Abstract
With overwhelming evidence of change in habitats, biologists today must assume that few, if any, study areas are natural and that biological variability is superimposed on trends rather than stationary means. Paleobiological data from the youngest sedimentary record, including death assemblages actively accumulating on modern land surfaces and seabeds, provide unique information on the status of present-day species, communities, and biomes over the last few decades to millennia and on their responses to natural and anthropogenic environmental change. Key advances have established the accuracy and resolving power of paleobiological information derived from naturally preserved remains and of proxy evidence for environmental conditions and sample age so that fossil data can both implicate and exonerate human stressors as the drivers of biotic change and permit the effects of multiple stressors to be disentangled. Legacy effects from Industrial and even pre-Industrial anthropogenic extirpations, introductions, (de)nutrification, and habitat conversion commonly emerge as the primary factors underlying the present-day status of populations and communities; within the last 2 million years, climate change has rarely been sufficient to drive major extinction pulses absent other human pressures, which are now manifold. Young fossil records also provide rigorous access to the baseline composition and dynamics of modern-day biota under pre-Industrial conditions, where insights include the millennial-scale persistence of community structures, the dominant role of physical environmental conditions rather than biotic interactions in determining community composition and disassembly, and the existence of naturally alternating states.
Keywords: biodiversity, ecology, conservation, paleobiology, paleoecology
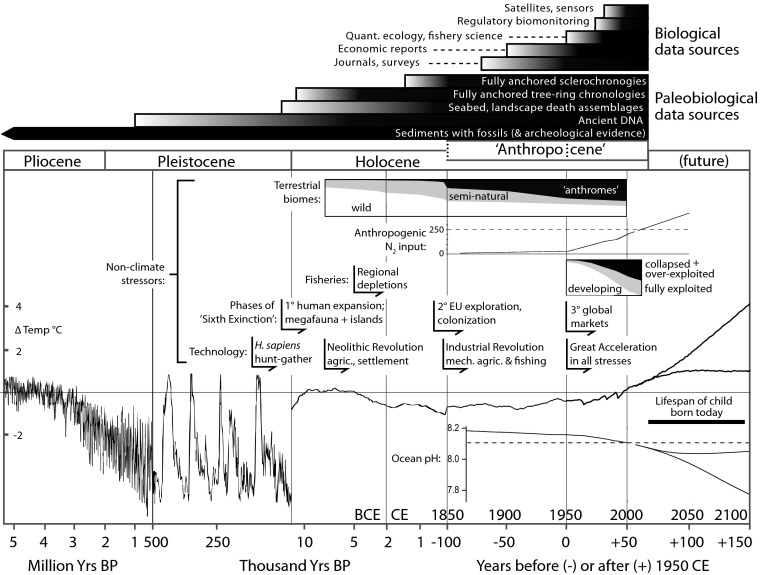
The Anthropocene is an informal term that is gaining wide currency for the present epoch of Earth’s history, when humans dominate a majority of natural processes globally (1, 2). The biological effects of climate change receive the greatest attention (3–6): secular warming, ocean acidification, and novel precipitation patterns are now pervasive (7, 8) (Fig. 1). However, many other human pressures—most particularly nutrification, habitat conversion, and the overexploitation and introduction of species—also intensified with the expansion of human populations and technology during the Industrial Revolution and the post-World War II “Great Acceleration” (2, 4, 17, 18) and are equally relevant to conservation and restoration efforts, as well as to basic ecological research. These pressures, moreover, started much earlier during preceding centuries to millennia at regional scales, both on land and in coastal seas, and have been accompanied by biological-stress syndromes (19), such as decreased body size, population size, trophic levels, and diversity, as well as functional and complete extinction of species (14, 20–24). These nonclimate factors are now as global as climate change (Fig. 1).
Fig. 1.
Historical trends in environmental conditions and technology over the last 5 million years, culminating in the Anthropocene, when human activities achieved a global signature. Most biological data (Top) are from studies conducted after World War II, and thus short-term variability has occurred in the context of trends rather than stationary means in climate, nutrients, and other human pressures. The deeper historical reach of paleobiological data challenge assumptions of stability and equilibrium and show that many populations, species, communities, and biomes had undergone significant changes in abundance, structure, and function at regional scales well before the mid-20th century. Note changes in scale along the x axis, where the present day is operationally set at 1950 CE (radiocarbon definition). Based on signals in sedimentary records and notwithstanding earlier effects on biota, geologists will likely formalize the start of the Anthropocene Epoch either at 1950 CE (i.e., when bomb-generated radionuclides appear and isotopically depleted industrial nitrates increase strongly) or at 1850 CE, with the culmination of the Industrial Revolution in Europe and North America, when atmospheric pCO2 first reached the upper limit of Holocene variability (the previous ∼12,000 y) (2, 9). Sources are as follows: ocean pH (adapted from ref. 8); temperature anomalies from a 1961–1990 baseline for the Plio-Pleistocene (reprinted from ref. 6 with permission from AAAS), Holocene to 1850 CE (10), and post-Industrial Revolution (HarCRUT3 data from ref. 11), all lowered by 0.3 °C to fit the 1986–2005 baseline used to project future changes (reprinted with permission from ref. 12); human cultural evolution (2); land-animal extinction phases, regional fisheries (13), and catch-based global data (reprinted with permission from ref. 14); land conversion (reprinted from ref. 15, by permission of the Royal Society); and anthropogenic reactive nitrogen input to biosphere (reprinted with permission from ref. 16), which now equals total natural N2 fixation (dashed line).
Today, even lakes in remote, high-altitude and -latitude settings receive atmospheric deposition of nitrates from industrial fertilizers (16, 25), and more than half of all land area inclusive of the Arctic has been converted to range, crop, or densely settled “anthromes” (15). The human footprint in marine settings has been more difficult to evaluate because these areas are further from direct observation, but seasonal hypoxia linked to the runoff of cultural nutrients occurs in all oceans and at more than 400 sites globally, having doubled each decade since the 1960s (26), and 32–57% of the world’s fisheries are assessed as overexploited or collapsed, with the remainder fully exploited (14). Both terrestrial and marine biologists now speak of a historically cumulative “Anthropocene defaunation,” a concept that includes many kinds of ecological disruption (e.g., refs. 27–30). More biological changes are almost certainly locked into place, much as additional decades of warming are inevitable from current levels of atmospheric CO2.
For biologists now fully embedded in the Anthropocene, the disconcerting working assumptions must be that (i) any area or biota under study today is not fully natural, given current human stresses and legacies of past stress, and (ii) interannual to -decadal variability is probably superimposed upon a trend rather than a stationary mean. These realizations underscore the arbitrary nature of most proposed ecological baselines, which are often defined by the first scientific observations (usually from the early 20th century, at best), by the oldest digitized records (1980s or younger), or by the personal experience of a scientist or manager (for discussion of the shifting baselines syndrome, see ref. 31) (Fig. 1). These realizations also suggest (iii) that many analytical subjects—species and populations, communities and habitats, landscapes and gradients, ecosystems and nutrient cycles—may well be in disequilibrial states, a fundamental challenge to experimentation, modeling, and prediction. Ecologists and conservation biologists increasingly recognize that unexpected relationships and outcomes often emerge as study durations exceed a decade, that directional change in climate and other environmental factors can compromise experimental controls, and that most field experiments incorporate legacy effects, such as species depletion or habitat change, that are only later appreciated (e.g., refs. 32–34).
The thesis of this Perspective is not simply that historical data of many types are needed, but that (i) the power of young fossil records to contribute critical data has been transformed in the last few decades by three revolutions in the earth sciences and (ii) paleobiologic analysis of records from the last few centuries and millennia is already challenging our understanding of ecological dynamics and contributing to conservation, restoration, and management efforts in a young field becoming known as conservation paleobiology (for reviews, see refs. 35–40). Paleobiology relies on a fundamentally different source of biological data from that of traditional ecology, drawing on naturally accumulated assemblages of skeletal and other dead remains rather than direct observation of living individuals and interactions (Fig. 1, Top). However, death assemblages forming on seafloors and land surfaces today, the precursors of fully buried fossil assemblages, include individuals drawn from still-standing populations. This overlap in the time frames of paleobiological and modern biological data provides a means of cross-validating shared data types (e.g., phenotype, DNA, abundance, range size), testing space-for-time assumptions, answering basic “before–after” questions, and building extended time series for extant (and recently extinct) species, communities, and ecosystems. Such time series are fundamental to framing and evaluating synthetic models, much as the integration of geohistorical and modern data has transformed our understanding of climate dynamics and is unveiling the interaction of climate and carbon cycles (e.g., ref. 41). Along with sedimentary cores, which are rarely taken [e.g., at long-term ecological research sites (LTERS)], death assemblages have been a severely underexploited source of biological data.
Integrating paleobiological, archeological, and biological data—and training—will thus become only more important as the 21st century unfolds. If we are to alter the trajectories of the most troubling biological trends in the modern world, or at the least manage biodiversity and ecosystem function in the certainty of continued climate and other change, we need to understand baseline dynamics, stressors, and biotic response on the centennial and longer time frames that encompass suspected natural and anthropogenic drivers.
Three Revolutions in the Earth Sciences That Have Transformed the Power of the Fossil Record
Fidelity of (Paleo)biological Information.
The first-order concern with all geohistorical records—ice cores, tree-rings, sediment cores, and biological remains in both natural and archeological contexts—is their fidelity, or faithfulness, to the original signal. Over the past two decades, taphonomic research on postmortem processes has dramatically increased the kinds of biological information that can be confidently extracted from fossil specimens, especially from the durable tissues that are most likely to be preserved under the more or less oxygenated conditions of soils, caves, lake floors, and seabeds (shells, bones, pollen, and other biomineralized or refractory organic tissues). Ancient DNA (aDNA) analysis can now exploit a larger array of materials and preservational settings than previously assumed, with a temporal scope to 1 Ma (million years ago) and full paleo-genomes now within reach (42). Diet, home range, growth rate, and other life-history information are regularly reconstructed for specimens from well-dated assemblages using many of the same isotopic, elemental, and histologic (e.g., sclerochronologic) methods applied to live-collected individuals, revealing both the vulnerability of species undergoing past population bottlenecks and their unsuspected trophic and life-history flexibility (43–46).
Taphonomic research has also quantified the reliability of paleoecological information at the community (assemblage) and metacommunity levels (for a review, see ref. 47). Concern with postmortem alteration of species richness and relative abundance has largely proven to be an oversimplification, notwithstanding the potential of interspecies differences in tissue durability, population turnover, and postmortem transportation to distort the record. In principle, the potential for postmortem bias of assemblage composition should be high where multiple generations of dead remains might accumulate before being sequestered by permanent burial, with habitats of different destructive intensity imposing a range of durations of such “time-averaging” (see table 1 in ref. 47). Despite these differences, research on the net effects of postmortem processes on community-level information, usually by comparing the naturally accumulating death assemblage with the local living assemblage, finds remarkably modest postmortem bias in a majority of cases, at least for the groups that have received the most intense evaluation (shelled mollusks, corals, land mammals, and wind-pollinated flora). Metaanalysis and modeling find that, in coastal soft-sediment habitats with minimal human modification, the difference in diversity between a time-averaged molluscan death assemblage and samples of a local, “nonaveraged” living assemblage can be predicted almost entirely from the contrast in temporal scale, much as biological samples from a large area differ from those from a small area (48, 49). Although death assemblages pool temporal variability and some spatial variability in local living assemblages, they still accurately detect environmental gradients and more closely approximate the species composition and abundance structure of the larger metacommunity, a stubborn data gap for biologists. Mammal death assemblages collected from open ground, raptor roosts, and marine flotsam have comparable ecological fidelity, with habitat- to regional-scale spatial resolution (50–52). Some clades with delicate skeletons have intrinsically poor preservation, of course, requiring analytic partitioning, and postmortem attenuation is stronger in some settings than others. Overall, however, rather than being compromised by myriad postmortem processes, death assemblages of key groups in undisturbed study areas have proven to be largely unbiased, albeit typically coarser, samples of their source communities. This fidelity is an extraordinary boon for the analysis of biodiversity, providing insights into species that are rare or otherwise difficult to sample, locally extirpated, or newly extinct (see examples in ref. 47).
A second key metaanalytic discovery is that strong live–dead discordance in species composition and relative abundance is a legacy of recent ecological change in the living assemblage: the living assemblage has apparently shifted away from its prior composition, which the time-averaged death assemblage remembers (53). This finding for marine mollusks overturns former assumptions that live–dead discordance is caused by poor postmortem preservation and seems to be general. For example, in Yellowstone National Park, elk have declined in the last decade in response to wolf reintroduction and are proportionately more abundant in death than in living assemblages; bison have increased in response to management changes and are proportionately more abundant alive than dead (ref. 54; see other examples in refs. 47 and 53, including the use of live-only species to detect new community states and species invasions). Only anthropogenic stressors seem to be capable of creating this magnitude of live–dead discordance, even in naturally variable environments such as lagoons, presumably because humans tend to produce a rapid, sustained change in environmental conditions (long-term press disturbance) or, in the case of introduced species, can overcome natural barriers to migration. Where human pressures are long-standing, or where postmortem destruction or deep burial is rapid (reducing the time frame for time averaging), baseline shifts may have occurred outside the memory of the local death assemblage, resulting in no live–dead discordance. This effect makes live–dead discordance a powerful and yet conservative method for detecting anthropogenic baseline shifts (more prone to false negatives than to false positives) (53).
Proxy Evidence of (Paleo)environmental Conditions.
For modern experimental and (geo)historical studies alike, testing for a biological response to environmental change requires environmental information that is accurate, spatially and temporally proximate, and not inferred from the populations under study. For periods before direct scientific observations—and in areas where monitoring is still limited—environmental conditions can be estimated from indirect, proxy variables that have been calibrated to direct measurements of conditions in modern environments. Data from tree rings, banded speleothems, and particulate charcoal in cores are examples of proxy records for past temperature and rainfall (e.g., ref. 35). Increasingly sophisticated analysis is reducing uncertainties in calibration and revealing the full magnitude of recent changes at the local to regional scales relevant to conservation and management (e.g., refs. 55 and 56).
The portfolio of methods is now diverse. Biological proxies remain important in all settings, for example indicator species of wetlands, seagrass, or ice cover and multispecies “transfer functions” used to infer temperature and salinity (57). However, as with niche models that use present-day bioclimatic associations to anticipate the future redistribution of species (see paleontologic tests of their reliability by refs. 58 and 59), there are concerns that the transfer–ecology approach is vulnerable to (i) failure of observed species’ distributions to reflect their full tolerances, (ii) inability to account for shifting biotic interactions and evolutionary adaptation, and (iii) assumptions of the statistical methods used (57, 60). Analysis of paleo-environmental conditions, including pollution and many aspects of ecosystem structure, has thus shifted increasingly from taxonomic to other biological, physical, and especially chemical evidence [isotopic and elemental ratios; environmental aDNA; molecular biomarkers of metabolism and organic-matter source; and indicator minerals of oxidation potential (Eh), pH, and salinity], achieving a new level of independence from ecological assumptions and expanding the dimensions of inference (e.g., refs. 45 and 61–66). For example, H isotope ratios of leaf waxes now supplement dust, pollen, and charcoal proxies of paleo-aridity, and all can survive transport to marine basins, permitting those sediments to capture the climate of both the ocean and the adjacent airshed (64). Elemental data from corals can establish that mortality is associated with the stress of anomalously high rather than low sea temperatures and can test for changes in levels and drivers of mortality (66).
Advances in Geochronology.
Testing hypotheses about biological change requires reliable information on the ages of specimens and assemblages. Decadal-, centennial-, or millennial-scale dating precision can suffice, depending on the question at hand. Recent advances in geochronology have broadened the scope of hypotheses that can be tested by (i) increasing the precision and accuracy of age estimates, (ii) expanding the list of materials that can be dated, and (iii) reducing the required mass of individual samples for analysis. For fossil records from the most recent ∼2 My, numerical age estimates rely on relatively short-lived natural radioisotopes and on cultural markers, such as nuclear bomb fallout, metal and persistent organic pollutants such as polychlorinated biphenyls (PCBs), and introduced species.
Radiocarbon remains the principal workhorse for records extending to ∼55 thousand years ago (ka) (67). The shift to accelerator mass spectrometry (AMS) 14C analysis during the 1990s improved the precision of age determinations to ±20–50 y (1 SD) for Holocene samples (the last 11.7 ka) and permitted analysis of carbon samples ∼one-tenth the mass previously required. Bomb production of 14C and dilution of 14C and 13C by fossil-fuel burning are persistent challenges for isolated young samples, but regional marine reservoir effects are now well-known and long tree-ring, speleothem, and coral records permit radiocarbon years to be calibrated to calendar years by accounting for natural variation in 14C production. New field, laboratory, and calibration protocols have similarly enhanced other methods. Amino acid racemization dating is applicable to carbonate shells up to ∼1 Ma in cool waters and can yield decadal resolution within the Holocene; U-Th dating is applicable to corals, speleothems, and bones up to ∼500 ka and provides decadal resolution within the last few thousand years; and optically stimulated luminescence is applicable to mineral grains deposited in terrestrial settings up to several hundred thousand years with resolution ±5–10% (66, 68–70). Naturally occurring 210Pb, which rains out rapidly from the atmosphere, remains a powerful method with approximately decadal resolution for sediments deposited within the past ∼150 y. Bomb-generated 137Cs is becoming more difficult to detect owing to radioactive decay, but longer lived plutonium isotopes and their daughters produced at the same time can take its place as a global geochemical marker.
Finally, both Bayesian and classical statistical inference are now used to interpolate sample ages between dated tie points within cores, typically with decadal resolution, providing more robust age models. Bayesian methods are also being used to temporally correlate records from widely separated areas, usually with a smaller loss in temporal acuity (e.g., two cores each capturing a local history with decadal resolution can now be correlated to each other with centennial precision) (71). These advances are all stunning improvements in age determination for individual specimens and host sediments, fully adequate to test for biological trends on decadal and longer scales.
Paleobiological Perspectives on Conservation, Management, and Ecological Theory
Persistence and Disassembly.
The ability of species and communities to persist over long periods is of great theoretical and practical interest, regardless of whether persistence reflects resistance to change in the face of environmental perturbation (robustness) or an ability to rebound from an altered state (resilience)—although the question of dynamics remains a key issue. At the community level, the historic range of variability of a community structure rather than of a particular species composition is now used as a dynamic target for management for federal lands in several countries (72), with paleoecological records from the last 3–4 ky used to establish the antiquity (“fundamental resilience”) of a forest type (73).
Evidence from the fossil record underscores the importance of scale and context. For example, over the last ∼6 ky of fairly stable climatic conditions, pollen analysis reveals the multimillennial persistence of terrestrial communities in many biomes and continents (73–75). Vegetation varied on decadal and centennial scales in concert with precipitation but maintained a structure and dominant functional type despite turnover in species and considerable pre-European land use. Some postcolonial, “secondary” forests have been similarly persistent for the last few centuries. In contrast, during strong climate changes between 50 and 6 ka, plant, insect, and mammal species exhibited individualistic shifts in their geographic range boundaries, meaning that species tended to relocate independently rather than as cohesive sets, resulting in novel or no-analog communities of still-extant species (refs. 76–78; in the sea, see refs. 79 and 80). Until human pressures emerged, climate-driven reshuffling of species entailed little evolutionary extinction (refs. 81 and 82; and see Unprecedented and Unsuspected Changes below).
Individualistic shifts in latitude, elevation, and bathymetry are now being detected by biologists, relying on the resurvey of an area that was first studied decades ago (e.g., refs. 34 and 83–85). Such modern-day corroborations are stimulating novel analyses of living populations in light of geologic history, finding, for example, that present-day endemism is most strongly correlated with the velocity of postglacial climate change rather than with a threshold temperature or particular direction of change (86) and that populations along the margin of a species’ range still exhibit low genetic diversity, a legacy of Quaternary expansion (87). Young fossil records alone show (i) just how pervasive changes in species’ distributions are likely to become, constituting both local losses and additions, (ii) that novel groupings are most likely to emerge near the edges rather than centers of biogeographic provinces or ranges (88, 89), (iii) that species most able to cross former boundaries and those showing strongest population declines are not random draws from their parent community (79, 90), and (iv) that species associations suggesting biotic interactions are rare and inconstant (91).
All of these dynamics can be expected as climate continues to change, arguably requiring new strategic approaches to management, conservation, and restoration (refs. 80 and 92; but see ref. 93). As a further challenge, many late 20th to early 21st century communities are geologically novel assemblages because of the introduced species they include, sometimes in key roles (e.g., ref. 80; and see Ranking Multiple Stressors below), placing a premium on having data of sufficient temporal scope to test whether dynamics and ecosystem processes differ fundamentally from those of communities assembled under natural conditions.
Regardless of how we choose to respond, the lessons from young fossil records are clear. To a first approximation—that is, with the exception of some obligate relationships—environmental conditions, including substratum type and disturbance regime, are better predictors than biotic interactions in determining which species assemble into and persist as a community. On decadal and longer time frames, stable conditions promote stable, persistent communities, and environmental change promotes community disassembly and reassembly into novel communities, probably for the same reasons that community composition varies spatially along environmental gradients. Once together, species may interact strongly or weakly, with more or less cascading effects: top-down changes—depletion or elimination of higher trophic levels—and loss of habitat-forming ecosystem engineers are typically anthropogenic and can dismantle a community in the absence of other environmental change (see Unprecedented and Unsuspected Changes below). The fossil record of the dynamics of extant species across the landscape or seascape indicates that the webs of biotic interactions observed in stable communities are allowed by stable environments rather than being a fundamental cause of community stability, as is evident from their modification or dissolution when species migrate (individualistically) in response to environmental change.
Alternative Stable States, Phase Shifts, and Recoveries.
The ability of populations and communities to alternate between discrete states under a single set of conditions has been difficult to demonstrate in modern systems (small-scale patch dynamics aside). Commonly, only a single shift can be recognized and is suspected to be anthropogenic, driven either by climate, habitat modification, and/or an increase or decrease in the density of a single species, particularly a top predator or ecosystem engineer (94–96). The rapid shift or collapse to a new state after some threshold stress is crossed (a nonlinear dynamic suggesting initial resistance), the ability to rebound to a former state after alleviation of stress (resilience), and the existence of warning signs for impending phase shifts, regardless of drivers, are important but challenging issues for field tests (but see ref. 97). Lags in response to stress and otherwise slow regime changes are particularly problematic for establishing cause and effect.
Lakes and coral reefs have received the greatest attention from biologists and are highly amenable to paleobiological analysis, which can test for patterns of change under fully or reasonably natural conditions where the existence of alternative stable states should be most clear. Lake and exceptionally high-resolution marine records of finfish reveal bimodal, decadal-to-centennial alternations in the abundance of key species that have persisted over millennia during pre-Industrial times (ref. 98; and see pollen studies cited above). Likely driven by climate, these alternations show that shifts in state are fully natural, perhaps with attractor states, and provide a baseline for evaluating the effects of commercial fishing and other human impacts. In the southern California pelagic system, for example, regular alternation in dominance by anchovies and sardines over the last ∼2 ka largely exonerate commercial harvesting as the primary driver of boom-and-bust populations of comparable decadal scale observed in the 20th century (99). In the NE Pacific and other oceans, strongly fluctuating populations of these species and other fish (salmon, hake) are linked to regional changes in temperature and productivity over past millennia up until climatic reorganization and commercial fishing within the last 100–150 y (98, 100). In tropical reefs, both historical documents and paleobiological analysis show that community structures have changed dramatically in the 20th century, in some cases to dominance by fleshy macroalgae or sponges (see next section). However, over the preceding 500 ky, different but remarkably consistent coral, red algal, and foraminiferal reef communities, all dominated by calcifiers, alternated on ∼100-ky time scales linked to the alternation of warm (highstand) and cooler, less favorable (lowstand) climatic regimes (101). Fossil records show that evolutionary survival entailed shifts of coral species both away from and back toward the core tropics (102) and that proximity to coral refugia during cool-water lowstands is the strongest correlate of modern-day richness in reef fish (ref. 103; for management implications, see ref. 104).
Alternating phases can thus emerge at multiple time scales. However, they are generally associated with evidence of change in environmental conditions, arguing against the existence of autogenically modulated alternating stable states in a strict sense, and some of the most impressive state changes within the last 2 My are unique to the Anthropocene (see next section). The power of fossil records for retrospective analysis could be productively focused on developing a stronger empirical understanding of possible harbingers of impending shifts, regardless of driver, such as increasing variance, decreasing resilience, and loss in trophic redundancy, and on anticipating community structures and compositions expected under future climate states (e.g., sponge-dominated reefs under ocean acidification) (105). Historical and paleobiological data that document rates and stages of past declines and recoveries (e.g., multidecadal to centennial perspectives of refs. 20, 23, 106, and 107) are also needed to frame expectations and parameterize regional models for restoration, moving beyond hypothetical diagrams of hysteresis. Compared with short-term assessments, metaanalysis using (geo)historical baselines reveals a lower frequency (10–50%) and magnitude of recovery, especially in species abundance and ecosystem structure, and finds that recoveries often require decades or more and attention to multiple stressors (ref. 106; and see Ranking Multiple Stressors below). This hard truth—even for marine systems, which are arguably less altered by human pressures than terrestrial systems (30)—needs to spur collaborative analyses and action, not discouragement or denial.
Unprecedented and Unsuspected Changes.
One of the key contributions of young fossil records is documentation of the unprecedented changes that have occurred in virtually all biomes within the last few centuries, especially evidence of sudden biotic collapse or shifts after millennia of stability or fluctuation around a stationary mean. Such data typically recalibrate our sense of scale, revealing large, unsuspected changes—mostly declines—(i) in the abundance and distribution of still-extant species and in the flexibility of their life-history and diet (e.g., refs. 31, 43, 44, and 108) and (ii) in the richness and complexity of communities and food webs (examples later in this section).
Fossil records do not carry the effort alone. Dramatic changes that occurred before professional scientific observations in an area can be revealed by written records—even the ancient Romans complained about declines in fish abundance (109)—and can be augmented by morphologic, isotopic, aDNA, and other analyses of archeological materials and museum-archived specimens (39, 110). Maps, photographs, and economic data can also draw back the veil on trends, especially for habitat and ecological changes at local-to-regional scales (23, 111–113), and experiments can test historical hypotheses of effect (e.g., ref. 114). In most instances, however, paleobiological analysis is needed to construct a documentary record that is sufficiently long, broad in scope (of taxa and environmental variables), and consistent in quality (determined by natural processes of fossil accumulation) to (i) verify or even recognize that change has occurred, (ii) evaluate the human and coupled human–natural stresses that might have caused or influenced biological change, and (iii) acquire a reasonable sample of variability and dynamics before the onset of those stresses.
Trophic simplification and diversity loss are common predictions of environmental stress, whether bottom-up (modification of nutrients and primary productivity, loss of habitat complexity, removal of habitat-former) or top-down (loss of consumers, especially predators). Top-down trophic cascades from the removal of apex consumers have now been recognized in all biomes but remain challenging for direct biological analysis: top-down effects can take years to decades to become evident due to long generation times and/or high motility of key species; processes commonly operate on much larger spatial scales than are amenable to experimentation; and populations of apex species are by now mostly reduced or extirpated (115).
Fossil records provide compelling evidence that, in the relatively recent past, many food webs were richer, with longer chains, more interactions, and different (mostly climate-controlled) dynamics of species substitution. For example, N-isotope analysis of 14C-dated bones shows that the Hawaiian petrel, a widespread and generalist oceanic predator, has fed at a significantly lower trophic level in the last 100 y than in the preceding 3,000 y, notwithstanding the arrival ∼950 y B.P. of humans to Pacific islands (116). Foraging segregation of petrel populations also decreased markedly, indicating less abundant prey. These changes suggest a rapid change in the composition of oceanic food webs well before direct scientific observation and are probably related to the development of commercial fishing. Shallow-water communities have also undergone significant simplification, mostly within the last few centuries, linked to the rise of sediment and nutrient runoff from colonial and industrial agriculture (refs. 117–119; but see ref. 120) and to top-down commercial extirpation of herbivores and filter-feeding invertebrates capable of keeping primary production in check (13). For example, the branching corals Acropora and Porites dominated Caribbean reefs for millennia (and for longer periods where records are sufficient) but, within the 20th century, have been widely replaced by turbidity-tolerant foliose Agaricia or noncalcifying taxa (refs. 121 and 122; and, in the Pacific, see ref. 118). Diatoms and other proxy records show that estuarine and lacustrine ecosystems have become more plankton-rich and detritus-based in recent decades to centuries, depending on the timing of watershed development and/or removal of key consumers, even when systems had been naturally eutrophic and episodically hypoxic over previous millennia (123–125).
Simplification of food webs and diversity loss are also evident in terrestrial records over the last 50 ka, usually with human involvement and entailing high extinction intensities (29, 126–128). Significant mammal and bird extinctions started with human expansion out of Africa in the Late Pleistocene, making initial contact with independently evolved faunas and eventually reaching Pacific islands by a few thousand years ago [approximately half of all mammal species >50 kg lost by ∼11 ka) (128); loss of ∼1,000 Pacific nonpasserine landbirds alone via pre-Industrial hunting, habitat change, and species introductions (129)]. A second phase of this “sixth extinction” began in the 15th century associated with European exploitation and agricultural colonialism (e.g., ref. 29), and a third, late 20th Century phase is underway related to the latest acceleration in human populations and global markets [manifest in the International Union for the Conservation of Nature (IUCN) Red List] (Fig. 1). The ecological consequences of regional species losses (and additions of introduced species) have been significant. For example, mammal food webs in Iberia exhibited relatively constant richness and structure for ∼800,000 y within the Pleistocene, with waning species being replaced by phylogenetically related species during each climate cycle, only to undergo dramatic reductions of richness and interactions in the Holocene (130). This change in structure and dynamics was not associated with a change in climate but occurred in two phases with (human-associated) extinction of mega-fauna and, as part of the Neolithic Revolution, the introduction of modern horse and cattle; the loss of specialist species actually increased connectivity, with humans as a new generalist predator (and see ref. 131).
Ranking Multiple Stressors.
Most regions today are under multiple anthropogenic pressures, but these variables occur in the context of natural processes such as orbital and other climate oscillations [El Niño Southern Oscillation (ENSO) and North Atlantic Oscillation (NAO)]. The potential for natural cycles and trends to amplify, mask, or reverse the effects of human pressure is thus a large concern in conservation. A related challenge is to disentangle the (probably compounding) effects of multiple human stressors. Rigorously determining the roles of different stressors—and of sequential application of stressors—requires datasets with sufficient temporal scope to look back past their onset, arguably a century at minimum even in remote areas and multiple centuries to millennia in many others. Given data of sufficient temporal scope, the roles of suspected stressors, human and otherwise, can be differentiated by comparing the timing, sign, and magnitude of changes in a target species or community or ecosystem (the response variable) with events in cultural history and with levels of variability and mean state before the onset of suspected activities.
The most contentious topic, among both biologists and paleobiologists, is the role of climate as a driver of biological change. In terms of species extinction, a fundamental tension exists between the persistence of most extant species despite strong Pleistocene climate variability and, on the other hand, the dramatic declines predicted for the future using bioclimatic envelope models and extrapolating from regional reductions in population sizes and genetic diversity (e.g., refs. 3, 4, 6, 82, and 84). Climate change was probably solely responsible for the extinction of some Eurasian and North American large-mammal species within the last 50 ky and may have reinforced the effects of hunting on others (132), modulating a simple “overkill” hypothesis for Pleistocene extinctions (and see refs. 126 and 128). If restricted to in situ evolution, many tetrapods will not survive projected warming unless rates of adaptation accelerate more than a thousandfold above those observed paleontologically (133).
Nonetheless, taking a global perspective of terrestrial vertebrate biodiversity over the last 2 My, the most compelling first-order pattern for elevated extinction is a close association with phases of human colonization and technological advance (e.g., ref. 127). The same can be said for marine mammals, although their extinctions arguably did not become significant until the Industrial Age (21, 23, 30, 31, 108). Among marine ectotherms, documented extinctions of corals, shelled mollusks, and fish are few, and those known are difficult to link to the present warming phase or to other warm pulses in the last 2 My (134). It is possible that, as with insects (82), the species most vulnerable to climate change were lost during the first strong Pleistocene excursions, leaving an inherently resilient subset able to survive subsequent climate changes up to the present day. Regional extirpation, short of global extinction and reflecting shifting ranges (see Persistence and Disassembly and Alternative Stable States, Phase Shifts, and Recoveries above), can still have large regional consequences, as shown by multicentennial and millennial disruptions of Pacific coral reef growth during past periods of high ENSO variability such as projected for the next century (135). The potential effects on biodiversity, community structure, and ecosystem function of the anthropogenic climate changes that are now unfolding (Fig. 1) thus should not be underestimated.
However, it would be equally wrong to underestimate the role of human pressures other than climate in creating the modern-day and future world. Overharvesting of wild species, species introductions, (de)nutrification, and other habitat modifications are intentionally excluded as “local effects” in many tests of climate impacts, but (i) are equally or more deeply rooted than post-Industrial warming, (ii) have become global since the 1950s (Fig. 1), and (iii) commonly emerge as the strongest correlates of biotic change at local to regional scales, both in biological and geohistorical analyses that consider multiple drivers (for examples, see refs. 40, 96, and the rest of this section). Such activities can have strong feedbacks on regional climate. For example, in New Zealand, pollen records show that wetland and lake landscapes today reflect changes in water balance driven by deforestation by pre-Industrial humans rather than recent or deep-past climate change (ref. 136 and in Europe, see ref. 137). The mixed results of climate-only studies may thus derive from unconsidered interacting variables (see discussions in refs. 6 and 17, and many others). Many biologists identify climate change as the greatest threat for future biodiversity loss but recognize that many changes today are smaller or different from expected, and they attribute these contradictions to other, usually anthropogenic factors (e.g., habitat conversion or invasive species that limit colonization).
Geohistorical analysis can create data of practical value while we strive to develop a general explanatory model for the roles of climate and other factors. For example, exploitation has figured in 95% of all depletions of animal species in estuaries, based on metaanalysis of nine suspected factors including climate change (20). Multiple factors (usually exploitation plus habitat loss) figure in ∼45% of losses and in 78% of recoveries of species with commercial, cultural, or other special value (for comparable analyses of reefs and recoveries, see refs. 106 and 117). Such multifactorial syntheses of data from written, archeological, and paleontological archives establish otherwise unknown baselines, provide an empirical foundation for identifying the factors that have contributed most to observed declines, allow areas to be ranked by degree of damage, and confirm that recoveries tend to require more factors than declines. In targeted areas, geohistorical data have resolved fundamental questions on native and nonnative species, altering restoration plans. For example, pollen analysis shows that plant species on the Galapagos Islands that were widespread and difficult to control are actually returning natives (ref. 138; for other examples, see refs. 40 and 47). Young fossil records also show that nonnative species that become dominant or habitat-transforming can have cascading effects on other groups—a particular concern given that many late 20th to early 21st century communities are novel assemblages because of the introduced species they include, sometimes in key roles (e.g., refs. 80 and 90).
Finally, paleontological data can be especially valuable for assessing multiple stressors on endangered species, where baseline data may be scarce and sampling living individuals is difficult or unethical (e.g., refs. 43, 44, and 108). For example, the critically endangered fish Totoaba macdonaldi in the upper Gulf of California has declined over the 20th century in both abundance and body size, and biologists suspected both direct fishing pressure and degradation of habitat in the Colorado River delta, owing to river damming. Sclerochronologic comparison of modern and 1,000- to 5,000-y-old otoliths established that Totoaba growth rates and thus sexual maturation slowed significantly coincident with river damming (139). Recovery of this traditional fishery thus cannot be achieved solely by relieving fishing pressure but will require restoration of some minimum river flow, with a binational trial flooding now underway.
Conclusions
Given extensive paleontological evidence for biotic change, the conclusion must be that, absent such long-term perspectives, most biological benchmarks—for abundance, distribution, variability, drivers, and dynamics—and rate estimates that are embedded within the last 50–100 y are probably far from natural. Natural may not be an achievable or desirable goal. However, paleobiological data for the recent past confront us with the true status of modern-day biota and with the very real potential that climate-driven changes will result in elevated extinctions rather than community disruptions alone, owing to the continued press of other human factors and damage already sustained. Paleontological data, derived both from fully buried fossil assemblages and from death assemblages that are still accumulating alongside living populations, constitute a powerful source of insights into the dynamics of extant (and recently extinct) species, communities, and ecosystems over the interdecadal to millennial time frames at which environments undergo natural and human-driven change. Improved environmental proxies, age-control, and confidence in paleobiological evidence mean that disparate data types should no longer impede the development of a rigorous “Biology in the Anthropocene” that squarely faces legacy effects, ongoing trends, and disequilibrial states as default conditions. We should in fact embrace the modern world as an unnatural experiment in progress, no matter how uncomfortable our eventual discoveries may prove to be, and commit to greater integration of modern and paleo approaches in both research and training. The fossil record’s future includes its ability to provide critical data and new time frames for conservation, management, and ecological theory.
Acknowledgments
I am grateful to two anonymous reviewers and M. Brenner, D. Jablonski, D. Kaufman, R. Terry, and K. Voorhies for exceptionally careful readings and constructive comments; S. Marcott for advice on compiling the temperature anomaly history in Fig. 1; and E. Gajos for artwork, which builds on an idea of C. Moritz and R. Agudo. Research was supported by National Science Foundation Grants EAR-0345897 and EAR-1124189.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Crutzen PJ. Geology of mankind. Nature. 2002;415(6867):23. doi: 10.1038/415023a. [DOI] [PubMed] [Google Scholar]
- 2.Steffen W, Grinevald J, Crutzen P, McNeill J. The Anthropocene: Conceptual and historical perspectives. Philos Trans A Math Phys Eng Sci. 2011;369(1938):842–867. doi: 10.1098/rsta.2010.0327. [DOI] [PubMed] [Google Scholar]
- 3.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecol Lett. 2012;15(4):365–377. doi: 10.1111/j.1461-0248.2011.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doney SC, et al. Climate change impacts on marine ecosystems. Annu Rev Mar Sci. 2012;4:11–37. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- 5.Poloczanska ES, et al. Global imprint of climate change on marine life. Nat Clim Chang. 2013;3(10):919–925. [Google Scholar]
- 6.Moritz C, Agudo R. The future of species under climate change: Resilience or decline? Science. 2013;341(6145):504–508. doi: 10.1126/science.1237190. [DOI] [PubMed] [Google Scholar]
- 7.Aronson RB, Thatje S, McClintock JB, Hughes KA. Anthropogenic impacts on marine ecosystems in Antarctica. Ann N Y Acad Sci. 2011;1223:82–107. doi: 10.1111/j.1749-6632.2010.05926.x. [DOI] [PubMed] [Google Scholar]
- 8.Bopp L, et al. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences. 2013;10(10):6225–6245. [Google Scholar]
- 9.Zalasiewicz J, Williams M, Waters CN. Can an Anthropocene series be defined and recognized? Geol Soc Lond Spec Publ. 2014;395:39–53. [Google Scholar]
- 10.Marcott SA, Shakun JD, Clark PU, Mix AC. A reconstruction of regional and global temperature for the past 11,300 years. Science. 2013;339(6124):1198–1201. doi: 10.1126/science.1228026. [DOI] [PubMed] [Google Scholar]
- 11.Brohan P, et al. Uncertainty estimates in regional and global observed temperature changes: A new dataset from 1850. J Geophys Res. 2006;111(D12):D12106. [Google Scholar]
- 12.IPCC . Summary for Policymakers. In: Stocker TF, et al., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 13.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293(5530):629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 14.Branch TA, Jensen OP, Ricard D, Ye Y, Hilborn R. Contrasting global trends in marine fishery status obtained from catches and from stock assessments. Conserv Biol. 2011;25(4):777–786. doi: 10.1111/j.1523-1739.2011.01687.x. [DOI] [PubMed] [Google Scholar]
- 15.Ellis EC. Anthropogenic transformation of the terrestrial biosphere. Philos Trans A Math Phys Eng Sci. 2011;369(1938):1010–1035. doi: 10.1098/rsta.2010.0331. [DOI] [PubMed] [Google Scholar]
- 16.Peñuelas J, et al. The human-induced imbalance between C, N and P in Earth's life system. Global Change Biology. 2013;18(1):3–6. [Google Scholar]
- 17.Vitousek PM, et al. Human domination of Earth's ecosystems. Science. 1997;277(5325):494–499. [Google Scholar]
- 18.Pusceddu A, et al. Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proc Natl Acad Sci USA. 2014;111(24):8861–8866. doi: 10.1073/pnas.1405454111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapport DJ, Regier HA, Hutchinson TC. Ecosystem behavior under stress. Am Nat. 1985;125(5):617–640. [Google Scholar]
- 20.Lötze HK, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312(5781):1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- 21.Rick TC, Erlandson JM, editors. Human Impacts on Ancient Marine Systems: A Global Perspective. Univ of California Press; Berkeley, CA: 2008. [Google Scholar]
- 22.Gedan KB, Silliman BR, Bertness MD. Centuries of human-driven change in salt marsh ecosystems. Annu Rev Mar Sci. 2009;1:117–141. doi: 10.1146/annurev.marine.010908.163930. [DOI] [PubMed] [Google Scholar]
- 23.Lötze HK, Worm B. Historical baselines for large marine animals. Trends Ecol Evol. 2009;24(5):254–262. doi: 10.1016/j.tree.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Strong DR, Frank KT. Human involvement in food webs. Annu Rev Environ Resour. 2010;35:1–23. [Google Scholar]
- 25.Catalan J, et al. Global change revealed by palaeolimnological records from remote lakes: A review. J Paleolimnol. 2013;49(3):513–535. [Google Scholar]
- 26.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321(5891):926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- 27.Jackson JBC. Colloquium paper: Ecological extinction and evolution in the brave new ocean. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11458–11465. doi: 10.1073/pnas.0802812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnosky AD, et al. Approaching a state shift in Earth’s biosphere. Nature. 2012;486(7401):52–58. doi: 10.1038/nature11018. [DOI] [PubMed] [Google Scholar]
- 29.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 30.McCauley DJ, et al. Marine defaunation: Animal loss in the global ocean. Science. 2015;347(6219):1255641. doi: 10.1126/science.1255641. [DOI] [PubMed] [Google Scholar]
- 31.Jackson JBC, Sala E, Alexander KE, editors. Shifting Baselines: The Past and the Future of Ocean Fisheries. Springer; Berlin: 2011. [Google Scholar]
- 32.Wootton JT, Pfister CA, Forester JD. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc Natl Acad Sci USA. 2008;105(48):18848–18853. doi: 10.1073/pnas.0810079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doak DF, et al. Understanding and predicting ecological dynamics: Are major surprises inevitable? Ecology. 2008;89(4):952–961. doi: 10.1890/07-0965.1. [DOI] [PubMed] [Google Scholar]
- 34.Rowe RJ, Finarelli JA, Rickart EA. Range dynamics of small mammals along an elevational gradient over an 80‐year interval. Glob Change Biol. 2010;16(11):2930–2943. [Google Scholar]
- 35.Swetnam TW, Allen CD, Betancourt JL. Applied historical ecology: Using the past to manage for the future. Ecol Appl. 1999;9(4):1189–1206. [Google Scholar]
- 36.Hayashida FM. Archaeology, ecological history, and conservation. Annu Rev Anthropol. 2005;34:43–65. [Google Scholar]
- 37.Willis KJ, Birks HJB. What is natural? The need for a long-term perspective in biodiversity conservation. Science. 2006;314(5803):1261–1265. doi: 10.1126/science.1122667. [DOI] [PubMed] [Google Scholar]
- 38.Dietl GP, Flessa KW. Conservation paleobiology: Putting the dead to work. Trends Ecol Evol. 2011;26(1):30–37. doi: 10.1016/j.tree.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Rick TC, Lockwood R. Integrating paleobiology, archeology, and history to inform biological conservation. Conserv Biol. 2013;27(1):45–54. doi: 10.1111/j.1523-1739.2012.01920.x. [DOI] [PubMed] [Google Scholar]
- 40.Dietl GP, et al. Conservation paleobiology: Leveraging knowledge of the past to inform conservation and restoration. Annu Rev Earth Planet Sci. 2015;43 [Google Scholar]
- 41.Foley AM, et al. Evaluation of biospheric components in Earth system models using modern and palaeo-observations: The state-of-the-art. Biogeosciences. 2013;10(12):8305–8328. [Google Scholar]
- 42.Shapiro B, Hofreiter M. A paleogenomic perspective on evolution and gene function: New insights from ancient DNA. Science. 2014;343(6169):1236573. doi: 10.1126/science.1236573. [DOI] [PubMed] [Google Scholar]
- 43.Chamberlain CP, et al. Pleistocene to recent dietary shifts in California condors. Proc Natl Acad Sci USA. 2005;102(46):16707–16711. doi: 10.1073/pnas.0508529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newsome SD, et al. The shifting baseline of northern fur seal ecology in the northeast Pacific Ocean. Proc Natl Acad Sci USA. 2007;104(23):9709–9714. doi: 10.1073/pnas.0610986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Limburg KE, et al. Tracking Baltic hypoxia and cod migration over millennia with natural tags. Proc Natl Acad Sci USA. 2011;108(22):E177–E182. doi: 10.1073/pnas.1100684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramakrishnan U, Hadly EA. Using phylochronology to reveal cryptic population histories: Review and synthesis of 29 ancient DNA studies. Mol Ecol. 2009;18(7):1310–1330. doi: 10.1111/j.1365-294X.2009.04092.x. [DOI] [PubMed] [Google Scholar]
- 47.Kidwell SM, Tomasovych A. Implications of time-averaged death assemblages for ecology and conservation biology. Annu Rev Ecol Evol Syst. 2013;44:539–563. [Google Scholar]
- 48.Tomasovych A, Kidwell SM. Fidelity of variation in species composition and diversity partitioning by death assemblages: Time-averaging transfers diversity from beta to alpha levels. Paleobiology. 2009;35(1):97–121. [Google Scholar]
- 49.Tomasovych A, Kidwell SM. Predicting the effects of temporal scaling on species composition, diversity, and rank abundance distributions in benthic assemblages. Paleobiology. 2010;36(4):672–695. [Google Scholar]
- 50.Terry RC. The dead do not lie: Using skeletal remains for rapid assessment of historical small-mammal community baselines. Proc Biol Sci. 2010;277(1685):1193–1201. doi: 10.1098/rspb.2009.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller JH, Druckenmiller P, Bahn V. Antlers on the Arctic Refuge: Capturing multi-generational patterns of calving ground use from bones on the landscape. Proc Biol Sci. 2013;280(1759):20130275. doi: 10.1098/rspb.2013.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pyenson ND. The high fidelity of the cetacean stranding record: Insights into measuring diversity by integrating taphonomy and macroecology. Proc Biol Sci. 2011;278(1724):3608–3616. doi: 10.1098/rspb.2011.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kidwell SM. Discordance between living and death assemblages as evidence for anthropogenic ecological change. Proc Natl Acad Sci USA. 2007;104(45):17701–17706. doi: 10.1073/pnas.0707194104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller JH. Ghosts of yellowstone: Multi-decadal histories of wildlife populations captured by bones on a modern landscape. PLoS ONE. 2011;6(3):e18057. doi: 10.1371/journal.pone.0018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marlon JR, et al. Long-term perspective on wildfires in the western USA. Proc Natl Acad Sci USA. 2012;109(9):E535–E543. doi: 10.1073/pnas.1112839109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly R, et al. Recent burning of boreal forests exceeds fire regime limits of the past 10,000 years. Proc Natl Acad Sci USA. 2013;110(32):13055–13060. doi: 10.1073/pnas.1305069110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birks HB, et al. Strengths and weaknesses of quantitative climate reconstructions based on late-Quaternary biological proxies. Open Ecol J. 2010;3(6):68–110. [Google Scholar]
- 58.Williams JW, et al. The ice age ecologist: Testing methods for reserve prioritization during the last global warming. Glob Ecol Biogeogr. 2013;22(3):289–301. [Google Scholar]
- 59.Davis EB, McGuire JL, Orcutt JD. Ecological niche models of mammalian glacial refugia show consistent bias. Ecography. 2014;37(11):1133–1138. [Google Scholar]
- 60.Huntley B. Reconstructing palaeoclimates from biological proxies: Some often overlooked sources of uncertainty. Quat Sci Rev. 2012;31(1):1–16. [Google Scholar]
- 61.Gooday AJ, et al. Historical records of coastal eutrophication-induced hypoxia. Biogeosciences. 2009;6(8):1707–1745. [Google Scholar]
- 62.Tripati AK, et al. 13C–18O isotope signatures and ‘clumped isotope’ thermometry in foraminifera and coccoliths. Geochim Cosmochim Acta. 2010;74(20):5697–5717. [Google Scholar]
- 63.Castañeda IS, Schouten S. A review of molecular organic proxies for examining modern and ancient lacustrine environments. Quat Sci Rev. 2011;30(21):2851–2891. [Google Scholar]
- 64.Sachse D, et al. Molecular paleohydrology: Interpreting the hydrogen-isotopic composition of lipid biomarkers from photosynthesizing organisms. Annu Rev Earth Planet Sci. 2012;40:221–249. [Google Scholar]
- 65.Rawlence NJ, et al. Using palaeoenvironmental DNA to reconstruct past environments: Progress and prospects. J Quat Sci. 2014;29(7):610–626. [Google Scholar]
- 66.Clark TR, et al. Discerning the timing and cause of historical mortality events in modern Porites from the Great Barrier Reef. Geochim Cosmochim Acta. 2014;138(1):57–80. [Google Scholar]
- 67.Jull AJ, Burr GS, Hodgins GW. Radiocarbon dating, reservoir effects, and calibration. Quat Int. 2013;299:64–71. [Google Scholar]
- 68.Kaufman DS, Manley WF. A new procedure for determining dl amino acid ratios in fossils using reverse phase liquid chromatography. Quat Sci Rev. 1998;17(11):987–1000. [Google Scholar]
- 69.Rhodes EJ. Optically stimulated luminescence dating of sediments over the past 200,000 years. Annu Rev Earth Planet Sci. 2011;39:461–488. [Google Scholar]
- 70.Price GJ, Feng YX, Zhao JX, Webb GE. Direct U–Th dating of vertebrate fossils with minimum sampling destruction and application to museum specimens. Quat Geochronol. 2013;18:1–8. [Google Scholar]
- 71.Blaauw M, et al. A Bayesian framework for age modeling of radiocarbon-dated peat deposits: Case studies from the Netherlands. Radiocarbon. 2007;49(2):357–367. [Google Scholar]
- 72.Keane RE, et al. The use of historical range and variability (HRV) in landscape management. For Ecol Manag. 2009;258:1025–1037. [Google Scholar]
- 73.Jackson ST. Conservation and resource management in a changing world: Extending historical range of variation beyond the baseline. In: Wiens JA, Hayward GD, Safford HD, Giffen C, editors. Historical Environmental Variation in Conservation and Natural Resource Management. Wiley; Chichester, UK: 2012b. pp. 92–109. [Google Scholar]
- 74.Willis KJ, Bhagwat SA. Questions of importance to the conservation of biological diversity: Answers from the past. Clim Past. 2010;6(6):759–769. [Google Scholar]
- 75.Willis KJ, Bailey RM, Bhagwat SA, Birks HJ. Biodiversity baselines, thresholds and resilience: Testing predictions and assumptions using palaeoecological data. Trends Ecol Evol. 2010;25(10):583–591. doi: 10.1016/j.tree.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Davis MB. Quaternary history and the stability of forest communities. In: West DC, Shugart HH, Botkin DB, editors. Forest Succession: Concepts and Application. Springer; New York: 1981. pp. 132–153. [Google Scholar]
- 77.Graham RW, et al. Spatial response of mammals to late Quaternary environmental fluctuations. Science. 1996;272(5268):1601–1606. doi: 10.1126/science.272.5268.1601. [DOI] [PubMed] [Google Scholar]
- 78.Williams JW, Jackson ST. Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ. 2007;5(9):475–482. [Google Scholar]
- 79.Roy K, Jablonski D, Valentine JW. Thermally anomalous assemblages revisited: Patterns in the extraprovincial latitudinal range shifts of Pleistocene marine mollusks. Geology. 1995;23(12):1071–1074. [Google Scholar]
- 80.Graham NA, et al. Coral reefs as novel ecosystems: Embracing new futures. Curr Opin Environ Sustain. 2014;7:9–14. [Google Scholar]
- 81.Stewart JR. The evolutionary consequence of the individualistic response to climate change. J Evol Biol. 2009;22(12):2363–2375. doi: 10.1111/j.1420-9101.2009.01859.x. [DOI] [PubMed] [Google Scholar]
- 82.Coope GR. Several million years of stability among insect species because of, or in spite of, Ice Age climatic instability? Philos Trans R Soc Lond B Biol Sci. 2004;359(1442):209–214, discussion 214. doi: 10.1098/rstb.2003.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moritz C, et al. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 2008;322(5899):261–264. doi: 10.1126/science.1163428. [DOI] [PubMed] [Google Scholar]
- 84.Cheung WW, et al. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009;10(3):235–251. [Google Scholar]
- 85.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333(6045):1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 86.Sandel B, et al. The influence of Late Quaternary climate-change velocity on species endemism. Science. 2011;334(6056):660–664. doi: 10.1126/science.1210173. [DOI] [PubMed] [Google Scholar]
- 87.Hill JK, Griffiths HM, Thomas CD. Climate change and evolutionary adaptations at species’ range margins. Annu Rev Entomol. 2011;56:143–159. doi: 10.1146/annurev-ento-120709-144746. [DOI] [PubMed] [Google Scholar]
- 88.Roy K. Analyzing temporal trends in regional diversity: A biogeographic perspective. Paleobiology. 2001;27(4):631–645. [Google Scholar]
- 89.Macken A, Reed E. Post-glacial reorganisation of a small mammal palaeocommunity in southern Australia reveals thresholds of change. Ecol Monogr. 2014;84(4):563–577. [Google Scholar]
- 90.Terry RC, Li CL, Hadley EA. Predicting small-mammal responses to climatic warming: Autecology, geographic range, and the Holocene fossil record. Glob Change Biol. 2011;17(10):3019–3034. [Google Scholar]
- 91.Blois JL, et al. A framework for evaluating the influence of climate, dispersal limitation, and biotic interactions using fossil pollen associations across the late Quaternary. Ecography. 2014;37(11):1095–1108. [Google Scholar]
- 92.Jackson ST, Hobbs RJ. Ecological restoration in the light of ecological history. Science. 2009;325(5940):567–569. doi: 10.1126/science.1172977. [DOI] [PubMed] [Google Scholar]
- 93.Murcia C, et al. A critique of the ‘novel ecosystem’ concept. Trends Ecol Evol. 2014;29(10):548–553. doi: 10.1016/j.tree.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 94.Knowlton N. Multiple ‘stable’ states and the conservation of marine ecosystems. Prog Oceanogr. 2004;60:387–396. [Google Scholar]
- 95.Folke C, et al. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 2004;35:557–581. [Google Scholar]
- 96.Rocha J, et al. Marine regime shifts: Drivers and impacts on ecosystems services. Phil Trans Roy Soc Biol Sci. 2015;370(1659):20130273. [Google Scholar]
- 97.Carpenter SR, et al. Early warnings of regime shifts: A whole-ecosystem experiment. Science. 2011;332(6033):1079–1082. doi: 10.1126/science.1203672. [DOI] [PubMed] [Google Scholar]
- 98.Finney BP, et al. Paleoecological studies on variability in marine fish populations: A long-term perspective on the impacts of climatic change on marine ecosystems. J Mar Syst. 2010;79(3):316–326. [Google Scholar]
- 99.Baumgartner TR, Soutar A, Ferreira-Bartrina V. Reconstruction of the history of Pacific sardine and northern anchovy populations over the last two millennia from sediments of the Santa Barbara Basin, California. CCOFI Rep. 1992;33:24–40. [Google Scholar]
- 100.Guiñez M, et al. Anchovy population and ocean-climate fluctuations in the Humboldt Current System during the last 700 years and their implications. Palaeogeogr Palaeoclimatol Palaeoecol. 2014;415:210–224. [Google Scholar]
- 101.Tager D, et al. Community dynamics of Pleistocene coral reefs during alternative climatic regimes. Ecology. 2010;91(1):191–200. doi: 10.1890/08-0422.1. [DOI] [PubMed] [Google Scholar]
- 102.Greenstein BJ, Pandolfi JM. Escaping the heat: Range shifts of reef coral taxa in coastal Western Australia. Glob Change Biol. 2008;14(3):513–528. [Google Scholar]
- 103.Pellissier L, et al. Quaternary coral reef refugia preserved fish diversity. Science. 2014;344(6187):1016–1019. doi: 10.1126/science.1249853. [DOI] [PubMed] [Google Scholar]
- 104.Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. Projecting coral reef futures under global warming and ocean acidification. Science. 2011;333(6041):418–422. doi: 10.1126/science.1204794. [DOI] [PubMed] [Google Scholar]
- 105.Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS. Could some coral reefs become sponge reefs as our climate changes? Glob Change Biol. 2013;19(9):2613–2624. doi: 10.1111/gcb.12212. [DOI] [PubMed] [Google Scholar]
- 106.Lötze HK, Coll M, Magera AM, Ward-Paige C, Airoldi L. Recovery of marine animal populations and ecosystems. Trends Ecol Evol. 2011;26(11):595–605. doi: 10.1016/j.tree.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 107.Mueller AD, et al. Recovery of the forest ecosystem in the tropical lowlands of northern Guatemala after disintegration of Classic Maya polities. Geology. 2010;38(6):523–526. [Google Scholar]
- 108.Estes JA, editor. Whales, Whaling, and Ocean Ecosystems. Univ of California Press; Berkeley, CA: 2006. [Google Scholar]
- 109.Lötze HK, Coll M, Dunne JA. Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean. Ecosystems (N Y) 2011;14(2):198–222. [Google Scholar]
- 110.Roy K, et al. Anthropogenic impacts and historical decline in body size of rocky intertidal gastropods in southern California. Ecol Lett. 2003;6(3):205–211. [Google Scholar]
- 111.Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science. 2007;315(5820):1846–1850. doi: 10.1126/science.1138657. [DOI] [PubMed] [Google Scholar]
- 112.McClenachan L. Documenting loss of large trophy fish from the Florida Keys with historical photographs. Conserv Biol. 2009;23(3):636–643. doi: 10.1111/j.1523-1739.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 113.Stein ED, et al. Historical ecology as a tool for assessing landscape change and informing wetland restoration priorities. Wetlands. 2010;30(3):589–601. [Google Scholar]
- 114.Altieri AH, Bertness MD, Coverdale TC, Herrmann NC, Angelini C. A trophic cascade triggers collapse of a salt-marsh ecosystem with intensive recreational fishing. Ecology. 2012;93(6):1402–1410. doi: 10.1890/11-1314.1. [DOI] [PubMed] [Google Scholar]
- 115.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 116.Wiley AE, et al. Millennial-scale isotope records from a wide-ranging predator show evidence of recent human impact to oceanic food webs. Proc Natl Acad Sci USA. 2013;110(22):8972–8977. doi: 10.1073/pnas.1300213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301(5635):955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 118.Roff G, et al. Palaeoecological evidence of a historical collapse of corals at Pelorus Island, inshore Great Barrier Reef, following European settlement. Proc Biol Sci. 2013;280(1750):20122100. doi: 10.1098/rspb.2012.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cramer KL, Jackson JB, Angioletti CV, Leonard-Pingel J, Guilderson TP. Anthropogenic mortality on coral reefs in Caribbean Panama predates coral disease and bleaching. Ecol Lett. 2012;15(6):561–567. doi: 10.1111/j.1461-0248.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- 120.Aronson RB, et al. Land use, water quality, and the history of coral assemblages at Bocas del Toro, Panamá. Mar Ecol Prog Ser. 2014;504:159–170. [Google Scholar]
- 121.Aronson RB, et al. Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology. 2004;85(7):1876–1891. [Google Scholar]
- 122.Pandolfi JM, Jackson JBC. Ecological persistence interrupted in Caribbean coral reefs. Ecol Lett. 2006;9(7):818–826. doi: 10.1111/j.1461-0248.2006.00933.x. [DOI] [PubMed] [Google Scholar]
- 123.Zillén L, et al. Past occurrences of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth Sci Rev. 2008;91(1):77–92. [Google Scholar]
- 124.Davidson TA, et al. Inferring past zooplanktivorous fish and macrophyte density in a shallow lake: Application of a new regression tree model. Freshw Biol. 2010;55(3):584–599. [Google Scholar]
- 125.Yasuhara M, Hunt G, Breitburg D, Tsujimoto A, Katsuki K. Human-induced marine ecological degradation: Micropaleontological perspectives. Ecol Evol. 2012;2(12):3242–3268. doi: 10.1002/ece3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grayson DK. The archaeological record of human impacts on animal populations. J World Prehist. 2001;15:1–68. [Google Scholar]
- 127.Burney DA, Flannery TF. Fifty millennia of catastrophic extinctions after human contact. Trends Ecol Evol. 2005;20(7):395–401. doi: 10.1016/j.tree.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 128.Koch PL, Barnosky AD. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 129.Duncan RP, Boyer AG, Blackburn TM. Magnitude and variation of prehistoric bird extinctions in the Pacific. Proc Natl Acad Sci USA. 2013;110(16):6436–6441. doi: 10.1073/pnas.1216511110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nenzén HK, Montoya D, Varela S. The impact of 850,000 years of climate changes on the structure and dynamics of mammal food webs. PLoS ONE. 2014;9(9):e106651. doi: 10.1371/journal.pone.0106651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yeakel JD, et al. Collapse of an ecological network in Ancient Egypt. Proc Natl Acad Sci USA. 2014;111(40):14472–14477. doi: 10.1073/pnas.1408471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lorenzen ED, et al. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature. 2011;479(7373):359–364. doi: 10.1038/nature10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Quintero I, Wiens JJ. Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol Lett. 2013;16(8):1095–1103. doi: 10.1111/ele.12144. [DOI] [PubMed] [Google Scholar]
- 134.Harnik PG, et al. Extinctions in ancient and modern seas. Trends Ecol Evol. 2012;27(11):608–617. doi: 10.1016/j.tree.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 135.Toth LT, et al. ENSO drove 2500-year collapse of eastern Pacific coral reefs. Science. 2012;337(6090):81–84. doi: 10.1126/science.1221168. [DOI] [PubMed] [Google Scholar]
- 136.Woodward C, Shulmeister J, Larsen J, Jacobsen GE, Zawadzki A. Landscape hydrology: The hydrological legacy of deforestation on global wetlands. Science. 2014;346(6211):844–847. doi: 10.1126/science.1260510. [DOI] [PubMed] [Google Scholar]
- 137.Cubizolle H, et al. Mire initiation, climatic change and agricultural expansion over the course of the Late-Holocene in the Massif Central mountain range (France): Causal links and implications for mire conservation. Quat Int. 2012;251:77–96. [Google Scholar]
- 138.Coffey EED, Froyd CA, Willis KJ. When is an invasive not an invasive? Macrofossil evidence of doubtful native plant species in the Galápagos Islands. Ecology. 2011;92(4):805–812. doi: 10.1890/10-1290.1. [DOI] [PubMed] [Google Scholar]
- 139.Rowell K, et al. Diverting the Colorado River leads to a dramatic life history shift in an endangered marine fish. Biol Conserv. 2008;141(4):1138–1148. [Google Scholar]