Abstract
A limited number of publications have documented the effects of acute alcohol administration among older adults. Among these, only a few have investigated sex differences within this population. The current project examined the behavioral effects of acute low- and moderate-dose alcohol on 62 older (ages 55–70) male and female, healthy, light to moderate drinkers. Participants were randomly assigned to one of three dose conditions: placebo (peak breath alcohol concentration [BrAC] of 0 mg/dL), low (peak BrAC of 40 mg/dL), and moderate (peak BrAC of 65 mg/dL). Tasks assessed psychomotor, set-shifting, and working memory performance. Better set-shifting abilities were observed among women, whereas men demonstrated more efficient working memory, regardless of dose. The moderate-dose group did not significantly differ from the placebo group on any task. However, the low-dose group performed better than the moderate-dose group across measures of set shifting and working memory. Relative to the placebo group, the low-dose group exhibited better working memory, specifically for faces. Interestingly, there were no sex by dose interactions. These data suggest that, at least for our study’s task demands, low and moderate doses of alcohol do not significantly hinder psychomotor, set-shifting, or working memory performance among older adults. In fact, low-dose alcohol may facilitate certain cognitive abilities. Furthermore, although sex differences in cognitive abilities were observed, these alcohol doses did not differentially affect men and women. Further investigation is necessary to better characterize the effects of sex and alcohol dose on cognition in older adults.
Keywords: alcohol, acute administration, older adults, sex differences, working memory task, trail making test
Introduction
Studies suggest that a lifestyle including moderate drinking may reduce older adults’ risk for health-related conditions such as coronary heart disease (Hvidtfeldt et al., 2010), osteoporosis (Poli et al., 2013), and mood disorders (e.g., depression and anxiety; Green, Perrin, & Polen, 2004). Despite numerous investigations of alcohol’s chronic effects, relatively little work has been directed to the study of acute alcohol effects among older adults. The few reports that are available vary in the age ranges and alcohol doses (see Davies & Bowen, 1999; Gilbertson, Prather, & Nixon, 2010) or fail to control for breath alcohol concentrations (BrACs; see Schaller et al., 2010 & Sengul et al., 2011), making systematic comparison difficult. Even when controlling for such factors, preliminary findings are not entirely consistent. In our laboratory, low-dose alcohol (i.e., target BrAC of 40 mg/dL) effects among older adults range from beneficial to detrimental. For example, investigation of psychomotor and set-shifting abilities with the Trail Making Tests (Reitan & Wolfson, 1993) reveal poorer (Gilbertson, Ceballos, Prather, & Nixon, 2009), similar, and enhanced (Boissoneault, Sklar, Prather, & Nixon, 2014) performance after alcohol administration, relative to placebo. These inconsistencies reflect the importance of conducting additional research.
Furthermore, the influence of sex on the behavioral effects of acute low- and moderate-dose alcohol among this population is understudied. Investigations observe sex differences in younger adults after acute alcohol administration. For example, reports suggest greater alcohol-related sub-clinical deficits in memory recall, divided attention, and motor skills among young women, relative to male cohorts (Miller, Weafer, & Fillmore, 2009; but see Weissenborn & Duka, 2003). However, it is unknown if aging adults show similar sex differences. Furthermore, because cognitive changes accompany normal aging (e.g., sub-clinical deficits in attention and working memory; Carriere, Cheyne, Solman, & Smilek, 2010; Gazzaley, Cooney, Rissman, & D’Esposito, 2005), the effects of sex and alcohol on this population may differ from those observed in younger adults. Therefore, examination of sex differences within various age groups is necessary to gain a better understanding of alcohol’s acute effects.
To address these gaps, the current study examined sex and acute alcohol effects on psychomotor, set-shifting, and working memory performance among older light to moderate drinkers. The Trail Making Tests Part A (TMT-A) and B (TMT-B) were used to assess psychomotor and set-shifting abilities, respectively. A task that required recognition of faces and scenes after a brief delay was used to investigate working memory function. Tasks were selected based on their demonstrated sensitivity for measuring the cognitive domains of interest and/or the effects of low and moderate-dose alcohol. For example, the TMT-A and B (Gilbertson et al., 2009) and the working memory task (WMT; Boissoneault et al., 2014) used in the current study have previously revealed interactions between age and alcohol.
Although sex differences in cognitive function among healthy older adults are not widely reported, previous investigations demonstrate that the TMT-A (Portin, Saarijärvi, Joukamaa, & Salokangas, 1995) and TMT-B (Ernst, 1987; Parsons, Rizzo, Zaag, McGee, & Buckwalter, 2005) are sensitive to sex effects among this population. Older men have exhibited superior performance on these tasks, relative to older women. Furthermore, working memory for faces may differ by sex. For example, researchers typically observe superior face recognition among women on tasks that require delayed response to previously shown faces, presented amongst distracting face stimuli (Herlitz & Yonker, 2002; Lewin, Wolgers, & Herlitz, 2001; Rehnman & Herlitz, 2007). Less is known about sex differences in face recognition among older populations. Thus, sex and dose effects on working memory for faces and scenes were investigated with a task that has been previously demonstrated to recruit working memory functions among older adults (Boissoneault et al., 2014; Gazzaley et al., 2005). Due to the relatively low BrACs targeted for the current study, the epoch of observable alcohol effects constrained the time available for behavioral assessment. Therefore, a single task was used to assess each cognitive domain.
Based on our previous findings, we predicted poorer behavioral performance after administration of a moderate alcohol dose (i.e., target BrAC of 65 mg/dL), relative to a low dose and placebo (i.e., target BrAC of 40 mg/dL and 0 mg/dL, respectively). Due to the paucity of empirical data concerning older adults and inconsistent findings for younger adults, we did not formulate hypotheses regarding the effects of low-dose alcohol or sex. Rather, we posed three empirical questions: 1) Would administration of the low dose facilitate behavioral performance (compared to the placebo and moderate dose), 2) Would older men and women perform differently on behavioral tasks, and 3) Would a dose by sex interaction be observed?
Materials and methods
Participants
Sixty-two older (ages 55–70) male (n = 26) and female (n = 36) healthy, non-problem drinkers were recruited from North Central Florida as part of a larger study assessing alcohol and aging. A portion of the participants (Ps) was included in previous reports (Boissoneault et al., 2014; Sklar, Boissoneault, Fillmore, & Nixon, 2014). Drinking patterns ranged from light to moderate alcohol consumption (i.e., ≤1 drink/day for women and ≤2 drinks/day for men, with a standard drink containing 0.6 fluid ounces of alcohol; USDA/USDHHS, 2010). All Ps consumed at least one alcoholic beverage per month, and consequently, are referred to as moderate drinkers hereafter. Ps had 12–18 years of education and were primarily Caucasian (~91%); approximately 2% identified as African-American and 3.5% ‘other’. Three and a half percent indicated Hispanic ethnicity. Ps provided written informed consent prior to the collection of screening and testing data and were compensated for participating. All procedures were approved by the University of Florida Medical Institutional Review Board.
Potential Ps completed questionnaires addressing demographics, affective states, and substance use. To confirm the absence of dementia, mental status was assessed using the mini-mental state examination (MMSE cutoff for inclusion: score ≥26; Folstein, Folstein, & McHugh, 1975) and Hopkins verbal learning test (HVLT cutoff for inclusion: total immediate recall >15; Benedict, Schretlen, Groninger, & Brandt, 1998). Following completion of paper-pencil reports and assessments, a self-reported medical history was obtained and the computerized Diagnostic Interview Schedule, version IV (c-DIS-IV) was administered to identify possible Axis I psychiatric disorders per the Diagnostic and Statistical Manual IV criteria (American Psychiatric Association, 1994; Robins et al., 2000). Ps were excluded if they: 1) met criteria for a current or lifetime Axis I disorder, including substance abuse; 2) endorsed a medical condition that could potentially interfere with neurocognitive testing (e.g., uncontrolled Type II diabetes, uncontrolled high blood pressure, etc.); 3) identified themselves as a smoker; and/or 4) reported the use of medication which contraindicated alcohol consumption (e.g., sedatives and muscle relaxants).
Laboratory Protocol
Ps were instructed to abstain from alcohol and sleep aids for 24 h, avoid the use of sedating allergy medications on the day of testing, and fast for 4 h prior to participation. Upon arrival, Ps were queried about their adherence to pre-testing guidelines. Additionally, a negative breath alcohol test and urine toxicology screen were required for participation. One hour prior to beverage administration, Ps consumed a small breakfast snack (~220 kcal).
Alcohol Administration
Ps were randomly assigned to one of three dose conditions: placebo (target peak BrAC = 0 mg/dL; n = 20, 12 women), low (target peak BrAC = 40 mg/dL; n = 20, 11 women), and moderate (target peak BrAC = 65 mg/dL; n = 22, 13 women). Beverages were mixed by unblinded, trained laboratory personnel, according to standard procedure (e.g., Fillmore, Blackburn, & Harrison, 2008). Ps and task administrators were blind to the assigned dose. Active dose groups received a volume of medical grade ethanol determined by a modified Widmark calculation (taking into consideration the age, sex, height, and weight of the individual, as appropriate) to reach the desired BrAC (Watson, Watson, & Batt, 1981). The total calculated alcohol dose was equally divided into two beverages. Each active and placebo beverage contained 183 mL of sugar-free, caffeine-free, diet soda (i.e., vehicle). Both beverages were consumed over a 5-min period.
BrACs were obtained at 10, 25, and 60 min post beverage-consumption (Intoxylizer® 400PA; CMI, Inc., Owensboro, KY). A “booster” beverage was administered soon after (~5 min) the second BrAC measure to sustain targeted BrACs throughout testing. For Ps in the active dose groups, BrACs at 25 min determined the “booster” contents. If BrACs were below 50% of the target, Ps received a 180-mL “booster” that contained the vehicle and half of the original alcohol dose. Otherwise, Ps received a “booster” of equal volume that contained only the vehicle solution. Consequently, six Ps received an active “booster” (3 men [2 low-dose group] & 3 women [3 low-dose group]). Previous implementation of this “booster” procedure demonstrated that the method maintains group appropriate BrACs across time (e.g., Gilbertson et al., 2009; Sklar, Gilbertson, Boissoneault, Prather, & Nixon, 2012; Sklar & Nixon, 2014). Importantly, Ps in the placebo group also received a 180-mL vehicle-only “booster” to maintain double-blind procedures.
All beverages were misted with ethanol, prior to initial and “booster” administration, to facilitate alcohol expectancy. To assess placebo effectiveness, Ps were asked to indicate their perceived dose assignment (i.e., active or placebo) at the end of the study session.
Cognitive Testing
Ps were administered the TMT-A and TMT-B (Reitan & Wolfson, 1993) 15 min post initial-beverage consumption to assess psychomotor and set-shifting ability, respectively. TMT administration was limited to the ascending limb of the BrAC curve, given the window of opportunity for observing dose effects and our previous work showing sensitivity on the ascending limb (e.g., Gilbertson et al., 2009). According to standard procedure, the TMT-A was always administered before the TMT-B. In an effort to examine working memory at BrAC plateau (immediately after peak), Ps performed a WMT (from Gazzaley et al., 2005) 35 min post initial-beverage consumption. Furthermore, task order was not counterbalanced across Ps to ensure that BrACs at the time of task administration were held constant within groups.
For the TMT-A, Ps were instructed to connect the dots in numerical order (i.e., 1 to 2, 2 to 3, etc.). For the TMT-B, Ps were instructed to connect the dots switching from numerical to alphabetical order (i.e., 1 to A, A to 2, 2 to B, etc.). Ps were made aware of mistakes and asked to proceed from their last correct move when one occurred. Instructions were given to perform as quickly and accurately as possible. The dependent variable of interest was time to completion. Errors were accounted for within completion time.
The WMT was provided by Gazzaley (personal communication, 2010). The task consisted of three instructional blocks (i.e., ‘passive condition’, ‘face condition’, and ‘scene condition’), each containing 20 trials of grey-scale stimuli. Ps were presented with all three blocks in a counterbalanced order. During each trial, Ps were shown a set of four images (two face stimuli and two scene stimuli) presented sequentially in a pseudo-random order. Stimuli were presented for 800 msec each with 200-msec interstimulus intervals. The ‘face condition’ required Ps to remember the faces and ignore the scenes, whereas the ‘scene condition’ required Ps to remember the scenes and ignore the faces. After each stimulus set, a fixation cross (+) was displayed on the screen for 9 sec. Following the delay, Ps were presented with a probe face or scene stimulus. Using a response pad and the index finger of their dominant hand, Ps indicated if the probe stimulus was present (‘right button’) or absent (‘left button’) in the preceding set. For the ‘passive condition’, Ps viewed the stimuli passively and indicated the direction of an arrow (left vs. right) presented at the end of each set. Due to particularly high performance measures (99.1% accuracy), the ‘passive condition’ provided little insight and was therefore excluded from experimental analyses. Ps were instructed to respond as quickly and accurately as possible.
For the WMT, efficiency was the primary dependent measure of interest. The current study derived efficiency by dividing the percent accurate by mean reaction time for accurate trials. Efficiency ratios were individually calculated for all Ps and averaged for group analysis. This measure has been previously shown to reveal subtle deficits that may not be obvious with end-point measures alone (Glenn & Parsons, 1990, 1992; Kaplan, 1988; Nixon, Lawton-Craddock, Tivis, & Ceballos, 2007).
Statistical analysis
Pearson correlations were conducted to identify potentially confounding relationships between descriptive and outcome variables. Demographics, affective measures, and substance use data were compared between sexes and dose groups using 2 (sex) × 3 (dose) ANOVAs.
A mixed-effects ANOVA was used to test for BrAC differences between sexes and active doses. To analyze behavioral outcomes for TMT-A and B, separate 2 (sex) × 3 (dose) ANOVAs were conducted. A mixed-effects ANOVA was performed to assess working memory efficiency. Dose group and sex were designated as between-participants factors and task condition (‘face condition’ vs. ‘scene condition’) as the within-participants factor. Where appropriate, Tukey’s method was used to probe main effects and interactions. Although not our primary measures of interest, analyses of accuracy and reaction time were conducted separately to explore their relative impact on those efficiency ratios revealing significant effects. Ps who had <50% accuracy on the WMT (5 Ps) performed at levels below chance. Thus, they were excluded from analyses to eliminate those who may not have understood the task demands. To remove the influence of outliers on TMT group averages, Ps who were 2.5 standard deviations from the mean (TMT-A & B; 1 & 2 Ps, respectively) were also excluded from analyses. SAS Version 9.3 (SAS Institute, Cary, NC) was used for all statistical operations.
Results
Demographics/Affect/Alcohol Use
Demographic, affective, and alcohol-use measures for men and women are presented by dose group in Table 1. As expected, there were no differences between dose groups for any variable of interest (p’s > 1). Women reported higher levels of state anxiety (State Anxiety Inventory; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) relative to men (p = 0.04). However, both sexes exhibited relatively low levels and state anxiety was not related to any dependent measures of interest (p’s > 0.10).
Table 1.
Descriptive Variables
| Men | Women | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Placebo | 40mg/dl | 65mg/dl | Placebo | 40mg/dl | 65mg/dl | |
| N=8 | N=9 | N=9 | N=12 | N=11 | N=13 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
|
| ||||||
| Age (years) | 62.13 (5.14) | 59.89 (5.01) | 62.22 (3.63) | 61.92 (4.17) | 60.36 (3.01) | 62.23 (5.36) |
|
| ||||||
| Education (years) | 15.63 (1.85) | 15.67 (1.87) | 16.89 (1.36) | 16.08 (1.51) | 16.18 (1.17) | 16.38 (1.61) |
|
| ||||||
| Verbal Agea | 19.00 (1.45) | 18.60 (1.08) | 19.36 (0.61) | 18.74 (1.72) | 19.29 (1.07) | 19.34 (1.18) |
|
| ||||||
| GDSb | 2.13 (2.64) | 2.56 (2.24) | 1.78 (2.54) | 2.42 (2.27) | 0.82 (1.25) | 2.31 (1.93) |
|
| ||||||
| STAIc* | 41.25 (5.97) | 42.78 (4.55) | 40.33 (5.27) | 43.83 (4.73) | 44.73 (8.74) | 46.85 (6.72) |
|
| ||||||
| QFId | 0.45 (0.29) | 0.31 (0.28) | 0.29 (0.22) | 0.39 (0.25) | 0.24 (0.15) | 0.23 (0.20) |
|
| ||||||
| HVLTe | 25.38 (5.80) | 23.56 (4.42) | 23.56 (3.75) | 27.33 (4.44) | 27.91 (3.24) | 25.31 (3.54) |
|
| ||||||
| MMSEf | 28.25 (0.46) | 28.89 (1.05) | 29.22 (0.67) | 29.25 (1.06) | 29.64 (0.50) | 29.31 (0.95) |
Shipley Institute of Living Scale-Verbal (SILS-V; Zachary, 1986),
Geriatric Depression Scale (Yesavage et al., 1982),
State Anxiety Inventory (Spielberger et al., 1983),
Quantity Frequency Index (average ounces of absolute ethanol consumed per day for the past 6 months; modified from Cahalan et al., 1969),
Hopkins Verbal Learning Test (Benedict et al., 1998),
Mini-mental State Examination (Folstein et al., 1975),
Significant sex effect [F1,56=4.32, p=.04; women (M=44.80) > men (M=41.45)].
Breath alcohol concentration
The BrAC curves for men and women in the active dose conditions are presented in Fig. 1. As expected, the BrACs for the moderate- and low-dose groups differed significantly (F[1,36] = 60.40, p < 0.0001). No main effect of sex (p > 0.62), sex by dose (p > 0.70), or sex by dose by time interaction (p > 0.93) was observed (all F’s < 1).
Figure 1.
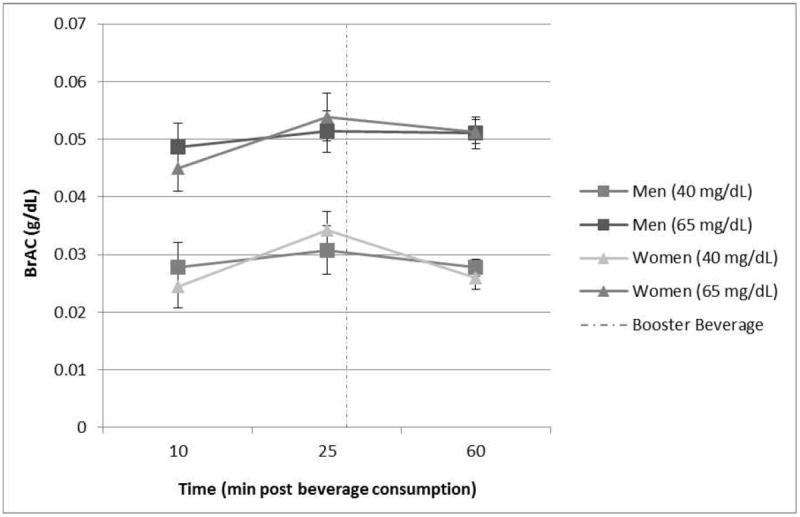
Breath Alcohol Concentration (BrAC): Sex and Dose. No sex differences were noted at either dose or at any time point (p’s > 0.40). Error bars depict standard error. Dashed line indicates time at “booster” beverage administration (~30 min).
Placebo effectiveness
Seventy-four percent of the placebo group reported that they had received alcohol at the end of the study session. Placebo effectiveness did not significantly differ between sexes (p > 0.39). Therefore, group differences are unlikely to be due to differential placebo effectiveness.
TMT-A
No significant main effect of sex (Mmen = 31.99 ± 7.66 sec; Mwomen = 31.29 ± 8.07 sec; p > 0.73) or dose (Mplacebo = 32.42 ± 7.74 sec; Mlow = 29.46 ± 6.77 sec; Mmoderate = 33.04 ± 8.68 sec; p > 0.31) was observed for time to complete TMT-A. Additionally, no sex by dose interaction was detected (p > 0.48).
TMT-B
Analyses revealed a main effect of dose for the TMT-B (F[2,54] = 3.32, p = 0.04, ; Fig. 2). Post hoc analyses revealed a trend toward better performance among the low-dose group, relative to the moderate-dose group (t35 = 2.32, p = 0.06). No differences between the placebo group and the low- or moderate-dose groups were detected (p’s > 0.10).
Figure 2.
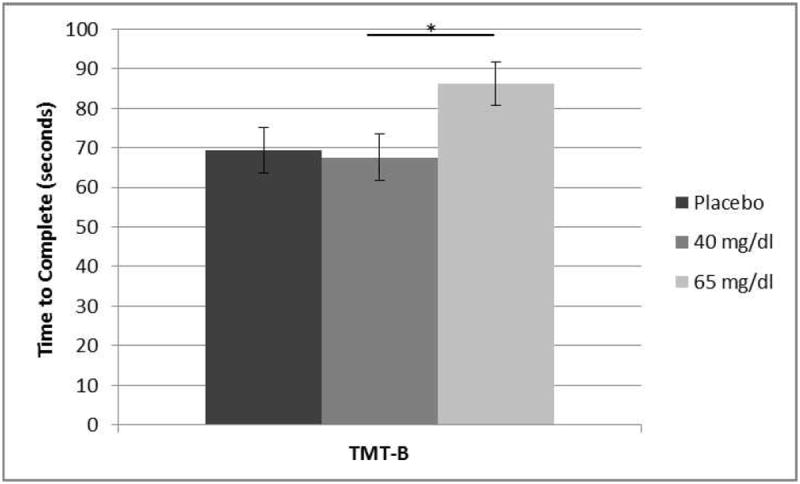
Trail Making Test B: Dose Main Effect. Time to complete TMT-B was significantly faster in the low-dose group (M = 67.51 ± 22.06 sec) vs. the moderate-dose group (M = 86.21 ± 34.16 sec). F2,54 = 3.32, p = 0.04, *t35 = 2.32, p = 0.06. No differences between the placebo group (M = 69.44 ± 18.22 sec) and the low or moderate-dose groups were detected. Error bars depict standard error.
A main effect of sex was also observed (F[1,54] = 5.30, p = 0.03, ; Fig. 3). Men took significantly longer to complete the task than did women. The dose by sex interaction did not achieve significance (p > 0.53).
Figure 3.
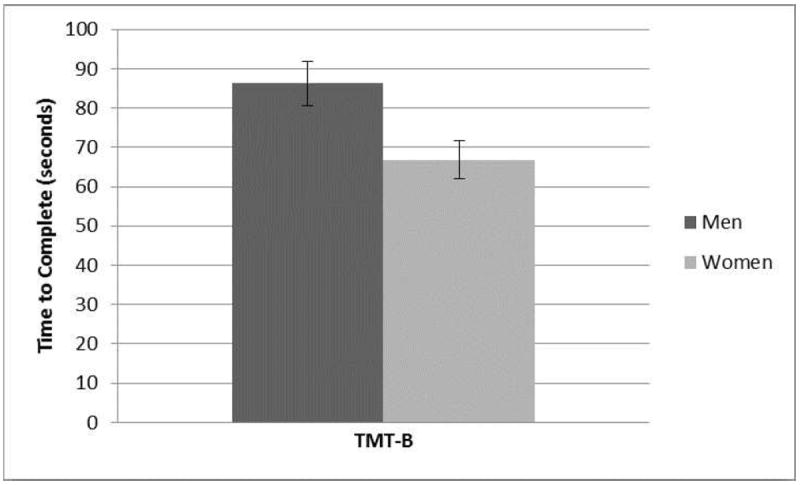
Trail Making Test B: Sex Main Effect. Time to complete TMT-B was significantly longer for men vs. women (M = 81.99 ± 30.18 sec vs. M = 66.79 ± 22.60 sec). Error bars depict standard error. F1,54 = 5.30, p = 0.03.
WMT
A main effect of dose was observed for WMT efficiency (F[2,51] = 4.42, p = 0.02, ). Post hoc analyses revealed that the low-dose group (M = 0.67 ± 0.15) responded more efficiently than the moderate-dose group (M = 0.54 ± 0.14; t35 = 2.82, p = 0.02). The placebo group (M = 0.60 ± 0.14) did not significantly differ from the low- or moderate-dose groups (p’s > 0.26).
A trend-level sex effect was observed (F[1,51] = 3.63, p = 0.06, ). Men (M = 0.64 ± 0.19) recorded higher efficiency scores than women (M = 0.57 ± 0.11). The sex by dose interaction term was not significant (p > 0.90).
Within-participant analyses yielded a main effect of condition (F[1,51] = 8.27, p = 0.006, ). Responding was more efficient under the ‘face condition’ (M = 0.63 ± 0.18) than the ‘scene condition’ (M = 0.58 ± 0.14). Furthermore, a dose by condition interaction was observed (F[2,51] = 4.46, p = 0.02, ; Fig. 4). Post hoc analyses revealed that the low-dose group responded more efficiently than the placebo (t36 = 2.46, p = 0.04) and moderate-dose groups (t36 = 3.68, p = 0.002) under the ‘face condition’. No significant difference was detected between the placebo and moderate-dose groups (p > 0.39). There were no dose effects within the ‘scene condition’ (p’s > 0.28).
Figure 4.
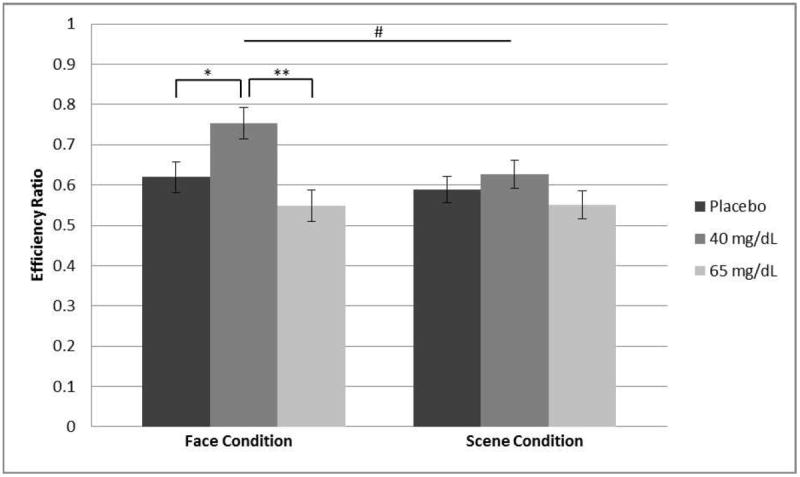
Working Memory Task Efficiency: Condition × Dose Interaction. The low-dose group (M = 0.75 ± 0.20) responded more efficiently (% accuracy/mean reaction time) than the placebo (M = 0.62 ± 0.13) and moderate-dose (M = 0.55 ± 0.17) groups under the face condition. No significant difference between dose groups was observed under the scene condition. Error bars depict standard error. #F2,51 = 4.46, p = 0.02, *t36 = 2.46, p = 0.04, **t36 = 3.68, p = 0.002.
As mentioned above, to better characterize findings concerning efficiency ratios, accuracy and reaction time were explored separately for the ‘face condition’. Alcohol dose had a significant effect on accuracy (F[2,51] = 5.23, p = 0.009, ) but not reaction time (Mplacebo = 1.45 ± 0.28 sec; Mlow = 1.33 ± 0.27 sec; Mmoderate = 1.53 ± 0.38 sec; p > 0.17). Post hoc analyses showed that the low-dose group (M = 87.34 ± 5.62% correct) was significantly more accurate than the moderate-dose group (M = 79.29 ± 8.15% correct; t35 = 3.27, p = 0.005). However, no significant differences between the placebo (M = 83.49 ± 7.50% correct) and the low- or moderate-dose groups were observed for this measure (p’s > 0.16). No sex effects or interactions (p’s > 0.11) were observed for accuracy (Mmen = 82.84 ± 8.77% correct; Mwomen = 83.79 ± 7.13% correct) or reaction time (Mmen = 1.38 ± 0.40 sec; Mwomen = 1.51 ± 0.25 sec).
Discussion
The present study assessed the acute effects of low- (target BrAC of 40 mg/dL) and moderate-dose (target BrAC of 65 mg/dL) alcohol on psychomotor, set shifting, and working memory performance among older men and women. Basic psychomotor skills, assessed by the TMT-A, were not subject to sex differences or the alcohol doses studied. In contrast, the more complicated TMT-B and the test for working memory were sensitive to both variables, although the pattern varied across tasks and condition.
As noted in the introduction, baseline sex differences in set-shifting ability have been previously reported in older adults. Investigations suggest superior TMT-B performance among older men, relative to older women (Ernst, 1987; Parsons et al., 2005). However, analysis of the TMT-B in the current study revealed better set-shifting skills among women, regardless of dose. Inconsistent findings may be partially explained by our sample’s light to moderate drinking habits, a variable not accounted for in the Ernst (1987) or Parsons et al. (2005) reports. Evidence suggests that drinking habits may influence cognitive function in older adults. Furthermore, this influence may differ by sex. For example, Dufouil, Ducimetière, & Alpérovitch (1997) investigated a group with comparable age to the current study (range: 59–71) and found a sex-specific relationship between daily alcohol consumption and TMT-B performance. Interestingly, light to moderate drinking, as opposed to abstaining, was associated with better set-shifting abilities among older women, but not older men. Given the sex-dependent associations between drinking habits and task performance, perhaps older moderate drinkers exhibit distinct sex differences in cognitive function, relative to abstainers and/or heavy drinkers. Future investigations may provide additional insight on the relationship between drinking patterns, sex, and cognition. Sex differences were also revealed for the WMT with men responding more efficiently than women, regardless of dose or task condition. Studies investigating younger adults largely suggest that women have superior working memory for faces, relative to men (Herlitz & Yonker, 2002; Lewin et al., 2001; Rehnman & Herlitz, 2007). Thus, our data suggest that sex differences in cognitive function may change with age.
To better understand these results, future studies might assess performance strategy (e.g., speed-accuracy tradeoffs). Importantly, process variables (e.g., efficiency; Kaplan, 1988) and the integration of multiple end-point measures (e.g., accuracy and reaction time) may provide more detailed information about strategy. For example, men and women in this study were equivalently accurate and had similar reaction times to accurate trials. However, efficiency, reflecting tradeoffs in accuracy and reaction time, revealed significant effects. Replications of these findings are necessary to more thoroughly characterize sex differences and similarities in cognitive abilities.
As mentioned above, analyses revealed dose effects for the TMT-B and the WMT. Taken together, our data suggest that alcohol doses consistent with a bout of social drinking (e.g., ~1–3 alcoholic beverages consumed over the course of an hour) do not significantly hinder psychomotor, set-shifting, or working memory performance, relative to placebo. Interestingly, low-dose alcohol had a positive effect on certain cognitive functions. However, among a different sample of older adults, low-dose alcohol resulted in poorer task performance (TMT-B), relative to placebo (Gilbertson et al., 2009). Specific factors contributing to inconsistent findings are not readily apparent.
Conflicting results emphasize the importance of constraining generalizations regarding the effects of these alcohol doses. The exact time of task administration in reference to alcohol consumption and the particular sample of interest may influence outcomes. Importantly, greater task complexity may yield additional information about the effects of dose on cognitive function (Dougherty et al., 1999; Jurado & Rosselli, 2007). Future acute alcohol studies may also benefit from measuring affective or cognitive states during task performance. For example, investigation may reveal an association between performance and alcohol’s effects on mood. Importantly, process variables may enhance detection of subtle behavioral effects. When the WMT source components (i.e., accuracy and reaction time) were disentangled, observations suggest that efficiency was the most sensitive measure for detecting alcohol and condition effects.
The effects of dose on WMT performance were largely driven by the ‘face condition’. Faces are often presumed to undergo preferential processing (Farah, Wilson, Drain, & Tanaka, 1998; Haxby, Hoffman, & Gobbini, 2000), even among older adults. For example, using the WMT employed in the current study, Gazzaley and colleagues (2008) found enhanced neural activation for faces, regardless of stimulus relevance. It may be that networks engaged in facial processing are differentially sensitive to alcohol, at least within certain dose ranges. Further work, using a variety of neurobehavioral methods (e.g., imaging, electrophysiology), is needed to clarify the association between specific doses and processing of faces versus scenes.
Finally, at least on these tasks, results suggest that sub-intoxicating doses of alcohol do not differentially affect healthy, older, moderate-drinking men and women. As noted previously, sex differences among older adults’ responses to alcohol are largely understudied. However, acute alcohol studies that assess younger adults suggest that women may be more vulnerable to the cognitive effects of acute intoxication (Mumenthaler, Taylor, O’Hara, & Yesavage, 1999). For example, relative to their pre-alcohol assessments, women have shown greater performance decrements than men on tasks of short-term memory (Niaura, Nathan, Frankenstein, Shapiro, & Brick, 1987) and divided attention (Mumenthaler et al., 1999). Although much of the literature suggests that women may be more susceptible to the effects of acute alcohol relative to men (e.g., Miller et al., 2009), this finding was not supported by the current study (see also Friedman, Robinson, & Yelland, 2011; Nixon, Prather, & Lewis, 2014; Weissenborn & Duka, 2003). Thus, the effects of sex and acute alcohol may differ with age. Future studies might enhance our understanding by elaborating on these findings across tasks and cognitive abilities.
Study Limitations
Limitations for the current study should be noted. First, this sample was not entirely representative of the older adult population. The homogenous racial makeup (~91% Caucasian), restricted age range, and health constraints (e.g., non-smokers) of the sample limit generalization. Future studies might include other age groups to study alcohol’s effects across adulthood. Second, the sample size was small. However, moderate to large effect sizes (Cohen, 1969) were observed for all significant findings. Thus, although a larger sample may have revealed additional group differences, those reported here are unlikely to be altered. Third, in an effort to assess BrACs consistent with a bout of social drinking, investigation was limited to sub-intoxicating doses. Sex differences among older adults may exist at BrACs not assessed in the current study. Thus, future work might explore a larger range of alcohol doses. Finally, to ensure study feasibility, investigation was restricted to a between-participants design and select cognitive tasks. A within-participants design may have introduced carryover effects and/or reduced sample size had we required a second testing session. That noted, the implementation of a within-participants design in future investigations might provide additional insight. As mentioned previously, the relatively low doses investigated in the current study resulted in a limited epoch for investigating alcohol’s observable effects. Given the time restrictions, a single task was used to assess each behavioral domain, constraining interpretation of cognitive function to these tasks. A more comprehensive test battery might enhance our understanding. In response to this limitation, we are conducting ongoing experiments that assess more complex behaviors (e.g., driving simulator performance).
Conclusions
Our results suggest that sub-intoxicating doses of alcohol may not negatively affect psychomotor, set-shifting, or working memory performance in older moderate drinkers. Interestingly, low-dose alcohol may facilitate performance on certain cognitive tasks, in controlled conditions. Although sex main effects were observed for certain measures, low- and moderate-dose alcohol did not differentially affect older men and women.
These data address an understudied question with increasing real-world application. The U.S. population of older adults continues to grow at a rapid rate; by the year 2030, this population is expected to double what it was in 2000. Given the prevalence of alcohol consumption among older adults (~59% for those age 55+), it has become essential to better understand alcohol’s acute effects on this population. To generalize findings to real-world scenarios, studies will need to apply more complex tasks.
Highlights.
The effects of sex and 2 acute-alcohol doses are studied in older moderate drinkers.
Psychomotor, set-shifting, and working memory performance are assessed.
Relative to placebo, low and moderate doses do not impair performance on any task.
The low-dose facilitates working memory abilities under certain task conditions.
Sub-intoxicating doses of alcohol do not differentially affect older men and women.
Acknowledgments
A portion of this work is reported in the MS thesis of the primary author, L. A. Hoffman (‘Sex Differences in the Behavioral Effects of Acute Moderate Alcohol Administration among Older Adults’). The authors thank the study subjects for their willingness to participate. Special thanks to Adam Gazzaley, MD, Ph.D., who generously provided working memory task stimuli and timing parameters. The authors acknowledge Ben Lewis, Ph.D., Jeff Boissoneault, Ph.D., Layla Lincoln, and Cole McCarty who assisted in the recruiting, screening, and conducting of laboratory sessions. This study was supported by NIAAA grant R01AA019802.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Boissoneault J, Sklar A, Prather R, Nixon SJ. Acute effects of moderate alcohol on psychomotor, set shifting, and working memory function in older and younger social drinkers. Journal of Studies on Alcohol and Drugs. 2014;75:870–879. doi: 10.15288/jsad.2014.75.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American Drinking Practices: A National Study of Drinking Behavior and Attitudes. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- Carriere JS, Cheyne JA, Solman GJ, Smilek D. Age trends for failures of sustained attention. Psychology and Aging. 2010;25:569–574. doi: 10.1037/a0019363. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. San Diego, CA: Academic Press; 1969. [Google Scholar]
- Davies BT, Bowen CK. Total body water and peak alcohol concentration: a comparative study of young, middle-age, and older females. Alcoholism: Clinical and Experimental Research. 1999;23:969–975. [PubMed] [Google Scholar]
- Dougherty DM, Moeller FG, Steinberg JL, Marsh DM, Hines SE, Bjork JM. Alcohol increases commission error rates for a continuous performance test. Alcoholism: Clinical and Experimental Research. 1999;23:1342–1351. [PubMed] [Google Scholar]
- Dufouil C, Ducimetière P, Alpérovitch A. Sex differences in the association between alcohol consumption and cognitive performance. EVA Study Group. Epidemiology of vascular aging. American Journal of Epidemiology. 1997;146:405–412. doi: 10.1093/oxfordjournals.aje.a009293. [DOI] [PubMed] [Google Scholar]
- Ernst J. Neuropsychological problem-solving skills in the elderly. Psychology and Aging. 1987;2:363–365. doi: 10.1037//0882-7974.2.4.363. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychological Review. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn JS, Harrison EL. Acute disinhibiting effects of alcohol as a factor in risky driving behavior. Drug and Alcohol Dependence. 2008;95:97–106. doi: 10.1016/j.drugalcdep.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman TW, Robinson SR, Yelland GW. Impaired perceptual judgment at low blood alcohol concentrations. Alcohol. 2011;45:711–718. doi: 10.1016/j.alcohol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D’Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gilbertson R, Ceballos NA, Prather R, Nixon SJ. Effects of acute alcohol consumption in older and younger adults: perceived impairment versus psychomotor performance. Journal of Studies on Alcohol and Drugs. 2009;70:242–252. doi: 10.15288/jsad.2009.70.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson R, Prather R, Nixon SJ. Acute alcohol administration and placebo effectiveness in older moderate drinkers: influences on cognitive performance. Journal of Studies on Alcohol and Drugs. 2010;71:345–350. doi: 10.15288/jsad.2010.71.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA. The role of time in neuropsychological performance: investigation and application in an alcoholic population. The Clinical Neuropsychologist. 1990;4:344–354. [Google Scholar]
- Glenn SW, Parsons OA. Neuropsychological efficiency measures in male and female alcoholics. Journal of Studies on Alcohol. 1992;53:546–552. doi: 10.15288/jsa.1992.53.546. [DOI] [PubMed] [Google Scholar]
- Green CA, Perrin NA, Polen MR. Gender differences in the relationships between multiple measures of alcohol consumption and physical and mental health. Alcoholism: Clinical and Experimental Research. 2004;28:754–764. doi: 10.1097/01.alc.0000125342.28367.a1. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Yonker JE. Sex differences in episodic memory: the influence of intelligence. Journal of Clinical and Experimental Neuropsychology. 2002;24:107–114. doi: 10.1076/jcen.24.1.107.970. [DOI] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Tolstrup JS, Jakobsen MU, Heitmann BL, Grønbaek M, O’Reilly E, et al. Alcohol intake and risk of coronary heart disease in younger, middle-aged, and older adults. Circulation. 2010;121:1589–1597. doi: 10.1161/CIRCULATIONAHA.109.887513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychology Review. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Kaplan E. A process approach to neuropsychological assessment. In: Boll T, Bryant B, editors. Clinical neuropsychology and brain function: Research, measurement, and practice. The Master lecture series. Vol. 7. Washington, DC: American Psychological Association; 1988. pp. 127–167. [Google Scholar]
- Lewin C, Wolgers G, Herlitz A. Sex differences favoring women in verbal but not in visuospatial episodic memory. Neuropsychology. 2001;15:165–173. doi: 10.1037//0894-4105.15.2.165. [DOI] [PubMed] [Google Scholar]
- Miller MA, Weafer J, Fillmore MT. Gender differences in alcohol impairment of simulated driving performance and driving-related skills. Alcohol and Alcoholism. 2009;44:586–593. doi: 10.1093/alcalc/agp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, Yesavage JA. Gender differences in moderate drinking effects. Alcohol Research & Health. 1999;23:55–64. [PMC free article] [PubMed] [Google Scholar]
- Niaura RS, Nathan PE, Frankenstein W, Shapiro AP, Brick J. Gender differences in acute psychomotor, cognitive, and pharmacokinetic response to alcohol. Addictive Behaviors. 1987;12:345–356. doi: 10.1016/0306-4603(87)90048-7. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Lawton-Craddock A, Tivis R, Ceballos N. Nicotine’s effects on attentional efficiency in alcoholics. Alcoholism: Clinical and Experimental Research. 2007;31:2083–2091. doi: 10.1111/j.1530-0277.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Prather RA, Lewis B. Sex differences in alcohol-related neurobehavioral consequences. In: Sullivan EV, Pfefferbaum A, editors. Alcohol and the nervous system; Handbook of clinical neurology, 3rd series. Oxford, UK: Elsevier; 2014. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Rizzo AR, Zaag CVD, McGee JS, Buckwalter JG. Gender differences and cognition among older adults. Aging Neuropsychology and Cognition. 2005;12:78–88. [Google Scholar]
- Poli A, Marangoni F, Avogaro A, Barba G, Bellentani S, Bucci M, et al. Moderate alcohol use and health: a consensus document. Nutrition Metabolism and Cardiovascular Diseases. 2013;23:487–504. doi: 10.1016/j.numecd.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Portin R, Saarijärvi S, Joukamaa M, Salokangas RK. Education, gender and cognitive performance in a 62-year-old normal population: results from the Turva Project. Psychological Medicine. 1995;25:1295–1298. doi: 10.1017/s0033291700033262. [DOI] [PubMed] [Google Scholar]
- Rehnman J, Herlitz A. Women remember more faces than men do. Acta Psychologica. 2007;124:344–355. doi: 10.1016/j.actpsy.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Robins LN, Cottler L, Bucholz KK, Compton W, North CS, Rourke KM. The diagnostic interview schedule, version IV. St. Louis, MO: Washington University; 2000. [Google Scholar]
- Schaller G, Kretschmer S, Gouya G, Haider DG, Mittermayer F, Riedl M, et al. Alcohol acutely increases vascular reactivity together with insulin sensitivity in type 2 diabetic men. Experimental and Clinical Endocrinology & Diabetes. 2010;118:57–60. doi: 10.1055/s-0029-1233453. [DOI] [PubMed] [Google Scholar]
- Sengul C, Cevik C, Ozveren O, Sunbul A, Oduncu V, Akgun T, et al. Acute alcohol consumption is associated with increased interatrial electromechanical delay in healthy men. Cardiology Journal. 2011;18:682–686. doi: 10.5603/cj.2011.0033. [DOI] [PubMed] [Google Scholar]
- Sklar AL, Boissoneault J, Fillmore MT, Nixon SJ. Interactions between age and moderate alcohol effects on simulated driving performance. Psychopharmacology. 2014;231:557–566. doi: 10.1007/s00213-013-3269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar AL, Gilbertson R, Boissoneault J, Prather R, Nixon SJ. Differential effects of moderate alcohol consumption on performance among older and younger adults. Alcoholism: Clinical and Experimental Research. 2012;36:2150–2156. doi: 10.1111/j.1530-0277.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar AL, Nixon SJ. Disruption of sensory gating by moderate alcohol doses. Psychopharmacology. 2014;231:4393–4402. doi: 10.1007/s00213-014-3591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans. 7. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. Journal of Studies on Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology. 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982-1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised Manual. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]


