Abstract
Up to 60 different proteins are recruited to the site of clathrin-mediated endocytosis in an ordered sequence. These accessory proteins have roles during all the different stages of clathrin-mediated endocytosis. First, they participate in the initiation of the endocytic event, thereby determining when and where endocytic vesicles are made; later they are involved in the maturation of the clathrin coat, recruitment of specific cargo molecules, bending of the membrane, and finally in scission and uncoating of the nascent vesicle. In addition, many of the accessory components are involved in regulating and coupling the actin cytoskeleton to the endocytic membrane. We will discuss the different accessory components and their various roles. Most of the data comes from studies performed with cultured mammalian cells or yeast cells. The process of endocytosis is well conserved between these different organisms, but there are also many interesting differences that may shed light on the mechanistic principles of endocytosis.
About 60 accessory proteins are involved in clathrin-mediated endocytosis. They participate in the initiation of endocytosis, clathrin coat maturation, cargo recruitment, membrane bending, and vesicle scission and uncoating.
Receptor-mediated endocytosis is the process by which eukaryotic cells concentrate and internalize cell surface receptors from the plasma membrane into small (∼50 nm– ∼100 nm diameter) membrane vesicles (Chen et al. 2011; McMahon and Boucrot 2011; Weinberg and Drubin 2012). This mechanism has been studied extensively in mammalian tissue culture cells and in yeast, and despite the evolutionary distance between yeast and mammalian cells the mechanism of receptor-mediated endocytosis in the respective cell types show remarkable similarities. Indeed many of the ∼60 endocytic accessory proteins (EAPs) found in yeast have homologs in mammalian cells, although both cell types also have unique EAPs (McMahon and Boucrot 2011; Weinberg and Drubin 2012).
In the following, we briefly describe known yeast and mammalian EAPs (Table 1). We then focus on recent efforts toward integrated models describing how EAPs are spatially and temporally organized to form endocytic vesicles. Many of these more recent studies have exploited advances in fluorescence microscopy and the use of fluorescent proteins (FPs) in live-cell imaging. We do not discuss the relationship between receptor-mediated endocytosis and signaling, which has been covered by recent reviews (Sigismund et al. 2012; see also Bökel and Brand 2013; Cosker and Segal 2014; Di Fiore and von Zastrow 2014).
Table 1.
Key endocytic proteins in mammals and in yeast
| Mammals | Yeast | Function | |
|---|---|---|---|
| Coat proteins | Clathrin | Chc1, Clc1 | Coat protein |
| AP-2 (4 subunits) | AP-2 (4 subunits) | Adaptor protein | |
| Epsin | Ent1/2 | Adaptor protein | |
| AP180 | Yap1801/2 | Adaptor protein | |
| CALM | – | Adaptor protein | |
| NECAP | – | Adaptor protein | |
| FCHo1/2 | Syp1 | Adaptor protein | |
| Eps15 | Ede1 | Scaffold protein | |
| Intersectin | Pan1 | Scaffold protein | |
| – | Sla1 | Scaffold protein | |
| – | End3 | Scaffold protein | |
| N-BAR proteins | Amphiphysin | Rvs161/167 | Membrane curvature sensor/generator |
| Endophilin | – | Membrane curvature sensor/generator | |
| BIN1 | – | Membrane curvature sensor/generator | |
| Dynamin | Dynamin1/2 | Vps1 | Mechanoenzyme, GTPase |
| Actin cytoskeleton | Actin | Act1 | Actin monomer |
| Arp2/3 complex | Arp2/3 complex | Actin filament nucleator | |
| ABP1 | Abp1 | Actin-binding protein | |
| Cortactin | – | Actin-binding protein | |
| Coronin | Crn1 | Actin-binding protein | |
| Cofilin | Cof1 | Actin depolymerizing protein | |
| Actin regulators | Myosin 1E | Myo3/5 | Actin motor |
| Myosin 6 | Actin motor | ||
| Hip1R, Hip1 | Sla2 | Actin-membrane coupler | |
| Syndapin | Bzz1 | BAR domain protein | |
| N-WASP | Las17 | Regulator of actin nucleation | |
| WIP/WIRE | Vrp1 | Regulator of actin nucleation | |
| SNX9 | – | Regulator of actin nucleation | |
| – | Bbc1 | Regulator of actin nucleation | |
| Other regulators | AAK1 | Ark1/Prk1 | Protein kinase |
| Auxilin, GAK | – | Uncoating factor | |
| Synaptojanin | Sjl2 | Lipid phosphatase | |
| OCRL1 | – | Lipid phosphatase |
The proteins are grouped into functional categories and the homologous proteins are listed on the same line.
A MODULAR DESIGN FOR YEAST AND MAMMALIAN ENDOCYTOSIS
In some respects, the endocytic machinery resembles a 3D jigsaw puzzle in which many of the pieces can interact with more than one of the other pieces and the size, composition, and shape of the jigsaw puzzle changes with time. Merely knowing the pieces does not tell you how the machine is organized.
Advances in live-cell fluorescence imaging techniques and the introduction of fluorescent proteins enabled the dynamics of endocytic accessory proteins to be monitored in live cells (Kirchhausen 2009). In yeast EAPs are recruited to, and dismissed from, sites of endocytosis in a stereotyped sequence relative to the inward movements of endocytic buds (Kaksonen et al. 2005; Carroll et al. 2012). Based on the spatiotemporal patterns of recruitment of endocytic accessory factors to sites of endocytosis, five distinct functional modules of proteins were identified: (1) an early module (Ede1, Syp1), (2) a coat module (clathrin, End3, Sla1, Pan1), (3) a WASP/Myo module (Bbc1, Myo5, Las17), (4) an amphiphysin module (Rvs161, Rvs167), and (5) an actin module (Sac6, Cap1, Cap2, Abp1, actin, Arp3) (Fig. 1). The precise role(s) of the different modules in endocytic bud formation are described in detail later in this review.
Figure 1.
Clathrin-mediated endocytosis in mammalian and yeast cells. The basic sequence of events is similar but the requirements for clathrin, actin, and dynamin differ between these organisms. The shape of the endocytic intermediates is approximately spherical in mammals, but tubular in yeast.
Unlike yeast cells, until recently it was not possible to replace endogenous genes in mammalian cells with their FP tagged cognates (but see Doyon et al. 2011). Instead low overexpression levels of endocytic accessory proteins were used, although this naturally raised concerns over potential artifacts caused by protein overexpression (Doyon et al. 2011; although see Aguet et al. 2013). Nonetheless, the recruitment of FP tagged endocytic accessory proteins was measured relative to single productive scission events in one cell type (mouse fibroblasts) with a temporal resolution of ∼2 sec (Merrifield et al. 2005; Taylor et al. 2011, 2012). Using cluster analysis to group protein-FP recruitment profiles, it was found that there were approximately seven natural groups of accessory protein recruitment kinetics corresponding to approximately four different protein modules: (1) the coat module, divided into (i) a clathrin submodule (epsin2, clathrin light chain, and NECAP), and (ii) an adaptor/F-BAR submodule (FCHo1/2, Eps15, AP2); (2) the NBAR domain module (endophilin2, amphiphysin2, and BIN1); (3) the actin module including (i) actin polymerization submodule (Abp1, cortactin, and Arp3), and (ii) actin depolymerization/suppression (cofilin, coronin1B, and SNX9); (4) the dynamin/myosin/N-WASP module (dynamin1/2, myosin/N-WASP, Eps8, Hip1R, myosin6, and syndapin2); (5) the GAK postscission module (GAK, ACK1, and OCRL1); (6) the Rab5a module (Rab5a and APPL1); and (7) the Fbp17/CIP4 module (Table 1) (Taylor et al. 2011).
The structural homologies between many mammalian and equivalent yeast EAPs are striking and the assembly dynamics of the EAPs appear overall rather similar. However, there are many intriguing differences in the spatiotemporal organization of the mammalian and yeast endocytic machinery. For instance, in mammalian cells clathrin is thought to be an integral part of the membrane-curvature generating mechanism required for clathrin-coated pit (CCP) invagination (Dannhauser and Ungewickell 2012; Kirchhausen 2012). In contrast, in yeast cells clathrin is not obligatory for receptor-mediated endocytosis (Payne et al. 1988). Rather, clathrin is recruited to the site of bud initiation when the membrane is still flat and membrane curvature is tightly correlated with actin polymerization (Kukulski et al. 2012). Finally, there are apparently striking differences in the mechanism of membrane scission in mammalian and yeast cells. In mammalian cells, membrane scission absolutely requires the large GTPase dynamin (Ferguson and De Camilli 2012) whereas in yeast cells actin, rather than an equivalent GTPase is important for scission (Kishimoto et al. 2011). The mechanistic similarities and differences between mammalian and yeast endocytosis are summarized in Figure 1.
Why are there differences in the organization of mammalian and yeast endocytosis? This may be attributable to physical constraints placed on the endocytic machinery in these two very different types of cells. For instance, it has been proposed that actin plays an obligatory role in walled yeast endocytosis because high membrane tension resulting from turgor pressure elicits more work to invaginate the membrane as compared with mammalian cells (Meckel et al. 2004; Aghamohammadzadeh and Ayscough 2009; Boulant et al. 2011). Indeed, there is evidence that membrane tension may modulate the requirement for actin in mammalian CME and yeast protoplasts wherein regimes of high membrane tension, generated by hyperosmotic challenge or physical stretching, invoke a requirement for actin (Aghamohammadzadeh and Ayscough 2009; Boulant et al. 2011). The differences in turgor pressure may also explain the differences in the shapes of endocytic membrane invaginations, which are roughly spherical in mammalian cells but tubular in yeast (Idrissi et al. 2012; Kukulski et al. 2012).
EAPs AND ENDOCYTIC SITE NUCLEATION: WHENCE THE PITS?
A simple question in the biology of CME is how CCPs start (Ehrlich et al. 2004; Antonescu et al. 2010; Henne et al. 2010; Nunez et al. 2011; Cocucci et al. 2012; Merrifield 2012; Umasankar et al. 2012). Much of the spatial and temporal regulation of endocytosis is probably conducted by regulating the initiation of CCP assembly. Many EAPs, including adaptor proteins, contain basic motifs adapted to bind negatively charged phosphatidylserine (PS) and phosphatidylinositol-4,5-bisphosphate (PIP2) concentrated in the inner leaflet of the plasma membrane (Antonescu et al. 2010). One might thus speculate a priori that the association of negatively charged phospholipids, adaptor proteins, receptor cargo, and coat proteins at the inner leaflet of the plasma membrane would be the first event in coated bud formation, and this has indeed been the central tenet in investigations of CCP nucleation.
First, the evidence for a role for negatively charged phospholipids in bud nucleation in both yeast and mammalian cells is well-established (Jost et al. 1998; Abe et al. 2008; Zoncu et al. 2009; Antonescu et al. 2010; Sun and Drubin 2012). In mammalian cells, depletion of plasma membrane PIP2 by pharmacological challenge with the Ca2+ ionophore ionomycin or by the engineered translocation of a PI5-phosphatase to the plasma membrane triggered a dramatic loss of CCPs and abolished CCP initiation events as measured using clathrin light chain-fluorescent protein (Clc-FP) or adaptor protein complex-2-fluorescent protein (AP2-FP) probes (Zoncu et al. 2009; although see Abe et al. 2008). By probing the cytoplasmic face of the plasma membrane with a GST-PH probe, gold labeling, and observation by EM, it was shown that PIP2 was significantly more concentrated at the rim of CCPs than on the surrounding, flat membrane or the apex of the CCP invagination (Fujita et al. 2009). It will be interesting to find, using alternative PI specific probes, whether other PIs are concentrated at CCPs with a similarly heterogeneous distribution.
Where does the PIP2 concentrated at CCPs come from? It seems unlikely that localized PIP2 production contributes to CCP initiation or maturation because no PIP2-kinases have been localized to CCPs (Sun et al. 2007; Antonescu et al. 2010). In contrast a variety of PIP2(5′)-phosphatases (OCRL, Sjn1, and Sjn2) do localize to CCPs and play important roles in CCP maturation and budding (Perera et al. 2006; Mao et al. 2009; Nakatsu et al. 2010, 2012; Taylor et al. 2011). A model has therefore emerged in which PIP2 diffusing in the plasma membrane is sequestered at the CCP nucleation site through interaction with a variety of PIP2-binding EAPs and wherein PIP2 turnover plays a key role in CCP maturation and scission (Antonescu et al. 2010). In a very recent development it was discovered that PI(3,4)P2 plays an essential role in the later stages of CCP invagination and transition from invaginated to Ω shaped CCPs (Posor et al. 2013). The PIP(3,4)P2 concentrated at CCPs is produced locally from PI(4,5)P2 by the concerted action of PI5 phosphatases and PI3K C2α and is required for recruitment of the BAR domain protein sorting nexin 9 (SNX9) (Posor et al. 2013). It should be noted that PI4P may also contribute to the recruitment of EAPs because PI(4,5)P2 and PI4P have some degree of overlap in defining the electrostatic properties of the inner leaflet of the plasma membrane (Hammond et al. 2012). The phosphoinositide PI4P has been associated with clathrin-coated vesicle (CCV) uncoating, so precisely how PIP2, PI(3,4)P2, and PI4P are coordinated at CCPs remains an intriguing area of investigation (Nakatsu et al. 2012; Posor et al. 2013). In yeast, negatively charged phospholipids play a similarly important role in CCP nucleation although here PS, rather than PIP2, is essential for endocytic bud site selection and initiation, whereas PIP2 is required for efficient invagination (Sun and Drubin 2012).
Second, the role of adaptor proteins in bud nucleation in both mammalian and yeast cells is generally clear (Motley et al. 2003; Antonescu et al. 2010; Cocucci et al. 2012), although some areas of controversy remain. In mammalian cells a plethora of adaptor proteins have been described (Fig. 1), although the AP2 complex is the best studied and most important. Knock- down of AP2, the main adaptor protein complex in mammalian cells, led to a dramatic loss of CCPs, abrogation of TfR (Motley et al. 2003) and EGFR internalization (Goh et al. 2010), and a correlate reduction in CCP nucleation events (Motley et al. 2003). However, a small population (∼1/12th the original) of CCPs nucleated in the apparent absence of AP2 and remained competent, for epidermal growth factor receptor (EGFR) internalization (Motley et al. 2003). It has been suggested that these CCPs retain small amounts of the residual AP2 complexes (Boucrot et al. 2010) which may be enough to nucleate CCP formation. Thus, whereas there remains some controversy, it seems likely that AP2 complexes play an essential role in the nucleation of most CCPs and AP2 may serve as a paradigm for the role played by adaptors in CCP nucleation. The distribution of PIP2 in the inner leaflet of the mammalian plasma membrane is uniform in nonexcitable cells (Antonescu et al. 2010) and AP2 has a relatively low affinity for PIP2, leading to the suggestion that AP2 “samples” the plasma membrane (Cocucci et al. 2012). On binding PIP2 the AP2 complex undergoes a dramatic structural rearrangement and unfurls to reveal cargo and clathrin-binding motifs (Jackson et al. 2010). If the AP2 complex then successfully binds receptor cargo and clathrin further PIP2-binding motifs are revealed, association with the membrane strengthens and nucleation of a nascent CCP begins (Jackson et al. 2010). In this model, the AP2 complex therefore acts as a coincidence detector directly linking PIP2, receptor cargo, and clathrin. Similarly, in yeast Ede1p (Eps15 homolog), clathrin, the AP2 complex, and Syp1p (FCHo homolog) are recruited early to nascent endocytic sites (Burston et al. 2009; Carroll et al. 2009; Reider et al. 2009). Deleting genes for Syp1, AP2 complex, or Yap1801/2 does not cause defects in endocytic dynamics but these proteins are important for the internalization of certain cargo (Burston et al. 2009; Carroll et al. 2009; Reider et al. 2009). The yeast scaffold protein Ede1p (Eps15 homolog) was found necessary for recruitment of the majority of early-arriving proteins barring the adaptor protein Yap1802p (Carroll et al. 2012).
Third, the (perhaps self-evident) role of clathrin in metazoan CME has been clearly shown and knockdown of clathrin heavy chain using siRNA led to a loss of coated pits and a concomitant decrease in both TfR and EGFR endocytosis (Motley et al. 2003; Huang et al. 2004). However, controlled elimination of clathrin heavy chain expression in chicken DT40 lymphocytes abrogated, but surprisingly did not entirely abolish, TfR recycling although the majority of cells died via apoptosis (Wettey and Jackson 2006). Unlike metazoan in yeast cells it was shown that clathrin is important, but not obligatory, for receptor-mediated endocytosis (Payne et al. 1988; Tan et al. 1993; Carroll et al. 2012). In CLC-1 knockout yeast, the later arriving proteins Sla2p (Hip1R homolog) and Ent1/2p (epsin homologs) were found to have longer lifetimes indicating that clathrin facilitates the transition from intermediate to late coat stages (Carroll et al. 2012).
Finally, the role of receptor cargo in CCP nucleation in mammalian cells has been clearly shown. Overexpression of the TfR did not increase the nucleation rate of CCPs but did increase the probability of CCPs successfully maturing (Loerke et al. 2009). However, an elegant experiment by Schmid and coworkers showed that local clustering of biotinylated TfR into tetramers using fluorescent streptavidin did promote CCP nucleation, suggesting that localized concentration of signaling motifs promoted CCP nucleation by recruiting adaptor proteins (Liu et al. 2010). The picture in yeast cells is less clearly defined and, unlike mammalian cells, the arrival of the receptor cargo, α-factor apparently occurred after the initiation of bud nucleation (Toshima et al. 2006).
Although the studies described have shown that anionic phospholipids, adaptor proteins, cargo, and coat proteins are necessary for bud initiation, the precise mechanism of the very earliest steps in CCP nucleation has remained obscure.
In mammalian cells, an elegant series of single molecule TIR-FM imaging experiments described the earliest 5 sec in the life of CCPs (Cocucci et al. 2012). Importantly, the stoichiometry of adaptors, clathrin, and receptor cargo could be inferred through careful quantification of the stepwise increases in FP tagged EAPs at nascent CCPs. It was shown that the nucleation of discrete, punctate CCPs occurred stochastically by minimal association of PIP2, 2AP2 molecule, and 1 clathrin triskelion (Cocucci et al. 2012), similar to predictions presented in earlier studies.
An alternative hypothesis to the “AP2-centric” model of CCP nucleation is that CCPs are nucleated by a specialized “nucleation complex” consisting of receptor cargo, PIP2, the adaptors AP2 and Eps15, and the F-BAR protein FCHo (Henne et al. 2010). This hypothesis in particular addressed the earliest stages of coat invagination and it was proposed that FCHo, a curvature-inducing F-BAR protein (Henne et al. 2007; Shimada et al. 2007) implicated in clathrin-mediated endocytosis (Sakaushi et al. 2007), might induce a “pimple” in the plasma membrane and so initiate the formation of a curved clathrin coat (Henne et al. 2010). However, subsequent experiments in zebrafish embryos and mammalian cultured cells suggested FCHo is nonessential for general CME (Shimada et al. 2007; Cocucci et al. 2012) and is rather an adaptor for BMP receptor (Umasankar et al. 2012) and/or facilitates CCP invagination (Gaidarov et al. 1999; Shimada et al. 2007; Cocucci et al. 2012).
In yeast cells a different strategy was used to capture the earliest events in bud formation. Correlative fluorescence microscopy and electron tomography (ET) was used to link the ultrastructure of endocytic buds to the relative time point in endocytic bud progression (Kukulski et al. 2012). Using Abp1-mRFP as a constant marker, cell lines coexpressing Ede1-GFP (Eps15), Sla1-GFP (Hip1R), and Rvs167-GFP (amphiphysin) were generated. By measuring the relative fluorescence of the Abp1-mRFP and corresponding GFP signal at randomly selected endocytic patches from the three cell lines, nine contiguous time windows were defined and mapped to the corresponding bud topology. When the early module or coat module was present in the absence of actin only flat membrane was found. After the initiation of actin polymerization, measured by Abp1-mRFP fluorescence, >99% of endocytic sites showed either an invagination or vesicle. These results suggest that curvature is not initiated at the point of coat recruitment but rather at the moment when actin polymerization begins at the endocytic site. Surprisingly, clathrin was found to be present on the membrane before the transition from a flat membrane to a highly curved invagination tip (Kukulski et al. 2012). This observation conflicts with the generally accepted dogma in which bud curvature is correlated with clathrin-coat growth (Ford et al. 2001, 2002).
THE CELL-WIDE SPATIAL AND TEMPORAL DYNAMICS OF CCP NUCLEATION
One of the great challenges of cell biology is to place molecular events with a spatial scale of nm and temporal scale <1 sec in the context of larger organelles with a spatial scale of μm and temporal scale >1 sec. The cell-wide nucleation of endocytic buds at the plasma membrane is a case in point and an area of active debate.
The nucleation of mammalian CCPs was first monitored using live-cell fluorescence microscopy (either TIRF or Epi) combined with the expression of clathrin-FP or AP2-FP, which allowed the spatiotemporal dynamics of CCP nucleation to be measured (Gaidarov and Keen 1999). The moment of nucleation of a CCP was taken as the earliest detection of a fluorescent clathrin or AP2 spot, which subsequently grew in fluorescence as the CCP matured (Gaidarov and Keen 1999), although the actual moment of nucleation probably occurred 10–20 sec earlier (Ehrlich et al. 2004). In one set of results, mammalian CCP nucleation was found to be pseudorandom and it was proposed that the departure from complete spatial randomness was caused by an exclusion zone around nascent CCPs (Ehrlich et al. 2004).
In some cellular contexts, the site of repeated mammalian CME events was found to be clearly nonrandom and occurred preferentially at “hot spots” (Gaidarov et al. 1999; Merrifield et al. 2005; Cao et al. 2011; Nunez et al. 2011; Taylor et al. 2011). Indeed, the first study to describe the live-cell imaging of CCPs using Clc-GFP revealed multiple CCVs emanating from the same site without the host clathrin-coated structure (CCS) fully dimming (Gaidarov et al. 1999). These results were subsequently confirmed using a TIRF-based assay to detect single productive scission events, which showed that both punctate and larger plaque-like clathrin structures (∼0.5–1 µm) in NIH-3T3 fibroblasts could host trains of multiple, quantized scission events (Merrifield et al. 2005; Taylor et al. 2011). Trains of scission events were spatially and temporally correlated with transient dimming of the host CCP, suggesting that only part of the coat participated in the budding event (Taylor et al. 2011). This would entail either remodeling of an existing flat clathrin lattice (den Otter and Briels 2011) or the addition of a curved domain to the flat lattice edge, as suggested by earlier EM images (Heuser 1980).
Even larger endocytic hotspots were revealed by live-cell confocal microscopy of Dyn2-GFP at the basal membrane of hepatocytes (Cao et al. 2011). In this instance, the endocytic hot spots occurred in ∼50% of cells with a lifetime of ∼10–30 min, were 2–10 µm in diameter, and could produce up to 10–15 vesicles/min, in contrast to the 1–2 vesicles/min supported by punctate CCPs. Ultrastructural analysis by EM revealed the hot spots comprised complex tubulovesicular networks with numerous budding clathrin coats. These structures are remarkably similar to the large, complex invaginations seen in neurons from dynamin knockout mice (Ferguson et al. 2007). It was suggested that the specialized endocytic plaques in hepatocytes may form transiently as clathrin and other endocytic factors concentrate above a threshold, although the trigger(s) responsible for the formation of such giant hot spots remain unclear.
It may be worth emphasizing here that the definition of “hot spot” is by no means fixed and apparently encompasses different, related phenomena. On the one hand, multiple CCVs can successively emerge in “trains” from fixed sites wherein the clathrin-FP signal never fully dims between budding events (Gaidarov et al. 1999; Merrifield et al. 2005; Cao et al. 2011; Taylor et al. 2011). On the other hand, the sequential nucleation of discrete CCPs can be both spatially and temporally correlated, suggesting that the plasma membrane has some form of “memory” for CCP nucleation (Nunez et al. 2011). Therefore that the term “hot spot” can mean both repeated endocytic events at the same CCS or repeated nucleation events of discrete CCPs at preferred sites at the plasma membrane. Precisely how these two different phenomena are related is currently unclear.
In yeast, the endocytic budding events are distributed in a nonrandom manner dependent on the cell-cycle state (Bi and Park 2012). Initially, in unbudded cells the endocytic sites are equally distributed throughout the cell, but when the budding starts the endocytic events become highly concentrated into the growing daughter cell. Later, during the cytokinesis, the endocytic sites concentrate to the cell division plane between the mother and daughter cells. This polarization of endocytic activity has an important role in the maintenance of the growth polarity (Jose et al. 2013).
At a smaller spatial scale the endocytic events in yeast appear random, except that they are excluded from plasma membrane regions that are in contact with cortical endoplasmic reticulum (Stradalova et al. 2012) and from eisosomes, stable microdomains of the fungal plasma membrane (Brach et al. 2011). The exclusion of endocytosis from these regions is most likely attributable to steric hindrance inhibiting bud nucleation.
COAT MATURATION AND THE PROPAGATION OF MEMBRANE CURVATURE
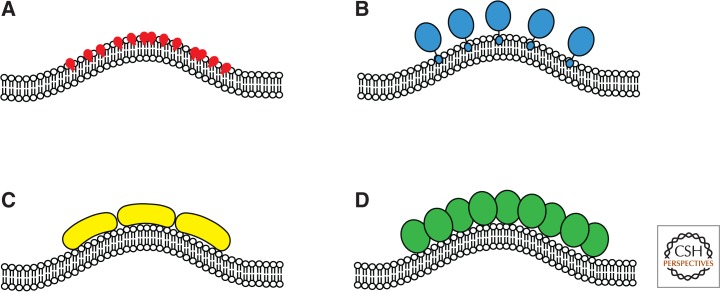
There is more than one mechanism to bend a membrane (Kirchhausen 2012; Shen et al. 2012) and therefore understanding precisely how EAPs initiate, promote, and maintain membrane curvature at the forming endocytic bud in vivo remains a challenging problem. Generating curvature in a phospholipid bilayer implies that some form of packing asymmetry exists between the two leaflets of the membrane (Kirchhausen 2012). In principle, there are at least four ways in which cytosolic EAPs may contribute to lipid asymmetry and membrane curvature during CCP invagination: (1) by locally clustering lipids with larger-than-average head groups through interaction with membrane-associated proteins (Zimmerberg and Kozlov 2006), by converting one lipid type to another (Kooijman et al. 2003) or by actively transporting a lipid type(s) from one leaflet to the other (Rauch and Farge 2000; Roelants et al. 2010); (2) by “floating” an amphipathic helix in the inner leaflet of the membrane (Ford et al. 2002) or by inserting a hydrophobic loop into the inner leaflet of the membrane (Plomann et al. 2010); (3) by association of a precurved membrane-binding protein domain (such as BAR, F-BAR, or i-BAR) with the membrane surface (Mim et al. 2012); or, most simply, (4) by membrane-associated protein crowding (Stachowiak et al. 2012). The different mechanisms for generating membrane curvature are summarized in Figure 2.
Figure 2.
Different mechanisms of membrane bending. (A) Local clustering of lipids with large head groups. (B) Insertion of an amphipathic helix or a loop into the membrane. (C) Binding of pre-curved proteins to the membrane surface. (D) Crowding of membrane-associated proteins.
Precisely which mechanism(s) plays a role in the different stages of CCP invagination and scission has been the subject of intense study and much of our understanding is based on in vitro reconstitution assays using purified components (Dannhauser and Ungewickell 2012; Ford et al. 2002). A variety of different EAPs have been shown to induce membrane curvature and lipid tubulation (Fig. 2) (Shen et al. 2012) but, perhaps surprisingly, the minimal components for CCP budding in vitro are lipid, an (synthetic) adaptor, clathrin and dynamin (Dannhauser and Ungewickell 2012). The synthetic adaptor used in these classic experiments was a mutant of epsin wherein the membrane bending ENTH domain was replaced with a histidine tag (H6-ΔENTH-epsin). Lipid vesicles, made from whole brain lipid, were doped with Ni2+-NTA-DOGS, which recruited H6-ΔENTH-epsin to the liposome surface. In turn H6-ΔENTH-epsin recruited clathrin, which, at 4°C, formed flat lattices reminiscent of clathrin plaques found in mammalian cells (Heuser 1980; Saffarian et al. 2009; Dannhauser and Ungewickell 2012). However, and quite remarkably, on warming to 37°C the plaques reorganized into clathrin-coated buds of uniform size, which could be released from the liposome surface by dynamin (Dannhauser and Ungewickell 2012). At 37°C, the plasma membrane is not a rigid sheet but undergoes low amplitude vibrations (Brown 2003) and although the characteristic length of membrane bending is short (∼50 nm) (Brown 2003), it remains an intriguing idea that the clathrin lattice may act as a Brownian ratchet and rectify thermally induced flexing of the membrane (Hinrichsen et al. 2006). This is an attractive idea because, presumably, the clathrin lattice could effectively integrate a variety of curvature-inducing mechanisms giving rise to the vectorial nature of CCP formation (Hinrichsen et al. 2006).
The degree of invagination of isolated mammalian CCPs is tightly correlated with the amount of clathrin coating on the budding membrane, as shown by thin-section EM (Ehrlich et al. 2004). From this, one might predict the state of curvature of individual CCPs, labeled with Clc-FP, based on the relative fluorescence of clathrin spots (Ehrlich et al. 2004). However, in many cell types at optical resolution such measurements are complicated by the proximity of clathrin-coated buds to neighboring clathrin-coated buds and/or flat clathrin lattices, at least at the basal surface of adherent cells (Heuser 1980; Hinrichsen et al. 2006). These are most likely endocytically active structures because, when individual scission events were detected in mammalian fibroblasts, it was shown that quantized scission events could be hosted by CCS of variable size and there was not a simple correlation between scission activity and the amplitude of Clc-FP signal (Merrifield et al. 2005; Taylor et al. 2011, 2012). At the time of writing, these findings have not been extended to explore the direct measurement of CCP curvature in real time and in live cells, although this is technically feasible (Sund et al. 1999).
MEMBRANE SCISSION
The final moment of endocytic vesicle formation is membrane scission wherein the thin membrane umbilicus linking an endocytic bud to the plasma membrane is severed (reviewed in Lenz et al. 2009; Campelo and Malhotra 2012). For mammalian cells, it is well established that membrane scission is controlled by the large GTPase dynamin and, because dynamin is perhaps one of the most extensively studied EAPs, rather than tackle the extensive literature, we will limit our discussion to a handful of recent key findings and refer the interested reader to several recent and extensive reviews (Mettlen et al. 2009; Ramachandran 2011; Schmid and Frolov 2011; Ferguson and De Camilli 2012; Morlot and Roux 2013; see also Johannes et al. 2014).
In the paradigm of dynamin action in mammalian cells, GTP-bound dynamin is recruited to the thin membrane neck of deeply invaginated CCPs where it executes membrane scission, most likely through GTP-hydrolysis-induced membrane compression (Morlot and Roux 2013). In a series of elegant biophysical studies using thin membrane tubules drawn from aspirated giant liposomes, Roux and colleagues showed that dynamin oligomerized on membrane tubules induced torque in the membrane tubule on the addition of GTP (Roux et al. 2006; Morlot and Roux 2013). It was shown that tubules sheared at the boundary between dynamin coated and uncoated sections of tubule (Morlot and Roux 2013). Membrane compression occurred rapidly following addition of GTP, as shown previously, but scission occurred much later with a longer delay following Poisson statistics. From these data, combined with careful modeling, the authors concluded that oligomerized dynamin compresses the membrane tubule to a diameter of ∼5 nm and developed stress at the boundary between the thin dynamin-coated section and thicker, uncoated adjacent regions. Critically, scission was found to be far more efficient if the membrane tubule was placed under tension, most likely because this eliminated the dissipation of dynamin-induced torque by membrane flow and instead favored membrane shearing (Morlot and Roux 2013). Most likely, a transient hemifused state leads to scission because the scission reaction is not leaky, as determined using elegant conductance measurements (Bashkirov et al. 2008). Overall, these results mesh well with recent studies publishing the crystal structure of dynamin, which provided detailed insight into the GTP-hydrolysis constriction mechanism of oligomerized dynamin (Chappie et al. 2011; Faelber et al. 2011, 2012; Ford et al. 2011).
The detailed analysis of the molecular mechanism of dynamin-induced membrane scission is a tour de force spread over several decades and involving many different labs (Mettlen et al. 2009; Ramachandran 2011; Schmid and Frolov 2011; Ferguson and De Camilli 2012; Morlot and Roux 2013). However, many questions remain concerning the mechanism of dynamin function and the roles of the numerous binding partners of dynamin including membrane curvature sensing/inducing BAR domain proteins such as amphiphysin and endophilin (Milosevic et al. 2011; Meinecke et al. 2013; Neumann and Schmid 2013) and proteins involved in actin polymerization/depolymerization (reviewed in Menon and Schafer 2013). Moreover, dynamin can also affect the earlier stages of CCP growth and invagination, although the precise mechanism by which it does so remains obscure (Loerke et al. 2009; Mettlen et al. 2009). Therefore the challenge remains to place the detailed molecular mechanism of dynamin membrane severing into the broader context of CCP maturation. One attractive idea is that dynamin works in concert with BAR domain proteins and the actin cytoskeleton to effect CCP invagination and that dynamin delivers the coup de grace to the resulting, strained membrane neck (Gu et al. 2010). This model is consistent with the discovery that in dynamin 1 + 2 double knockout fibroblasts CCPs invaginate into the cytoplasm atop long membrane tubules coated with the BAR domain protein endophilin and actin, showing that invagination and actin polymerization still occur in the absence of dynamin but in a deregulated manner (Ferguson et al. 2009). This is congruent with a model in which dynamin is recruited after actin and endophilin have formed a thin membrane neck. More recently it was shown that dynamin GTPase mutants modulate actin dynamics at invaginating CCPs (Taylor et al. 2012) and, intriguingly, that dynamin itself can bind to actin and displace the capping protein gelsolin (Gu et al. 2010). These results suggest that there may be a yet more intimate relationship between dynamin and actin polymerization during CCP invagination and membrane scission. However, mammalian endocytosis does not always require actin polymerization.
Importantly, this model of the mammalian endocytic scission mechanism is consistent with a broad range of findings, but despite the compelling evidence that dynamin is necessary for the budding of CCPs some puzzling facts remain. It seems unlikely that dynamin-mediated scission represents a universal mechanism of vesicle budding because vesicles can bud from other membrane surfaces in the cell in a dynamin-independent manner (Campelo and Malhotra 2012). Moreover, in yeast, the dynamin homolog Vps1 is required for efficient endocytosis but is dispensable for endocytic vesicle budding per se, whereas the actin cytoskeleton is not (Aghamohammadzadeh and Ayscough 2009; Smaczynska-de et al. 2012). In mammalian cells the opposite is true and dynamin is essential for endocytosis, whereas the actin cytoskeleton is only essential in some cell types or under certain conditions (Aghamohammadzadeh and Ayscough 2009; Boulant et al. 2011).
A second mechanism for effecting membrane scission is by the generation of a line tension in the membrane neck (Liu et al. 2006, 2009). Here, selective hydrolysis of PIP2 in the developing bud by the PI5′ phosphatase synaptojanin [in mammalian cells (Chang-Ileto et al. 2011)] or Sjl2 [in yeast cells (Stefan et al. 2005)] leads to a line tension between PI4P enriched in the bud and PIP2 enriched in the tubular invagination, which remains protected by BAR domain proteins (in yeast) and dynamin (in mammalian cells) (Chang-Ileto et al. 2011). In yeast cells, this mechanism alone may be sufficient to effect scission of the deep, tubular invaginations formed, perhaps because a turgor pressure of ∼1 MPa can help squeeze the nascent bud making a dynamin-like constriction mechanism redundant in the final stages of scission (Aghamohammadzadeh and Ayscough 2009). In mammalian cells, the same PIP2 hydrolysis mechanism most likely works in synergy with dynamin constriction and serves two roles: first to contribute to membrane instability by generating a line tension (Liu et al. 2006) and, second, to recruit the PI4P-binding G-cyclin-associated kinase (GAK), necessary for CCV uncoating, to the nascent bud (Lee et al. 2006). In this scenario, the coincidence of dynamin-constriction-mediated instability and a line tension would presumably work together to effect more efficient scission.
Overall, as for the mechanism(s) of membrane curvature, evolution has apparently blended these different mechanisms of membrane scission in different ways to suit the mechanochemical properties of the host cell. It will be of interest to explore this theme further in other types of walled cells, such as plant stromal guard cells, which have remarkably high turgor pressure of up to ∼4 MPa (Meckel et al. 2005).
UNCOATING
Following scission, CCVs rapidly shed their clathrin coat through the coordinated action of the ATPase/chaperone Hsc70 (Chappell et al. 1986) and neuronal J-domain kinase (Ahle and Ungewickell 1990) or ubiquitous paralog auxilin2 (cyclin-G-associated kinase, GAK) (Greener et al. 2000; Umeda et al. 2000).
Live-cell imaging studies revealed GAK was recruited to sites of scission after dynamin (Lee et al. 2006; Massol et al. 2006; Taylor et al. 2011) and that GAK recruitment to budding CCPs required GAK’s PI-binding PTEN domain (Lee et al. 2005; Massol et al. 2006). Full-length GAK was found to bind most tightly to monophosphorylated PIs, notably PI4P (Lee et al. 2005; Massol et al. 2006), which prompted the hypothesis that conversion of PIP2 to PI4P by a PI5′ phosphatase might act as the trigger recruiting GAK to newly scissioned vesicles (Massol et al. 2006). Indeed it was already known that the 5′-PI phosphatase synaptojanin is required for CCV uncoating (Verstreken et al. 2003) and knockout in mice of auxilin1 + 2 (Yim et al. 2005; Lee et al. 2008) or synaptojanin (Cremona et al. 1999) similarly led to accumulation of synaptic CCVs. Loss of PIP2 from the newly scissioned vesicle via synaptojanin-mediated PIP2 dephosphorylation would explain why CCVs also lose their AP2 shell because binding of AP2 to the membrane is mediated by PIP2 (Honing et al. 2005). This led to a model in which the coordinated action of synaptojanin (to generate PI4P and abolish AP-membrane interaction) and auxilin/GAK/Hsc70 (to remove clathrin) act together to irreversibly shed the clathrin/adaptor coat of newly formed CCVs (Massol et al. 2006).
One feature of this model that remained unclear was how auxilin / GAK recruitment and synaptojanin recruitment were coordinated. It was previously established that the BAR domain protein endophilin targets synaptojanin to newly scissioned vesicles in Drosophila (Verstreken et al. 2002). This was later confirmed by the generation of endophilin knockout mice by the de Camilli lab (Milosevic et al. 2011). Here, knockout of all three endophilin isoforms led to the accumulation of CCVs, but not CCPs, in mouse synapses (Milosevic et al. 2011).
CONCLUSIONS AND FUTURE DIRECTIONS
One of the most fascinating properties of the endocytic machinery is that, quite unlike any man-made machine, the machinery self-assembles in a stereotyped manner in the absence of any one “manager” and despite stochastic variation inherent in the small numbers of molecules involved (Sirotkin et al. 2010). Also, the endocytic machinery is remarkably robust in the sense that particular components can be ablated or mutated and the machinery will often still work, albeit with altered kinetics (Kaksonen et al. 2005). Understanding the design principles underlying the function of this prototypical self-assembling, a robust molecular machine remains a major challenge in the field.
Most of the experiments described in this review were designed to investigate the properties and mechanistic action of one or a few specific components of the endocytic machinery, often in isolation, and these types of experiments have been essential in understanding how the endocytic machinery works. However, the field has reached a stage of maturity in which diverse studies on different EAPs can be integrated into concise, predictive models. In an elegant paper, Drubin (an experimentalist) and Oster (a theorist) and colleagues integrated ultrastructural, biochemical, and live-cell imaging data of the endocytic machinery in yeast into a mechanochemical model of endocytosis (Liu et al. 2009). The model helps explain how, at each moment of the invagination and scission process, feedback loops between changes in membrane geometry, membrane composition, and EAP recruitment seamlessly set the scene for the next moment in the process. Perhaps the future of studies in endocytosis, and membrane trafficking in general, is to develop and rigorously test these types of models and ask how subtle changes in endocytic membrane trafficking may contribute to the development of different disease pathologies over the lifetime of an individual.
Building such predictive models will require systematic, quantitative data on the endocytic machinery including both reductionist data about individual components obtained from in vitro systems and more holistic data from intact cells. Combining these types of approaches will likely be essential for a comprehensive mechanistic model of the endocytic process.
Footnotes
Editors: Sandra L. Schmid, Alexander Sorkin, and Marino Zerial
Additional Perspectives on Endocytosis available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Abe N, Inoue T, Galvez T, Klein L, Meyer T 2008. Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. J Cell Sci 121: 1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghamohammadzadeh S, Ayscough KR 2009. Differential requirements for actin during yeast and mammalian endocytosis. Nat Cell Biol 11: 1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguet F, Antonescu CN, Mettlen M, Schmid SL, Danuser G 2013. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev Cell 26: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahle S, Ungewickell E 1990. Auxilin, a newly identified clathrin-associated protein in coated vesicles from bovine brain. J Cell Biol 111: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonescu CN, Danuser G, Schmid SL 2010. Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis. Mol Biol Cell 21: 2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, Frolov VA 2008. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell 135: 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Park HO 2012. Cell polarization and cytokinesis in budding yeast. Genetics 191: 347–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bökel C, Brand M 2014. Endocytosis and signaling during development. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E, Saffarian S, Zhang R, Kirchhausen T 2010. Roles of AP2 in clathrin-mediated endocytosis. PloS ONE 5: e10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T 2011. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol 13: 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach T, Specht T, Kaksonen M 2011. Reassessment of the role of plasma membrane domains in the regulation of vesicular traffic in yeast. J Cell Sci 124: 328–337. [DOI] [PubMed] [Google Scholar]
- Brown FL 2003. Regulation of protein mobility via thermal membrane undulations. Biophys J 84: 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston HE, Maldonado-Baez L, Davey M, Montpetit B, Schluter C, Wendland B, Conibear E 2009. Regulators of yeast endocytosis identified by systematic quantitative analysis. J Cell Biol 185: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo F, Malhotra V 2012. Membrane fission: The biogenesis of transport carriers. Ann Rev Biochem 81: 407–427. [DOI] [PubMed] [Google Scholar]
- Cao H, Krueger EW, McNiven MA 2011. Hepatocytes internalize trophic receptors at large endocytic “hot spots.” Hepatology 54: 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SY, Stirling PC, Stimpson HE, Giesselmann E, Schmitt MJ, Drubin DG 2009. A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms. Dev Cell 17: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SY, Stimpson HE, Weinberg J, Toret CP, Sun Y, Drubin DG 2012. Analysis of yeast endocytic site formation and maturation through a regulatory transition point. Mol Biol Cell 23: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Ileto B, Frere SG, Chan RB, Voronov SV, Roux A, Di Paolo G 2011. Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Dev Cell 20: 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell TG, Welch WJ, Schlossman DM, Palter KB, Schlesinger MJ, Rothman JE 1986. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell 45: 3–13. [DOI] [PubMed] [Google Scholar]
- Chappie JS, Mears JA, Fang S, Leonard M, Schmid SL, Milligan RA, Hinshaw JE, Dyda F 2011. A pseudoatomic model of the dynamin polymer identifies a hydrolysis-dependent powerstroke. Cell 147: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Irani NG, Friml J 2011. Clathrin-mediated endocytosis: The gateway into plant cells. Curr Opin Plant Biol 14: 674–682. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Aguet F, Boulant S, Kirchhausen T 2012. The first five seconds in the life of a clathrin-coated pit. Cell 150: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cosker KE, Segal RA 2014. Neuronal signaling through endocytosis. Cold Spring Harb Perspect Biol 6: a020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, et al. 1999. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99: 179–188. [DOI] [PubMed] [Google Scholar]
- Dannhauser PN, Ungewickell EJ 2012. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat Cell Biol 14: 634–639. [DOI] [PubMed] [Google Scholar]
- den Otter WK, Briels WJ 2011. The generation of curved clathrin coats from flat plaques. Traffic 12: 1407–1416. [DOI] [PubMed] [Google Scholar]
- *.Di Fiore PP, von Zastrow M 2014. Endocytosis, signaling, and beyond. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon JB, Zeitler B, Cheng J, Cheng AT, Cherone JM, Santiago Y, Lee AH, Vo TD, Doyon Y, Miller JC, et al. 2011. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat Cell Biol 13: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118: 591–605. [DOI] [PubMed] [Google Scholar]
- Faelber K, Posor Y, Gao S, Held M, Roske Y, Schulze D, Haucke V, Noe F, Daumke O 2011. Crystal structure of nucleotide-free dynamin. Nature 477: 556–560. [DOI] [PubMed] [Google Scholar]
- Faelber K, Held M, Gao S, Posor Y, Haucke V, Noe F, Daumke O 2012. Structural insights into dynamin-mediated membrane fission. Structure 20: 1621–1628. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P 2012. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, 2007. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 316: 570–574. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona O, et al. 2009. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 17: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291: 1051–1055. [DOI] [PubMed] [Google Scholar]
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT 2002. Curvature of clathrin-coated pits driven by epsin. Nature 419: 361–366. [DOI] [PubMed] [Google Scholar]
- Ford MG, Jenni S, Nunnari J 2011. The crystal structure of dynamin. Nature 477: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T 2009. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci 106: 9256–9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I, Keen JH 1999. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol 146: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I, Santini F, Warren RA, Keen JH 1999. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol 1: 1–7. [DOI] [PubMed] [Google Scholar]
- Goh LK, Huang F, Kim W, Gygi S, Sorkin A 2010. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol 189: 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener T, Zhao X, Nojima H, Eisenberg E, Greene LE 2000. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J Biol Chem 275: 1365–1370. [DOI] [PubMed] [Google Scholar]
- Gu C, Yaddanapudi S, Weins A, Osborn T, Reiser J, Pollak M, Hartwig J, Sever S 2010. Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J 29: 3593–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF 2012. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337: 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Kent HM, Ford MG, Hegde BG, Daumke O, Butler PJ, Mittal R, Langen R, Evans PR, McMahon HT 2007. Structure and analysis of FCHo2 F-BAR domain: A dimerizing and membrane recruitment module that effects membrane curvature. Structure 15: 839–852. [DOI] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT 2010. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 328: 1281–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J 1980. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol 84: 560–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen L, Meyerholz A, Groos S, Ungewickell EJ 2006. Bending a membrane: How clathrin affects budding. Proc Natl Acad Sci 103: 8715–8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S, Ricotta D, Krauss M, Spate K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ 2005. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell 18: 519–531. [DOI] [PubMed] [Google Scholar]
- Huang F, Khvorova A, Marshall W, Sorkin A 2004. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem 279: 16657–16661. [DOI] [PubMed] [Google Scholar]
- Idrissi FZ, Blasco A, Espinal A, Geli MI 2012. Ultrastructural dynamics of proteins involved in endocytic budding. Proc Natl Acad Sci 109: E2587–E2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LP, Kelly BT, McCoy AJ, Gaffry T, James LC, Collins BM, Honing S, Evans PR, Owen DJ 2010. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141: 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johannes L, Wunder C, Bassereau P 2014. Bending “on the rocks”—A cocktail of biophysical modules to build endocytic pathways. Cold Spring Harb Perspect Biol 6: a016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose M, Tollis S, Nair D, Sibarita JB, McCusker D 2013. Robust polarity establishment occurs via an endocytosis-based cortical corralling mechanism. J Cell Biol 200: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M, Simpson F, Kavran JM, Lemmon MA, Schmid SL 1998. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr Biol 8: 1399– 1402. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123: 305–320. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T 2009. Imaging endocytic clathrin structures in living cells. Trend Cell Biol 19: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T 2012. Bending membranes. Nat Cell Biol 14: 906–908. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Sun Y, Buser C, Liu J, Michelot A, Drubin DG 2011. Determinants of endocytic membrane geometry, stability, and scission. Proc Natl Acad Sci 108: E979–E988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman EE, Chupin V, de Kruijff B, Burger KN 2003. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic 4: 162–174. [DOI] [PubMed] [Google Scholar]
- Kukulski W, Schorb M, Kaksonen M, Briggs JA 2012. Plasma membrane reshaping during endocytosis is revealed by time-resolved electron tomography. Cell 150: 508–520. [DOI] [PubMed] [Google Scholar]
- Lee DW, Zhao X, Zhang F, Eisenberg E, Greene LE 2005. Depletion of GAK/auxilin 2 inhibits receptor-mediated endocytosis and recruitment of both clathrin and clathrin adaptors. J Cell Sci 118: 4311–4321. [DOI] [PubMed] [Google Scholar]
- Lee DW, Wu X, Eisenberg E, Greene LE 2006. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J Cell Sci 119: 3502–3512. [DOI] [PubMed] [Google Scholar]
- Lee DW, Zhao X, Yim YI, Eisenberg E, Greene LE 2008. Essential role of cyclin-G-associated kinase (auxilin-2) in developing and mature mice. Mol Biol Cell 19: 2766–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz M, Morlot S, Roux A 2009. Mechanical requirements for membrane fission: Common facts from various examples. FEBS Lett 583: 3839–3846. [DOI] [PubMed] [Google Scholar]
- Liu J, Kaksonen M, Drubin DG, Oster G 2006. Endocytic vesicle scission by lipid phase boundary forces. Proc Natl Acad Sci 103: 10277–10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sun Y, Drubin DG, Oster GF 2009. The mechanochemistry of endocytosis. PLoS Biol 7: e1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AP, Aguet F, Danuser G, Schmid SL 2010. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J Cell Biol 191: 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G, Schmid SL 2009. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol 7: e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Balkin DM, Zoncu R, Erdmann KS, Tomasini L, Hu F, Jin MM, Hodsdon ME, De Camilli P 2009. A PH domain within OCRL bridges clathrin-mediated membrane trafficking to phosphoinositide metabolism. EMBO J 28: 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol RH, Boll W, Griffin AM, Kirchhausen T 2006. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci 103: 10265–10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E 2011. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533. [DOI] [PubMed] [Google Scholar]
- Meckel T, Hurst AC, Thiel G, Homann U 2004. Endocytosis against high turgor: Intact guard cells of Vicia faba constitutively endocytose fluorescently labelled plasma membrane and GFP-tagged K-channel KAT1. Plant J 39: 182–193. [DOI] [PubMed] [Google Scholar]
- Meckel T, Hurst AC, Thiel G, Homann U 2005. Guard cells undergo constitutive and pressure-driven membrane turnover. Protoplasma 226: 23–29. [DOI] [PubMed] [Google Scholar]
- Meinecke M, Boucrot E, Camdere G, Hon WC, Mittal R, McMahon HT 2013. Cooperative recruitment of dynamin and BIN/amphiphysin/Rvs (BAR) domain-containing proteins leads to GTP-dependent membrane scission. J Biol Chem 288: 6651–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M, Schafer DA 2013. Dynamin: Expanding its scope to the cytoskeleton. Int Rev Cell Mol Biol 302: 187–219. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ 2012. Fishing for clathrin-coated pit nucleators. Nat Cell Biol 14: 452–454. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D 2005. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121: 593–606. [DOI] [PubMed] [Google Scholar]
- Mettlen M, Pucadyil T, Ramachandran R, Schmid SL 2009. Dissecting dynamin’s role in clathrin-mediated endocytosis. Biochem Soc Trans 37: 1022–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O’Toole E, Ferguson S, Cremona O, et al. 2011. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron 72: 587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mim C, Cui H, Gawronski-Salerno JA, Frost A, Lyman E, Voth GA, Unger VM 2012. Structural basis of membrane bending by the N-BAR protein endophilin. Cell 149: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlot S, Roux A 2013. Mechanics of dynamin-mediated membrane fission. Ann Rev Biophys 42: 629–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A, Bright NA, Seaman MN, Robinson MS 2003. Clathrin-mediated endocytosis in AP2-depleted cells. J Cell Biol 162: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu F, Perera RM, Lucast L, Zoncu R, Domin J, Gertler FB, Toomre D, De Camilli P 2010. The inositol 5-phosphatase SHIP2 regulates endocytic clathrin-coated pit dynamics. J Cell Biol 190: 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu F, Baskin JM, Chung J, Tanner LB, Shui G, Lee SY, Pirruccello M, Hao M, Ingolia NT, Wenk MR, et al. 2012. PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J Cell Biol 199: 1003–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Schmid SL 2013. Dual role of BAR domain-containing proteins in regulating dynamin-2 catalyzed vesicle release. J Biol Chem 288: 25119–25128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez D, Antonescu C, Mettlen M, Liu A, Schmid SL, Loerke D, Danuser G 2011. Hotspots organize clathrin-mediated endocytosis by efficient recruitment and retention of nucleating resources. Traffic 12: 1868–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GS, Baker D, van Tuinen E, Schekman R 1988. Protein transport to the vacuole and receptor-mediated endocytosis by clathrin heavy chain-deficient yeast. J Cell Biol 106: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D 2006. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci 103: 19332–19337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomann M, Wittmann JG, Rudolph MG 2010. A hinge in the distal end of the PACSIN 2 F-BAR domain may contribute to membrane-curvature sensing. J Mol Biol 400: 129–136. [DOI] [PubMed] [Google Scholar]
- Posor Y, Eichhorn-Gruenig M, Puchkov D, Schoneberg J, Ullrich A, Lampe A, Muller R, Zarbakhsh S, Gulluni F, Hirsch E, et al. 2013. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 499: 233–237. [DOI] [PubMed] [Google Scholar]
- Ramachandran R 2011. Vesicle scission: Dynamin. Sem Cell Dev Biol 22: 10–17. [DOI] [PubMed] [Google Scholar]
- Rauch C, Farge E 2000. Endocytosis switch controlled by transmembrane osmotic pressure and phospholipid number asymmetry. Biophys J 78: 3036–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider A, Barker SL, Mishra SK, Im YJ, Maldonado-Baez L, Hurley JH, Traub LM, Wendland B 2009. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J 28: 3103–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants FM, Baltz AG, Trott AE, Fereres S, Thorner J 2010. A protein kinase network regulates the function of aminophospholipid flippases. Proc Natl Acad Sci 107: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A, De Camilli P 2006. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441: 528–531. [DOI] [PubMed] [Google Scholar]
- Saffarian S, Cocucci E, Kirchhausen T 2009. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol 7: e1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaushi S, Inoue K, Zushi H, Senda-Murata K, Fukada T, Oka S, Sugimoto K 2007. Dynamic behavior of FCHO1 revealed by live-cell imaging microscopy: Its possible involvement in clathrin-coated vesicle formation. Biosci Biotechnol Biochem 71: 1764–1768. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Frolov VA 2011. Dynamin: Functional design of a membrane fission catalyst. Ann Rev Cell Dev Biol 27: 79–105. [DOI] [PubMed] [Google Scholar]
- Shen H, Pirruccello M, De Camilli P 2012. SnapShot: Membrane curvature sensors and generators. Cell 150: e1301–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, et al. 2007. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 129: 761–772. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G, Di Fiore PP 2012. Endocytosis and signaling: Cell logistics shape the eukaryotic cell plan. Physiol Rev 92: 273–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin V, Berro J, Macmillan K, Zhao L, Pollard TD 2010. Quantitative analysis of the mechanism of endocytic actin patch assembly and disassembly in fission yeast. Mol Biol Cell 21: 2894–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaczynska-de R II, Allwood EG, Mishra R, Booth WI, Aghamohammadzadeh S, Goldberg MW, Ayscough KR 2012. Yeast dynamin Vps1 and amphiphysin Rvs167 function together during endocytosis. Traffic 13: 317–328. [DOI] [PubMed] [Google Scholar]
- Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC 2012. Membrane bending by protein-protein crowding. Nat Cell Biol 14: 944–949. [DOI] [PubMed] [Google Scholar]
- Stefan CJ, Padilla SM, Audhya A, Emr SD 2005. The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Mol Cell Biol 25: 2910–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradalova V, Blazikova M, Grossmann G, Opekarova M, Tanner W, Malinsky J 2012. Distribution of cortical endoplasmic reticulum determines positioning of endocytic events in yeast plasma membrane. PloS ONE 7: e35132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Drubin DG 2012. The functions of anionic phospholipids during clathrin-mediated endocytosis site initiation and vesicle formation. J Cell Sci 125: 6157–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Carroll S, Kaksonen M, Toshima JY, Drubin DG 2007. PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J Cell Biol 177: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund SE, Swanson JA, Axelrod D 1999. Cell membrane orientation visualized by polarized total internal reflection fluorescence. Biophys J 77: 2266–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PK, Davis NG, Sprague GF, Payne GS 1993. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol 123: 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Perrais D, Merrifield CJ 2011. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol 9: e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Lampe M, Merrifield CJ 2012. A feedback loop between dynamin and actin recruitment during clathrin-mediated endocytosis. PLoS Biol 10: e1001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima JY, Toshima J, Kaksonen M, Martin AC, King DS, Drubin DG 2006. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent α-factor derivatives. Proc Natl Acad Sci 103: 5793–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umasankar PK, Sanker S, Thieman JR, Chakraborty S, Wendland B, Tsang M, Traub LM 2012. Distinct and separable activities of the endocytic clathrin-coat components Fcho1/2 and AP-2 in developmental patterning. Nat Cell Biol 14: 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda A, Meyerholz A, Ungewickell E 2000. Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur J Cell Biol 79: 336–342. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ 2002. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell 109: 101–112. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ 2003. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron 40: 733–48. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Drubin DG 2012. Clathrin-mediated endocytosis in budding yeast. Trend Cell Biol 22: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettey FR, Jackson AP 2006. Using DT40 to study clathrin function. Subcell Biochem 40: 119–143. [DOI] [PubMed] [Google Scholar]
- Yim YI, Scarselletta S, Zang F, Wu X, Lee DW, Kang YS, Eisenberg E, Greene LE 2005. Exchange of clathrin, AP2 and epsin on clathrin-coated pits in permeabilized tissue culture cells. J Cell Sci 118: 2405–2413. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Kozlov MM 2006. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol 7: 9–19. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P 2009. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136: 1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]