Significance
Alzheimer’s disease (AD) is a devastating disease that results in the progressive cognitive deficits of elderly and has become one of major social and economic burdens worldwide. There is no effective drug or therapy to prevent or halt the progressive cognitive dysfunctions due to the complex mechanisms such as accumulation of amyloid-β (Aβ), increase in oxidative stress, and formation of neurofibrillary tangle that drive the development of the disease. We found here that Edaravone, a drug that has been used for ischemic stroke, is able to prevent and treat AD by targeting multiple pathways of AD pathogenesis and rescuing the cognitive deficits of a mouse model of AD. Our study suggests Edaravone is a promising drug candidate for AD.
Keywords: Alzheimer’s disease, Edaravone, amyloid-β, BACE1, oxidative stress
Abstract
Alzheimer’s disease (AD) is one of most devastating diseases affecting elderly people. Amyloid-β (Aβ) accumulation and the downstream pathological events such as oxidative stress play critical roles in pathogenesis of AD. Lessons from failures of current clinical trials suggest that targeting multiple key pathways of the AD pathogenesis is necessary to halt the disease progression. Here we show that Edaravone, a free radical scavenger that is marketed for acute ischemic stroke, has a potent capacity of inhibiting Aβ aggregation and attenuating Aβ-induced oxidation in vitro. When given before or after the onset of Aβ deposition via i.p. injection, Edaravone substantially reduces Aβ deposition, alleviates oxidative stress, attenuates the downstream pathologies including Tau hyperphosphorylation, glial activation, neuroinflammation, neuronal loss, synaptic dysfunction, and rescues the behavioral deficits of APPswe/PS1 mice. Oral administration of Edaravone also ameliorates the AD-like pathologies and memory deficits of the mice. These findings suggest that Edaravone holds a promise as a therapeutic agent for AD by targeting multiple key pathways of the disease pathogenesis.
Alzheimer’s disease (AD) is the most common form of dementia among the elderly, and the incidence increases with the aging population worldwide, causing a huge social and economic burden for families and societies (1, 2). Accumulating evidence indicates that amyloid-β (Aβ) and its oligomers play central roles in the pathogenesis of AD (3). Despite significant progress that has been made toward understanding the pathogenesis of AD in recent years, no efficient disease-modifying therapeutics are available for the management of AD (4). In recent years, a number of drug candidates targeting Aβ through immunotherapy or using secretase inhibitors have proceeded to clinical trials but all failed to improve cognitive functions in patients (5). Clearly, lessons have been learned through failed clinical trials, indicating that a drug targeting a single target or pathway does not work on this complex disease (6). Aβ, overproduced and accumulated in AD brains, triggers subsequent pathological events such as synaptic degeneration, Tau-hyperphosphorylation, oxidative stress, neuroinflammation, neurite degeneration, and neuronal loss (7, 8). These secondary pathological events can form vicious cycles themselves and accelerate the disease progression (9–11). Therefore, we proposed that it is critical to discover novel drugs, which target multiple key pathways in the pathogenesis of AD, to improve or halt the progression of the disease (6).
As a series of new drugs for AD failed in clinical trials, it is necessary to choose drugs with both an established safety profile and a mechanism-based rationale for future clinical trials. One approach is to screen current drugs approved by regulatory bodies for other indications and reposition them for AD (12). In the present study, we took such an approach and investigated the potential therapeutic effect of Edaravone, an oxygen radical scavenger that is currently used for the treatment of acute ischemic stroke (13, 14). Oxidative imbalance is a manifestation of AD even preceding Aβ deposition and neurofibrillary tangle (NFT) (15). Aβ is a highly redox active peptide that generates reactive oxygen species (ROS) (16, 17). ROS is one of the key factors, which promote several Aβ-driven vicious cycles and propagate the pathogenesis of AD (9–11). Previous study found that Edaravone was able to attenuate Aβ-induced oxidative stress and neurotoxicity (18, 19). Aβ accumulation and aggregation into amyloid plaques in the brain are considered to trigger the AD pathogenesis. In the present study, we found that Edaravone can interact with Aβ and is competent in inhibiting Aβ aggregation and disaggregating preformed Aβ fibrils, suggesting that Edaravone is a scavenger for both ROS and Aβ. In animal models, we found that Edaravone, given before or after the onset of Aβ deposition, reduced Aβ burden in the brain and cerebral arterioles by inhibiting Aβ deposition and reducing BACE1 processing of the amyloid-β precursor protein (APP), attenuated oxidative stress and neuroinflammation, inhibited Tau hyperphosphorylation, protected brain neurons from loss and synaptic degeneration, and finally rescued the cognitive deficits of aged APPswe/PS1dE9 (APP/PS1) mice.
Results
Edaravone Inhibits Aβ Aggregation and Antagonizes Aβ Neurotoxicity in Vitro.
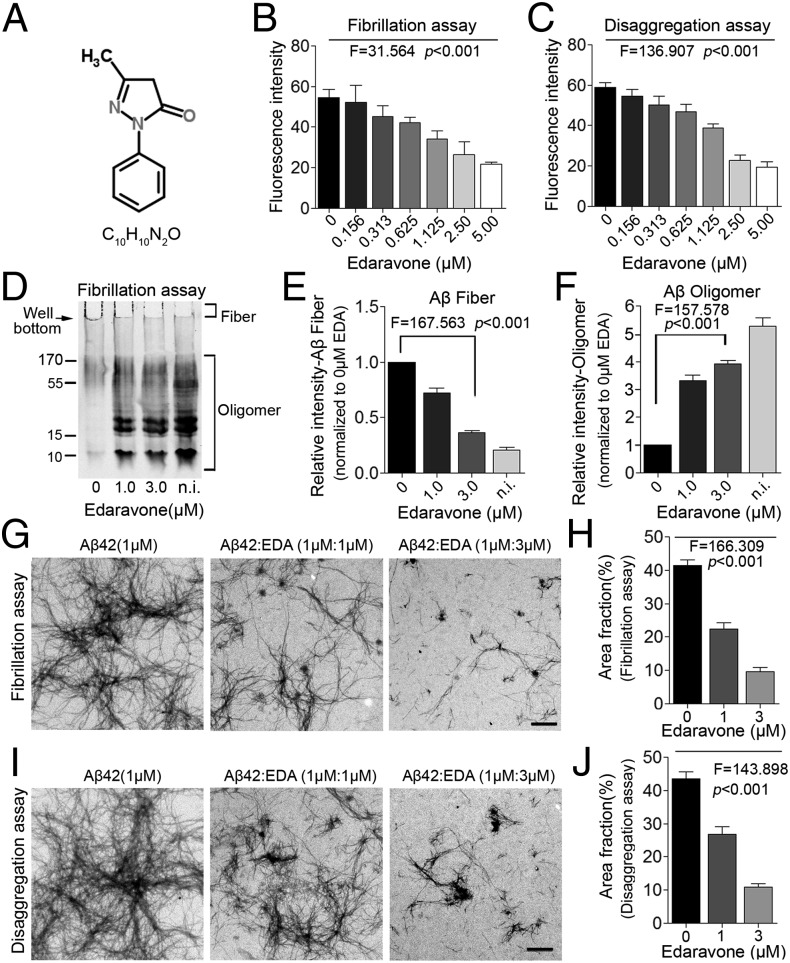
Previous studies suggested that several natural antioxidants such as curcumin and grape-derived polyphenolics can inhibit aggregation of Aβ (20, 21). Based on these findings, we speculate that Edaravone (the structure shown in Fig. 1A), an oxygen radical scavenger, might also be able to interfere with Aβ aggregation. Using Thioflavin T (ThT) fluorescence assay, we found that Edaravone, when incubated with Aβ monomers or preformed Aβ fibrils, dose-dependently reduced Aβ fibrillation-induced fluorescence intensity (Fig. 1 B and C). Western blot assays further showed that Edaravone inhibited the formation of Aβ fibrils during incubation (Fig. 1 D and E), which was revealed by the bands on the bottom of the loading wells without moving to the gel (arrow in Fig. 1D). Loaded with a fresh sample without prior incubation, soluble oligomers are the predominant Aβ species, and only faint fibrils were seen in the loading well (Fig. 1D). Edaravone preincubated with Aβ dose-dependently increased the soluble Aβ oligomer species (Fig. 1F). Moreover, transmission electron microscopy (TEM) assays visually confirmed that Edaravone suppressed the fibrillation of Aβ and disaggregated the preformed Aβ fibrils (Fig. 1 G–J). To elucidate the Edaravone binding epitope in Aβ42, we did Aβ42 fragment competition assays using ThT fluorescence measurement. We found that only the peptide of the Aβ42 fragment amino acid (aa) 13–18, among seven peptides that cover the entire Aβ aa sequence, had an ability to increase the Aβ fibril fluorescence, which was otherwise suppressed by Edaravone (Fig. S1A), indicating this peptide may compete for the binding site of Edaravone in Aβ42 and suggesting Edaravone may bind on the Aβ42 sequence aa 13–18. No peptides interfered with the formation of Aβ42 fibrils (Fig. S1B) or formed any fibrils themselves (Fig. S1C). The putative Edaravone binding epitope aa 13–18 is within the β strand region of Aβ42 (Fig. S1D).
Fig. 1.
Edaravone inhibits Aβ aggregation and disaggregates preformed Aβ fibrils in vitro. (A) Molecular structure of Edaravone. (B and C) ThT fluorescence assays for effect of Edaravone on Aβ aggregation (B) and disaggregation of preformed Aβ fibrils (C) (n = 3 per assay). (D–F) Western blot for effect of Edaravone on Aβ aggregation (D), and quantitative analyses of Aβ fiber (E) and soluble Aβ oligomer (F) (n = 3 per assay). (G and H) TEM images and quantitative analyses for effect of Edaravone on Aβ fibrillation (G and H) and disaggregation of preformed Aβ fibrils (I and J) (n = 8 per group). (Scale bar, 1 μm.) EDA, Edaravone; n.i., nonincubation. One-way ANOVA and trend test. Error bar, SEM.
Based on the above findings, we examined whether Edaravone can protect neurons from Aβ-induced neurotoxicity. In human neuroblastoma SH-SY5Y cells, Edaravone dose-dependently protected neurons from cell death (Fig. S2 A and B) and neurite collapse (Fig. S2C) triggered by Aβ. Furthermore, using neonatal primary cortical neurons as another cell model, we also found the protective effects of Edaravone against neurite collapse (Fig. S2 D and E), cell death (Fig. S2 F and G), and ROS production (Fig. S2H) triggered by Aβ. These results suggest that Edaravone has a potent capacity of inhibiting Aβ aggregation and neutralizing the toxicity of Aβ in vitro.
Edaravone Improves Cognitive Deficits Before and After Onset of Aβ Deposition.
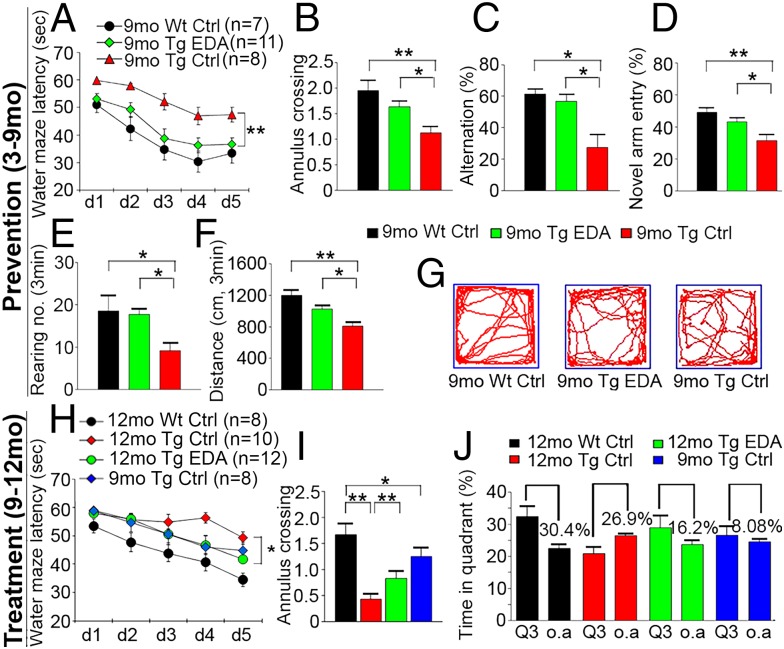
Based on its properties related to oxidative stress and Aβ aggregation, we next investigated the preventive and treatment effects of Edaravone on AD-type pathologies and cognitive deficits in APP/PS1 mice. All in vivo data presented below were from female AD mice except those specifically indicated. Compared with the normal saline control or the baseline control of APP/PS1 mice, the mice in the prevention and treatment groups performed better in the Morris water maze (Fig. 2 and Fig. S3), as reflected by significant reductions in the escape latency time (Fig. 2 A and H) and distance to platform (Fig. S3B) in progressive platform learning trials, greater numbers of annulus crossing (Fig. 2 B and I), and more time spent in target quadrant in the probe trial (Fig. 2J and Fig. S3D) in Edaravone-treated mice. As shown in Fig. 2A, the learning deficit in APP/PS1 mice at 9 mo of age was rescued by preventive medication of Edaravone, and the escaping time in Edaravone-treated mice was similar to WT controls. By 12 mo of age, although the escaping latency in the treatment group was longer than that in WT mice (Fig. 2H), it was similar to the baseline control of 9-mo-old APP/PS1 mice. There was no difference in swimming speed among control and experimental groups (Fig. S3C). The mice in the prevention group also performed better in Y-maze tests than the saline-treated control and the baseline controls (Fig. 2 C and D and Fig. S3 E–G). In spontaneous alternation tests, the mice treated with Edaravone showed a higher spontaneous alternation percentage (Fig. 2C) and greater total entries into three arms (Fig. S3E). Additionally, in novel arm exploration tests, Edaravone-treated mice showed more entries into and more time spent in the novel arm (Fig. 2D and Fig. S3G) and greater total entries (Fig. S3F). We also found a higher number of rearing (Fig. 2E) and a longer distance traveled (Fig. 2 F and G) in the prevention group in the open field test. No difference was found in the number of grooming behaviors among the different groups (Fig. S3H). In male APP/PS1 mice, Edaravone treatment from 9 to 12 mo of age also improved behavioral performances (Fig. S4 A–C). These results indicate that Edaravone can prevent or halt cognitive decline in aged APP/PS1 mice.
Fig. 2.
Edaravone improves behavioral performances of APP/PS1 mice. (A–G) Behavioral tests and quantitative analyses in the prevention experiment. (A) Escape latency during platform trials in Morris water maze. (B) Number of annulus crossing in probe test. (C and D) Percentage of alternation (C) and novel arm entry (D) in Y-maze test. (E and F) Number of rearing (E) and distance traveled (F) in open field test. (G) Representative tracing graphs of open field test. (H–J) Morris water maze tests in the treatment experiment. (H) Escape latency during platform trials. (I) Number of annulus crossing in probe test. (J) Quantitative analysis of time spent in quadrants. Q3, quadrant where the platform is located; o.a., all other quadrants. *P < 0.05, **P < 0.01 (two-way or one-way ANOVA). Error bar, SEM.
Edaravone Reduces Aβ Burden of APP/PS1 Mice.
To investigate whether Edaravone affects Aβ deposition in APP/PS1 mice, we performed Congo red staining for compact amyloid plaques and Aβ immunostaining (6E10) for total amyloid plaques. Compared with APP/PS1 controls, the mice treated with Edaravone in the prevention and treatment groups showed significantly lower amyloid plaque burden in the brain than the control group (Fig. 3 A, B, D, and E and Fig. S5 A and B). We further examined cerebral amyloid angiopathy (CAA). Based on the extent to which Aβ is deposited on the blood vessels, we categorized the severity of CAA into four grades from 0 (no CAA) to 3 (severe CAA) (Fig. 3G). We found that Edaravone significantly reduced proportion of severe CAA in both the prevention and treatment groups (Fig. 3H). The number of microhemorrhage profiles in the treatment group was also significantly reduced compared with the control (Fig. S2 C and D). ELISA tests also showed a significant decline in the levels of total Aβ, Aβ40, and Aβ42 in TBS, SDS, and formic acid (FA) fractions of brain homogenates (Fig. 3 C and F) in both the prevention and treatment groups compared with their respective controls. Edaravone treatment was also effective in reducing the brain Aβ burden of male APP/PS1 mice (Fig. S4 D–H). These data indicate that Edaravone can reduce both the parenchymal and vascular Aβ burden in brain.
Fig. 3.
Edaravone ameliorates amyloid deposition in APP/PS1 mice. (A and D) Congo red staining and 6E10 immunohistochemical staining in the prevention (A) and treatment (D) experiments. Insets show the representative plaque at higher magnification. (Scale bar, 1 mm.) (B and E) Comparison of Congo red- or 6E10-positive plaques in the neocortex (NC) and hippocampus (HC) in the prevention (B) or treatment (E) experiments. (C and F) ELISA of Aβ40 and Aβ42 in TBS, SDS, and formic acid (FA) fraction of brain homogenates in the prevention (C) or treatment (F) experiments. (G) Cerebral amyloid angiopathy (CAA) was visualized using double immunofluorescence of 1a4 (smooth muscle actin antibody) and 6E10 (Aβ antibody) and scored according to the four-level scale for the severity of CAA. [Scale bar, 20 (Upper) and 5 μm (Lower).] (H) Comparison of CAA scores. *P < 0.05, **P < 0.01 (Student t test). Error bar, SEM.
Edaravone Inhibits Amyloidogenic Processing of APP in APP/PS1 Mice.
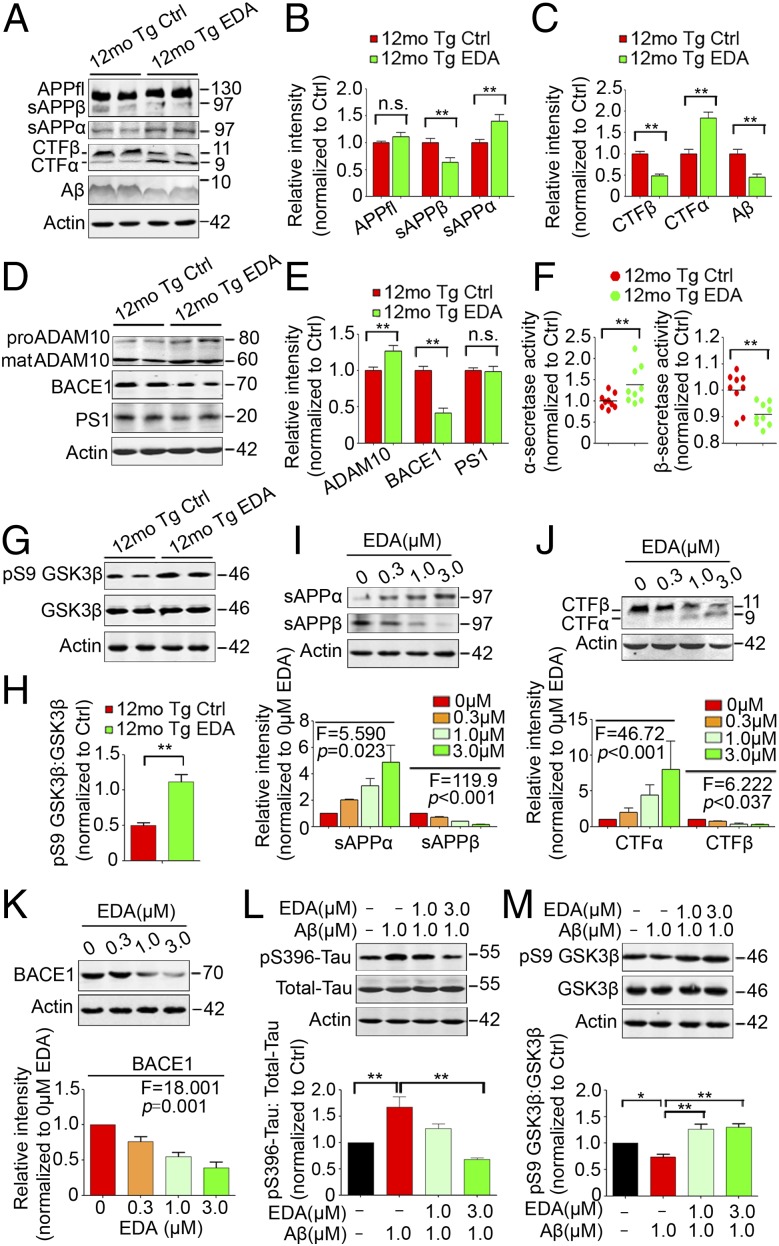
Edaravone had no effect on the expression of total APP (Fig. 4 A and B), however, it significantly reduced β-cleavage and increased α-cleavage of APP in the brains of APP/PS1 mice. The mice treated with Edaravone showed significantly decreased levels of β-cleavage products (CTFβ and sAPPβ) and Aβ production, and increased levels of CTFα and sAPPα in the treatment experiment (Fig. 4 A–C). Consistent with the in vivo data, the treatment of SH-SY5Y-APP695 cells with Edaravone dose-dependently increased the levels of CTFα and sAPPα and decreased the levels of CTFβ and sAPPβ (Fig. 4 I and J). Moveover, Edaravone treatment increased the expression of disintegrin and metalloprotease 10 (ADAM10) and α-secretase activity and decreased β-site APP cleaving enzyme 1 (BACE1) expression and activity, but had no effect on the expression of presenlin 1 (PS1) (Fig. 4D–F). Consistent with the results in vivo, Edaravone dose-dependently suppressed BACE1 expression in SH-SY5Y-APP695 cells (Fig. 4K). Edaravone had no effect on the expression of Aβ-degrading enzymes neprilysin (NEP) and insulin-degrading enzyme (IDE) or the Aβ blood–brain barrier (BBB)-transporting molecules low density lipoprotein receptor-related protein (LRP) and receptor for advanced glycation end products (RAGE) (Fig. S5 E and F).
Fig. 4.
Effects of Edaravone on APP processing, Tau phosphorylation and GSK3β activation. (A–E) Western blots and quantitative analysis for APP and APP metabolites (A–C) and ADAM10, BACE1, and PS1 (D and E) in brain homogenates. (F) α- and β-secretase activities in brain homogenates. (G and H) Western blots and quantitative analysis for pS9 GSK3β and total GSK3β in brain homogenates. (I–K) Western blots and quantitative analysis for sAPPα (I), sAPPβ (I), CTFβ (J), CTFα (J), and BACE1 (K) in SH-SY5Y-APP695 cell lysate. (L and M) Western blots and quantitative analysis for pSer396-Tau and total Tau (L), pS9 GSK3β and total GSK3β (M) in SH-SY5Y cell lysate. n.s., nonsignificant. *P < 0.05, **P < 0.01 (n = 8–9 per group for brain samples, Student t test; n = 3 for cell samples, one-way ANOVA and Tukey’s test). Error bar, SEM.
Edaravone Rescues Neuronal and Dendritic Loss and Attenuates Inflammation in the Brains of APP/PS1 Mice.
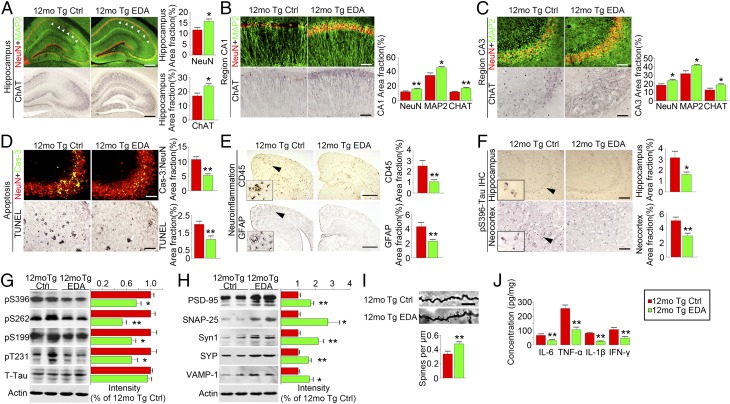
Compared with saline-treated APP/PS1 controls, APP/PS1 mice in both the prevention and treatment groups showed markedly increased positive-staining area fractions of NeuN (neurons), MAP2 (dendrites), and ChAT (cholinergic neurons) immunostainings (Fig. 5 A–C and Fig. S5 A and B) and significantly reduced apoptosis detected by caspase-3 immunofluorescence and TUNEL staining (Fig. 5D) in the hippocampus. Microgliosis (detected by CD45 antibody) and astrocytosis (detected by GFAP antibody) in the treatment group were significantly decreased (Fig. 5E). Additionally, the levels of proinflammatory cytokines, including TNF-α, IFN-γ, IL-1β, and IL-6, in brain homogenates in the treatment group were also lower than APP/PS1 controls (Fig. 5J). Importantly, we found that synapse-associated protein expression was significantly increased in brain homogenates of Edaravone-treated APP/PS1 mice in both the prevention (Fig. S6 C–E) and treatment (Fig. 5H) groups, and the number of dendritic spines detected by Golgi staining in the hippocampus was also significantly increased by Edaravone (Fig. 5I).
Fig. 5.
Edaravone attenuates neuronal loss and AD-type pathologies in APP/PS1 mice. APP/PS1 mice aged 9 mo were treated with Edaravone (12-mo Tg EDA, n = 9) or saline (12-mo Tg Ctrl, n = 7) for 3 mo. (A–C) MAP2, NeuN, and ChAT immunostaining and quantification in hippocampus and its subregions. [Scale bar, 400 (A) and 100 μm (B and C).] (D) Neuronal apoptosis detected by activated caspase-3 (cas-3) immunofluorescence (Top) in CA3 of hippocampus and by TUNEL staining (Middle) in neocortex. [Scale bar, 100 (Top) and 200 μm (Middle).] (E) Immunostaining and quantification of microgliosis and astrocytosis. (Scale bar, 1 mm.) (F) Quantification of Tau phosphorylation using pSer396-Tau immunohistochemistry. (G) Western blot and quantification for phosphorylated Tau at multiple sites including pS396-, pS262-, pS199-, pT231-Tau, and total Tau (T-tau) in brain homogenates. (H) Western blot and quantification for synapse-associated proteins including SNAP25, PSD95, Synapsin I (Syn I), VAMP1, and synaptophysin (SYP) in brain homogenates. (I) Representative photomicrograph and quantification of dendritic spines intensity of basal segment of CA1 hippocampal neuron (three mice per group and seven neurons per mouse). (Scale bar, 10 μm.) (J) Quantification of IL-1β, IL-6, TNF-α, and IFN-γ in brain homogenates. *P < 0.05, **P < 0.01 (Student t test). Error bar, SEM.
Edaravone Attenuates Tau-Phosphorylation in APP/PS1 Mice.
Edaravone significantly improved Tau pathology in the prevention and treatment experiments (Fig. 5 F and G and Fig. S7). The area fractions of Tau-phospho-Ser396–positive neurons in the subregions of hippocampus and neocortex of Edaravone-treated APP/PS1 mice were significantly lower than those of the controls (Fig. 5F and Fig. S7 A and B). Western blots further showed that Tau-phosphorylation at multiple sites, including serine 396, 262, 199, and threonine 231, was consistently and significantly diminished in the brain of APP/PS1 mice in both the prevention (Fig. S7 C and D) and treatment groups (Fig. 5G). In addition, we found that the phosphorylation at Ser9 of GSK3β, an enzyme well known for its role in the phosphorylation of Tau and the pathogenesis of AD (22), was significantly increased in the treatment group (Fig. 4 G and H). To examine the underlying mechanism, we treated SH-SY5Y cells with Aβ in the presence and absence of Edaravone. Indeed, Edaravone dose-dependently inhibited the elevation of phosphorylation of Tau at Ser396 site induced by Aβ (Fig. 4L) and increased the ratio of pSer9-GSK3β to total GSK3β in vitro (Fig. 4M). These findings indicate that the suppression of Tau-hyperphosphorylation of Edaravone is via its effect on Aβ.
Effect of Edaravone on Oxidative Stress in the Brains of APP/PS1 Mice.
We performed studies on oxidative stress markers in the brains of APP/PS1 mice treated with Edaravone. Compared with the controls, the activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and hydroxyl radical scavenging were significantly increased (Fig. S8 A–C), and the levels of lipid peroxidation products malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), protein peroxidation products 2,4-dinitrophenylhydrazine (DNPH), and 3-nitral tyrosine (3-NT) were significantly reduced in the brain of Edaravone-treated mice (Fig. S8 D–J). Furthermore, we found this decrease in 3-NT levels was more significant in the hippocampus than in the whole brain and cortex (Fig. S5 K–N). As expected, oxidative stress levels in the brains of Edaravone-treated APP/PS1 mice were significantly reduced.
Effect of Oral Edaravone Treatment in APP/PS1 Mice.
Based on the pharmacokinetic result, the bioavailability of oral Edaravone was 38% of the i.v. delivery (Fig. S9A), we fed APP/PS1 mice with Edaravone at 33.2 mg/kg/d between the ages of 3 and 12 mo. The Morris water maze test by 12 mo of age showed that oral Edaravone prominently attenuated the cognitive deficits, as reflected by reduced escaping latency in platform testing and increased annulus crossing during the probing test (Fig. S9 B and C). Oral intake of Edaravone also markedly alleviated Aβ plaque burden in the hippocampus and neocortex (Fig. S9 D–G). The Aβ levels in the brain were also significantly reduced by the oral intake (Fig. S9 H–J).
Discussion
In the present study, we found that Edaravone is a potent drug that can rescue the phenotypes of several major AD hallmarks in an AD mouse model. Injection of Edaravone before or after the onset of Aβ deposition in these mice suppressed Aβ burden by up to 40–50%, reduced the pathology of phosphorylated Tau in neurons by more than 40%, suppressed neuroinflammation and neuronal apoptosis, preserved neuronal structures including dendritic spine integrity and synaptic proteins, and most importantly, rescued the cognitive impairment of APP/PS1 mice. These results are surprising and intriguing as the structure of Edaravone (5-methyl-2-phenyl-2.4-dihydro-3H-pyrozol-3-one; Fig. 1A) is simple, with only 174.2 D in size, and consists of a pyrozol ring linked with a phenyl group, a ketone group, and a methyl group. However, our investigation revealed that Edaravone can target Aβ via binding the aa sequence 13–18 and suppresses Aβ-induced pathological cascades. These results can well explain its potent functions in AD, as this region of Aβ represents the structure for β sheet formation (23). In addition, the lipophilic nature of both phenyl and methyl groups of Edaravone render its excellent property of penetrating BBB (24) for its interaction with Aβ in the brain.
Finding effective drugs for the prevention and treatment of AD remains a holy grail for doctors and scientists. AD is a very complex disease caused by complicated interaction between genetic and environmental factors (25). Therefore, the drug candidates targeting Aβ by either immunotherapies or inhibitors of β- or γ-secretases are not successful due to the complicate nature of AD pathogenesis and the serious side effects of secretase inhibitors that interfere with their physiological functions or immunotherapy that causes neural inflammation (5, 26, 27). We propose that an effective intervention for AD should target multiple pathways that are able to break the multiple cascades of signals driving the pathogenesis of AD such as amyloidogenesis, oxidative stress, and GSK3β-Tau phosphorylation. We discovered that Edaravone appears to meet this criteria of having multiple functions on AD pathogenesis.
In the present study, we revealed an interaction of Edaravone with Aβ with several classic techniques. First, we used TEM, ThT, and Western blot assays to determine the effect of Edaravone on Aβ fibrillation. Edaravone indeed can efficiently suppress the formation of Aβ fibrils and dissolve the preformed Aβ fibrils, indicating that Edaravone acts on Aβ. Second, applying primary neurons or cell lines as a model, we found that Edaravone can suppress Aβ-induced neurotoxicity such as neurite collapse and cell death to a large extent. Third, multiple animal experiments including preventive medication, therapeutic medication, and oral administration showed that Edaravone significantly reduces amyloid plaques in the hippocampus and neocortex, CAA, and the Aβ levels in the brain. More importantly, we also found that Edaravone suppresses the expression of BACE1, sAPPβ, and CTFβ in vivo and in cultured human neuroblastoma-derived neurons, suggesting that the interaction between Edaravone and Aβ can break the positive feed-forward loop of Aβ-driven BACE1-mediated amyloidogenesis (28–30). As both oxidative stress and Aβ are positive regulators that drive the up-regulation of BACE1 via the activation of JNK (31) and GSK3β (32), respectively, it is likely that Edaravone suppresses BACE1 expression and amyloidogenesis by attenuation of oxidative stress and Aβ-induced GSK3β phosphorylation.
The intraneuronal NFT caused by hyperphosphorylation of Tau protein is another hallmark of AD (33) and consistently correlated with the cognitive dysfunctions in patients (34, 35). It is well recognized that the formation of NFT is a down-stream event of Aβ toxicity that is caused by Tau hyperphosphorylation triggered by Aβ-induced activation of several kinases such as Cdk5 (36), GSK3β (37) and PKA (37). In the present study, we found that Edaravone can suppress elevated phosphorylation of Tau at several sites in vivo and in vitro in response to Aβ, as examined by Western blot and by immunostaining methods. Consistently, Edaravone can increase the activation pS9-GSK3β which self-inactivates GSK3β kinase activities (38). These findings suggest that Edaravone acts on Aβ and suppresses its toxicity of triggering Tau hyperphosphorylation by inhibiting the GSK3β pathway.
As expected, we also found that Edaravone is a strong neuroprotective agent that protects neurons from neurite degeneration, dendritic spine shrinkage, down-regulation of synaptic proteins, apoptosis, and loss of ChAT in APP/PS1 mice and in neurons in response to Aβ. It is likely that the strong neuroprotection by Edaravone is through its dual functions in blocking Aβ aggregation/deposition and scavenging free radicals generated in the AD brain. This note is supported by our findings that Edaravone suppresses several oxidative stress markers such as oxidized lipids and proteins and increases antioxidant markers such as SOD and GSH-Px in the brain of AD mice and the discovery that Edaravone acts on Aβ aggregation and fibrillation.
CAA is a common disorder caused by the deposition of Aβ in the vessel wall of small arterial vessels of the brain, leading to cerebral hemorrhage, ischemia, and infarction (39). No specific effective drug for CAA is available at present. In both animal and clinical studies of immunotherapies, CAA is aggravated during the clearance of brain Aβ with subsequent increase of cerebral microhemorrhages (40, 41), which are related to formation of immune complex of antibody and Aβ (5). In the present study, we found that CAA is substantially reduced, with a reduction in parenchymal Aβ deposition and without an increase in microhemorrhage. ROSs are a key contributor to CAA formation, CAA-induced vessel dysfunction, and CAA-related microhemorrhage, implying that the ROS scavenger is an important therapeutic for CAA (42). These findings suggest that Edaravone would also be a promising drug candidate for CAA.
In summary, we uncovered an application of the ischemic stroke drug Edaravone in the therapy for AD and CAA by targeting multiple key AD pathways including Aβ, oxidative stress, and GSK3β-Tau phosphorylation. Edaravone would be effective in both the prevention and treatment for AD, representing a future direction of drug discovery for AD by simultaneously blocking multiple cascades leading to disease pathogenesis. Because Edaravone is currently used for stroke and proven safe in humans, the promising data from our current study warrant a large-scale clinical trial to test its efficacy in sporadic and familial AD.
Materials and Methods
See SI Materials and Methods for detailed descriptions.
The research protocol was approved by the institutional review boards of both the Third Military Medical University and the University of South Australia. The effects of Edaravone on Aβ aggregation and disaggregation were examined using ThT, Western blot, and TEM methods (43). Edaravone binding epitope mapping was performed by the competition assay using the ThT-activated fluorescence method. The effects of Edaravone on neuronal toxicity, neurite growth, APP processing, BACE1 expression, Tau phosphorylation, and GSK3β activation in response to Aβ were examined in SH-SY5Y (Chinese Academy of Sciences) and SH-SY5Y-APP695 cells and primary mouse cortical neurons. APP/PS1 transgenic mice at either 3 or 9 mo of age were used to test the effectiveness of Edaravone on AD as a prevention or treatment, via either i.p. injection or oral administration. The behavioral performance of mice treated with Edaravone was tested using the Morris water maze, Y-maze, and open field protocols. The changes in brain Aβ burden, CAA severity, amyloidogenic APP processing and Aβ metabolism, α- and β-secretase activities, oxidative stress, neuroinflammation, Tau phosphorylation, neurodegeneration, and neuronal loss were assessed with histological and biochemical methods. Unless otherwise stated, the results are presented as mean ± SEM. Statistical comparisons between two groups were tested using Student t test, or Mann–Whitney u test, as applicable. The comparisons among groups were tested using one-way or two-way ANOVA, and the trend analysis was performed when necessary. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants 81270423, 30973144, 81200988, and 81000549, National Health and Medical Research Council of Australia Grants 1020567 and 1021409, Ministry of Science and Technology of China Grant 2011CB944200, Chongqing Science and Technology Committee Grant CSTC2012JJA10111, CSTC2010BA5004, and China Postdoctoral Science Foundation Grants 2012M521861 and 2013T60955. X.-F.Z. is a visiting professor of Kunming Medical University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422998112/-/DCSupplemental.
References
- 1.Kalaria RN, et al. World Federation of Neurology Dementia Research Group Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Burden of Disease Collaborators The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korczyn AD. The amyloid cascade hypothesis. Alzheimers Dement. 2008;4(3):176–178. doi: 10.1016/j.jalz.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: The challenge of the second century. Sci Transl Med. 2011;3(77):sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ. Immunotherapy for Alzheimer disease: The challenge of adverse effects. Nat Rev Neurol. 2012;8(8):465–469. doi: 10.1038/nrneurol.2012.118. [DOI] [PubMed] [Google Scholar]
- 6.Wang YJ. Alzheimer disease: Lessons from immunotherapy for Alzheimer disease. Nat Rev Neurol. 2014;10(4):188–189. doi: 10.1038/nrneurol.2014.44. [DOI] [PubMed] [Google Scholar]
- 7.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2(7):a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44(1):181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Zawia NH, Lahiri DK, Cardozo-Pelaez F. Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med. 2009;46(9):1241–1249. doi: 10.1016/j.freeradbiomed.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guglielmotto M, Giliberto L, Tamagno E, Tabaton M. Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front Aging Neurosci. 2010;2:3. doi: 10.3389/neuro.24.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamagno E, Guglielmotto M, Monteleone D, Tabaton M. Amyloid-β production: Major link between oxidative stress and BACE1. Neurotox Res. 2012;22(3):208–219. doi: 10.1007/s12640-011-9283-6. [DOI] [PubMed] [Google Scholar]
- 12.Corbett A, et al. Drug repositioning for Alzheimer’s disease. Nat Rev Drug Discov. 2012;11(11):833–846. doi: 10.1038/nrd3869. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida H, et al. Neuroprotective effects of edaravone: A novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12(1):9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S, et al. Edaravone for acute ischaemic stroke. Cochrane Database Syst Rev. 2011;(12):CD007230. doi: 10.1002/14651858.CD007230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark TA, et al. Oxidative stress and its implications for future treatments and management of Alzheimer disease. Int J Biomed Sci. 2010;6(3):225–227. [PMC free article] [PubMed] [Google Scholar]
- 16.De Felice FG, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, et al. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38(24):7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 18.Zhang GL, et al. Edaravone ameliorates oxidative damage associated with Aβ25-35 treatment in PC12 cells. J Mol Neurosci. 2013;50(3):494–503. doi: 10.1007/s12031-013-9973-z. [DOI] [PubMed] [Google Scholar]
- 19.He F, et al. Inhibitory effects of edaravone in β-amyloid-induced neurotoxicity in rats. Biomed Res Int. 2014;2014:370368. doi: 10.1155/2014/370368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, et al. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28(25):6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flaherty DB, Soria JP, Tomasiewicz HG, Wood JG. Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J Neurosci Res. 2000;62(3):463–472. doi: 10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Colletier JP, et al. Molecular basis for amyloid-beta polymorphism. Proc Natl Acad Sci USA. 2011;108(41):16938–16943. doi: 10.1073/pnas.1112600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: From bench to bedside. Cardiovasc Ther. 2008;26(2):101–114. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 25.Hampel H, et al. German Task Force on Alzheimer’s Disease (GTF-AD) The future of Alzheimer’s disease: The next 10 years. Prog Neurobiol. 2011;95(4):718–728. doi: 10.1016/j.pneurobio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Yan R, Vassar R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014;13(3):319–329. doi: 10.1016/S1474-4422(13)70276-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golde TE, Koo EH, Felsenstein KM, Osborne BA, Miele L. γ-Secretase inhibitors and modulators. Biochim Biophys Acta. 2013;1828(12):2898–2907. doi: 10.1016/j.bbamem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buggia-Prevot V, Sevalle J, Rossner S, Checler F. NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42. J Biol Chem. 2008;283(15):10037–10047. doi: 10.1074/jbc.M706579200. [DOI] [PubMed] [Google Scholar]
- 29.Guglielmotto M, et al. Amyloid-β₄₂ activates the expression of BACE1 through the JNK pathway. J Alzheimers Dis. 2011;27(4):871–883. doi: 10.3233/JAD-2011-110884. [DOI] [PubMed] [Google Scholar]
- 30.Chami L, et al. Nuclear factor-κB regulates βAPP and β- and γ-secretases differently at physiological and supraphysiological Aβ concentrations. J Biol Chem. 2012;287(29):24573–24584. doi: 10.1074/jbc.M111.333054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chami L, Checler F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Mol Neurodegener. 2012;7:52. doi: 10.1186/1750-1326-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ly PT, et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Invest. 2013;123(1):224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloom GS. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 34.Han SD, et al. Alzheimer’s Disease Neuroimaging Initiative Beta amyloid, tau, neuroimaging, and cognition: Sequence modeling of biomarkers for Alzheimer’s Disease. Brain Imaging Behav. 2012;6(4):610–620. doi: 10.1007/s11682-012-9177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunderland T, et al. Longitudinal stability of CSF tau levels in Alzheimer patients. Biol Psychiatry. 1999;46(6):750–755. doi: 10.1016/s0006-3223(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 36.Noble W, et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38(4):555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- 37.Carlyle BC, et al. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc Natl Acad Sci USA. 2014;111(13):5036–5041. doi: 10.1073/pnas.1322360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen Y, et al. Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing. J Neurosci. 2008;28(10):2624–2632. doi: 10.1523/JNEUROSCI.5245-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gahr M, Nowak DA, Connemann BJ, Schönfeldt-Lecuona C. Cerebral Amyloidal Angiopathy—a disease with implications for neurology and psychiatry. Brain Res. 2013;1519:19–30. doi: 10.1016/j.brainres.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 40.Racke MM, et al. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci. 2005;25(3):629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patton RL, et al. Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer’s disease patients: A biochemical analysis. Am J Pathol. 2006;169(3):1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han BH, et al. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc Natl Acad Sci USA. 2015;112(8):E881–E890. doi: 10.1073/pnas.1414930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang YJ, et al. p75NTR regulates Abeta deposition by increasing Abeta production but inhibiting Abeta aggregation with its extracellular domain. J Neurosci. 2011;31(6):2292–2304. doi: 10.1523/JNEUROSCI.2733-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.