii | 0 | 1 (0.3) | 1 (0.2) |
| Pantoea dispersa | 0 | 1 (0.3) | 1 (0.2) |
| Gram-negative anaerobic bacteria | |||
| Bacteroides spp | 0 | 0 | 0 |
| Fungi | |||
| Candida albicansa | 1 (2) | 9 (2) | 10 (2) |
| Candida dubliniensisa | 0 | 1 (0.3) | 1 (0.2) |
| Candida glabrataa | 0 | 1 (0.3) | 1 (0.2) |
| Candida lusitaniaea | 0 | 1 (0.3) | 1 (0.2) |
| Candida parapsilosisa | 0 | 7 (2) | 7 (2) |
| Malassezia pachydermatis | 0 | 2 (0.5) | 2 (0.5) |
| Yeastc | 0 | 3 (0.8) | 3 (0.7) |
| Organisms | 44 | 408 | 452 |
Number of times the organism was retrieved from blood culture (% of sepsis cases attributed to each organism)
Indicates a commensal species of bacteria or fungus
Includes Steptococcus mitis(2) and nonspeciated viridans streptococci(8)
Individual organisms not speciated
Rates of early-onset sepsis, death, and sepsis-related death were inversely proportional to GA and BW (Table II). In extremely low birth weight infants, rates of E. coli and GBS-related early-onset sepsis were 14.0 and 7.6 per 1000 live births, respectively. Rates of E. coli-related early onset sepsis were also higher in VLBW infants (10.7 v.4.3 per 1000 live births). GBS-related early-onset sepsis rates were higher than E. coli in infants 1500–2000 grams (2.0 v. 1.0 per 1000 live births) and in those >2000 grams BW (0.02 v. 0.07 per 1000 live births).
Table II.
A Comparison of Early-Onset and Late-Onset Sepsis, 2004–2013
| Early-Onset Sepsis (42 episodes in 42 infants) | Late-Onset Sepsis (368 episodes in 298 infants) | |
|---|---|---|
| GA (weeks)a | 30 ± 6 | 29 ± 5 |
| BW (grams)a | 1601 ± 1108 | 1283 ± 869 |
| VLBWb | 25 (60) | 215 (72) |
| Maleb | 22 (52) | 161 (54) |
| Inbornb | 40 (95) | 232 (78) |
| Sepsis ratec | 0.9 | 33.3 |
| <1000 grams BW | 25.4 | 190.8 |
| <1500 grams BW | 17.2 | 136.1 |
| 1500–2000 grams BW | 3 | 18.4 |
| >2000 grams BW | 0.3 | 0.8 |
| 22–24 weeks’ GA | 29.7 | 259.4 |
| 25–29 weeks’ GA | 13.9 | 176.7 |
| 30–36 weeks’ GA | 2.7 | 22.7 |
| >36 weeks’ GA | 0.3 | 10.9 |
| Length of stay (days)a | 45.4 ± 49.9 | 81.8 ± 58.1 |
| Deathb | 13 (31) | 64 (21) |
| <1000 grams BW | 10 (53) | 48 (30) |
| <1500 grams BW | 11 (44) | 57 (27) |
| 1500–2000 grams BW | 1 (25) | 2 (9) |
| >2000 grams BW | 1 (8) | 5 (8) |
| 22–24 weeks’ GA | 6 (55) | 24 (32) |
| 25–29 weeks’ GA | 5 (45) | 31 (28) |
| 30–36 weeks’ GA | 1 (11) | 6 (10) |
| >36 weeks’ GA | 1 (10) | 3 (7) |
| Sepsis-related deathb | 9 (21) | 34 (9) |
| <1000 grams BW | 7 (37) | 28 (12) |
| <1500 grams BW | 7 (30) | 31 (10) |
| 1500–2000 grams BW | 1 (25) | 1 (4) |
| >2000 grams BW | 1 (8) | 2 (3) |
| 22–24 weeks’ GA | 6 (55) | 14 (13) |
| 25–29 weeks’ GA | 1 (9) | 16 (10) |
| 30–36 weeks’ GA | 1 (11) | 3 (4) |
| >36 weeks’ GA | 1 (10) | 1 (2) |
Presented as mean ± 1 standard deviation
Presented as N (%)
Early-onset sepsis rates presented as cases per 1000 live births; late-onset sepsis rates presented as cases per 1000 NICU admissions
A significant decrease in the overall rate of early-onset sepsis was observed from 1979–2013 (linear time, p=0.0003; quadratic time, p=0.001; cubic time, p=0.002; quartic time, p=0.01), but early-onset sepsis rates did not change significantly from 2004–2013 (p>0.05; Figure, A). A significant decline in GBS-related early-onset sepsis was estimated from 1979–2013 (linear time, p=0.004; quadratic time, p=0.01; cubic time, p=0.01; and quartic time, p=0.01), and from 2004–2013 (linear time, p=0.02; quadratic time, p=0.02; cubic time, p=0.02; and quartic time, p=0.02). E. coli sepsis rates remained relatively static from 1979–2013 and from 2004–2013 (p>0.05 for linear or polynomial effects of time).
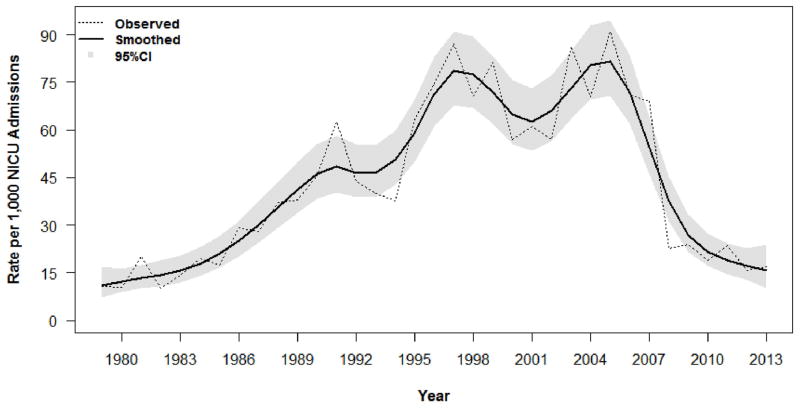
Figure. A, Early-and B, Late-onset sepsis rates per 1000 live births from 1979–2013.
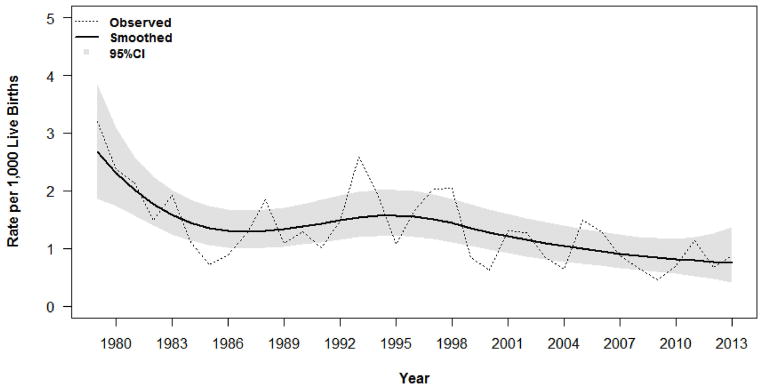
The observed rates are presented as dashed lines. A non-parametric method was used to estimate infection rates in a more dynamic manner using Smoothing Spline Poisson Regression (represented by a solid line), with approximate Bayesian 95% CI for the smoothed rates (represented as the gray shaded area).
Late-onset sepsis
From 2004–2013, 368 episodes of late-onset sepsis yielding 408 organisms were identified in 298 infants. In all cases of late-onset sepsis, CoNS were the most common organisms isolated (31%), followed by S aureus (17%), Enterococcus faecalis (13%), and E. coli (11%) (Table I). Late-onset sepsis occurred, on average, on day of life 33 ± 32, and a commensal species of bacteria or fungus was identified in 52% of cases. Rates of late-onset sepsis, death, and sepsis-related death were inversely proportional to GA and BW (Table II). Late-onset sepsis episodes attributed to Pseudomonas aeruginosa were associated with the highest sepsis-related mortality (56%), followed by E. coli (20%), Klebsiella pneumoniae (13%), and S aureus (12%). Sepsis-related death occurred in only 0.9% of episodes of CoNS-related late-onset sepsis.
The overall rate of late-onset sepsis followed a cubic polynomial trajectory for time (linear time, p=0.04; quadratic time, p=0.04; and cubic time p<0.001), increasing significantly between 1979 and 2005, and reaching a peak of 90.9 cases per 1000 NICU admissions (79 BSI) in 2005 (Figure, B). Unit-wide initiatives aimed at reducing healthcare-associated infections subsequently were designed and implemented in the NICU. The majority focused on reducing CLABSI10, although concurrent initiatives aimed at improving the rate of appropriate hand hygiene, eliminating the practice of drawing blood cultures via indwelling central lines, and the early introduction of enteral nutrition were also conducted. Rates of late-onset sepsis subsequently showed a significant decrease from 2004–2013 (linear time, p=0.0002; quadratic time, p=0.0003; cubic time, p=0.0002) (Figure, B). Late-onset sepsis rates attributed to CoNS followed a similar trajectory, decreasing significantly from 2004–2013 and following a quadratic polynomial for time (linear time, p=0.0001; quadratic time, p<0.0001). Rates of late-onset sepsis attributed to S aureus followed a skewed bell-curve pattern, with an initial increase from 1979–2005 followed by a significant decrease from 2004–2013 (linear time, p<0.0001; quadratic time, p<0.0001). No significant trends in E. coli-related late-onset sepsis rates were observed, including during the last decade (p>0.05 for linear or polynomial effects of time).
Infection prevention efforts implemented in 2008 and 2009 resulted in a major shift in the microbiology of sepsis in the YNHH NICU beginning in 2010. From 2004–2009, 31% of all BSI in the YNHH NICU were attributed to CoNS. This declined to 3% from 2010–2013 (Table III), and no episodes of CoNS-related sepsis were identified in our NICU during 2012 and 2013. In order to assess this observation further, we compared episodes of late-onset sepsis from 2004–2009 (306 episodes in 239 infants) with those from 2010–2013 (“post-intervention”; 62 episodes in 59 infants).
Table III.
The Microbiology of All Sepsis Episodes in Infants Born at Yale-New Haven Hospital, 1928 to 2013
| Percent in each study period* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1928–32 | 1933–43 | 1944–57 | 1958–65 | 1966–78 | 1979–88 | 1989–03 | 2004–09 | 2010–13 | |
| Number of sepsis episodes: | 39 | 44 | 62 | 73 | 239 | 147 | 520 | 332 | 78 |
| Gram-positive aerobic and facultative bacteria | 28 | 9 | 13 | 3 | 5 | 3 | 8 | 13 | 21 |
| Staphylococcus aureus | |||||||||
| Coagulase-negative Staphylococcus species | 0 | 0 | 0 | 1 | 1 | 8 | 29 | 31 | 3 |
| Beta-hemolytic streptococci | |||||||||
| Group B | 0 | 5 | 6 | 1 | 32 | 37 | 12 | 7 | 15 |
| Group D | 0 | 0 | 2 | 10 | 4 | 8 | 9 | 13 | 5 |
| Non-grouped | 38 | 36 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Viridans Streptococcus species | 0 | 2 | 0 | 3 | 1 | 3 | 1 | 1 | 0 |
| Streptococcus pneumoniae | 5 | 11 | 5 | 3 | 1 | 1 | 0 | 0 | 0 |
| Listeria monocytogenes | 0 | 2 | 2 | 0 | 1 | 1 | <1 | 0 | 0 |
| Gram-negative aerobic and facultative bacteria | |||||||||
| Escherichia coli | 26 | 25 | 37 | 45 | 32 | 20 | 11 | 10 | 36 |
| Klebsiella-Enterobacter spp | 0 | 0 | 0 | 11 | 12 | 3 | 11 | 10 | 5 |
| Pseudomonas spp | 3 | 0 | 21 | 15 | 2 | 3 | 3 | 3 | 7 |
| Haemophilus spp | 0 | 0 | 0 | 1 | 4 | 5 | 1 | <1 | 3 |
| Salmonella spp | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 |
| Gram-negative anaerobic and facultative bacteria | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 |
| Fungi | 0 | 0 | 0 | 0 | 2 | 1 | 8 | 6 | 3 |
| Other | 0 | 9 | 3 | 5 | 5 | 1 | 6 | 3 | 2 |
Percent of all sepsis episodes (early and late-onset) attributed to each species of bacteria or fungus
No significant changes in the GA (29 ± 6 weeks v. 29 ± 5 weeks; p=0.77), BW (1421 ± 1023 grams v. 1248 ± 824 grams; p=0.23), and percentage of male infants (49% v. 59%; p=0.40) were observed between periods. The percentage of cases of late-onset sepsis categorized as commensal species-related (56% v. 40%; p=0.03) and central line-associated (62% v. 34%; p<0.001) decreased significantly and fewer infants (27% v. 5%; p<0.001) experienced more than one episode of late-onset sepsis in the post-intervention period. The cumulative durations of central line use (23.5 ± 26.6 days v.17.6 ± 15.7 days; p=0.02) and antibiotic exposure (12.1 ± 15.4 days v. 5.8 ± 7.4 days; p<0.001) prior to the onset of sepsis declined post-intervention despite no significant change in the time of occurrence of late-onset sepsis between periods (day of life 33.7 ± 32.4 v. day of life 28.0 ± 29.3; p=0.16). The number of late-onset sepsis evaluations (including empiric antibiotic prescription) also declined significantly from an annual rate of 16.7 evaluations per 1000 patient days (257 evaluations per year) to 6.8 evaluations per 1000 patient days (106 evaluations per year) post-intervention (rate difference: −9.83; 95% CI: −11.54, −8.12). Length of stay (80.8 ± 57.0 days v. 83.9 ± 63.1 days; p=0.78) and death attributed to any cause (20% v. 29%; p=0.13) did not change between periods, but rate of death in infants with sepsis increased significantly from 7% of late-onset sepsis episodes to 19% of episodes post-intervention (p=0.003).
DISCUSSION
The epidemiology of early and late-onset neonatal sepsis at YNHH continues to evolve, undergoing significant transformation over the last decade. We believe that the majority of these changes in our NICU are attributable to the implementation of targeted infection prevention initiatives for both early- and late-onset sepsis.
In 1979, the rate of early-onset sepsis at YNHH was 3.2 cases per 1000 live births, declining to approximately 1 case per 1000 live births after the implementation of universal screening for maternal GBS colonization and intrapartum antibiotic prophylaxis for GBS carriers.5,6 This rate has remained relatively static over the last decade, although significant changes in the microbiology and epidemiology of early-onset sepsis have been observed. GBS had been the most common organism responsible for early-onset sepsis in our NICU for nearly 40 years, representing 42% of all cases from 1966–1978, 55% from 1979–1988, and 47% from 1989–2003, and E. coli was responsible for 21%, 14%, and 23% of cases, respectively.4–6 From 2004–2013, E. coli emerged as the most common organism responsible for early-onset sepsis in our NICU, accounting for 45% of cases compared with 36% of cases due to GBS. In addition to intrapartum GBS prophylaxis, this shift may be partially attributed to significant changes in the demographics of the population at risk.
From 1979–1988, the mean GA and BW of inborn infants at YNHH with early-onset sepsis were 35 weeks and 2440 grams, with 22% classified as VLBW.5 From 1989–2003, infants with early-onset sepsis in the YNHH NICU had mean GA and BW of 34 weeks and 2388 grams, and 37% were VLBW.6 From 2004–2013, the mean GA and BW decreased to 30 weeks and 1601 grams, with 60% classified as VLBW. In a cohort of 389 infants with early-onset sepsis born between 2006–2009, the Neonatal Research Network reported E. coli as the most common pathogen in VLBW infants, with a rate of 5.1 infections per 1000 live births compared with 2.1 per 1000 live births for GBS.13 Early-onset sepsis at YNHH is no longer primarily a disease of the term and late-preterm infant due to GBS, but instead occurs most commonly in the VLBW preterm infant and more likely is due to E. coli.
These observations stress the need for continued efforts to improve GBS screening and testing and the need to develop and implement novel strategies for GBS prevention. Similarly, approaches to reduce early-onset E. coli transmission and infection also are warranted with particular attention paid to current maternal antimicrobial regimens and E. coli antimicrobial-resistance patterns. Finally, continued local and national surveillance of trends in the organisms responsible for early-onset sepsis and their antimicrobial susceptibility patterns are necessary to permit prompt, accurate assessment and intervention, and to ensure that empiric, post-natal antimicrobial regimens remain effective.
For nearly 20 years, CoNS were the organisms most responsible for late-onset sepsis in our NICU.6 CoNS are known pathogens for late-onset sepsis5,6,9 and CLABSI15,16, as well as common blood culture contaminants.17,18 Healy et al, after applying strict clinical criteria for CoNS-related bacteremia in their NICU, deemed 54 of 149 episodes (36%) contaminants.17 Twenty-six years earlier, Freeman et al. observed that CoNS had emerged as the leading cause of nosocomial BSI in the NICU at Children’s Hospital, Boston and speculated as to whether this increase represented true bacteremia or contamination.18 The authors recognized the lack of a consensus definition for CoNS-related bacteremia, the subjective nature of clinical criteria in the NICU population, and difficulties in obtaining blood cultures in preterm infants as major pitfalls in making this differentiation.18 They commented that the emergence of a larger cohort of high-risk, VLBW infants had complicated matters, noting that physicians were more likely to consider a positive blood culture a true infection, irrespective of the organism(s), if obtained from a VLBW infant.18
A similar trend was observed in the YNHH NICU as our patient population began to change. Freedman et al. first documented the emergence of Staphylococcus epidermidis as a pathogen in the YNHH NICU in the 1960s and 1970s.4 In their case series, 2 episodes were described in what was then a rare population of infants who were >30 days of life, had undergone recent bowel surgery, and were receiving total parenteral nutrition via a central vascular catheter.4 A decade later, Gladstone et al. chose to create a separate category termed “late, late onset-sepsis” (>30 days of life) to account for this rapidly growing at-risk population.5 Infants in this category primarily were VLBW and were noted to have the highest proportion of intravascular catheter use, commensal species-related sepsis, and sepsis-associated mortality.5 By the 1990s, 79% of cases of sepsis in our inborn NICU population were categorized as late or late, late-onset and CoNS had surpassed GBS as the most common organisms responsible for neonatal sepsis.6 Rates of late-onset sepsis continued to increase5,6 making these infections common and, to some extent, considered part of the associated risk involved in caring for a highly susceptible patient population.
In the midst of a rapidly growing problem, data from randomized controlled trials and quality improvement efforts aimed at reducing CLABSI began to emerge.19–21 NICU-specific prevalence studies22 and evidenced-based prevention guidelines with NICU-specific recommendations were eventually published23, and centers began to report dramatic success in reducing all BSI by targeting CLABSI.24 We implemented similar strategies for CLABSI prevention in 200810 that coincided with a hospital-wide initiative to improve hand hygiene and a NICU-based effort to expedite the introduction and advancement of enteral nutrition. The same year, the Centers for Disease Control and Prevention published a modified surveillance definition for CoNS-related bacteremia in an effort to reduce reporting, and unnecessary treatment, of false-positive cultures.12 The New York State Perinatal Collaborative later observed a 40% reduction in CLABSI rates merely by implementing this change of definition.25 In 2009, recognizing that a successful intervention to reduce BSI should target both true- and false-positive blood cultures, we altered practice to eliminate sampling of blood via indwelling catheters and to initiate the practice of obtaining two peripheral blood cultures at the time of sepsis evaluation.
We speculate that the combined results of these efforts greatly reduced the number of true- and false-positive blood cultures in our NICU and, in doing so, altered not only the actual but also the perceived burden of infection among our staff. This is reflected in the decreased rate of late-onset sepsis evaluations and antibiotic exposure that we have recently observed despite no major changes in the demographics of our patient population. As observed by Freeman et al.18, it is understandable why a clinician might interpret any positive blood culture as a true BSI in the setting of a vulnerable patient population with a high rate of sepsis and non-specific clinical and laboratory signs of infection. It is therefore possible that a substantial decrease in the baseline rate of infection in a NICU might alter the interpretation of a single positive blood culture for CoNS from true infection to presumed contaminant.
We observed an increase in late-onset sepsis-related mortality, defined as the percentage of total episodes of sepsis resulting in death. This observation is most likely related to the significant reduction of CoNS-related late-onset sepsis episodes, which were associated with <1% mortality. A decrease in CoNS-related sepsis resulted in a reduction in the overall number of sepsis cases without greatly impacting the number of those resulting in death. With the near eradication of CoNS, the microbiology of late-onset sepsis in our NICU now is comprised mostly of virulent pathogens with high sepsis-associated mortality (eg, E. coli, S. aureus, Pseudomonas aeruginosa), so although an infant is less likely to become infected, those that become infected are more likely to die.
Our current investigation has limitations. We included only blood-culture positive sepsis thereby underestimating the true burden of infection in the NICU population. We also only included positive cultures from infants who were hospitalized in the NICU and therefore could not estimate the true rate of neonatal sepsis in the non-NICU population. We chose to adhere to the prior definition for commensal-species related bacteremia in an effort to maintain consistency in reporting over the study period that may have overestimated our rate of CoNS-related bacteremia. Alternatively, we also eliminated the practice of drawing blood cultures via central venous catheters which may have overestimated the rate of late-onset sepsis in previous study periods (or underestimates the rate currently). Additionally, the implementation of several interventions over a short period of time makes it impossible to discern which, if any, was responsible for the changes that were observed or if this decline was due to random chance alone. We can therefore only speculate that our interventions were responsible for the observed trends in late-onset sepsis.
Acknowledgments
Funded by the National Center for Research Resources and the National Center for Advancing Translational Science (UL1 RR024139), the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research.
ABBREVIATIONS
- BSI
blood stream infections
- BW
birth weight
- CI
confidence intervals
- CLABSI
central line associated blood stream infections
- CoNS
coagulase-negative staphylococci
- GA
gestational age
- GBS
Group B streptococcus
- NICU
neonatal intensive care unit
- VLBW
very low birth weight
- YNHH
Yale-New Haven Hospital
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As va service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dunham EC. Septicemia in the newborn. Am J Dis Child. 1933;45:229–53. [Google Scholar]
- 2.Nyhan WL, Fousek MD. Septicemia of the newborn. Pediatrics. 1958;22:268–78. [PubMed] [Google Scholar]
- 3.Gluck L, Wood HF, Fousek MD. Septicemia of the newborn. Pediatr Clin North Am. 1966;13:1131–47. doi: 10.1016/s0031-3955(16)31911-3. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RM, Ingram DI, Gross I, Ehrenkranz RA, Warshaw JB, Baltimore RS. A half century of neonatal sepsis at Yale 1928 to 1978. Am J Dis Child. 1981;135:140–4. doi: 10.1001/archpedi.1981.02130260032010. [DOI] [PubMed] [Google Scholar]
- 5.Gladstone IM, Ehrenkranz RA, Edberg SC, Baltimore RS. A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience. Pediatr Infect Dis J. 1990;9:819–25. doi: 10.1097/00006454-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics. 2005;116:595–602. doi: 10.1542/peds.2005-0552. [DOI] [PubMed] [Google Scholar]
- 7.Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 8.Baltimore RS, Huie SM, Meek JI, Schuchat A, O’Brien KL. Early-onset neonatal sepsis in the era of group B streptococcal prevention. Pediatrics. 2001;108:1094–8. doi: 10.1542/peds.108.5.1094. [DOI] [PubMed] [Google Scholar]
- 9.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late- onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 10.Bizzarro MJ, Sabo B, Noonan M, Bonfiglio MP, Northrup V, Diefenbach K. A Quality Improvement Initiative to Reduce Central Line Associated Bloodstream Infections in a Neonatal Intensive Care Unit. Infect Control Hosp Epidemiol. 2010;31:241–8. doi: 10.1086/650448. [DOI] [PubMed] [Google Scholar]
- 11.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early Onset Neonatal Sepsis: The Burden of Group B Streptococcal and E. coli Disease Continues. Pediatrics. 2011;127:817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong Gu. Gss: General Smoothing Splines. R package version 2.1-0. 2013 http://CRAN.R-project.org/package=gss.
- 15.Donowitz LG, Haley CE, Gregory WW, Wenzel RP. Neonatal intensive care unit bacteremia: emergence of gram-positive bacteria as major pathogens. Am J Infect Control. 1987;15:141–7. doi: 10.1016/0196-6553(87)90137-4. [DOI] [PubMed] [Google Scholar]
- 16.Goldmann DA. Coagulase-negative staphylococci: interplay of epidemiology and bench research. Am J Infect Control. 1990;18:211–21. doi: 10.1016/0196-6553(90)90187-w. [DOI] [PubMed] [Google Scholar]
- 17.Healy CM, Baker CJ, Palazzi DL, Campbell JR, Edwards MS. Distinguishing true coagulase-negative Staphylococcus infections from contaminants in the neonatal intensive care unit. J Perinatol. 2013;33:52–8. doi: 10.1038/jp.2012.36. [DOI] [PubMed] [Google Scholar]
- 18.Freeman J, Platt R, Sidebottom DG, Leclair JM, Epstein MF, Goldmann DA. Coagulase-negative staphylococcal bacteremia in the changing neonatal intensive care unit population. Is there an epidemic? JAMA. 1987;258:2548–52. [PubMed] [Google Scholar]
- 19.Bishop-Kurylo D. The clinical experience of continuous quality improvement in the neonatal intensive care unit. J Perinat Neonatal Nurs. 1998;12:51–7. doi: 10.1097/00005237-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Camara D. Minimizing risks associated with peripherally inserted central catheters in the NICU. Am J Matern Child Nurs. 2001;26:17–21. doi: 10.1097/00005721-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Garland JS, Alex CP, Mueller CD, Otten D, Shivpuri C, Harris MC, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics. 2001;107:1431–6. doi: 10.1542/peds.107.6.1431. [DOI] [PubMed] [Google Scholar]
- 22.Sohn AH, Garrett DO, Sinkowitz-Cochran RL, Grohskopf LA, Levine GL, Stover BH, et al. Pediatric Prevention Network. Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001;139:821–7. doi: 10.1067/mpd.2001.119442. [DOI] [PubMed] [Google Scholar]
- 23.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002;23:759–69. doi: 10.1086/502007. [DOI] [PubMed] [Google Scholar]
- 24.Aly H, Herson V, Duncan A, Herr J, Bender J, Patel K, et al. Is bloodstream infection preventable among premature infants? A tale of two cities. Pediatrics. 2005;115:1513–8. doi: 10.1542/peds.2004-1785. [DOI] [PubMed] [Google Scholar]
- 25.Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, et al. New York State Regional Perinatal Care Centers. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127:436–4. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]