Abstract
The marine environment covers almost three quarters of the planet and is where evolution took its first steps. Extremophile microorganisms are found in several extreme marine environments, such as hydrothermal vents, hot springs, salty lakes and deep-sea floors. The ability of these microorganisms to support extremes of temperature, salinity and pressure demonstrates their great potential for biotechnological processes. Hydrolases including amylases, cellulases, peptidases and lipases from hyperthermophiles, psychrophiles, halophiles and piezophiles have been investigated for these reasons. Extremozymes are adapted to work in harsh physical-chemical conditions and their use in various industrial applications such as the biofuel, pharmaceutical, fine chemicals and food industries has increased. The understanding of the specific factors that confer the ability to withstand extreme habitats on such enzymes has become a priority for their biotechnological use. The most studied marine extremophiles are prokaryotes and in this review, we present the most studied archaea and bacteria extremophiles and their hydrolases, and discuss their use for industrial applications.
Keywords: enzymes, marine extremophiles, hydrolases
1. Extremophiles from Marine Habitats: A Source of Bioproducts
The oceans cover more than 70% of the surface of the planet Earth and contain a vast biological diversity, accounting for more than 95% of the whole biosphere. Marine habitats can be divided into coastal and open ocean environments of various natures, supporting marine life with a wide heterogeneity of microorganisms. They represent the largest reservoir of biodiversity on the planet, and have a great potential for the development of new natural products including enzymes. However, in the prokaryotic group only 1%–10% of the species has been described [1].
Extreme environments combine a range of physical parameters such as pressure, temperature, pH, salinity, oxidative stress, radiation, chemicals (oxygen, H2S, CH4) and metals (Fe, Cu, Mo, Zn, Cd, Pb and others). Some of these chemicals are toxic, however extremophiles microorganisms are able to adapt due to their highly flexible metabolism, allowing them to survive and thrive in these extreme conditions [2]. Over the last few decades, extremophiles have attracted the attention of research centers in the search for new bioactive substances, such as enzymes and biocides to be used in major sectors of the world economy, including the agricultural, chemical, food, textile, pharmaceutical, bioenergy and cosmetic industries.
The global market for industrial enzymes was nearly US$ 4.8 billion in 2013, and it is expected to reach US$ 7.1 billion by 2018, with a compound annual growth rate (CAGR) of 8% over the five-year period, according to BCC Research [3]. Besides their economic value, microbial enzymes are applied in technologies employing eco-friendly processes [4]. Microorganisms are easily cultivated in bioreactors with controllable growth conditions such as pH, temperature, aeration, medium composition and other parameters, leading to high reproducibility. In contrast, enzymes isolated from plant and animals present a series of limitations like soil composition, light incidence, seed homogeneity, pathogen control and other issues that make the reproducibility of these processes more difficult [5].
Competition for space and nutrients in the marine environment constitutes a selective force leading to evolution and generating multiple enzyme systems to adapt to the different environments. Marine microorganisms can be found in extreme conditions such as hypersaline habitats, high pressures and extreme temperature. Many marine extremophiles are capable of overcoming such extreme conditions and are a source of enzymes with special characteristics. Therefore, these microorganisms are of great interest for industrial processes, mainly in biocatalysis [6]. This vast variation in marine habitats has led to the development of new hydrolases with novel specificities and properties including tolerance to extreme conditions used in industrial processes [7,8]. Metagenomic studies have revealed that extremophile prokaryotes from marine habitats are a source of novel genes and consequently a source of new bioproducts, including enzymes and other active metabolites. Therefore, it is important to study and understand these microorganisms in order to be able to use the biochemical, ecological, evolutionary and industrial potential of these marine microbes [9,10].
Hydrolases from extremophiles have advantages when compared to chemical biocatalysts. Their catalyses are clean processes, ecologically friendly, highly specific and take place in mild reaction conditions. These hydrolases can also be active in the presence of organic solvents, an important feature for the preparation of single-isomer chiral drugs. Various applications have been described for these hydrolases. A metagenome library was created from the brine: seawater interface of the Urania hypersaline basin. One esterase presented high enantioselectivity toward an ester of the important chiral synthon solketal, an important drug intermediate. The esterase hydrolyzed solketal acetate, producing building blocks for the synthesis of pharmaceuticals, such as anti-AIDS drugs [11]. Lipases from psychrophiles can be used for the synthesis of a wide range of nitrogenized compounds that are used for the production of pharmaceuticals such as amines and amides. This procedure was studied in Candida Antarctica, a patented eukaryote [12].
Other hydrolases with medical applications are peptidases. They are useful catalysts for inorganic synthesis and have many industrial applications in the pharmaceutical field, such as anti-inflammatory and digestive agents. Peptidases can be isolated from marine extremophiles [10], such as from Thermatoga maritime, which is a hyperthermophilic isolate from a marine geothermal area near Vulcano, Italy, and has a homomultimeric peptidase (669 kDa) based on 31 kDa subunits, named Maritimacin [13]. This enzyme was found to have a structural and gene sequence similarity to bacteriocin from the mesophilic bacterium Brevibacterium linens which inhibits the growth of certain Gram-positive bacteria [14]. The thermophilic Pyrococcus horikoshii has an intracellular peptidase (PH1704) with remarkable stability. Recently this enzyme, a cysteine peptidase, was shown to be the first allosteric enzyme that has negative cooperativity with chloride ions (Cl−). The discovery of new allosteric sites is very important for pharmaceutical developments [15]. Peptidases from halophiles have been used in peptide synthesis and an example is the extracellular peptidase from Halobacterium halobium which was exploited for efficient peptide synthesis in Water-N′-N′-dimethylformamide [16].
Given the promising potential for the biotechnological applications of these organisms, this work presents the recent advance in the knowledge of hydrolases from marine extremophiles and their biotechnological potential. Peptidases, lipases, amylases, cellulases and other cell wall-degrading hydrolases in halophiles, thermophiles, psychrophiles and piezophiles will be the main focus of this review.
2. Extremophiles
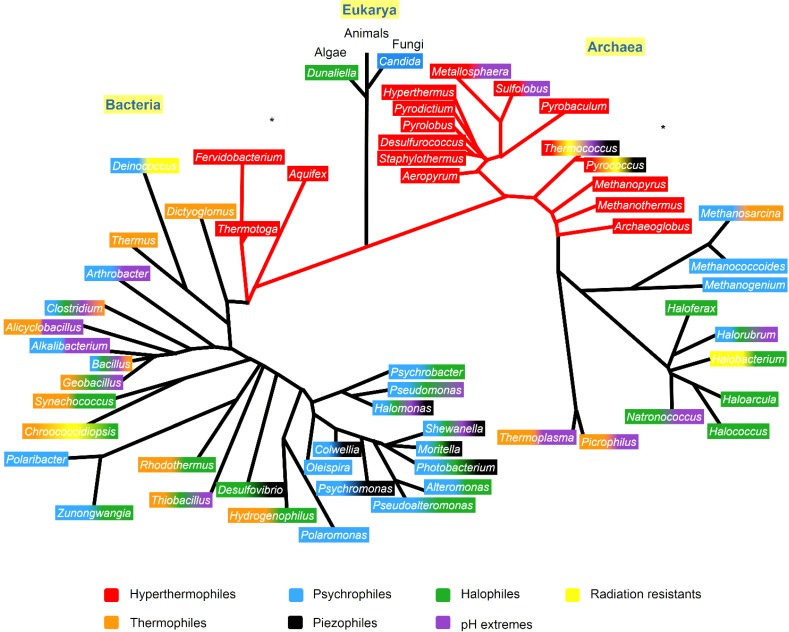
Extremophile organisms are classified as living organisms able to survive and proliferate in environments with extreme physical (temperature, pressure, radiation) and geochemical parameters (salinity, pH, redox potential). Polyextremophile microorganisms are those that can survive in more than one of these extreme conditions. The vast majority of extremophile organisms belong to the prokaryotes, and are therefore, microorganisms belonging to the Archaea and Bacteria domains [17,18]. A phylogenetic tree showing the microorganisms of different genera and their extremophilic characteristics is presented in Figure 1.
Figure 1.
Phylogenetic tree showing the extremophiles and the resistant characteristics that appear in at least one species of each genera, identified with the color code. The phylogenetic tree was based on Woese et al. [28], Lang et al. [29] and Dereeper et al. [30]. The * indicates the phylogenetic branch that were according to Lang et al. [29].
Extremophile microorganisms are classified according to the extreme environments in which they grow and the major types are summarized in Table 1. Different structural and metabolic characteristics are acquired by these organisms so that they can survive in these environments [19,20]. Because of the ability to withstand extreme situations, possible industrial applications of extremophiles have been widely investigated [21,22,23,24,25].
Table 1.
Extremophile microorganisms and their environments (adapted from Horikoshi and Bull [17]).
| Extremophile Microorganism | Favorable Environment to Growth |
|---|---|
| Acidophile | Optimum pH for growth—Below 3 |
| Alkaliphile | Optimum pH for growth—Above 10 |
| Halophile | Requires at least 1M salt for growth |
| Hyperthermophile | Optimum growth at temperatures above 80 °C |
| Thermophile | Grows at temperatures between 60 °C and 85 °C |
| Eurypsychrophile (psychrotolerant) | Grows at temperatures above 25 °C, but also grow bellow 15 °C |
| Stenopsychrophile (psychrophile) | Grows at temperatures between 10 °C and 20 °C |
| Piezophile | Grows under high pressure—Above 400 atm (40 MPa) |
| Endolithic | Grows inside rocks |
| Hipolith | Grows on rocks and cold deserts |
| Oligotroph | Able to grow in environments of scarce nutrients |
| Radioresistant | Tolerance to high doses of radiation |
| Metallotolerant | Tolerance to high levels of heavy metals |
| Toxitolerant | Tolerates high concentrations of toxic agents (eg. Organic solvents) |
| Xerophile | Grows in low water availability, resistant to desiccation |
One of the most well known applications of an extremophile is the DNA polymerase of the extremophile Thermus aquaticus (Taq polymerase) [26] which is widely used in the polymerase chain reaction (PCR). The stability and enzymatic activity of extremophiles and their extremozymes are useful alternatives to conventional biotechnological processes [27].
2.1. Thermophiles
The thermophile marine microorganisms include several groups such as the phototrophic bacteria (cyanobacteria, green and purple bacteria), bacteria domains (Actinobacteria sp., Bacillus sp., Clostridium sp., Desulfotomaculum sp., Thermus sp., Thiobacillus sp., fermenting bacteria, spirochetes and numerous other genres) and the archaea domains (Pyrococcus sp., Sulfolobus sp., Thermococcus sp., Thermoplasma sp. and methanogenic) [31,32,33]. The maximum temperature that hyperthermophile organisms have been observed to tolerate is around 120 °C [34,35].
Thermophiles have several mechanisms to support extreme temperatures. It is believed that the thermostability of cellular components such as ATP, amino acids, and peptides may exceed 250 °C, suggesting that the maximum temperature for life goes beyond the temperatures that have been observed until now [36,37]. The proteins of organisms adapted to extreme temperatures generally have similar three-dimensional structures of mesophilic organisms but the amino acid content is different from ordinary proteins and the number of charged residues on their surfaces is much greater than nonadapted organisms. In addition, such proteins often have shorter loops, thus preventing the occurrence of nonspecific interactions due to their increased flexibility at high temperatures [38,39].
Extreme thermophile bacteria produce thermostable proteins that can be readily crystallized to obtain stable enzymes for structural and functional studies. Proteins from hyper/thermophiles require sufficient structural rigidity to resist unfolding. This is an important feature to characterize antidrug targets. A classical instance is the bacteria Thermus thermophilus that was originally isolated from a thermal vent within a hot spring in Izu, Japan, and is frequently used in genetic manipulation studies. The DNA gyrase from this extremophile has been used as an antidrug target model. DNA gyrase is a type IIA topoisomerase that introduces negative supercoils into closed circular bacterial DNA using ATP hydrolysis. It is an important antibacterial target that is sensitive to the widely-used fluoroquinolone drugs [40,41].
The thermal hypothesis determines that a G:C pair and its contents are related to thermostability. This is observed for several thermophilic bacteria. Geobacillus thermoleovorans CCB US3UF5 is a thermophilic bacterium that was isolated from a hot spring in Malaysia and is a source for thermostable enzymes. The bacteria contains a circular chromosome of 3,596,620 bp with a mean G:C content of 52.3% [42]. However, a study reported a comparative analyses of G:C composition and optimal growth temperature with 100 prokaryote genomes (Archaea and Bacteria domains) that failed to demonstrate this correlation (G:C/thermostability). Moreover the authors related that the G:C content of structural RNA (16S and 23S) is strongly correlated with optimal temperature and it is higher at high temperatures [43]. An increased number of disulfide bonds improve stability within thermophilic proteins and play a role in preventing the alteration of the quaternary structure [44].
Ether lipids are always present in thermophile archaea without exception, but mesophilic archaea also have ether lipids. The presence of isoprenoid chains in archaea membranes is associated with two properties to maintain the thermostability of the lipid membrane: A high permeability barrier and a liquid crystalline state. Bacterial membranes only keep these states at the transition phase of temperature [45].
Accordingly, enzymes adapted to higher temperatures bring advantages to industrial processes, promoting faster reactions, high solubility of the substrate, a lower risk for contamination of the system, and also lowering the solution viscosity and increasing the miscibility of the solvent [46]. Several thermophiles and thermostable enzymes are used in the biorefinery industry, first and second generation biofuels, paper and bleaching industries [47,48,49].
2.2. Psychrophiles
Earth is primarily a cold marine planet: 90% of the water in the oceans has temperatures of 5 °C and 20% of the terrestrial region of the Earth is permafrost (frozen soils), glaciers and ice sheets, polar sea ice and snow covered regions [50]. These regions have cold-adapted microorganisms which have a restricted temperature range for growth. Stenopsychrophiles (formerly true psychrophiles) have an upper temperature limit of 20 °C for growth. However, the majority of isolates are eurypsychrophile (formerly psychrotolerant) and have a broader temperature range, tolerating warmer environments [51]. Psychrophile microorganisms are often found in other extremely cold environments such as deep oceans, caves, land surfaces and even in the upper atmosphere [52]. They have been described performing DNA synthesis at −20 °C and active metabolism at −25 °C [53,54].
Various stenopsychrophiles isolated from Antarctic have been studied; for example the genera Arthrobacter, Colwellia, Gelidibacter, Glaciecola, Halobacillus, Halomonas, Hyphomonas, Marinobacter, Planococcus, Pseudoalteromonas, Pseudomonas, Psychrobacter, Psychroflexus, Psychroserpens, Shewanella and Sphingomona. Methanogens, members of Archaea, are the only group known to have individual species able to grow in a very wide temperature range from subzero to 122 °C [55,56].
The adaptations and mechanisms related to life in icy environments include responses to cold shock and RNA chaperones. Cold shock proteins (CSPs) act as cold-adaptive proteins in psychrophiles. They are small proteins that bind to RNA to preserve its single-stranded conformation and contain a nucleic-acid-binding domain, known as the cold shock domain (CSD), and also they have additional roles besides serving as RNA chaperones [57,58]. Small RNA-binding proteins (RBPs) can facilitate cold adaptation but together with the CSPs they have other functions in bacteria. RNA helicases are regulated during cold growth and are capable of unwinding secondary structures in an ATP-dependent manner in some psychrophiles [58]. The presence of dihydrouridine can enhance tRNA flexibility and is elevated in some psychrophilic bacteria and archaea [59].
Other factors involved in cold adaptation are the production of secondary cold active metabolites, enzymes that are activated and induced by cold, antifreeze proteins, and the production of pigments and membrane fluidity [60,61,62]. From the structural point of view, the proteins of these organisms have a higher content of α-helix relative to the β-sheets, which is considered to be an important factor to maintain flexibility even at low temperatures [63]. Also the cytoplasmic membranes of these microorganisms contain a higher proportion of unsaturated fatty acids (52%) compared to mesophilic (37%) and thermophilic (10%) organisms, favoring the maintenance of the semi-fluid state of membranes [64]. Marine psychrophiles participate in biogeochemical cycling, polar food web and produce a wide variety of enzymes including amylases, cellulases, peptidases, lipases, xylanases and other classes of enzymes [50,65]. High rates of catalysis at low temperatures are generally achieved by the flexible structure and low stability of cold-active enzymes. The most common adaptive feature of cold-active enzymes is a reaction rate (kcat) that is largely independent of temperature [56,66].
Furthermore, enzymes adapted to lower temperatures allow efficient production at low cost, save energy, and are important in thermal protection, resulting in improvements in the quality of various products [67]. Enzymes suited to low temperatures are used in the food, cosmetics, pharmaceutical, and biofuels industries; also they are applied to substances for molecular biology, nanotechnology, in the manufacturing of household detergents, in the cleaning of animal wastes, with peptidases for cleaning contact lenses and pectinases to extract and clear fruit juices [67,68]. A clear example of the benefit generated by the industrial application of cold adapted microorganisms is the use in the hydrolysis of lactose at low temperatures in the process of milk storage. This bioprocess has enabled the production of milk for patients with milk intolerance and is now being patented for use on a large scale by Nutrilab NV (Bekkevoort, Belgium) [69].
2.3. Halophiles
Halophiles are microorganisms that require salt (NaCl) for growth, and they can be found in lakes, oceans, salt pans or salt marshes. Moreover about 25% of the available land on Earth is in the form of saline deposits [70]. According to the optimal salt concentration for growth, they are classified in three categories: (i) extreme halophile—Grows in an environment with 3.4–5.1 M (20% to 30%) NaCl; (ii) moderate halophile—Grows in an environment with 0.85–3.4 M (3% to 25%) NaCl; and (iii) slightly halophile—Grows in an environment with 0.2–0.85 M (1% to 5%) NaCl [31]. Halotolerant microorganisms do not show an absolute requirement for salt to grow but grow well in high salt concentrations [71].
Members of the family Halobacteriaceae have been isolated from different habitats including alkaline and salt lakes, marine salterns, the Dead Sea and saline soils. Deep hypersaline anoxic basins (DHABs) or deep-sea hypersaline anoxic lakes (DHALs) are extreme habitats that have been discovered on the sea floor in different oceanic regions, such as the Gulf of Mexico, the Red Sea and the Eastern Mediterranean Sea. DHABs are composed by dissolution of evaporitic deposits, entrapped in the sea floor, forming a very stable brine and sharply stratified in water columns, a chemocline. The brines enclosed in these basins are characterized by hypersalinity, 5–10 times higher than seawater, a lack of oxygen and highly reducing conditions, high pressure (around 350 atm–35 MPa) and absence of light. These physicochemical parameters make the DHABs one of the most extreme environments of the planet and they have also ensured that those habitats have been maintained isolated for thousands of years [11,72,73].
Since the discovery of the first Mediterranean DHAB named Tyro in 1983, six others have been unveiled: l’Atalante, Bannock, Discovery, Medee, Thetis, and Urania [74], and these habitats are a source of anaerobic halophilic microorganisms. Prokaryotes from the Bacteria and Archaea domains belonging to new taxonomic lineages were discovered in high abundance in DHABs by 16S rRNA libraries and fluorescent in situ hybridization (FISH). Microbiologically DHABs of the Eastern Mediterranean are the most studied. Halorhabdus utahensis constitutes 33% of the total archaeal community and tolerates up to 0.8 M MgCl2 [75]. In the Mediterranean Ridge, a DHAL named Kryos has been identified. This lake is filled with MgCl2-rich, athalassohaline brine (salinity > 470 practical salinity units). Two groups of halophilic euryarchaeal divisions (MSBL1 and HC1) account for ~85% of the rRNA-containing archaeal clones analyzed in the 2.27–3.03 M MgCl2 layer [74]. An earlier study assumed that 2.3 M of MgCl2 was the upper limit of concentration for life to survive [76], despite the fact that halophilic archaea have been identified in deep hypersaline anoxic basins composed of saturating concentrations of MgCl2 [73,77]. Antunes et al. [78] recently published a long list of microorganisms in a microbiological review of these unique deep-sea anoxic environments.
In addition, some halophiles are thermostable and tolerant to a wide range of pH. The metabolic diversity of halophiles is widely spread, comprising the anoxic phototrophic, aerobic heterotrophic, fermenter, denitrifying, sulfate reducers and methanogenic organisms [79].
Halophiles have developed different adaptive strategies to support the osmotic pressure induced by the high NaCl concentrations in the environments they inhabit. Some extremely halophilic bacteria accumulate inorganic ions (K+, Na+, Cl−) in the cytoplasm, which is a type of “salt-in” strategy to balance the osmotic pressure of the environment, and they have also developed specific proteins that are stable and active in the presence of salts [80,81,82]. Also, the halophile organisms contain enzymes that maintain their activity at high salt concentrations, alkaline pH and high temperatures [71]. Most proteins and enzymes denature when suspended in high salt concentrations. Halophilic proteins bind significant amounts of salt and water. This characteristic is dependent on the number of acidic amino acids on the surface of the protein.
The function of electrostatic interactions in the stability and folding of halophilic proteins has been investigated and is an important determinant of haloadaptation. The intracellular K+ ions of haloarchaea have been found to be extremely high, near to 5 M [83]. Based on the comparative analyses of halophile and non-halophile proteomes, the amino acid composition of halophilic enzymes is in general characterized by an abundant content of acidic amino acid, a high proportion of aspartic and glutamic acids, a low frequency of lysine, and a high occurrence of amino acids with a low hydrophobic character. Structural analyses between halophilic and mesophilic proteins reveal that the major differences are concentrated on the surface of the protein. These characteristics allow cooperation with electrostatic interactions and the presence of a higher number of salt bridges [84]. The stability of the enzymes depends on the negative charge on the surface of the protein due to acidic amino acids, the hydrophobic groups in the presence of high salt concentrations and the hydration of the protein surface due to carboxylic groups present in aspartic and glutamic acids. In addition, negative surface charges are thought to be important for the solvation of halophilic proteins, to prevent denaturation, aggregation and precipitation [85,86].
Moderate halophiles use other haloadaptations based on biosynthesis and/or accumulation in the cytoplasm of high amounts of specific organic osmolytes, which function as osmoprotectants, providing osmotic balance and maintaining low intracellular salt concentrations without interfering in the normal metabolism of the cell. The osmolyte could be obtained by direct uptake from the environment [85]. These solutes can act as stabilizers for biological structures and allow the cells to adapt not only to salts but also to heat, desiccation, cold or even freezing conditions [87]. Many halophilic bacteria accumulate ectoine or hydroxyectoine as the predominant compatible solutes. Other types of osmolyte include glycine, betaine and other neutral glycerols [71].
One of the adaptation mechanism developed is the lipid composition. Structural adaptations have been observed in the S-layers of halophiles. The extreme halophile contains sulfated glucuronic acid residues and a higher degree of glycosylation, leading to an increased density in surface charges. This characteristic demonstrates an adaptation in response to the higher salt concentrations experienced by Halobacterium salinarum. Moreover, in Haloarchaea, some S-layer glycoproteins are enriched in acidic residues [88].
The halophiles have been used in biodegradation of organic pollutants, in desalinization of wastewater, in nanotechnology, in production of biopolymers and as osmoprotectors [89,90]. The halotolerance of hydrolases derived from halophilic bacteria can be exploited wherever enzymatic transformations are required to function under physical and chemical conditions, such as in the presence of organic solvents and extremes in temperature and salt content. Many halophiles can secrete extracellular hydrolytic enzymes, such as amylases, lipases, peptidases, xylanases and cellulases that are thermostable and adaptable to a wide range of pH [80].
2.4. Piezophiles
High hydrostatic pressure is one of the physical parameters in deep-ocean environments and it plays a selective role in the distribution of life on the planet. The oceans, which have an average depth of 3800 m and an average pressure of 380 atmosphere (atm) or 38 MPa, make up ~95% of the biosphere. In the deepest parts of the oceans, pressures of 700 to 1100 atm (70 to 110 MPa) prevent the growth of most microorganisms. Moreover, the temperature in the deep-sea is typically within the 1–3 °C range. However, there are hydrothermal vent habitats where high pressures and high temperatures are found, and in these regions marine microbes might be exposed to temperatures and pressures ranging from 1–300 °C and 1–1100 atm (0.1–110 MPa), respectively [91].
The effects of high hydrostatic pressure on microbial metabolisms occurs in the cellular structures and cellular processes such as cell division and motility [92]. Microorganisms called piezophiles (previously named barophile), such as deep-sea bacteria or archaea, live in high pressure environments and are of interest to various sectors of biotechnology [93]. The Mariana Trench is the deepest part of the ocean found on the planet; it has a maximum depth of 11 km and a pressure of 1100 atm (110 MPa). This extreme habitat harbors organisms that can grow in standard pressure and temperature and strict piezophiles, like Moritella yayanosii and Shewanella benthica, that have pressure growth conditions of between 700 and 800 atm (70 ~80 MPa), but not less than 500 atm (50 MPa) [18,94].
Microorganisms which possess optimal growth rates at pressures above atmospheric pressure are classified as piezophilic; and as piezotolerant those that grow at high pressure, as well as at atmospheric pressure but they do not have optimal growth rates at pressures above one [91]. Bacterial piezophiles are mainly psychrophiles belonging to five genera of γ-proteobacteria, Photobacterium, Shewanella, Colwellia, Psychromonas and Moritella, while piezophilic Archaea are mostly (hyper)thermophiles from Thermococcales [95].
The physiological adaptations required for growth under these extreme conditions are substantial and involve a combination of modifications of gene structure and regulation. The adaptation mechanisms of piezophiles are under investigation. Whether piezophilic adaptation requires the modification of a few genes, or metabolic pathways, or a more profound reorganization of the genome has not yet been fully elucidated [95]. As with psychrophiles, piezophiles contain lipids with highly unsaturated fatty acids [96]. Other adaptation mechanisms against the high pressures include reduction of cell division, modification of membrane and transport proteins and accumulation of osmolytes, which stabilize the proteins [94,97,98]. The occurrence of elongated helices in the 16S rRNA genes to increase adaptation to growth at elevated pressure has also been described [99].
2.5. Polyextremophiles
Microorganisms in their natural habitats are thought to experience stress during their life cycle. Many extremophiles inhabit environments with more than one extreme parameter, for example, extremophiles that thrive in the depth of the oceans or close to hot springs. In the first situation, if the extremophiles are found in ocean mud, they could be piezophiles or psychrophiles, but if they were found close to a hydrothermal vent, they could be piezophiles, thermophiles or acidophiles, due to the minerals released in the chimney, or even, if they were found in DHABs, they could be piezophiles, psychrophiles or halophiles.
In an effort to provide a comprehensive look at the extremes of temperature and pH, Capece et al. [100] tabulated over 200 extremophile species found in the literature. They are called thermoacidophiles, thermoalkaliphiles, psychroacidophiles and psychroalkaliphiles. Since membrane fluidity decreases at low temperatures, the lower permeability to protons (H+) becomes an advantage for acidophiles and alkaliphiles. The pH homeostasis is controlled by H+ movements across the membrane. The presence of psychroacidophiles and psychroalkaliphiles has not yet been found in nature; only the presence of psychrotolerant alkaliphiles, such as Alkalibacterium psychrotolerans has been observed [100,101].
However, the presence of thermoacidophiles is well documented. High temperatures increase the permeability to H+ resulting in a lethal cytoplasmic acidification [102]. Modifications in RNA codon thermostability and a neutral surface charge in proteins prevent the occurrence of an acid hydrolysis. The existence of thermoacidophiles over 100 °C has not yet been observed [100,103]. The survival of thermoalkaliphiles is thought to be related more to the buffering capacity to maintain a stable intracellular cytoplasm than the maintenance of a bioenergetic gradient [100,102,104].
High salt concentrations allied to temperature extremes have been observed in psychrohalophiles and thermohalophiles. Many environments in polar sea ice are cold brine solutions, thus some degree of halophily is typical in most psychrophiles. Indeed, cold adaptation and salt adaptation have common approaches [100]. Thermohalophiles are rare. Their uncharged proteins became extremely unstable in hypersaline solutions, and are denatured by solvents at high temperatures and by decreasing the electrostatic interactions required to maintain the native folding [105,106]. Thermococcus waiotapuensis is the most hyper-thermohalophilic organism discovered to date, however, the basis of its biochemical stability is still unclear [107].
The correlation of temperature and pressure is also another parameter involved in extremophilic survivability. Increases in volume are favored at high temperatures but disfavored at high pressures. Thermopiezophile protein adaptations are synergistic, comprising a small surface charge with a strongly hydrophobic core. Other biochemical responses are the induction of both heat-shock and cold-shock response pathways reinforcing the synergistic reaction [100,108]. The cold-shock response is extremely important in psychropiezophiles, since they do not benefit from the synergistic temperature and pressure approach. They also incorporate monosaturated and polysaturated fatty acids to prevent membrane crystallization, which is caused by both extremes of temperature, a characteristic already incorporated in piezophiles [100,109].
Halo-acidophiles and halo-alkaliphiles are both found in nature. For halo-acidophiles, high extracellular concentrations allow a more favorable efflux of H+. Halo-alkaliphiles are more commonly found, because the monovalent cations of salts are essential for pH homeostasis and energetic coupling [110,111]. On other hand, for halo-alkaliphiles, the less favorable influx of H+, caused by high salt concentrations, leads to a lethal alkylation of the cytoplasm, and becomes a limitation to growth. Halo-alkaliphiles are able to maintain a gradient of about 1.0–1.5 units [102,112]. Finally, high pressures promote negative volume changes by the dissociation of acids and the protonation of amine groups in proteins. This leads to an acidification of the solution, creating an environment where piezo-acidophiles can be found. On the other hand, the alkalinification of the environment associated to high-pressures creates a propitious site for piezo-alkaliphiles. However, both of these organisms still need to be further investigated for systematic identification and characterization [100,113].
Polyextremophilic enzymes have been applied in the food, detergent, chemical, pulp and paper industries. A thermo-alkali-stable enzyme from Bacillus halodurans TSEV1 has applicability in pre bleaching of paper pulp and recently has been expressed in Pichia pastoris for the production of oligosaccharides [114,115]. Another strain of B. halodurans PPKS-2 produced an alkaliphilic, halotolerant, detergent and thermostable mannanase. This strain grows in agro wastes and can be applied for mannanase production on an industrial scale for detergent and pulp and paper bleaching [116]. The Antarctic cold-adapted halophilic Archeon Halorubrum lacusprofundi produces a recombinant polyextremophilic enzyme that is active in cold temperatures, high salinity and is stable in aqueous-organic mixed solvents. This enzyme is suitable for applications in synthetic chemistry [117].
3. Biocatalysis: Bioengineering and Other Strategies
The application of hydrolases in industrial processes sometimes fails due to the lack of robustness, stability and undesirable properties [5]. Recent experimental advances, associated to novel bioinformatic tools and protein engineering, has allowed the development of more efficient hydrolases for industrial purposes [46,118,119]. Since the introduction of site-directed mutagenesis and other integrated modern techniques such as synthetic biology and system biology, it is now possible to modify the characteristics of these enzymes, such as enhancing their stability and specificity [120,121].
Anaerobic, extremophilic and marine bacteria are a source of enzymes with superior chances of success in biotechnological processes. A great deal of laboratory effort has been concentrated on their production and characterization. Furthermore, the design of novel enzymes as well as molecular approaches such as enzyme evolution and metagenomic approaches can be used to identify and develop novel biocatalysts from uncultured bacteria—A treasure-trove of unknown proteins. Some characteristics of extremophiles are important for obtaining recombinants with specific properties. The advantage of psychrophile enzymes is their reduced energy consumption. Thus, psychrophiles could be used in the production of thermolabile proteins for genetic engineering [60].
Stabilization of hydrolytic enzymes is of interest due their potential applications in the medical, chemical and pharmaceutical industries. Methods to stabilize proteins include protein engineering or chemical modifications [122,123]. In the past two decades, protein engineering has become a powerful means to alter or improve enzymatic catalysis [124,125], and is divided into two methods: (1) directed evolution—A random mutagenesis is applied to a protein; and (2) rational protein design—Knowledge of the structure and function of the protein is necessary in order to change its characteristics. These methods have been successfully applied to increase protein activity, selectivity or thermostability [126]. The protein engineering technique is helping to improve the production of chemicals, such as optically pure tertiary alcohols and drugs, like the profen family (NSAIDs) [127,128]. Directed evolution has been reported to be laborious and costly, however it does provide the means for selecting mutants with improved properties [122,123]. Allying chemical modifications to recombinant DNA technology can generate improvements in enzyme stability and efficiency, preventing changes in the active-site and in targeting enzyme surface groups [129,130].
Mutations could be induced by physical and chemical methods such as UV-irradiation, γ-rays, fast neutron irradiation, nitrosoguanidine (NTG), diethyl sulfate and nitrous acid which have been applied to breed lipase-producing microorganisms. With these simple methods, the lipase yield can be improved by 1 to 10-fold; however, the low positive mutation rate, the long periodicity and the laborious screening has limited its widespread use [131].
Enhancing the stability of extremophilic enzymes is very beneficial because this could maintain the high activity at low/high physical-chemical parameters for prolonged periods of time, a characteristic that can be exploited in many industrial processes. However, basic issues involved in the stabilization mechanisms need to be analysed before any modifications can be made. These issues include the type and size of the protein, the structure and size of the modifying reagent, the chemical reactions involved in the modification procedures and the conditions of such modifications [122,123].
Siddiqui et al. [132] demonstrated that chemical modifications could be useful in providing details of structure, function and stability of proteins, turning them into potential guides for future target studies, including an attempt to convert mesophilic enzymes into cold-adapted ones. Tadeo et al. [84] demonstrated that it is possible to decrease the salt dependence of a halophilic protein to the level of a mesophilic one, and engineering the protein in an inverse form, from mesophilic to an obligate halophilic form, suggesting that the halophilicity is related exclusively to surface residues.
On the other hand, searching for enzymes that already exist in nature could be faster and more straightforward than the engineering routes. Metagenomic techniques could be a powerful tool for making such discoveries, by trying to find novel genes and enzymes from uncultured microorganisms [133]. Metagenomic analysis can be either sequence driven, for example, accessing the 16S rRNA, recA or other conserved sequences, or function driven, expressing features such as a specific enzyme activity or antibiotic production [134]. The 16S rRNA metagenomic studies provide an enormous diversity of free-living marine microorganisms from different environments and include the discovery of new enzymes of microbial origin [135]. A list of marine enzymes discovered by metagenomics can be found in Kennedy et al. [136].
Some advantages can be applied to functional metagenomics over the sequence methods. The functional approach recognizes the genes by their function, rather than their sequences, avoiding incorrect annotations or similar sequences of gene products with multiple functions. When you search for novel functions or a complete new class of genes, this could be crucial [136]. Currently platform databases, such as KEGG (Kyoto Encyclopedia of Genes and Genomes) and GenomeNet, are helping in the interpretation of a large amount of data generated by metagenomic output analyses. A large number of bioinformatic tools are now available for Data Mining of metagenomic sequences, based on a series of features [136,137]. Combining different approaches, like activity-based mining with tailoring of robust and selective enzymes, would give a biotechnological process a chance to take over from chemical catalysis, transforming industrial chemistry into a more eco-friendly process [138].
Currently all these techniques are powerful tools for biocatalysis. Miyake et al. [139] constructed a recombinant protein expression system using the psychrophilic bacterium Shewanella sp., isolated from Antarctic seawater. This bacterium grows at temperatures close to 0 °C. The enzyme produces β-lactamase reaching 91 mg/liter of culture at 4 °C and 139 mg/liter of culture at 18 °C. In another study, mesophilic enzymes were expressed in psychrophilic microorganisms for efficient 3-hydroxypropionaldehyde production from glycerol, using Shewanella livingstonensis Ac10 as a selected host [140]. A heat-sensitive esterase and two chaperones (Cpn60 and Cpn10) from a psychrophilic bacterium, Oleispira antarctica RB-8 T were simultaneously expressed in E. coli. The resulting enzyme from the recombinant strains was more active (180 fold) than the E. coli strain grown at 37 °C and was also active at low temperatures [141].
Other more suitable techniques can be applied in the search for novel enzymes from rare microorganisms, such as single cell genomics, metatranscriptomics and metaproteomics. The single cell genomics allow the study of the entire biochemical process of a single uncultured cell; the metatranscriptomics can access only the transcriptionally active genes in their specific population; and the metaproteomics can analyze the enzymes directly involved in a particular biochemical pathway [136].
Systems Biology is another integrated strategy used in industrial biotechnology. A quantitative analysis of biological systems is carried out using a mathematical model via computer simulation. Cellular metabolisms are analyzed and optimized for application in the development of strains and bioprocesses [121]. “Omics” technologies have been employed in studies such as: Transcriptome (genome-wide study of mRNA expression levels), proteome (analysis of structure and function of proteins and their interactions), metabolome (measurement of all metabolites to access the complete metabolic response to a stimulus), fluxome analysis (metabolic flux) and also in advanced mathematical modeling tools, such as genome-scale metabolic modeling [5,121].
Synthetic Biology is a field that has been growing in the last few years and is in many ways related to genetic engineering. It is an integrated and interdisciplinary theme including bioinformatics, microbiology, molecular biology, systems biology and biology in order to design and construct new biologic functions and systems not found in nature or improving certain functions through the creation of a synthetic genome [120].
4. Hydrolases and Their Biotechnological Potential
Currently more than 500 products are produced using enzymes and about 150 industrial processes benefit from the use of enzymes or catalysts from microorganisms. Moreover, more than 3000 enzymes are known and approximately 65% are hydrolases used in the detergent, textile, pulp, paper and starch industries and almost 25% of these are used for food processing [142,143]. Studies show that the diversity of extremophile microorganisms may be higher than we think [31,144]. However, the characterization and use of such a diversity of enzymes becomes complicated due to the difficulty of isolating and growing these microorganisms [142].
By definition, hydrolases are enzymes that catalyze reactions with the substrate through the hydrolysis of chemical bonds. Hydrolases are enzymes classified as Class 3 (EC 3) by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB) that keeps an updated list of all the enzymes described in a database, available at ExplorEnz [145].
5. Amylases from Marine Extremophiles
Starch comprises an abundant source of available energy and is present in almost all higher plants. Starch is a polymer composed of glucose molecules that form a so-called straight-chain amylose via the α 1–4 linked type. The association of α 1–4 with the α 1–6 type branches creates amylopectin, the largest part of the starch molecule [146].
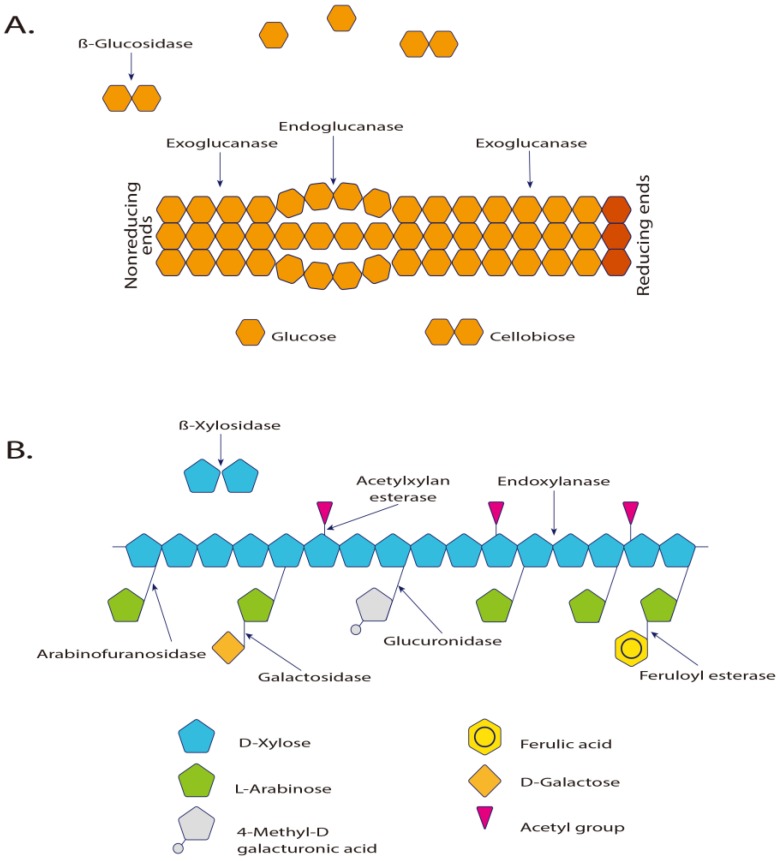
Amylases are enzymes, which hydrolyze the starch molecules to glucose monomers and can be classified according to the specificity of the substrate in which they operate, as showed in Table 2 [5,147]. Figure 2 illustrates the function of the three major amylolytic enzymes.
Table 2.
Classification of Amylases.
| Enzyme | Classification | Cleavage | Product | ||
|---|---|---|---|---|---|
| Amylases | Endoamylase | α-amylases | EC 3.2.1.1 | Internal α-1,4 | Dextrins |
| Exoamylases | β-amylase or maltase | EC 3.2.1.2 | Outer regions of α-1,4 | Maltose | |
| Glucoamylase | EC3.2.1.3 | β-cyclodextrin and glucose | |||
| α-glucosidase | EC 3.2.1.20 | ||||
| Debranching | Pullulanases | EC 3.2.1.41 | α-1,6 linkages | Maltotriose | |
| Isoamylases | EC 3.2.1.68 | Pullulan | Malto-oligosaccharides | ||
| Dextrinases | EC 3.2.1.142 | α-1,6 linkages | Maltose |
Figure 2.
The three major amylolytic enzymes [5].
The amylolytic enzymes are one of the most interesting enzymes for industrial processes. The α-amylase of different species of the genus Bacillus are the amylases that are the most applied in biotechnological processes, because of their thermophilic properties and high conversion rates [148]. The research for extremozymes and their special characteristics for industrial processes has been expanded [149], and over the years several stable amylases from thermophiles, psychrophiles, alkaliphiles, acidophiles and halophiles have been reported [150,151,152]. They are found in different genera of marine extremophile archaea and bacteria from the surface to deep sea locations, and include Desulfurococcus sp., Pseudoalteromonas sp., Pyrococcus sp., Rhodothermus sp. and Thermococcus sp. [153,154,155,156]. Geobacillus sp. is an isolate from a geothermal vent, and has a remarkable alpha-amylase stability between 80 °C to 140 °C [157].
Several industrial processes can be performed with the use of amylases. The endoamylases, which have optimum activity for temperatures between 70 °C and 95 °C have been applied in the production of ethanol from corn starch, rice or as a pretreatment for forming sugars (saccharification) for fermentation [158,159]. Amylases are also used in the food industry for the production of glucose syrups, fructose and maltose, reduced viscosity of syrups, reducing the turbidity of juices and also for alcohol fermentation. They are also involved in the textile, papermaking, detergent, chemical, pharmaceutical and petroleum industries [5,160,161].
Thermostable amylases are important for starch hydrolysis under high temperatures, accelerating the reactions and reducing possible contaminations [148]. Many companies sell different thermostable and broad-range pH operating enzymes. This is the case of Fuelzyme®—Verenium Corporation (San Diego, CA, USA), an alpha-amylase which was originated from Thermococcus sp. isolated from a deep-sea hydrothermal vent. Fuelzyme® is applied to mash liquefaction during ethanol production, releasing dextrins and oligosaccharides with better solubility and with low molecular weight. It operates in a pH range of 4.0–6.5 and temperatures above 110 °C [162]. However, Fuelzyme® and Spezyme® (DuPont-Genencor Science) are only used for biofuel production. The use of a blend of these commercially available amylases and others from Bacillus sp. has been suggested to make industrial starch processing more efficient, and suitable for downstream applications [163].
Pullulanases are commonly associated to glucoamylases for the saccharification of starch, and there is a growing demand in industry for them [164]. Marine sources have been naturally appointed as providers of such enzymes [165]. Thermostable pullulanases from type II (amylopullulanases) are being used in the process of starch liquefaction and saccharification combined [166,167]. The hyperthermophilic archaea Staphylothermus marinus, an isolate from the deep-sea associated with geothermal activity or hydrothermal vents, presents an optimum growth at 98 °C. A new thermostable amylopullulanase of the glycosyl hydrolase family from S. marinus, has recently been described with degradation activity towards pullulan and cyclodextrin at 105 °C [168]. One of the most thermostable and thermo-active pullulanases type II was described for Pyrococcus furiosus and Thermococcus litoralis, with activity ranging from 130 °C to 140 °C in the presence of 5 mM Ca++ [169].
On the other hand, cold adapted amylases also have their uses in the detergents, textile and food industries, due to their considerable energy savings and reduction of bacterial contamination [150]. The most studied cold active alpha-amylase originates from Alteromonas haloplanctis, which is synthesized at 0–2 °C and has been successfully expressed in Escherichia coli [170]. The alpha-amylase from Pseudoalteromonas haloplanktis (AHA) (former Alteromonas haloplanctis) showed 80% of initial activity at 4.5 M of NaCl at 10 °C [171]. Structural features in AHA have been studied through site-directed mutagenesis and chemical modification, including a modification performed that provides support for the importance of arginine residues, instead of lysine, which enhances the enzyme thermostability to cold adaptation; however, it decreases their activity [132]. Recently, Zunongwangia profunda was isolated from the deep-sea and presented a cold adapted and salt tolerant alpha-amylase, one of the very few alpha-amylases that can tolerate both cold and salt conditions [172]. An alpha-amylase was found in an isolate from the genus Bacillus on a marine salt farm, with a hyperthermostable enzyme acting at 110 °C as optimum operating temperature [173]. The Halomonas sp. strain AAD21 was found to produce a halo and thermostable alpha-amylase [174]. The Holoarcula sp. stain S-1, produced an extracellular organic solvent-tolerant alpha-amylase that was stable and active in benzene, toluene and chloroform, with a maximal activity at 50 °C in 4.3 M of NaCl and pH 7.0 [175]. Extreme halophiles and piezophiles are not common sources of amylases. Some other examples of extremophile producers of amylases are summarized in Table 3.
Table 3.
Extreme amylases from marine extremophiles.
| Microorganism | Domain | Natural Isolation Site | Metabolism | Enzyme | Type | Reference |
|---|---|---|---|---|---|---|
| Pyrococcus furiosus (recombinant) | Archaea | Thermal marine sediments | Hyperthermophile | Amylase Endoamylase | α-amylase | [176] |
| Fervidobacterium pennavorans V5 (recombinant) | Bacteria | Hot springs, Azores islands | Hyperthermophile | Amylase debranching | Pullulanase type I | [177] |
| Pseudoalteromonas haloplanktis | Bacteria | Antarctica | Psycrophille | Amylase Endoamylas | α-amylase | [171] |
| Halothermothrix orenii | Bacteria | Tunisian salt lake | Halophile/poliextremophile | Amylase Endoamylase | α amylase | [178] |
| Haloferax mediterranei | Archaea | Saltern, Spain | Extreme halophile | Amylase Endoamylase | a-amylase | [179] |
6. Cell Wall-Degrading Hydrolases from Marine Extremophiles
Along with starch, the cell wall is another element present in the structure of all plants and which also maintains an energy reserve, although rarely used and of difficult access. This element consists of three major polymers: Cellulose, hemicellulose and lignin. Cellulose is the most abundant macromolecule on Earth and one of the major constituents of plants, formed by β-1,4-linked glucose molecules. Due to its compactness and crystalline disposition in nature, cellulose is very resistant to hydrolysis and degradation. Hemicelluloses are non-cellulosic polysaccharides composed of complex carbohydrate polymers, where xylan and glucomannan are the main components. Lignin, with hemicellulose and pectin, fills the spaces between the cellulose fibers acting as a bonding material between the cell wall components [180].
Cellulases are complex hydrolases capable of degrading insoluble cellulose polymers, present in plants, fungi and bacteria. Cellulases are among the enzymes that are the most produced for industrial purposes and it is expected that within a few years, their production will increase further, due to their use in biofuel conversion [181]. Regarding the hydrolysis of xylan, a wide variety of enzymes become necessary, and they differ in their specificity and mechanism of action [5,182]. Table 4 summarizes the classification of cellulases and xylanases. Figure 3 illustrates the dynamics of polysaccharide catalysis.
Table 4.
Classification of cell wall-degrading hydrolases.
| Enzyme | Classification | Cleavage | Product | ||
|---|---|---|---|---|---|
| Cellulases | Endoglucanases | Endo-β-1,4-glucanase | EC 3.2.1.4 | Intramolecular bonds of β-1,4-glycosidic | New chain ends |
| Exoglucanases | β-glucosidase | EC 3.2.1.21 | Ends of the cellulose | Glucose or soluble cellulose | |
| Exo-β-1,4-glucan cellobiohydrolase | EC 3.2.1.91 | Glycosidic terminals | Cellobiose | ||
| Xylanases | β-1,4-endoxylanase | EC 3.2.1.8 | Internal glycosidic linkages along heteroxylan main skeleton | Polymerization degree of the substrate | |
| α-D-xylosidase | EC 3.2.1.177 | Small xylo-oligosaccharides and xylobiose | Xylose |
Figure 3.
(A) Enzymes involved in the hydrolysis of cellulose and (B) in xylan hydrolysis [183]. Exo-β-1,4-glucanase and cellobiohydrolase hydrolyze the glycosidic terminals releasing cellobiose units. The β-glucosidases act directly on cellulose, hydrolyzing it to glucose. The 1,4-β-glucosidase is essential to complete the hydrolysis process of the cellulose [5]. The α-d-xylosidases are exoglycosidases that act in the non-reducing end, hydrolyzing small xylo-oligosaccharides and xylobioses, releasing xylose.
Cellulolytic microorganisms have developed complex forms of cellulolytic systems which actively hydrolyze the cellulose fibrils, and are capable of producing cellulolytic enzymes with additional functions. Furthermore, they are organized in the form of multiprotein complexes such as cellulosomes and xylanossomas [180,183,184]. The use of different vegetables such as sugarcane, corn, beets, among others for the bioenergy industry generates residues that could be reused. Biotechnological research has been stimulated to develop technologies for second-generation ethanol production from plant biomass containing lignocellulose [184,185]. Extremophile microorganisms, especially thermophilic and alkaliphiles, are widely used in lignocellulolytic processes. The thermophilic and psychrophilic cellulases have also been used in different industrial processes in the food and fermented beverages, textile, pulp and paper and animal feed industries [47,186,187]. Glycoside hydrolases are commonly found in marine thermophile-microorganisms, like Pyrococcus sp., Thermococcus sp., and Thermotoga sp. [156,188,189].
Beta-glycosidase from the hyperthermophilic bacteria Thermotoga maritima, used in transglycosylation reactions, is thermostable and resistant to a large number of proteolytic denaturants found in nature and the presence of alcohol or organic compounds stimulates its activities [190]. Thermostable xylanases are being largely used in the paper bleaching industry [191]. Xylosidases and xylanases are expressed in Thermotoga neapolitana during the bio-production of hydrogen, using many different carbohydrates as feedstock [192]. Recombinant xylanase is being improved to achieve more extremophilic characteristics, like XynB from T. maritima, which has been successfully expressed in E. coli, exhibiting thermo and alkaline stability, an attractive characteristic for bleaching kraft pulp in the paper industry [193]. Aiming for crystalline cellulose hydrolysis, a variant designed from a beta-1,4-endoglucanase (EGPh) of P. horikoshii exhibited stronger activity than the wild type EGPh [194].
Psychrophiles are a source of these enzymes. The first cold-active and alkali-stable β-glucosidase was isolated from Martelella mediterranea. This enzyme retains 80% of its activity at pH 11.0 and 50% at 4 °C [195]. A deep-sea mud Exiguobacterium oxidotolerans also presents a cold active beta-glycosidase, maintaining 61% of its maximum activity at 10 °C and a pH range from 6.6 to 9.0 [196]. The Antarctic bacterium P. haloplanktis CelG gene was purified and expressed in E. coli for kinetic and structural optimization purposes [197]. Psychrophilic xylanases, like TAH3a from P. haloplanktis and MSY-2 from Flavobacterium sp. have also been used in the baking industry [198,199]. Ideal enzymes for treating milk should work well at 4–8 °C and pH 6.7–6.8, and this is still highly desired in industrial applications. Arthrobacter sp. is an Antarctic isolate that produces a β-d-galactosidase capable of working at 4–8 °C, and it retains 15% of its maximum activity at 0 °C [200].
The slightly halophilic bacterium Alteromonas macleodii has been isolated from the Pacific Ocean, Mediterranean Sea, English Channel, Black Sea and Gulf of Thailand. The activities for beta-d-galactosidase, alpha-d-galactosidase and beta-d-glucosidase were found using a sample isolated from the Black Sea [201].
7. Marine Extremophilic Proteases
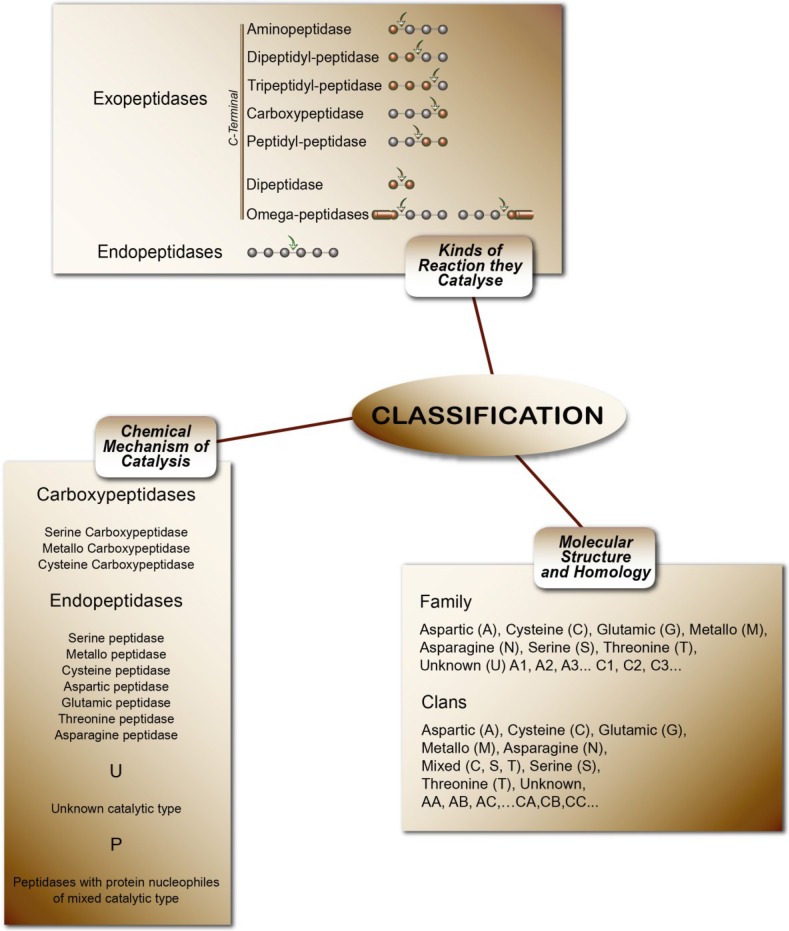
Peptidases or proteases are proteolytic enzymes that catalyze the hydrolysis of peptide bonds on proteins or peptides. Peptidases are classified in subclass 3.4 (peptide-hydrolases). The rating uses three criteria: I—Chemical mechanism of catalysis. In this system, peptidases are classified according to their catalytic type in serine, cysteine, threonine, aspartic, glutamic, asparagine, metallo and unknown catalytic (S, C, T, A, G, N, M, and U, respectively) and P for peptidases with protein nucleophiles of mixed catalytic types. II—Type of reaction they catalyze. These are subdivided into exo-peptidases (EC 3.4.11-19), when enzymes cleave terminal amino acids, and endo-peptidases (EC 3.4.21-99), when they hydrolyze peptide bonds in the middle of the polypeptide chain. III—Molecular structure and homology. This is the most modern classification concept, based on amino acid sequences and three-dimensional structures. The peptidases are classified in families and clans. In the families, the classification of peptidases is grouped by amino acid sequence comparisons: 251 families of peptidases can be found in MEROPS Release 9. Currently the number of peptidases described and indexed in the MEROPS database exceeds more than 4000 enzymes [202]. Figure 4 summarizes the classification of peptidases.
Figure 4.
The classifications of peptidases [5].
Peptidases are enrolled in different biological processes such as regulation, localization, modulation and activities of protein interactions [203]. An important function of peptidases, is to perform posttranslational processing events, leading to the activation or inactivation of proteins, including enzymes [204]. Peptidases are also a potential drug target for microbial diseases, taking part in pathogenesis, inactivating the host immune defense mediators, in the processing of host or parasite proteins and in the digestion of host proteins. These features make peptidases very valuable to the pharmaceutical industries [5]. Other innumerous applications of peptidases are in the detergent, cosmetic, chemical and food industries [5,142].
Most peptidases from extremophilic archaea belong to the serine peptidases family, although, other families are also represented. Hyperthermophilic peptidases include serine peptidases, cysteine peptidases and the threonine-dependent proteasomes. Pyrococus sp. are archaeon hyperthermophiles, strictly anaerobes and obligate heterotrophs and are found in diverse habitats such as thermal marine sediments and shallow hydrothermal vents [205]. P. furiosus was isolated from deep-sea vents and volcanic marine mud of Italy [206]. An intracellular peptidase from P. horikoshii is an oligomeric cysteine peptidase [15] similar to the P. furiosus intracellular peptidase I [207]. In this genus, pyrolysin, a serine peptidase, metalopeptidases and ATP-dependent peptidases such as Lon A and subunits of proteasome have been characterized [208]. Thermococcus litoralis is a hyperthermophile archaea that is found around deep-sea hydrothermal vents as well as near shallow submarine thermal springs and oil wells. A proline dipeptidase named prolidase cleaves dipeptides having proline at the C-terminus and a nonpolar residue (Met, Leu, Val, Phe, Ala) at the amino terminus [209].
Psychrophiles are a source of peptidases and other hydrolases. The potential of the enzymes produced by them has been reviewed [65,210,211]. The diversity of extremophile microorganisms is a valuable resource in the search for new proteolytic enzymes, mainly those active at low temperatures derived from psychrophiles [69,186,212]. S. livingstonensis is a psychrophilic, gram-negative, isolate from Antarctic coastal areas that produces a serine alkaline peptidase [213]. Colwellia psychrerythraea is an obligate psychrophile, Gram-negative bacteria that can be found in cold marine environments including in Arctic and Antarctic sea-ice. The bacteria produce a peptidase of the family M1 aminopeptidase. The enzyme with a molecular mass of 71 kDa displayed an optimum temperature at 19 °C [214].
Natrialba magadii is an extremophile archaea that lives in alkaline hypersaline conditions (pH 9.5, 3.5 M NaCl). A Lon peptidase from this halophile was cloned and sequenced by Sastre et al. [215]. The ATP-dependent Lon peptidase is universally distributed in bacteria, eukaryotic organelles and archaea. In this species, like other archaea, there is a Lon B type serine peptidase, with a molecular mass of 85 kDa. Haloferax volcanii, a haloarchaeon isolated from the Dead Sea, also presents a Lon Peptidase. In a study by Cerletti et al. [216] of this species, the group demonstrated that a suboptimal cellular level of the Lon protein affected its growth rate, cell shape, cell pigmentation, lipid composition and sensitivity to various antibiotics. An extracellular serine peptidase with 45 kDa was characterized in the same species by Gimenez et al. [217]. Natrialba asiatica, which was isolated from a beach in Japan, and H. mediterranei isolated from seawater evaporation ponds near Alicante, Spain produced a halolysin, a serine peptidase [218]. Other extracellular and intracellular peptidase classes including metalopeptidases, and 20S proteasomes/threonine peptidases are also found in the group (for review see De Castro et al. [219]). Table 5 summarizes other peptidases found in marine extremophiles.
Table 5.
Peptidases from marine extremophiles.
| Peptidase | Extremophile | Habitats | Reference |
|---|---|---|---|
| Serine peptidase | Fervidobacterium pennavorans | Hot spring | [230] |
| Sulfolobus solfataricus | Volcanic hot spring | [231] | |
| Thermococcus litoralis | Deep-sea hydrothermal vent Shallow submarine thermal springs and oil wells | [232] | |
| Thermoanaerobacter yonseiensis | Geothermal hot stream at Sileri on Java island, Indonesia | [34,233] | |
| Cysteine peptidase | Aciduliprofundum boonei | Marine hydrothermal vent | [234] |
| Haloferax volcanii | Dead Sea, the Great Salt Lake, and oceanic environments with high NaCl | [235] | |
| Metallocarboxypeptidase | Caldithrix abyssi | Deep-sea hydrothermal chimneys | [234] |
Halophilic microorganisms present some advantages in fermentation processes that occur in the presence of salt. The high salt tolerance of extreme halophiles enables their cultivation under non-sterile and thus cost-reducing conditions [220]. Some peptidases such as bromelain, papain, and pepsin have been used as biocatalysts of protein hydrolysis in fish sauce fermentation. These peptidases are not stable in high salt concentration. In contrast, peptidases from extreme halophiles require salt to carry out their activities. In addition, halophilic peptidases have a wide application in processing activities for the food, leather and detergent industries [220,221].
The Deinococcus genus has been isolated from a variety of habitats including Antarctica, water and activated sludge, hot springs and aquifers [222,223,224]. The fresh water radioactive site isolate, Deinococcus aquiradiocola, proved to be a proteolytic, capable of hydrolyzing gelatin and casein [225]. Deinococcus indicus, an arsenic-resistant bacterium isolated from an aquifer, has been positively characterized for casein and gelatin hydrolysis [226]. Some species have been isolated from marine habitats such as Deinococcus geothermalis from deep-ocean subsurfaces [227,228]. Pietrow et al. [229] described the production of the thermo-alkali-stable peptidase from D. geothermalis. The peptidase presented stability at 60 °C and pH 9.0; these properties are appropriate for the detergent industry.
8. Lipases and Esterases from Marine Extremophiles
Lipases and esterases are hydrolases that catalyze the cleavage and formation of ester bonds (EC 3.1) and belong to Carboxylic Ester Hydrolases (EC 3.1.1) (Table 6). Both types of enzymes belong to the family of serine hydrolases and share structural and functional characteristics, such as an α/β-hydrolase fold in their core structure, and have a characteristic catalytic triad (serine, aspartic/glutamic and histidine). Lipases are mainly active against water-insoluble substrates, such as triglycerides composed of long-chain fatty acids (C ≥ 8). Esterases preferentially hydrolyze “simple” esters and usually only triglycerides composed of fatty acids shorter than C 8. Based on amino acid sequences, esterases and lipases have been grouped into eight families. Enzymes in Family 1 are called true lipases and are further classified into six subfamilies. Enzymes belonging to Families 2–8 are esterases. The genome analysis of bacteria has shown putative lipases/esterases genes that are not included in any family [236,237].
Table 6.
Lipases and esterases according the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB) [145].
| Lipases | Systematic Name | E.C. | Reaction Catalysed |
|---|---|---|---|
| Carboxylesterase (other names: Esterases, serina esterases, etc.) | Carboxylesterase | 3.1.1.1 | carboxylic ester + H2O = alcohol + carboxylate |
| Triacylglycerol lipase (other names: Lipase; triglyceride lipase, etc.) | Triacylglycerol acylhydrolase | 3.1.1.3 | triacylglycerol + H2O = diacylglycerol + carboxylate |
The biotechnological applications of lipases have a broad spectrum of use including in the food, dairy, pharmaceutical, cosmetic, agrochemical, biosurfactant, detergent and paper industries [238,239,240,241]. Biodiesel production has been one of the major sectors stimulating the search for lipases [242,243]. Biodiesel is composed of methyl-esterified fatty acids derived from transesterification of triglycerides by enzymatic action, providing a number of advantages such as a reduction of the operational process in the manufacture and separation of glycerol by-products. For the environment, the advantages are countless, such as the reduction of particles emissions, low toxicity and high biodegradability [5,244]. The genus Pseudomonas is the most exploited for lipases [245]. The industrial use of lipases and esterases from extremophile microorganisms has been increasing steadily in recent years [246], mainly with the use of thermostable enzymes from thermophiles and psychrophiles [247,248,249].
The Thermococcus genus has been isolated from black smokers (hydrothermal vents) or freshwater springs, with 1%–3% salt (NaCl) concentration, other marine hydrothermal areas and oil reservoirs. Thermococcus sibiricus is a hyperthermophile from a high-temperature Samotlor oil reservoir (Western Siberia). Genomic analysis revealed that T. sibiricus contains 15 genes that encode for lipases/esterases. Four of these putative enzymes contain signal peptides suggesting that they are extracellular enzymes [250].
A thermostable esterase gene from the aquatic hyperthermophilic Archaeoglobus fulgidus DSM 4304 was cloned in E. coli. The esterase was tested with various acyl chains of ρ-nitrophenol and the highest activity was found for ρ-nitrophenyl butyrate at 80 °C [251]. The archaeon Pyrobaculum calidifontis VA1, also a hyperthermophilic isolated from a hot spring in the Philippines, presented a thermostable carboxylesterase, with an optimum operation at 90 °C, and organic tolerance, supporting concentrations up to 80% [252]. Other thermostable esterases have been described in S. solfataricus P1. Arylesterase, the least understood among the esterases, showed high stability against denaturing agents at an optimum temperature and pH of 94 °C and 7.0, respectively [253]. Esterases have been purified and studied from the extremely Sulfolobus acidocaldarius [254] and Sulfolobus shibatae [255]. A thermostable esterase gene from P. furiosus has been cloned in E. coli, purified and studied [256].
Lipases from psychrophile microorganisms are some of the most widely used classes of enzymes in biotechnology applications, organic chemistry, detergent industry, for bioremediation purposes and in the food industry [5,12,240]. A cold-adapted lipase (M37) from Photobacterium lipolyticum, previously isolated from an intertidal flat of the Yellow Sea in Korea, maintains activity between 5 °C to 25 °C, and was expressed in E. coli at 18 °C [257]. The lipase M37 has been tested as an alternative to C. antarctica lipase B (Novozym435®—Novozymes Corporation (Bagsvaerd, Denmark), in the production of biodiesel. Novozym435 is unstable in a medium containing high concentrations of methanol, required for efficient biodiesel production [258,259].
C. antarctica was discovered to be a lipase producer in the 1980’s and it was found that two types of lipases (A and B) were being produced. Since then, innumerous applications have been described, such as the production of non-steroidal anti-inflammatory drugs (NSAIDs), biofuel and organic compounds [260,261,262]. Lipase B is commonly used for desymmetrization, ester synthesis and production of peracids [130,263]. Due to such abilities, a great effort has been made to improve the stability of lipase B through chemical modifications and directed evolution [130,261,264].
Several Antarctic isolates from Terra Nova Bay, including Pseudoalteromonas sp., Psychrobacter sp. and Vibrio sp. have exhibited cold adapted lipases and esterases. The enzymes of six of them work better at a 4 to 15 °C range, with pH changing from 5 to 9.0 and the NaCl concentrations between 1% and 7.0% [265]. Until now, only a few esterases from psychrophilic microorganisms have been cloned and characterized [156]. The cold-adapted esterase from Pseudoalteromonas arctica shows optimum activity at 25 °C, but retains more than 50% of its activity at 0 °C [266]. In addition, after the complete genome of P. haloplanktis was sequenced, a new cold-active lipase (Lip1) that configures a new lipolytic family was found [237]. Other psychrophilic cold-adapted lipases and esterases are summarized in Table 7.
Table 7.
Lipases and esterases from psychrophiles.
| Prokaryote | Habitat | Lipase/Esterase | Reference |
|---|---|---|---|
| Desulfotalea psychrophila (sulfate-reducing bacteria) | Marine sediments that are permanently cold | Esterase | [267] |
| Pseudoalteromonas haloplanktis | Marine Antarctic | Lipase | [237] |
| Psychrobacter sp. wp37 | Deep-sea sediments | Lipase | [268] |
| Colwellia psychrerythraea 34H (recombinant in E. coli) | Cold marine environments including Arctic and Antarctic sea ice | Lipase | [269] |
One halotolerant strain identified as Salinivibrio sp. strain SA-2 was isolated from a hypersaline brackish water with 14% salinity (Garmsar-Iran) and presented a thermostable lipase, retaining 90% of its activity at 80 °C in a pH range of 7.5–8 for 30 min, tolerating a range of 0 to 3.0 M NaCl concentrations [270]. The Haloarcula marismortui is an extreme halophilic archaeon isolated from the Dead Sea, a hypersaline lake, producing different enzymes such as alkaline phosphatase, lipases, alcohol dehydrogenases and other bio-products [271]. An esterase from H. marismortui was expressed in E. coli and has been characterized as a salt-dependent esterase, with maximum activity at 3.0 M of KCl and no activity in the absence of this salt [272]. A lipase was isolated from Natronococcus sp., an extremely halophilic archaeon [273]. According to Ferrer et al. [11], five esterase genes were found in the Urania deep-sea hypersaline anoxic basins (Eastern Mediterranean), through a screening in the metagenome expression library. After expression in E. coli at least two were able to function in an extreme condition, with high-pressure and saline affinity, and one possesses an adaptive structure, showing halotolerance and conferring a wide range of catalytic activities. These esterases may possibly take part in the production of intermediate pharmaceuticals, synthesizing optically pure biological active substances.
9. Conclusions and Future Perspectives
The study of marine extremophiles has been significantly reinforced using the modern techniques of bioengineering and molecular biology. Such interactions have favored a better understanding of these microorganisms and their biotechnological applications. The evolutionary adaptation to extreme conditions has facilitated the selection of a more tolerant metabolism, of enzymes and other products with special features not found in any other prokaryotes. Good examples are hydrolases with the ability to operate in temperatures as low as those of the microorganisms isolated from Antarctica to those active at high temperatures such as the hyperthermophilic microorganisms isolated from thermal fractures; enzymes that act in high concentrations of salts found in anoxic basin environments, or high pressure, are found in several polyextremophiles. These properties make these unique microorganisms a source of genes that may be mapped by bio-prospecting metagenomic studies and used for cloning and expression in other organisms to obtain enzymes with properties suitable for industrial bioprocesses. However, although the studies related to the marine extremophiles cited here have increased greatly over the past decade, there are still other extremozymes in promising habitats such as deep sea floors, and also in the Red Sea that have so far attracted few studies and are open for exploration. Marine extremophiles are a group of prokaryotic organisms that are being intensively studied worldwide. Although major advances have been made recently, our knowledge of the physiology, metabolism, enzymology and genetics of this fascinating group of microorganisms is still limited. Therefore, an important growth is expected to take place in this sector, pursuing a better understanding of the application of these hydrolases from marine extremophiles.
Acknowledgments
The authors wish to acknowledge the Brazilian National Council for Scientific and Technological Development (MCT-CNPq), Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ) and Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support. This manuscript was reviewed by a professional science editor and by a native English-speaking copy editor to improve readability.
Author Contributions
The authors contributed equally to this work.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Bull A.T., Ward A.C., Goodfellow M. Search and discovery strategies for biotechnology: The paradigm shift. Microbiol. Mol. Biol. Rev. 2000;64:573–606. doi: 10.1128/MMBR.64.3.573-606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nath I.V.A., Bharathi P.A.L. Diversity in transcripts and translational pattern of stress proteins in marine extremophiles. Extremophiles. 2011;15:129–153. doi: 10.1007/s00792-010-0348-x. [DOI] [PubMed] [Google Scholar]
- 3.BCC Research . Global Markets for Enzymes in Industrial Applications. BCC Research; Wellesley, MA, USA: 2014. p. 146. BIO030H. [Google Scholar]
- 4.Díaz-Tenaa E., Rodríguez-Ezquerroa A., Marcaidea L.N.L.L., Bustinduyb L.G., Sáenzb A.E. Use of Extremophiles Microorganisms for Metal Removal. Procedia Eng. 2013;63:67–74. doi: 10.1016/j.proeng.2013.08.197. [DOI] [Google Scholar]
- 5.Vermelho A.B., Noronha E.F., Filho E.X., Ferrara M.A., Bon E.P.S. Diversity and biotechnological applications of prokaryotic enzymes. In: Rosenberg E., DeeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. The Prokaryotes. Springer-Verlag Heidelberg; Berlin, Germany: 2013. pp. 213–240. [Google Scholar]
- 6.Chandrasekaran M., Kumar S.R. Marine microbial enzymes. In: Werner H., Roken S., editors. Biotechnology. Volume 9. EOLSS; Paris, France: 2010. pp. 47–79. [Google Scholar]
- 7.Fulzele R., Desa E., Yadav A., Shouche Y., Bhadekar R. Characterization of novel extracellular protease produced by marine bacterial isolate from the Indian Ocean. Braz. J. Microbiol. 2011;42:1364–1373. doi: 10.1590/S1517-83822011000400018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuel P., Raja A., Prabakaran P. Investigation and application of marine derived microbial enzymes: Status and prospects. Int. J. Ocean. Mar. Ecol. Syst. 2012;1:1–10. [Google Scholar]
- 9.Russo R., Giordano D., Riccio A., di Prisco G., Verde C. Cold-adapted bacteria and the globin case study in the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125. Mar. Genomics. 2010;3:125–131. doi: 10.1016/j.margen.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Trincone A. Potential biocatalysts originating from sea environments. J. Mol. Catal. B-Enzym. 2010;66:241–256. doi: 10.1016/j.molcatb.2010.06.004. [DOI] [Google Scholar]
- 11.Ferrer M., Golyshina O.V., Chernikova T.N., Khachane A.N., Martins Dos Santos V.A., Yakimov M.M., Timmis K.N., Golyshin P.N. Microbial enzymes mined from the Urania deep-sea hypersaline anoxic basin. Chem. Biol. 2005;12:895–904. doi: 10.1016/j.chembiol.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Joseph B., Ramteke P.W., Thomas G. Cold active microbial lipases: Some hot issues and recent developments. Biotechnol. Adv. 2008;26:457–470. doi: 10.1016/j.biotechadv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Hicks P.M., Rinker K.D., Baker J.R., Kelly R.M. Homomultimeric protease in the hyperthermophilic bacterium Thermotoga maritima has structural and amino acid sequence homology to bacteriocins in mesophilic bacteria. FEBS Lett. 1998;440:393–398. doi: 10.1016/S0014-5793(98)01451-3. [DOI] [PubMed] [Google Scholar]
- 14.Valdes-Stauber N., Scherer S. Nucleotide sequence and taxonomical distribution of the bacteriocin gene lin cloned from Brevibacterium linens M18. Appl. Environ. Microbiol. 1996;62:1283–1286. doi: 10.1128/aem.62.4.1283-1286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhan D., Sun J., Feng Y., Han W. Theoretical study on the allosteric regulation of an oligomeric protease from Pyrococcus horikoshii by Cl-Ion. Molecules. 2014;19:1828–1842. doi: 10.3390/molecules19021828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J., Dordick J.S. Unusual salt and solvent dependence of a protease from an extreme halophile. Biotechnol. Bioeng. 1997;55:471–479. doi: 10.1002/(SICI)1097-0290(19970805)55:3<471::AID-BIT2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Horikoshi K., Bull A.T. Prologue: Definition, categories, distribution, origin and evolution, pioneering studies, and emerging fields of extremophiles. In: Horikoshi K., Antranikaian G., Bull A.T., Robb F.T., Stetter K.O., editors. Extremophiles Handbook. Springer; Tokyo, Japan: 2011. pp. 4–15. [Google Scholar]
- 18.Stan-Latter H. Physico-chemical boundaries of life. In: Stan-Latter H., Fendrihan S., editors. Adaption of Microbial Life to Environmental Extremes. Springer-Verlag; New York, NY, USA: 2012. pp. 1–14. [Google Scholar]
- 19.Horikoshi K., Antranikaian G., Bull A.T., Robb F.T., Stetter K.O. Extremophiles Handbook. Springer; Tokyo, Japan: 2011. p. 1247. [Google Scholar]
- 20.Seckbach J., Oren A., Stan-Latter H. Polyextremophiles: Life Under Multiple Forms of Stress. Springer; Dordrecht, The Netherlands: 2013. p. 634. [Google Scholar]
- 21.Arora R., Bell E.M. Biotechnological applications of extremophiles: Promise and prospects. In: Bell E.M., editor. Life at Extremes: Environments, Organisms and Strategies for Survival. CAB International; Dunbeg, UK: 2012. pp. 498–521. [Google Scholar]
- 22.Gabani P., Singh O.V. Radiation-resistant extremophiles and their potential in biotechnology and therapeutics. Appl. Microbiol. Biotechnol. 2013;97:993–1004. doi: 10.1007/s00253-012-4642-7. [DOI] [PubMed] [Google Scholar]
- 23.Karan R., Capes M.D., Dassarma S. Function and biotechnology of extremophilic enzymes in low water activity. Aquat. Biosyst. 2012;8:4. doi: 10.1186/2046-9063-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morozkina E.V., Slutskaia E.S., Fedorova T.V., Tugai T.I., Golubeva L.I., Koroleva O.V. Extremophilic microorganisms: Biochemical adaptation and biotechnological application (review) Prikl. Biokhim. Mikrobiol. 2010;46:5–20. [PubMed] [Google Scholar]
- 25.Singh O.V. Extremophiles: Sustainable Resources and Biotechnological Implications. Wiley-Blackwell; Hoboken, NJ, USA: 2012. p. 456. [Google Scholar]
- 26.Gelfand D.H., Stoffel S., Lawyer F.C., Saiki R.K. Purified Thermostable Enzyme. US 4,889,818. 1989 Dec 26;
- 27.Rampelotto P.H. Biotechnological applications of extremophiles. Curr. Biotechnol. 2013;2:273–274. doi: 10.2174/221155010204131218105352. [DOI] [Google Scholar]
- 28.Woese C.R., Kandler O., Wheelis M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang J.M., Darling A.E., Eisen J.A. Phylogeny of bacterial and archaeal genomes using conserved genes: Supertrees and supermatrices. PLoS ONE. 2013;8:e62510. doi: 10.1371/journal.pone.0062510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pikuta E.V., Hoover R.B., Tang J. Microbial extremophiles at the limits of life. Crit. Rev. Microbiol. 2007;33:183–209. doi: 10.1080/10408410701451948. [DOI] [PubMed] [Google Scholar]
- 32.Rothschild L.J., Mancinelli R.L. Life in extreme environments. Nature. 2001;409:1092–1101. doi: 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- 33.Stetter K.O. History of discovery of hyperthermophiles. In: Horikoshi K., Antranikaian G., Bull A.T., Robb F.T., Stetter K.O., editors. Extremophiles Handbook. Springer; Tokyo, Japan: 2011. pp. 404–426. [Google Scholar]
- 34.Canganella F., Wiegel J. Anaerobic thermophiles. Life. 2014;4:77–104. doi: 10.3390/life4010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takai K., Nakamura K., Toki T., Tsunogai U., Miyazaki M., Miyazaki J., Hirayama H., Nakagawa S., Nunoura T., Horikoshi K. Cell proliferation at 122 degrees C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc. Natl. Acad. Sci. USA. 2008;105:10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fushida S., Mizuno Y., Masuda H., Toki T., Makita H. Concentrations and distributions of amino acids in black and white smoker fluids at temperatures over 200 °C. Org. Geochem. 2014;66:98–106. doi: 10.1016/j.orggeochem.2013.11.008. [DOI] [Google Scholar]
- 37.White R.H. Hydrolytic stability of biomolecules at high temperatures and its implication for life at 250 degrees C. Nature. 1984;310:430–432. doi: 10.1038/310430a0. [DOI] [PubMed] [Google Scholar]
- 38.Colletier J.P., Aleksandrov A., Coquelle N., Mraihi S., Mendoza-Barbera E., Field M., Madern D. Sampling the conformational energy landscape of a hyperthermophilic protein by engineering key substitutions. Mol. Biol. Evol. 2012;29:1683–1694. doi: 10.1093/molbev/mss015. [DOI] [PubMed] [Google Scholar]
- 39.Tehei M., Madern D., Franzetti B., Zaccai G. Neutron scattering reveals the dynamic basis of protein adaptation to extreme temperature. J. Biol. Chem. 2005;280:40974–40979. doi: 10.1074/jbc.M508417200. [DOI] [PubMed] [Google Scholar]
- 40.Aung H.L., Samaranayaka C.U., Enright R., Beggs K.T., Monk B.C. Characterisation of the DNA gyrase from the thermophilic eubacterium Thermus thermophilus. Protein. Expr. Purif. 2015;107:62–67. doi: 10.1016/j.pep.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Hidalgo A., Berenguer J. Biotechnological applications of Thermus thermophilus as host. Curr. Biotechnol. 2013;2:304–312. doi: 10.2174/18722083113076660030. [DOI] [Google Scholar]
- 42.Sakaff M.K.L.M., Rahman A.Y.A., Saito J.A., Hou S.B., Alam M. Complete genome sequence of the thermophilic bacterium Geobacillus thermoleovorans CCB_US3_UF5. J. Bacteriol. 2012;194:1239–1239. doi: 10.1128/JB.06580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurst L.D., Merchant A.R. High guanine-cytosine content is not an adaptation to high temperature: A comparative analysis amongst prokaryotes. Proc. Biol. Sci. 2001;268:493–497. doi: 10.1098/rspb.2000.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed C.J., Lewis H., Trejo E., Winston V., Evilia C. Protein adaptations in archaeal extremophiles. Archaea. 2013;2013:373275. doi: 10.1155/2013/373275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koga Y. Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea. 2012;2012:789652. doi: 10.1155/2012/789652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liszka M.J., Clark M.E., Schneider E., Clark D.S. Nature versus nurture: Developing enzymes that function under extreme conditions. Annu. Rev. Chem. Biomol. Eng. 2012;3:77–102. doi: 10.1146/annurev-chembioeng-061010-114239. [DOI] [PubMed] [Google Scholar]
- 47.Klippel B., Antranikian G. Lignocellulose converting enzymes from thermophiles. In: Horikoshi K., Antranikaian G., Bull A.T., Robb F.T., Stetter K.O., editors. Extremophiles Handbook. Springer; Tokyo, Japan: 2011. pp. 444–474. [Google Scholar]
- 48.Turner P., Mamo G., Karlsson E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell. Fact. 2007;6:9. doi: 10.1186/1475-2859-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeoman C.J., Han Y., Dodd D., Schroeder C.M., Mackie R.I., Cann I.K. Thermostable enzymes as biocatalysts in the biofuel industry. Adv. Appl. Microbiol. 2010;70:1–55. doi: 10.1016/S0065-2164(10)70001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Junge K., Christner B., Staley J.T. Diversity of psychrophilic bacteria from sea ice—and glacial ice communities. In: Horikoshi K., Antranikaian G., Bull A.T., Robb F.T., Stetter K.O., editors. Extremophiles Handbook. Springer; Tokyo, Japan: 2011. pp. 794–815. [Google Scholar]
- 51.Cavicchioli R. Cold-adapted archaea. Nat. Rev. Microbiol. 2006;4:331–343. doi: 10.1038/nrmicro1390. [DOI] [PubMed] [Google Scholar]
- 52.Margesin R., Miteva V. Diversity and ecology of psychrophilic microorganisms. Res. Microbiol. 2011;162:346–361. doi: 10.1016/j.resmic.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Bakermans C., Bergholz P.W., Rodrigues D., Vishnevetskaya T.A., Ayala-del-Río H.L., Tiedje J. Genomic and expression analyses of cold-adapted microorganisms. In: Miller R.V., Whyte L.G., editors. Polar Microbiology: Life in a Deep Freeze. ASM Press; Washington, DC, USA: 2011. pp. 126–155. [Google Scholar]
- 54.Mykytczuk N.C., Foote S.J., Omelon C.R., Southam G., Greer C.W., Whyte L.G. Bacterial growth at −15 °C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 2013;7:1211–1226. doi: 10.1038/ismej.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurosawa N., Sato S., Kawarabayasi Y., Imura S., Naganuma T. Archaeal and bacterial community structures in the anoxic sediment of Antarctic meromictic lake Nurume-Ike. Polar Sci. 2010;4:421–429. doi: 10.1016/j.polar.2010.04.002. [DOI] [Google Scholar]
- 56.Siddiqui K.S., Williams T.J., Wilkins D., Yau S., Allen M.A., Brown M.V., Lauro F.M., Cavicchioli R. Psychrophiles. Annu. Rev. Earth Planet. Sci. 2013;41:87–115. doi: 10.1146/annurev-earth-040610-133514. [DOI] [Google Scholar]
- 57.Jones P.G., Inouye M. The cold-shock response--a hot topic. Mol. Microbiol. 1994;11:811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 58.Lim J., Thomas T., Cavicchioli R. Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtonii. J. Mol. Biol. 2000;297:553–567. doi: 10.1006/jmbi.2000.3585. [DOI] [PubMed] [Google Scholar]
- 59.Noon K.R., Guymon R., Crain P.F., McCloskey J.A., Thomm M., Lim J., Cavicchioli R. Influence of temperature on tRNA modification in Archaea: Methanococcoides burtonii (optimum growth temperature [Topt], 23 °C) and Stetteria hydrogenophila (Topt, 95 °C) J. Bacteriol. 2003;185:5483–5490. doi: 10.1128/JB.185.18.5483-5490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casanueva A., Tuffin M., Cary C., Cowan D.A. Molecular adaptations to psychrophily: The impact of “omic” technologies. Trends Microbiol. 2010;18:374–381. doi: 10.1016/j.tim.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 61.De Maayer P., Anderson D., Cary C., Cowan D.A. Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep. 2014;15:508–517. doi: 10.1002/embr.201338170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorv J.S., Rose D.R., Glick B.R. Bacterial ice crystal controlling proteins. Scientifica. 2014;2014:976895. doi: 10.1155/2014/976895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madigan M.T., Martinko J.M., Dunlap P.V., Clarck D.P. Brock Biology of Microorganisms. 14th ed. Benjamin Cummings; San Francisco, NC, USA: 2014. p. 1136. [Google Scholar]
- 64.Deming J.W. Extremophiles: Cold environments. In: Schaechter M., editor. The Desk Encyclopedia of Microbiology. Academic Press; Oxford, UK: 2009. pp. 147–157. [Google Scholar]
- 65.Joshi S., Satyanarayana T. Biotechnology of cold-active proteases. Biology. 2013;2:755–783. doi: 10.3390/biology2020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siddiqui K.S., Cavicchioli R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006;75:403–433. doi: 10.1146/annurev.biochem.75.103004.142723. [DOI] [PubMed] [Google Scholar]
- 67.Fendriham S., Negoiţă T.G. Psychrophilic microorganisms as important source for biotechnological processes. In: Stan-Latter H., Fendrihan S., editors. Adaption of Microbial Life to Environmental Extremes. Springer-Verlag; New York, NY, USA: 2012. pp. 133–172. [Google Scholar]
- 68.Huston A.L. Biotechnological aspects of cold adapted enzymes. In: Margesin R., Schinner F., Marx J.-C., Gerday C., editors. Psychrophiles: From Biodiversity to Biotechnology. Springer; Heidelberg-Berlin, Germany: 2008. pp. 347–364. [Google Scholar]
- 69.Feller G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica. 2013;2013:512840. doi: 10.1155/2013/512840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson R.B., Carpenter S.R., Dahm C.N., McKnight D.M., Naiman R.J., Postel S.L., Running S.W. Water in a changing world. Ecol. Appl. 2001;11:1027–1045. doi: 10.1890/1051-0761(2001)011[1027:WIACW]2.0.CO;2. [DOI] [Google Scholar]
- 71.Siglioccolo A., Paiardini A., Piscitelli M., Pascarella S. Structural adaptation of extreme halophilic proteins through decrease of conserved hydrophobic contact surface. BMC Struct. Biol. 2011;11:50. doi: 10.1186/1472-6807-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Javor B. Hypersaline Environments. Springer Heidelberg; Berlin, Germany: 1989. Deep sea hypersaline basins; pp. 176–188. [Google Scholar]
- 73.Van der Wielen P.W., Bolhuis H., Borin S., Daffonchio D., Corselli C., Giuliano L., D’Auria G., de Lange G.J., Huebner A., Varnavas S.P., et al. The enigma of prokaryotic life in deep hypersaline anoxic basins. Science. 2005;307:121–123. doi: 10.1126/science.1103569. [DOI] [PubMed] [Google Scholar]
- 74.Yakimov M., La Cono V., Ferrer M., Golyshin P., Giuliano L. Metagenomics of deep hypersaline anoxic basins. In: Nelson K.E., editor. Encyclopedia of Metagenomics. Springer; New York, NY, USA: 2014. pp. 1–9. [Google Scholar]
- 75.Mapelli F., Borin S., Daffonchio D. Microbial diversity in deep hypersaline anoxic basins. In: Stan-Lotter H., Fendrihan S., editors. Adaption of Microbial Life to Environmental Extremes. Springer; Vienna, Austria: 2012. pp. 21–36. [Google Scholar]
- 76.Hallsworth J.E., Yakimov M.M., Golyshin P.N., Gillion J.L., D’Auria G., de Lima Alves F., La Cono V., Genovese M., McKew B.A., Hayes S.L., et al. Limits of life in MgCl2-containing environments: Chaotropicity defines the window. Environ. Microbiol. 2007;9:801–813. doi: 10.1111/j.1462-2920.2006.01212.x. [DOI] [PubMed] [Google Scholar]
- 77.McGenity T.J., Oren A. Hypersaline environments. In: Bell E.M., editor. Life at Extremes: Environments, Organisms and Strategies for Survival. CAB International; Dunbeg, UK: 2012. pp. 402–437. [Google Scholar]
- 78.Antunes A., Ngugi D.K., Stingl U. Microbiology of the Red Sea (and other) deep-sea anoxic brine lakes. Environ. Microbiol. Rep. 2011;3:416–433. doi: 10.1111/j.1758-2229.2011.00264.x. [DOI] [PubMed] [Google Scholar]
- 79.Oren A. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst. 2008;4:2. doi: 10.1186/1746-1448-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreno M.L., Pérez D., García M.T., Mellado E. Halophilic bacteria as a source of novel hydrolytic enzymes. Life. 2013;3:38–51. doi: 10.3390/life3010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ginzburg M., Sachs L., Ginzburg B.Z. Ion metabolism in a Halobacterium. I. Influence of age of culture on intracellular concentrations. J. Gen. Physiol. 1970;55:187–207. doi: 10.1085/jgp.55.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lanyi J.K., Silverman M.P. The state of binding of intracellular K + in Halobacterium cutirubrum. Can. J. Microbiol. 1972;18:993–995. doi: 10.1139/m72-154. [DOI] [PubMed] [Google Scholar]
- 83.Roberts M.F. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005;1:5. doi: 10.1186/1746-1448-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tadeo X., Lopez-Mendez B., Trigueros T., Lain A., Castano D., Millet O. Structural basis for the aminoacid composition of proteins from halophilic archaea. PLoS Biol. 2009;7:e1000257. doi: 10.1371/journal.pbio.1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DasSarma S., DasSarma P. Els. John Wiley & Sons; Chichester, UK: 2012. Halophiles; pp. 1–11. [Google Scholar]
- 86.Tokunaga H., Arakawa T., Tokunaga M. Engineering of halophilic enzymes: Two acidic amino acid residues at the carboxy-terminal region confer halophilic characteristics to Halomonas and Pseudomonas nucleoside diphosphate kinases. Protein Sci. 2008;17:1603–1610. doi: 10.1110/ps.035725.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delgado-Garcia M., Valdivia-Urdiales B., Aguilar-Gonzalez C.N., Contreras-Esquivel J.C., Rodriguez-Herrera R. Halophilic hydrolases as a new tool for the biotechnological industries. J. Sci. Food Agric. 2012;92:2575–2580. doi: 10.1002/jsfa.5860. [DOI] [PubMed] [Google Scholar]
- 88.Eichler J. Facing extremes: Archaeal surface-layer (glyco)proteins. Microbiology. 2003;149:3347–3351. doi: 10.1099/mic.0.26591-0. [DOI] [PubMed] [Google Scholar]
- 89.Enache M., Popescu G., Itoh T., Kamekura M. Halophilic microorganisms from man-made and natural hypersaline environments: Physiology, ecology, and biotechnological potential. In: Stan-Latter H., Fendrihan S., editors. Adaption of Microbial Life to Environmental Extremes. Springer-Verlag; New York, NY, USA: 2012. pp. 173–197. [Google Scholar]
- 90.Le Borgne S., Paniagua D., Vazquez-Duhalt R. Biodegradation of organic pollutants by halophilic bacteria and archaea. J. Mol. Microbiol. Biotechnol. 2008;15:74–92. doi: 10.1159/000121323. [DOI] [PubMed] [Google Scholar]
- 91.Abe F., Horikoshi K. The biotechnological potential of piezophiles. Trends Biotechnol. 2001;19:102–108. doi: 10.1016/S0167-7799(00)01539-0. [DOI] [PubMed] [Google Scholar]
- 92.Bartlett D.H., Bidle K.A. Membrane-based adaptations of deep-sea piezophiles. In: Seckbach J., editor. Enigmatic Microorganisms and Life in Extreme Environments. Springer; Dordrecht, The Netherlands: 1999. pp. 503–512. [Google Scholar]
- 93.Mota M.J., Lopes R.P., Delgadillo I., Saraiva J.A. Microorganisms under high pressure-adaptation, growth and biotechnological potential. Biotechnol. Adv. 2013;31:1426–1434. doi: 10.1016/j.biotechadv.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Kato C. High pressure and prokaryotes. In: Horikoshi K., Antranikaian G., Bull A.T., Robb F.T., Stetter K.O., editors. Extremophiles Handbook. Springer; Tokyo, Japan: 2011. pp. 658–668. [Google Scholar]
- 95.Oger P., Cario A. [The high pressure life of piezophiles] Biol. Aujourdhui. 2014;208:193–206. doi: 10.1051/jbio/2014023. [DOI] [PubMed] [Google Scholar]
- 96.Bartlett D.H. Pressure effects on in vivo microbial processes. Biochim. Biophys. Acta. 2002;1595:367–381. doi: 10.1016/S0167-4838(01)00357-0. [DOI] [PubMed] [Google Scholar]
- 97.Lauro F.M., Bartlett D.H. Prokaryotic lifestyles in deep sea habitats. Extremophiles. 2008;12:15–25. doi: 10.1007/s00792-006-0059-5. [DOI] [PubMed] [Google Scholar]
- 98.Simonato F., Campanaro S., Lauro F.M., Vezzi A., D’Angelo M., Vitulo N., Valle G., Bartlett D.H. Piezophilic adaptation: A genomic point of view. J. Biotechnol. 2006;126:11–25. doi: 10.1016/j.jbiotec.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 99.Lauro F.M., Chastain R.A., Blankenship L.E., Yayanos A.A., Bartlett D.H. The unique 16S rRNA genes of piezophiles reflect both phylogeny and adaptation. Appl. Environ. Microbiol. 2007;73:838–845. doi: 10.1128/AEM.01726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Capece M.C., Clark E., Saleh J.K., Halford D., Heinl N., Hoskins S., Rothschild L.J. Polyextremophiles and the constraints for terrestrial habitability. In: Seckbach J., Oren A., Stan-Latter H., editors. Polyextremophiles: Life under Multiple Forms of Stress. Volume 27. Springer; Dordrecht, The Netherlands: 2013. pp. 3–60. [Google Scholar]
- 101.Yumoto I., Hirota K., Nodasaka Y., Yokota Y., Hoshino T., Nakajima K. Alkalibacterium psychrotolerans sp. nov., a psychrotolerant obligate alkaliphile that reduces an indigo dye. Int. J. Syst. Evol. Microbiol. 2004;54:2379–2383. doi: 10.1099/ijs.0.63130-0. [DOI] [PubMed] [Google Scholar]
- 102.Padan E., Bibi E., Ito M., Krulwich T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta. 2005;1717:67–88. doi: 10.1016/j.bbamem.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baker-Austin C., Dopson M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007;15:165–171. doi: 10.1016/j.tim.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 104.Wiegel J. Anaerobic alkaliphiles and alkaliphilic poly-extremophiles. In: Horikoshi K., Antranikaian G., Bull A.T., Robb F.T., Stetter K.O., editors. Extremophiles Handbook. Springer; Tokyo, Japan: 2011. pp. 81–97. [Google Scholar]
- 105.Fukuchi S., Yoshimune K., Wakayama M., Moriguchi M., Nishikawa K. Unique amino acid composition of proteins in halophilic bacteria. J. Mol. Biol. 2003;327:347–357. doi: 10.1016/S0022-2836(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 106.Zaccai G. Molecular adaptations to life in high salt: Lessons from Haloarcula marismortui. In: Gargaud M., López-García P., Martin H., editors. Origins and Evolution of Life: An Astrobiological Perspective. Cambridge University Press; New York, NY, USA: 2011. pp. 375–388. [Google Scholar]
- 107.Gonzalez J.M., Sheckells D., Viebahn M., Krupatkina D., Borges K.M., Robb F.T. Thermococcus waiotapuensis sp. nov., an extremely thermophilic archaeon isolated from a freshwater hot spring. Arch. Microbiol. 1999;172:95–101. doi: 10.1007/s002030050745. [DOI] [PubMed] [Google Scholar]
- 108.Vanlint D., Michiels C.W., Aertsen A. Piezophysiology of the model bacterium Escherichia coli. In: Horikoshi K., Antranikaian G., Bull A.T., Robb F.T., Stetter K.O., editors. Extremophiles Handbook. Springer; Tokyo, Japan: 2011. pp. 669–686. [Google Scholar]
- 109.Kato C., Li L., Nogi Y., Nakamura Y., Tamaoka J., Horikoshi K. Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl. Environ. Microbiol. 1998;64:1510–1513. doi: 10.1128/aem.64.4.1510-1513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blum J.S., Bindi A.B., Buzzelli J., Stolz J.F., Oremland R.S. Bacillus arsenicoselenatis, sp. nov., and Bacillus selenitireducens, sp. nov.: Two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch. Microbiol. 1998;171:19–30. doi: 10.1007/s002030050673. [DOI] [PubMed] [Google Scholar]
- 111.Mesbah N.M., Hedrick D.B., Peacock A.D., Rohde M., Wiegel J. Natranaerobius thermophilus gen. nov., sp. nov., a halophilic, alkalithermophilic bacterium from soda lakes of the Wadi An Natrun, Egypt, and proposal of Natranaerobiaceae fam. nov. and Natranaerobiales ord. nov. Int. J. Syst. Evol. Microbiol. 2007;57:2507–2512. doi: 10.1099/ijs.0.65068-0. [DOI] [PubMed] [Google Scholar]
- 112.Mesbah N.M., Wiegel J. Halophiles exposed concomitantly to multiple stressors: Adaptive mechanisms of halophilic alkalithermophiles. In: Ventosa A., Oren A., Ma Y., editors. Halophiles and Hypersaline Environments. Springer; Berlin, Germany: 2011. pp. 249–273. [Google Scholar]
- 113.Abe F., Kato C., Horikoshi K. Pressure-regulated metabolism in microorganisms. Trends Microbiol. 1999;7:447–453. doi: 10.1016/S0966-842X(99)01608-X. [DOI] [PubMed] [Google Scholar]
- 114.Kumar V., Satyanarayana T. Thermo-alkali-stable xylanase of a novel polyextremophilic Bacillus halodurans TSEV1 and its application in biobleaching. Int. Biodeter. Biodegr. 2012;75:138–145. doi: 10.1016/j.ibiod.2012.09.007. [DOI] [Google Scholar]
- 115.Kumar V., Satyanarayana T. Generation of xylooligosaccharides from microwave irradiated agroresidues using recombinant thermo-alkali-stable endoxylanase of the polyextremophilic bacterium Bacillus halodurans expressed in Pichia pastoris. Bioresour. Technol. 2015;179:382–389. doi: 10.1016/j.biortech.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 116.Vijayalaxmi S., Prakash P., Jayalakshmi S.K., Mulimani V.H., Sreeramulu K. Production of extremely alkaliphilic, halotolerent, detergent, and thermostable mannanase by the free and immobilized cells of Bacillus halodurans PPKS-2. Purification and characterization. Appl. Biochem. Biotechnol. 2013;171:382–395. doi: 10.1007/s12010-013-0333-9. [DOI] [PubMed] [Google Scholar]
- 117.Karan R., Capes M.D., DasSarma P., DasSarma S. Cloning, overexpression, purification, and characterization of a polyextremophilic beta-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. BMC Biotechnol. 2013;13:3. doi: 10.1186/1472-6750-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bommarius A.S., Blum J.K., Abrahamson M.J. Status of protein engineering for biocatalysts: How to design an industrially useful biocatalyst. Curr. Opin. Chem. Biol. 2011;15:194–200. doi: 10.1016/j.cbpa.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 119.Kumar A., Singh S. Directed evolution: Tailoring biocatalysts for industrial applications. Crit. Rev. Biotechnol. 2013;33:365–378. doi: 10.3109/07388551.2012.716810. [DOI] [PubMed] [Google Scholar]
- 120.Chen Z., Wilmanns M., Zeng A.P. Structural synthetic biotechnology: From molecular structure to predictable design for industrial strain development. Trends Biotechnol. 2010;28:534–542. doi: 10.1016/j.tibtech.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 121.Otero J.M., Nielsen J. Industrial systems biology. Biotechnol. Bioeng. 2010;105:439–460. doi: 10.1002/bit.22592. [DOI] [PubMed] [Google Scholar]
- 122.Venkatesh R., Sundaram P.V. Upward shift of thermotolerance of cold water fish and mammalian trypsins upon chemical modification. Ann. N. Y. Acad. Sci. 1998;864:512–516. doi: 10.1111/j.1749-6632.1998.tb10370.x. [DOI] [PubMed] [Google Scholar]
- 123.Venkatesh R., Sundaram P.V. Modulation of stability properties of bovine trypsin after in vitro structural changes with a variety of chemical modifiers. Protein Eng. 1998;11:691–698. doi: 10.1093/protein/11.8.691. [DOI] [PubMed] [Google Scholar]
- 124.Arnold F.H. Combinatorial and computational challenges for biocatalyst design. Nature. 2001;409:253–257. doi: 10.1038/35051731. [DOI] [PubMed] [Google Scholar]
- 125.Bornscheuer U.T., Huisman G.W., Kazlauskas R.J., Lutz S., Moore J.C., Robins K. Engineering the third wave of biocatalysis. Nature. 2012;485:185–194. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- 126.Reetz M.T. Laboratory evolution of stereoselective enzymes: A prolific source of catalysts for asymmetric reactions. Angew. Chem. Int. Ed. 2011;50:138–174. doi: 10.1002/anie.201000826. [DOI] [PubMed] [Google Scholar]
- 127.Kourist R., Bornscheuer U.T. Biocatalytic synthesis of optically active tertiary alcohols. Appl. Microbiol. Biotechnol. 2011;91:505–517. doi: 10.1007/s00253-011-3418-9. [DOI] [PubMed] [Google Scholar]
- 128.Kourist R., Dominguez de Maria P., Miyamoto K. Biocatalytic strategies for the asymmetric synthesis of profens—Recent trends and developments. Green Chem. 2011;13:2607–2618. doi: 10.1039/c1gc15162b. [DOI] [Google Scholar]
- 129.Eijsink V.G., Bjork A., Gaseidnes S., Sirevag R., Synstad B., van den Burg B., Vriend G. Rational engineering of enzyme stability. J. Biotechnol. 2004;113:105–120. doi: 10.1016/j.jbiotec.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 130.Siddiqui K.S., Cavicchioli R. Improved thermal stability and activity in the cold-adapted lipase B from Candida antarctica following chemical modification with oxidized polysaccharides. Extremophiles. 2005;9:471–476. doi: 10.1007/s00792-005-0464-1. [DOI] [PubMed] [Google Scholar]
- 131.Shu Z.-Y., Jiang H., Lin R.-F., Jiang Y.-M., Lin L., Huang J.-Z. Technical methods to improve yield, activity and stability in the development of microbial lipases. J. Mol. Catal. B: Enzym. 2010;62:1–8. doi: 10.1016/j.molcatb.2009.09.003. [DOI] [Google Scholar]
- 132.Siddiqui K.S., Poljak A., Guilhaus M., De Francisci D., Curmi P.M., Feller G., D’Amico S., Gerday C., Uversky V.N., Cavicchioli R. Role of lysine versus arginine in enzyme cold-adaptation: Modifying lysine to homo-arginine stabilizes the cold-adapted alpha-amylase from Pseudoalteramonas haloplanktis. Proteins. 2006;64:486–501. doi: 10.1002/prot.20989. [DOI] [PubMed] [Google Scholar]
- 133.Ferrer M., Martinez-Abarca F., Golyshin P.N. Mining genomes and “metagenomes” for novel catalysts. Curr. Opin. Biotechnol. 2005;16:588–593. doi: 10.1016/j.copbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 134.Handelsman J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004;68:669–685. doi: 10.1128/MMBR.68.4.669-685.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lopez-Lopez O., Cerdan M.E., Gonzalez-Siso M.I. New extremophilic lipases and esterases from metagenomics. Curr. Protein Pept. Sci. 2014;15:445–455. doi: 10.2174/1389203715666140228153801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kennedy J., Flemer B., Jackson S.A., Lejon D.P., Morrissey J.P., O’Gara F., Dobson A.D. Marine metagenomics: New tools for the study and exploitation of marine microbial metabolism. Mar. Drugs. 2010;8:608–628. doi: 10.3390/md8030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kotera M., Moriya Y., Tokimatsu T., Kanehisa M., Goto S. KEGG and GenomeNet, new developments, metagenomic analysis. In: Nelson K.E., editor. Encyclopedia of Metagenomics. Springer; New York, NY, USA: 2014. pp. 1–11. [Google Scholar]
- 138.Blaser H.U. Enantioselective catalysis in fine chemicals production. Chem. Commun. 2003;3:293–296. doi: 10.1039/b209968n. [DOI] [PubMed] [Google Scholar]
- 139.Miyake R., Kawamoto J., Wei Y.L., Kitagawa M., Kato I., Kurihara T., Esaki N. Construction of a low-temperature protein expression system using a cold-adapted bacterium, Shewanella sp. strain Ac10, as the host. Appl. Environ. Microbiol. 2007;73:4849–4856. doi: 10.1128/AEM.00824-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tajima T., Fuki K., Kataoka N., Kudou D., Nakashimada Y., Kato J. Construction of a simple biocatalyst using psychrophilic bacterial cells and its application for efficient 3-hydroxypropionaldehyde production from glycerol. AMB Express. 2013;3:69. doi: 10.1186/2191-0855-3-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferrer M., Chernikova T.N., Yakimov M.M., Golyshin P.N., Timmis K.N. Chaperonins govern growth of Escherichia coli at low temperatures. Nat. Biotechnol. 2003;21:1266–1267. doi: 10.1038/nbt1103-1266. [DOI] [PubMed] [Google Scholar]
- 142.Adrio J.L., Demain A.L. Microbial enzymes: Tools for biotechnological processes. Biomolecules. 2014;4:117–139. doi: 10.3390/biom4010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Binod P., Palkhiwala P., Gaikaiwari R., Nampoothiri K.M., Duggal A., Dey K., Pandey A. Industrial enzymes—Present status and future perspectives for India. J. Sci. Ind. Res. India. 2013;72:271–286. [Google Scholar]
- 144.Kumar L., Awasthi G., Singh B. Extremophiles: A novel source of industrially important enzymes. Biotechnol. Appl. Biochem. 2011;10:1–15. [Google Scholar]
- 145.NC-IUBMB. The Enzyme List Class 3—Hydrolases. [(acessed on 27 November 2014)]. ExplorEnz. Available online: http://www.enzyme-database.org/index.php.
- 146.Zeeman S.C., Kossmann J., Smith A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010;61:209–234. doi: 10.1146/annurev-arplant-042809-112301. [DOI] [PubMed] [Google Scholar]
- 147.Castro A.M., Ribeiro B.D. Methods for detection of amylolytic activities. In: Vermelho A.B., Couri S., editors. Methods to Determine Enzymatic Activity. Bentham Science; Sharjah, UAE: 2013. pp. 100–124. [Google Scholar]
- 148.Prakash O., Jaiswal N. alpha-Amylase: An ideal representative of thermostable enzymes. Appl. Biochem. Biotechnol. 2010;160:2401–2414. doi: 10.1007/s12010-009-8735-4. [DOI] [PubMed] [Google Scholar]
- 149.Feller G., Gerday C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003;1:200–208. doi: 10.1038/nrmicro773. [DOI] [PubMed] [Google Scholar]
- 150.Kuddus M., Roohi, Arif J.M., Ramteke P.W. An overview of cold-active microbial α-amylase: Adaptation strategies and biotechnological potentials. Biotechnology. 2011;10:246–258. doi: 10.3923/biotech.2011.246.258. [DOI] [Google Scholar]
- 151.Niehaus F., Bertoldo C., Kahler M., Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- 152.Sharma A., Satyanarayana T. Microbial acid-stable α-amylases: Characteristics, genetic engineering and applications. Process Biochem. 2013;48:201–211. doi: 10.1016/j.procbio.2012.12.018. [DOI] [Google Scholar]
- 153.Brown I., Dafforn T.R., Fryer P.J., Cox P.W. Kinetic study of the thermal denaturation of a hyperthermostable extracellular alpha-amylase from Pyrococcus furiosus. Biochim. Biophys. Acta. 2013;1834:2600–2605. doi: 10.1016/j.bbapap.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 154.Duffner F., Bertoldo C., Andersen J.T., Wagner K., Antranikian G. A new thermoactive pullulanase from Desulfurococcus mucosus: Cloning, sequencing, purification, and characterization of the recombinant enzyme after expression in Bacillus subtilis. J. Bacteriol. 2000;182:6331–6338. doi: 10.1128/JB.182.22.6331-6338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gomes I., Gomes J., Steiner W. Highly thermostable amylase and pullulanase of the extreme thermophilic eubacterium Rhodothermus marinus: Production and partial characterization. Bioresour. Technol. 2003;90:207–214. doi: 10.1016/S0960-8524(03)00110-X. [DOI] [PubMed] [Google Scholar]
- 156.Trincone A. Marine biocatalysts: Enzymatic features and applications. Mar. Drugs. 2011;9:478–499. doi: 10.3390/md9040478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gurumurthy D.M., Neelagund S.E. Molecular characterization of industrially viable extreme thermostable novel alpha-amylase of Geobacillus sp. Iso5 isolated from geothermal spring. J. Pure Appl. Microbiol. 2012;6:1759–1773. [Google Scholar]
- 158.Anto H., Trivedi U.B., Patel K.C. Glucoamylase production by solid-state fermentation using rice flake manufacturing waste products as substrate. Bioresour. Technol. 2006;97:1161–1166. doi: 10.1016/j.biortech.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 159.Sun H., Zhao P., Ge X., Xia Y., Hao Z., Liu J., Peng M. Recent advances in microbial raw starch degrading enzymes. Appl. Biochem. Biotechnol. 2010;160:988–1003. doi: 10.1007/s12010-009-8579-y. [DOI] [PubMed] [Google Scholar]
- 160.Kyaw N., de Mesquita R.F., Kameda E., Neto J.C., Langone M.A., Coelho M.A. Characterization of commercial amylases for the removal of filter cake on petroleum wells. Appl. Biochem. Biotechnol. 2010;161:171–180. doi: 10.1007/s12010-009-8773-y. [DOI] [PubMed] [Google Scholar]
- 161.Sivaramakrishnan S., Gangadharan D., Nampoothiri K.M., Pandey A. Amylases from microbial sources—An overview on recent developments. Food Technol. Biotechnol. 2006;44:173–184. [Google Scholar]
- 162.Callen W., Richardson T., Frey G., Miller C., Kazaoka M., Mathur E., Short J. Amylases and Methods for Use in Starch Processing. US 8,338,131. 2012 Dec 25;
- 163.Nedwin G.E., Sharma V., Shetty J.K. Alpha-Amylase Blend for Starch Processing and Method of Use Thereof. US 8,545,907. 2013 Oct 1;
- 164.Hii S.L., Tan J.S., Ling T.C., Ariff A.B. Pullulanase: Role in starch hydrolysis and potential industrial applications. Enzyme Res. 2012;2012:921362. doi: 10.1155/2012/921362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernandes P. Marine enzymes and food industry: Insight on existing and potential interactions. Front. Mar. Sci. 2014;1:46. doi: 10.3389/fmars.2014.00046. [DOI] [Google Scholar]
- 166.Lévêque E., Janeček Š., Haye B., Belarbi A. Thermophilic archaeal amylolytic enzymes. Enzym. Microb. Tech. 2000;26:3–14. doi: 10.1016/S0141-0229(99)00142-8. [DOI] [Google Scholar]
- 167.Vieille C., Zeikus G.J. Hyperthermophilic enzymes: Sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 2001;65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li X., Li D., Park K.H. An extremely thermostable amylopullulanase from Staphylothermus marinus displays both pullulan- and cyclodextrin-degrading activities. Appl. Microbiol. Biotechnol. 2013;97:5359–5369. doi: 10.1007/s00253-012-4397-1. [DOI] [PubMed] [Google Scholar]
- 169.Brown S.H., Kelly R.M. Characterization of amylolytic enzymes, having both alpha-1,4 and alpha-1,6 hydrolytic activity, from the thermophilic Archaea Pyrococcus furiosus and Thermococcus litoralis. Appl. Environ. Microbiol. 1993;59:2614–2621. doi: 10.1128/aem.59.8.2614-2621.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feller G., Le Bussy O., Gerday C. Expression of psychrophilic genes in mesophilic hosts: Assessment of the folding state of a recombinant alpha-amylase. Appl. Environ. Microbiol. 1998;64:1163–1165. doi: 10.1128/aem.64.3.1163-1165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Srimathi S., Jayaraman G., Feller G., Danielsson B., Narayanan P.R. Intrinsic halotolerance of the psychrophilic alpha-amylase from Pseudoalteromonas haloplanktis. Extremophiles. 2007;11:505–515. doi: 10.1007/s00792-007-0062-5. [DOI] [PubMed] [Google Scholar]
- 172.Qin Y., Huang Z., Liu Z. A novel cold-active and salt-tolerant alpha-amylase from marine bacterium Zunongwangia profunda: Molecular cloning, heterologous expression and biochemical characterization. Extremophiles. 2014;18:271–281. doi: 10.1007/s00792-013-0614-9. [DOI] [PubMed] [Google Scholar]
- 173.Pancha I., Jain D., Shrivastav A., Mishra S.K., Shethia B., Mishra S., V P.M., Jha B. A thermoactive alpha-amylase from a Bacillus sp. isolated from CSMCRI salt farm. Int. J. Biol. Macromol. 2010;47:288–291. doi: 10.1016/j.ijbiomac.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 174.Uzyol K.S., Sarıyar-Akbulut B., Denizci A.A., Kazan D. Thermostable α-amylase from moderately halophilic Halomonas sp. AAD21. Turk. J. Biol. 2012;36:327–338. [Google Scholar]
- 175.Fukushima T., Mizuki T., Echigo A., Inoue A., Usami R. Organic solvent tolerance of halophilic alpha-amylase from a Haloarchaeon, Haloarcula sp. strain S-1. Extremophiles. 2005;9:85–89. doi: 10.1007/s00792-004-0423-2. [DOI] [PubMed] [Google Scholar]
- 176.Laderman K.A., Asada K., Uemori T., Mukai H., Taguchi Y., Kato I., Anfinsen C.B. Alpha-amylase from the hyperthermophilic archaebacterium Pyrococcus furiosus. Cloning and sequencing of the gene and expression in Escherichia coli. J. Biol. Chem. 1993;268:24402–24407. [PubMed] [Google Scholar]
- 177.Bertoldo C., Duffner F., Jorgensen P.L., Antranikian G. Pullulanase type I from Fervidobacterium pennavorans Ven5: Cloning, sequencing, and expression of the gene and biochemical characterization of the recombinant enzyme. Appl. Environ. Microbiol. 1999;65:2084–2091. doi: 10.1128/aem.65.5.2084-2091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bhattacharya A., Pletschke B.I. Review of the enzymatic machinery of Halothermothrix orenii with special reference to industrial applications. Enzyme Microb. Technol. 2014;55:159–169. doi: 10.1016/j.enzmictec.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 179.Perez-Pomares F., Bautista V., Ferrer J., Pire C., Marhuenda-Egea F.C., Bonete M.J. Alpha-amylase activity from the halophilic archaeon Haloferax mediterranei. Extremophiles. 2003;7:299–306. doi: 10.1007/s00792-003-0327-6. [DOI] [PubMed] [Google Scholar]
- 180.Tomme P., Warren R.A., Gilkes N.R. Cellulose hydrolysis by bacteria and fungi. In: Stafford R., editor. Advances in Microbial Physiology. Volume 37. Academic Press; London, UK: 1995. pp. 1–81. [DOI] [PubMed] [Google Scholar]
- 181.Wilson D.B. Aerobic microbial cellulase systems. In: Himmel M.E., editor. Biomass Recalcitrance: Deconstructing the Plant Cell Wall for Bioenergy. Blackwell Publishing; Oxford, UK: 2008. pp. 374–392. [Google Scholar]
- 182.Collins T., Gerday C., Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 2005;29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 183.Ratanakhanokchai K., Waeonukul R., Pason P., Pason C., Kyu K.L., SakkaK, Kosugi A., Mori Y. Paenibacillus curdlanolyticus strain B-6 multienzyme complex: A novel system for biomass utilization. In: Matovic M.D., editor. Biomass Now—Cultivation and Utilization. Intech; Rijeka, Croatia: 2013. pp. 369–394. [Google Scholar]
- 184.Maki M., Leung K.T., Qin W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int. J. Biol. Sci. 2009;5:500–516. doi: 10.7150/ijbs.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Naik S.N., Goud V., Rout P.K., Dalai A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sust. Energ. Rev. 2010;14:578–597. doi: 10.1016/j.rser.2009.10.003. [DOI] [Google Scholar]
- 186.Kasana R.C. Proteases from psychrotrophs: An overview. Crit. Rev. Microbiol. 2010;36:134–145. doi: 10.3109/10408410903485525. [DOI] [PubMed] [Google Scholar]
- 187.Kumar V., Satyanarayana T. Thermoalkaliphilic microbes. In: Seckbach J., Oren A., Stan-Latter H., editors. Polyextremophiles: Life under Multiple Forms of Stress. Volume 27. Springer Science +Business Media; Dordrecht, The Netherlands: 2013. pp. 271–298. [Google Scholar]
- 188.Duffaud G.D., McCutchen C.M., Leduc P., Parker K.N., Kelly R.M. Purification and characterization of extremely thermostable beta-mannanase, beta-mannosidase, and alpha-galactosidase from the hyperthermophilic eubacterium Thermotoga neapolitana 5068. Appl. Environ. Microbiol. 1997;63:169–177. doi: 10.1128/aem.63.1.169-177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Matsui I., Sakai Y., Matsui E., Kikuchi H., Kawarabayasi Y., Honda K. Novel substrate specificity of a membrane-bound beta-glycosidase from the hyperthermophilic archaeon Pyrococcus horikoshii. FEBS Lett. 2000;467:195–200. doi: 10.1016/S0014-5793(00)01156-X. [DOI] [PubMed] [Google Scholar]
- 190.Goyal K., Selvakumar P., Hayashi K. Characterization of a thermostable beta-glucosidase (Bg1B) from Thermotoga maritima showing transglycosylation activity. J. Mol. Catal. B-Enzym. 2001;15:45–53. doi: 10.1016/S1381-1177(01)00003-0. [DOI] [Google Scholar]
- 191.Subramaniyan S., Prema P. Biotechnology of microbial xylanases: Enzymology, molecular biology, and application. Crit. Rev. Biotechnol. 2002;22:33–64. doi: 10.1080/07388550290789450. [DOI] [PubMed] [Google Scholar]
- 192.Ooteghem V.S. Process for Generation of Hydrogen Gas from Various Feedstocks Using Thermophilic Bacteria. US 6,942,998. 2005 Sep 13;
- 193.Jiang Z.Q., Deng W., Zhu Y.P., Li L.T., Sheng Y.J., Hayashi K. The recombinant xylanase B of Thermotoga maritima is highly xylan specific and produces exclusively xylobiose from xylans, a unique character for industrial applications. J. Mol. Catal. B-Enzym. 2004;27:207–213. doi: 10.1016/j.molcatb.2003.11.012. [DOI] [Google Scholar]
- 194.Kang H.J., Uegaki K., Fukada H., Ishikawa K. Improvement of the enzymatic activity of the hyperthermophilic cellulase from Pyrococcus horikoshii. Extremophiles. 2007;11:251–256. doi: 10.1007/s00792-006-0033-2. [DOI] [PubMed] [Google Scholar]
- 195.Mao X., Hong Y., Shao Z., Zhao Y., Liu Z. A novel cold-active and alkali-stable beta-glucosidase gene isolated from the marine bacterium Martelella mediterranea. Appl. Biochem. Biotechnol. 2010;162:2136–2148. doi: 10.1007/s12010-010-8988-y. [DOI] [PubMed] [Google Scholar]
- 196.Chen S., Hong Y., Shao Z., Liu Z. A cold-active β-glucosidase (Bgl1C) from a sea bacteria Exiguobacterium oxidotolerans A011. World J. Microb. Biot. 2010;26:1427–1435. doi: 10.1007/s11274-010-0317-7. [DOI] [Google Scholar]
- 197.Garsoux G., Lamotte J., Gerday C., Feller G. Kinetic and structural optimization to catalysis at low temperatures in a psychrophilic cellulase from the Antarctic bacterium Pseudoalteromonas haloplanktis. Biochem. J. 2004;384:247–253. doi: 10.1042/BJ20040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Collins T., Hoyoux A., Dutron A., Georis J., Genot B., Dauvrin T., Arnaut F., Gerday C., Feller G. Use of glycoside hydrolase family 8 xylanases in baking. J. Cereal Sci. 2006;43:79–84. doi: 10.1016/j.jcs.2005.08.002. [DOI] [Google Scholar]
- 199.Dornez E., Verjans P., Arnaut F., Delcour J.A., Courtin C.M. Use of psychrophilic xylanases provides insight into the xylanase functionality in bread making. J. Agric. Food Chem. 2011;59:9553–9562. doi: 10.1021/jf201752g. [DOI] [PubMed] [Google Scholar]
- 200.Hildebrandt P., Wanarska M., Kur J. A new cold-adapted beta-D-galactosidase from the Antarctic Arthrobacter sp. 32c—Gene cloning, overexpression, purification and properties. BMC Microbiol. 2009;9:151. doi: 10.1186/1471-2180-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Varbanets L.D., Avdeeva L.V., Borzova N.V., Matseliukh E.V., Gudzenko A.V., Kiprianova E.A., Iaroshenko L.V. The Black Sea bacteria—Producers of hydrolytic enzymes. Mikrobiol. Z. 2011;73:9–15. [PubMed] [Google Scholar]
- 202.Rawlings N.D., Waller M., Barrett A.J., Bateman A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids. Res. 2014;42:503–509. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lopez-Otin C., Matrisian L.M. Emerging roles of proteases in tumour suppression. Nat. Rev. Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 204.Rawlings N.D., Bateman A. Pepsin homologues in bacteria. BMC Genomics. 2009;10:437. doi: 10.1186/1471-2164-10-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gunbin K.V., Afonnikov D.A., Kolchanov N.A. Molecular evolution of the hyperthermophilic archaea of the Pyrococcus genus: Analysis of adaptation to different environmental conditions. BMC Genomics. 2009;10:639. doi: 10.1186/1471-2164-10-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Stetter K.O. History of discovery of the first hyperthermophiles. Extremophiles. 2006;10:357–362. doi: 10.1007/s00792-006-0012-7. [DOI] [PubMed] [Google Scholar]
- 207.Halio S.B., Bauer M.W., Mukund S., Adams M., Kelly R.M. Purification and characterization of two functional forms of intracellular protease PfpI from the hyperthermophilic archaeon Pyrococcus furiosus. Appl. Environ. Microbiol. 1997;63:289–295. doi: 10.1128/aem.63.1.289-295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ward D.E., Shockley K.R., Chang L.S., Levy R.D., Michel J.K., Conners S.B., Kelly R.M. Proteolysis in hyperthermophilic microorganisms. Archaea. 2002;1:63–74. doi: 10.1155/2002/503191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Atomi H. Recent progress towards the application of hyperthermophiles and their enzymes. Curr. Opin. Chem. Biol. 2005;9:166–173. doi: 10.1016/j.cbpa.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 210.Cavicchioli R., Siddiqui K.S., Andrews D., Sowers K.R. Low-temperature extremophiles and their applications. Curr. Opin. Biotechnol. 2002;13:253–261. doi: 10.1016/S0958-1669(02)00317-8. [DOI] [PubMed] [Google Scholar]
- 211.Gerday C., Aittaleb M., Bentahir M., Chessa J.P., Claverie P., Collins T., D’Amico S., Dumont J., Garsoux G., Georlette D., et al. Cold-adapted enzymes: From fundamentals to biotechnology. Trends Biotechnol. 2000;18:103–107. doi: 10.1016/S0167-7799(99)01413-4. [DOI] [PubMed] [Google Scholar]
- 212.Cristobal H.A., Lopez M.A., Kothe E., Abate C.M. Diversity of protease-producing marine bacteria from sub-antarctic environments. J. Basic Microbiol. 2011;51:590–600. doi: 10.1002/jobm.201000413. [DOI] [PubMed] [Google Scholar]
- 213.Kulakova L., Galkin A., Kurihara T., Yoshimura T., Esaki N. Cold-active serine alkaline protease from the psychrotrophic bacterium Shewanella strain ac10: Gene cloning and enzyme purification and characterization. Appl. Environ. Microbiol. 1999;65:611–617. doi: 10.1128/aem.65.2.611-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Huston A.L., Methe B., Deming J.W. Purification, characterization, and sequencing of an extracellular cold-active aminopeptidase produced by marine psychrophile Colwellia psychrerythraea strain 34H. Appl. Environ. Microbiol. 2004;70:3321–3328. doi: 10.1128/AEM.70.6.3321-3328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sastre D.E., Paggi R.A., de Castro R.E. The Lon protease from the haloalkaliphilic archaeon Natrialba magadii is transcriptionally linked to a cluster of putative membrane proteases and displays DNA-binding activity. Microbiol. Res. 2011;166:304–313. doi: 10.1016/j.micres.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 216.Cerletti M., Martinez M.J., Gimenez M.I., Sastre D.E., Paggi R.A., de Castro R.E. The LonB protease controls membrane lipids composition and is essential for viability in the extremophilic haloarchaeon Haloferax volcanii. Environ. Microbiol. 2014;16:1779–1792. doi: 10.1111/1462-2920.12385. [DOI] [PubMed] [Google Scholar]
- 217.Gimenez M.I., Studdert C.A., Sanchez J.J., De Castro R.E. Extracellular protease of Natrialba magadii: Purification and biochemical characterization. Extremophiles. 2000;4:181–188. doi: 10.1007/s007920070033. [DOI] [PubMed] [Google Scholar]
- 218.Kamekura M., Seno Y., Dyall-Smith M. Halolysin R4, a serine proteinase from the halophilic archaeon Haloferax mediterranei; gene cloning, expression and structural studies. Biochim. Biophys. Acta. 1996;1294:159–167. doi: 10.1016/0167-4838(96)00016-7. [DOI] [PubMed] [Google Scholar]
- 219.De Castro R.E., Maupin-Furlow J.A., Gimenez M.I., Herrera Seitz M.K., Sanchez J.J. Haloarchaeal proteases and proteolytic systems. FEMS Microbiol. Rev. 2006;30:17–35. doi: 10.1111/j.1574-6976.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- 220.Vidyasagar M., Prakash S., Mahajan V., Shouche Y.S., Sreeramulu K. Purification and characterization of an extreme halothermophilic protease from a halophilic bacterium Chromohalobacter sp. TVSP101. Braz. J. Microbiol. 2009;40:12–19. doi: 10.1590/S1517-83822009000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hough D.W., Danson M.J. Extremozymes. Curr. Opin. Chem. Biol. 1999;3:39–46. doi: 10.1016/S1367-5931(99)80008-8. [DOI] [PubMed] [Google Scholar]
- 222.Asker D., Awad T.S., Beppu T., Ueda K. Deinococcus misasensis and Deinococcus roseus, novel members of the genus Deinococcus, isolated from a radioactive site in Japan. Syst. Appl. Microbiol. 2008;31:43–49. doi: 10.1016/j.syapm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 223.Asker D., Awad T.S., McLandsborough L., Beppu T., Ueda K. Deinococcus depolymerans sp. nov., a gamma- and UV-radiation-resistant bacterium, isolated from a naturally radioactive site. Int. J. Syst. Evol. Microbiol. 2011;61:1448–1453. doi: 10.1099/ijs.0.013482-0. [DOI] [PubMed] [Google Scholar]
- 224.Kampfer P., Lodders N., Huber B., Falsen E., Busse H.J. Deinococcus aquatilis sp. nov., isolated from water. Int. J. Syst. Evol. Microbiol. 2008;58:2803–2806. doi: 10.1099/ijs.0.2008/001206-0. [DOI] [PubMed] [Google Scholar]
- 225.Asker D., Awad T.S., Beppu T., Ueda K. Deinococcus aquiradiocola sp. nov., isolated from a radioactive site in Japan. Int. J. Syst. Evol. Microbiol. 2009;59:144–149. doi: 10.1099/ijs.0.65762-0. [DOI] [PubMed] [Google Scholar]
- 226.Suresh K., Reddy G.S., Sengupta S., Shivaji S. Deinococcus indicus sp. nov., an arsenic-resistant bacterium from an aquifer in West Bengal, India. Int. J. Syst. Evol. Microbiol. 2004;54:457–461. doi: 10.1099/ijs.0.02758-0. [DOI] [PubMed] [Google Scholar]
- 227.Kimura H., Asada R., Masta A., Naganuma T. Distribution of microorganisms in the subsurface of the manus basin hydrothermal vent field in Papua New Guinea. Appl. Environ. Microbiol. 2003;69:644–648. doi: 10.1128/AEM.69.1.644-648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liedert C., Peltola M., Bernhardt J., Neubauer P., Salkinoja-Salonen M. Physiology of resistant Deinococcus geothermalis bacterium aerobically cultivated in low-manganese medium. J. Bacteriol. 2012;194:1552–1561. doi: 10.1128/JB.06429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pietrow O., Panek A., Synowiecki J. Extracellular proteolytic activity of Deinococcus geothermalis. Afr. J. Biotechnol. 2013;12:4020–4027. [Google Scholar]
- 230.Friedrich A.B., Antranikian G. Keratin degradation by Fervidobacterium pennavorans, a novel thermophilic anaerobic species of the order thermotogales. Appl. Environ. Microbiol. 1996;62:2875–2882. doi: 10.1128/aem.62.8.2875-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Burlini N., Magnani P., Villa A., Macchi F., Tortora P., Guerritore A. A heat-stable serine proteinase from the extreme thermophilic archaebacterium Sulfolobus solfataricus. Biochim. Biophys. Acta. 1992;1122:283–292. doi: 10.1016/0167-4838(92)90406-4. [DOI] [PubMed] [Google Scholar]
- 232.Klingeberg M., Hashwa F., Antranikian G. Properties of extremely thermostable proteases from anaerobic hyperthermophilic bacteria. Appl. Microbiol. Biotechnol. 1991;34:715–719. [Google Scholar]
- 233.Jang H.J., Kim B.C., Pyun Y.R., Kim Y.S. A novel subtilisin-like serine protease from Thermoanaerobacter yonseiensis KB-1: Its cloning, expression, and biochemical properties. Extremophiles. 2002;6:233–243. doi: 10.1007/s00792-001-0248-1. [DOI] [PubMed] [Google Scholar]
- 234.Lloyd K.G., Schreiber L., Petersen D.G., Kjeldsen K.U., Lever M.A., Steen A.D., Stepanauskas R., Richter M., Kleindienst S., Lenk S., et al. Predominant archaea in marine sediments degrade detrital proteins. Nature. 2013;496:215–218. doi: 10.1038/nature12033. [DOI] [PubMed] [Google Scholar]
- 235.Seth-Pasricha M., Bidle K.A., Bidle K.D. Specificity of archaeal caspase activity in the extreme halophile Haloferax volcanii. Environ. Microbiol. Rep. 2013;5:263–271. doi: 10.1111/1758-2229.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Arpigny J.L., Jaeger K.E. Bacterial lipolytic enzymes: Classification and properties. Biochem. J. 1999;343:177–183. doi: 10.1042/0264-6021:3430177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.de Pascale D., Cusano A.M., Autore F., Parrilli E., di Prisco G., Marino G., Tutino M.L. The cold-active Lip1 lipase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125 is a member of a new bacterial lipolytic enzyme family. Extremophiles. 2008;12:311–323. doi: 10.1007/s00792-008-0163-9. [DOI] [PubMed] [Google Scholar]
- 238.Gupta R., Gupta N., Rathi P. Bacterial lipases: An overview of production, purification and biochemical properties. Appl. Microbiol. Biotechnol. 2004;64:763–781. doi: 10.1007/s00253-004-1568-8. [DOI] [PubMed] [Google Scholar]
- 239.Hasan F., Shah A.A., Hameed A. Industrial applications of microbial lipases. Enzyme Microb. Technol. 2006;39:235–251. doi: 10.1016/j.enzmictec.2005.10.016. [DOI] [Google Scholar]
- 240.Jaeger K.E., Eggert T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002;13:390–397. doi: 10.1016/S0958-1669(02)00341-5. [DOI] [PubMed] [Google Scholar]
- 241.Sangeetha R., Arulpandi I., Geetha A. Bacterial lipases as potential industrial biocatalysts: An overview. Res. J. Microbiol. 2011;6:1–24. doi: 10.3923/jm.2011.1.24. [DOI] [Google Scholar]
- 242.Charpe T.W., Rathod V.K. Biodiesel production using waste frying oil. Waste Manag. 2011;31:85–90. doi: 10.1016/j.wasman.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 243.Gupta P., Upadhyay L.S.B., Shrivastava R. Lipase catalyzed-transesterification of vegetable oils by lipolytic bacteria. Res. J. Microbiol. 2011;6:281–288. doi: 10.3923/jm.2011.839.850. [DOI] [Google Scholar]
- 244.Bajaj A., Lohan P., Jha P.N., Mehrotra R. Biodiesel production through lipase catalyzed transesterification: An overview. J. Mol. Catal. B: Enzym. 2010;62:9–14. doi: 10.1016/j.molcatb.2009.09.018. [DOI] [Google Scholar]
- 245.Reetz M.T., Jaeger K.E. Overexpression, immobilization and biotechnological application of Pseudomonas lipases. Chem. Phys. Lipids. 1998;93:3–14. doi: 10.1016/S0009-3084(98)00033-4. [DOI] [PubMed] [Google Scholar]
- 246.Fucinos P., Gonzalez R., Atanes E., Sestelo A.B., Perez-Guerra N., Pastrana L., Rua M.L. Lipases and esterases from extremophiles: Overview and case example of the production and purification of an esterase from Thermus thermophilus HB27. Methods Mol. Biol. 2012;861:239–266. doi: 10.1007/978-1-61779-600-5_15. [DOI] [PubMed] [Google Scholar]
- 247.Andualema B., Gessesse A. Microbial lipases and their industrial applications: Review. Biotechnol. Appl. Biochem. 2012;11:100–118. [Google Scholar]
- 248.Schafer T., Antranikian G., Royter M., Hoff T. Lipases from Thermophilic Anaerobes. US 7,972,831. 2011 Jul 5;
- 249.Vind J., Knötzel J.C.F., Borch K., Svendsen A., Callisen T.H., Yaver D., Bjornvad M.E., Hansen P.K., Lamsa M. Lipase Variants. 8,187,854. US. 2012 May 29;
- 250.Mardanov A.V., Ravin N.V., Svetlitchnyi V.A., Beletsky A.V., Miroshnichenko M.L., Bonch-Osmolovskaya E.A., Skryabin K.G. Metabolic versatility and indigenous origin of the archaeon Thermococcus sibiricus, isolated from a siberian oil reservoir, as revealed by genome analysis. Appl. Environ. Microbiol. 2009;75:4580–4588. doi: 10.1128/AEM.00718-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kim S.B., Lee W., Ryu Y.W. Cloning and characterization of thermostable esterase from Archaeoglobus fulgidus. J. Microbiol. 2008;46:100–107. doi: 10.1007/s12275-007-0185-5. [DOI] [PubMed] [Google Scholar]
- 252.Hotta Y., Ezaki S., Atomi H., Imanaka T. Extremely stable and versatile carboxylesterase from a hyperthermophilic archaeon. Appl. Environ. Microbiol. 2002;68:3925–3931. doi: 10.1128/AEM.68.8.3925-3931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Park Y.J., Yoon S.J., Lee H.B. A novel thermostable arylesterase from the archaeon Sulfolobus solfataricus P1: Purification, characterization, and expression. J. Bacteriol. 2008;190:8086–8095. doi: 10.1128/JB.00803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Arpigny J.L., Jendrossek D., Jaeger K.E. A novel heat-stable lipolytic enzyme from Sulfolobus acidocaldarius DSM 639 displaying similarity to polyhydroxyalkanoate depolymerases. FEMS Microbiol. Lett. 1998;167:69–73. doi: 10.1111/j.1574-6968.1998.tb13209.x. [DOI] [PubMed] [Google Scholar]
- 255.Huddleston S., Yallop C.A., Charalambous B.M. The identification and partial characterisation of a novel inducible extracellular thermostable esterase from the archaeon Sulfolobus shibatae. Biochem. Biophys. Res. Commun. 1995;216:495–500. doi: 10.1006/bbrc.1995.2650. [DOI] [PubMed] [Google Scholar]
- 256.Ikeda M., Clark D.S. Molecular cloning of extremely thermostable esterase gene from hyperthermophilic archaeon Pyrococcus furiosus in Escherichia coli. Biotechnol. Bioeng. 1998;57:624–629. doi: 10.1002/(SICI)1097-0290(19980305)57:5<624::AID-BIT15>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 257.Ryu H.S., Kim H.K., Choi W.C., Kim M.H., Park S.Y., Han N.S., Oh T.K., Lee J.K. New cold-adapted lipase from Photobacterium lipolyticum sp. nov. that is closely related to filamentous fungal lipases. Appl. Microbiol. Biotechnol. 2006;70:321–326. doi: 10.1007/s00253-005-0058-y. [DOI] [PubMed] [Google Scholar]
- 258.Bosley J.A., Peilow A.D. Preparation of Immobilized Lipase by Adsorption of Lipase and a Non-Lipase Protein on a Support. US 5232843. 1993 Aug 3;
- 259.Yang K.S., Sohn J.H., Kim H.K. Catalytic properties of a lipase from Photobacterium lipolyticum for biodiesel production containing a high methanol concentration. J. Biosci. Bioeng. 2009;107:599–604. doi: 10.1016/j.jbiosc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 260.Gotor-Fernández V., Busto E., Gotor V. Candida antarctica Lipase B: An ideal biocatalyst for the preparation of nitrogenated organic compounds. Adv. Synth. Catal. 2006;348:797–812. doi: 10.1002/adsc.200606057. [DOI] [Google Scholar]
- 261.Qin B., Liang P., Jia X., Zhang X., Mu M., Wang X.-Y., Ma G.-Z., Jin D.-N., You S. Directed evolution of Candida antarctica lipase B for kinetic resolution of profen esters. Catal. Commun. 2013;38:1–5. doi: 10.1016/j.catcom.2013.03.040. [DOI] [Google Scholar]
- 262.Singh N., Jha M.K., Sarma A.K. A critical review of enzymatic transesterification: A sustainable technology for biodiesel production. In: Kumar S., Sarma A.K., Tyagi S.K., Yadav Y.K., editors. Recent Advances in Bioenergy Research. Volume 3. SSS-NIRE; Kapurthala, India: 2014. pp. 298–312. [Google Scholar]
- 263.Nielsen T.B., Ishii M., Kirk O. Lipases A and B from the yeast Candida antarctica. In: Margesin R., Schinner F., editors. Biotechnological Applications of Cold-Adapted Organisms. Springer-Verlag; Heidelberg, Germany: 1999. pp. 49–61. [Google Scholar]
- 264.Widersten M. Protein engineering for development of new hydrolytic biocatalysts. Curr. Opin. Chem. Biol. 2014;21:42–47. doi: 10.1016/j.cbpa.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 265.Lo Giudice A., Michaud L., de Pascale D., de Domenico M., di Prisco G., Fani R., Bruni V. Lipolytic activity of Antarctic cold-adapted marine bacteria (Terra Nova Bay, Ross Sea) J. Appl. Microbiol. 2006;101:1039–1048. doi: 10.1111/j.1365-2672.2006.03006.x. [DOI] [PubMed] [Google Scholar]
- 266.Al Khudary R., Venkatachalam R., Katzer M., Elleuche S., Antranikian G. A cold-adapted esterase of a novel marine isolate, Pseudoalteromonas arctica: Gene cloning, enzyme purification and characterization. Extremophiles. 2010;14:273–285. doi: 10.1007/s00792-010-0306-7. [DOI] [PubMed] [Google Scholar]
- 267.Rabus R., Ruepp A., Frickey T., Rattei T., Fartmann B., Stark M., Bauer M., Zibat A., Lombardot T., Becker I., et al. The genome of Desulfotalea psychrophila, a sulfate-reducing bacterium from permanently cold Arctic sediments. Environ. Microbiol. 2004;6:887–902. doi: 10.1111/j.1462-2920.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 268.Zeng X., Xiao X., Wang P., Wang F. Screening and characterization of psychrotrophic lipolytic bacteria from deep-sea sediments. J. Microbiol. Biotechnol. 2004;14:952–958. [Google Scholar]
- 269.Do H., Lee J.H., Kwon M.H., Song H.E., An J.Y., Eom S.H., Lee S.G., Kim H.J. Purification, characterization and preliminary X-ray diffraction analysis of a cold-active lipase (CpsLip) from the psychrophilic bacterium Colwellia psychrerythraea 34H. Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 2013;69:920–924. doi: 10.1107/S1744309113019428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Amoozegar M.A., Salehghamari E., Khajeh K., Kabiri M., Naddaf S. Production of an extracellular thermohalophilic lipase from a moderately halophilic bacterium, Salinivibrio sp. strain SA-2. J. Basic Microbiol. 2008;48:160–167. doi: 10.1002/jobm.200700361. [DOI] [PubMed] [Google Scholar]
- 271.Córdova-López J., Camacho-Córdova D.I., Camacho-Ruiz R.M., Mateos J.C., Rodríguez J. Haloarcula marismortui, eighty-four years after its discovery in the Dead Sea, Review. IJERT. 2014;3:1257–1267. [Google Scholar]
- 272.Muller-Santos M., de Souza E.M., Pedrosa Fde O., Mitchell D.A., Longhi S., Carriere F., Canaan S., Krieger N. First evidence for the salt-dependent folding and activity of an esterase from the halophilic archaea Haloarcula marismortui. Biochim. Biophys. Acta. 2009;1791:719–729. doi: 10.1016/j.bbalip.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 273.Corral P., Gutierrez M.C., Castillo A.M., Dominguez M., Lopalco P., Corcelli A., Ventosa A. Natronococcus roseus sp. nov., a haloalkaliphilic archaeon from a hypersaline lake. Int. J. Syst. Evol. Microbiol. 2013;63:104–108. doi: 10.1099/ijs.0.036558-0. [DOI] [PubMed] [Google Scholar]