Significance
Precambrian fossils are essential for understanding the emergence of complex life. New analytical tools and new fossil discoveries are now changing the picture, allowing us to refine and extend our knowledge about the early fossil record. High-resolution data from 3.46-Ga Apex chert microbiota help us to test rigorous criteria for studying the early fossil record. Preservational windows in the 1.88-Ga Gunflint chert allow us to posit novel cellular forms, and emphasize the critical role played by the fossil record in understanding early biodiversity. Micromapping of 3.43-Ga Strelley Pool sandstone reveals microfossils preserved between sand grains from the earliest known shoreline, reminding us that many kinds of ancient habitat have yet to be explored in this way.
Keywords: early life, microfossils, astrobiology, paleontology, biogeochemistry
Abstract
New analytical approaches and discoveries are demanding fresh thinking about the early fossil record. The 1.88-Ga Gunflint chert provides an important benchmark for the analysis of early fossil preservation. High-resolution analysis of Gunflintia shows that microtaphonomy can help to resolve long-standing paleobiological questions. Novel 3D nanoscale reconstructions of the most ancient complex fossil Eosphaera reveal features hitherto unmatched in any crown-group microbe. While Eosphaera may preserve a symbiotic consortium, a stronger conclusion is that multicellular morphospace was differently occupied in the Paleoproterozoic. The 3.46-Ga Apex chert provides a test bed for claims of biogenicity of cell-like structures. Mapping plus focused ion beam milling combined with transmission electron microscopy data demonstrate that microfossil-like taxa, including species of Archaeoscillatoriopsis and Primaevifilum, are pseudofossils formed from vermiform phyllosilicate grains during hydrothermal alteration events. The 3.43-Ga Strelley Pool Formation shows that plausible early fossil candidates are turning up in unexpected environmental settings. Our data reveal how cellular clusters of unexpectedly large coccoids and tubular sheath-like envelopes were trapped between sand grains and entombed within coatings of dripstone beach-rock silica cement. These fossils come from Earth’s earliest known intertidal to supratidal shoreline deposit, accumulated under aerated but oxygen poor conditions.
How good is the earliest fossil record? How can we best measure and analyze it? These questions matter because of the vast duration of Precambrian time (ca. 4.55–0.54 Ga), which spans nearly 90% of Earth history and contains most of the major evolutionary transformations in the history of life. There is a pressing need for reliable Precambrian fossil remains to help decode the emergence of complex life in terms of pattern, rate, and process.
The remarkable quality of the Precambrian fossil record has been slow to emerge. For decades, the views of Darwin (1) that “no fossil wholly soft may be preserved” and consequently that Precambrian life had existed but went wholly unpreserved held sway. A further 50 y of innovation in microscopy and petrography was needed to discover early life, as shown by remarkable preservation of cells in phosphate of the 1.0-Ga Torridon Group (2), although another century passed before the significance of this particular taphonomic window became clear (3). Over the last 60 y, attention has focused primarily on microcrystalline quartz (called “chert”) for high-quality preservation of cellular organic materials, most famously within the 1.88-Ga Gunflint chert (e.g., ref. 4). Early discoveries were followed by decades of intense research on stromatolitic cherts, algal mats, and organic-rich sediments, including a description of Earth’s “oldest microfossils” from the 3.46-Ga Apex chert (5, 6).
Early life research is currently advancing on numerous fronts, in many ways driven forward by debates concerning the validity of claims for the earliest life. To understand the state of play, it is therefore necessary to understand the context and development of these debates. Four major areas of advancement are now changing our understanding: (i) prokaryotic phylogeny and cell diversity, (ii) diverse bacterial environments (including extremophile habitats) traced from the present and back into deep time, (iii) new analytical techniques, and (iv) testing of criteria for recognizing ancient fossil bacteria. Below, we use three case studies (the 1.88-Ga Gunflint chert, the 3.46-Ga Apex chert, and the 3.43-Ga Strelley Pool arenite) to show how research into this field can flourish with the application of new techniques and approaches.
The Gunflint Chert: New Analytical Techniques
The Gunflint chert provides a test case for the value of modern analytical techniques. Such techniques include laser Raman microspectroscopy, secondary ion mass spectrometry (SIMS, time-of-flight–SIMS, and NanoSIMS), focused ion beam milling combined with transmission electron microscopy (FIB-TEM) and also combined with 3D scanning electron microscopy (3D-FIB-SEM), and synchrotron radiation, plus confocal laser scanning microscopy. Where appropriately applied, such techniques allow for 3D mapping of morphology, and even the mapping of metabolic pathways at nanoscales (7–9).
Since geological context is essential to such research, we have freshly mapped the remarkably preserved sediments exposed at the Schreiber Beach locality, Lake Superior, Ontario (Fig. S1). Here can be seen an ancient shoreline, with a weathered basement of Archean lava, locally eroded into rounded boulders. During a marine transgression, these boulders were draped by layers of carbonaceous chert and dolomitic carbonate and then by banded ironstone.
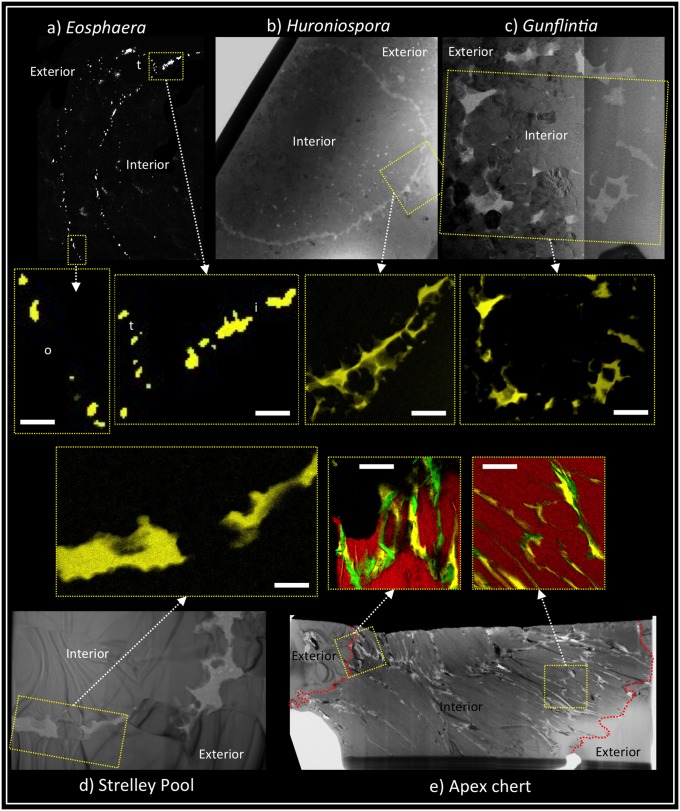
Small tubular filaments of Gunflintia are abundant within this deposit (Fig. S2 A and B) and were originally compared with oscillatoriacean cyanobacteria owing to the presence of cell-like septation (4). Later work, however, suggested that much, if not all, of the septation could have arisen from decay of nonseptate tubular sheaths (10, 11). Fresh thin sections through these stromatolitic carbonaceous cherts reveal numerous Gunflintia specimens seemingly provided with cell-like septation (Fig. S2 A and B). The 3D FIB-SEM reconstructions of specimens replicated by pyrite, however, show no such features (Fig. S2 C and D), nor do specimens wholly preserved in carbonaceous matter (Fig. S2 E and F; see also refs. 8 and 9). Indeed, the septate appearance arises as a decay artifact. In Gunflintia, this may have been the result of saprophytic activity and decomposition, hinted at by the presence of closely associated rounded cell-like bodies of possible heterotrophs (Fig. S2 C and D) (9). Loss of phylogenetically informative characters through decay recalls problems encountered with the fossil record of metazoans (12). However, with Precambrian microfossils, there is an important additional factor to consider: the mimicking of phylogenetically informative characters arising from mineral growth. In the Gunflint chert, our FIB-TEM analysis shows how wall embayment and “sculpture” can arise from the growth of small silica (chert) spherulites (e.g., Fig. 1B) (8). Not all preservation changes are negative, however. Different modes of decomposition found between filamentous Gunflintia and the rounded Huroniospora (Fig. 1) provide information of potential phylogenetic significance. While Gunflintia has a readily degradable organic sheath, Huroniospora bears excystment-like openings (13) with lesser decay and retains a higher fidelity of biochemical signals after pyritization, suggesting a more robust, cyst-like polymer (9). New high spatial resolution techniques are therefore contributing critical information to the debate about fossils known for 60 y. Many of these techniques were first applied within paleontology to debates concerning some of the earliest signs of life, but they are now finding applications elsewhere in the fossil record.
Fig. 1.
Comparison of carbonaceous material between Precambrian microfossils and pseudofossils. (A−C) Microfossils from the 1.88-Ga Gunflint chert. (A) Eosphaera showing thin (ca. 100 nm) plausible outer cell membrane (o), thicker (ca. 200 nm) inner cyst-like sphere (i), plus part of a thin (<100 nm) tubercle cell membrane (t). (B) Huroniospora, a probable cyst showing a thick (ca. 500 nm) carbonaceous wall partly impregnated with silica crystals. (C) Gunflintia, a probable sheath showing a thick (maximum 500 nm) wall partially disrupted by silica crystal growth. (D) A probable sheath from the 3.43-Ga Strelley Pool Formation showing a thick (ca. 500 nm) wall partially disrupted by silica crystal growth. (E) Pseudofossil comparable to Primaevifilum spp. from the 3.46-Ga Apex chert, showing carbon (and closely associated iron) of variable thickness interleaved between aluminosilicate and quartz grains with no evidence for cellular architecture. Scale bar is 500 nm for the seven false color elemental maps in center of figure. Yellow, carbon; black, silica; red, aluminosilicate; green, iron. A−E obtained using FIB-SEM (A) and bright-field TEM (B−E); center false color elemental maps obtained using SEM–energy dispersive X-ray spectroscopy (maps from A) and energy-filtered TEM (maps from B−E). B–D are modified with permission from ref. 8.
The Gunflint Chert: Mapping Out Novel Cellular Forms
Should very ancient fossils be shoehorned into modern groups when they do not readily fit? Or should new higher groupings be invented to accommodate them? There is no parsimonious resolution to this apparent dichotomy. Current work on prokaryote genomics highlights the enormous metabolic diversity that can exist within aqueous habitats (14), requiring us to consider numerous different forms of cell membrane, and to explore the ways in which both their organic and inorganic products as well as their metabolic pathways may yet be discovered in the geological record (Fig. S3).
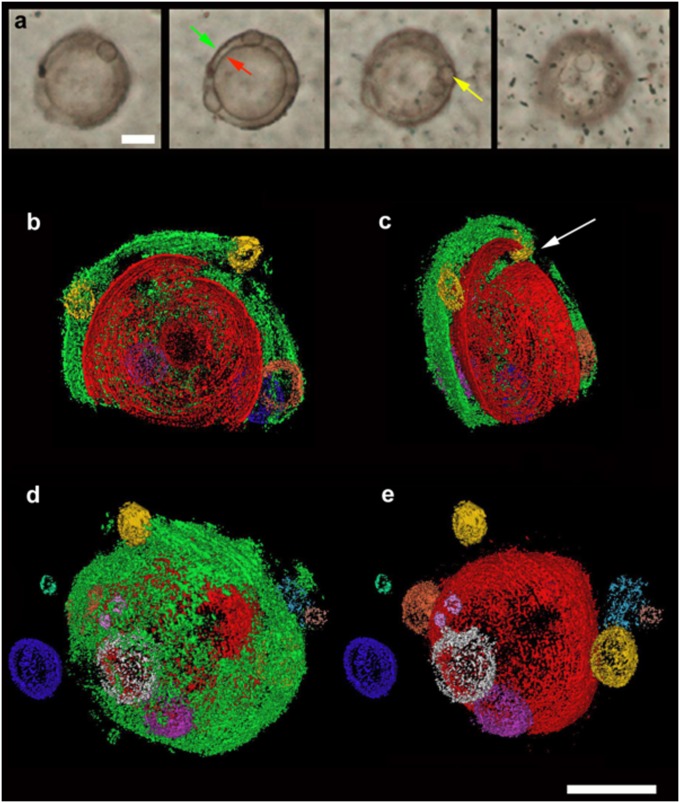
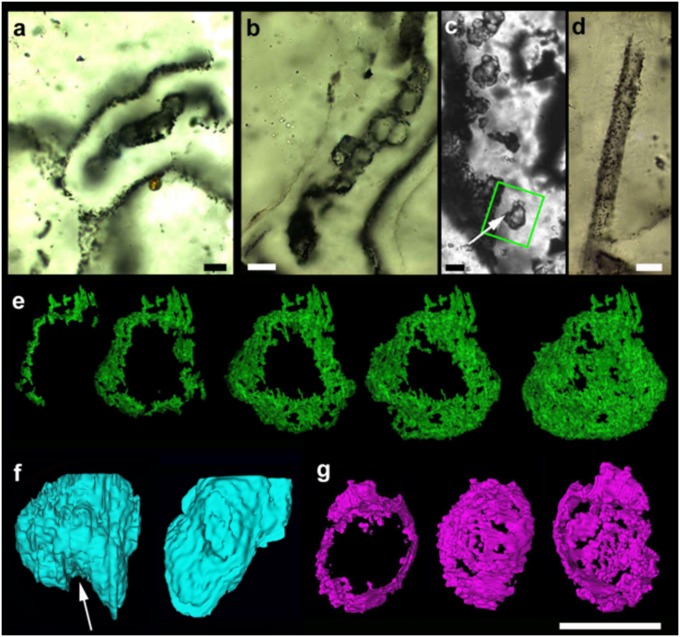
The case of the Gunflint microfossil Eosphaera tyleri Barghoorn 1965 from the Schreiber Beach locality (4) (n = ∼160) is particularly interesting. Barghoorn and Tyler’s investigations revealed a complex sphere-within-sphere construction, ∼28–32 μm in diameter, with a thicker walled inner sphere enclosed within a thinner walled outer sphere (Fig. 2A and Fig. S4). Each sphere is separated by a regular intervallar space containing from 0 to 15 small tubercle-like spheroids without regular arrangement. This construction makes Eosphaera tyleri among the most morphologically complex fossils yet known from rocks older than ∼1.2 Ga. To help reconstruct its architecture and taphonomy in more detail, a small volume of thin section enclosing an optically visible specimen was analyzed using FIB-SEM serial sectioning (Fig. 2 B–E and Fig. S4). This technique confirms a much more delicate construction than seen in the closely associated Gunflintia and Huroniospora (compare Fig. 1 and Fig. S2) (cf. 8, 9). The 3D reconstruction shows an inner sphere (Fig. 2, red, 20 μm), enclosed by an outer sphere (Fig. 2, green, 30 μm) plus a scatter of ∼10 rounded internal ellipsoidal to spherical tubercles (Fig. 2, ca. 1–7 μm in diameter) set within a regular intervallar space about 5 μm wide. The tubercles may reflect the growth sequence of the organism, with tubercle shape altering through ontogeny.
Fig. 2.
Exceptional preservation and novel morphology of 1.88-Ga complex carbonaceous microfossil Eosphaera tyleri from the Gunflint chert, Schreiber Beach, Ontario. (A) Four levels of optical focus through a thin section in nonstromatolitic microfabric, showing a well-preserved Eosphaera complete with inner sphere (red arrow) and outer sphere (green arrow) plus several rounded tubercles (e.g., yellow arrow) within the intervallar space. (B−E) The 3D reconstructions (from FIB-SEM sequential slicing) of a different Eosphaera specimen (see Fig. S4). Note the thicker and more robust inner sphere (red, 20 μm across) with linear rupture (beneath white arrow), thinner and more membranous outer sphere (green, 30 μm across), and about 10 hollow, spherical to elliptical cell-like tubercles (various colors including yellow, 1–5.8 μm) plus two external tubercles (blue, <7 μm; pale green at left, 1.8 μm). (B and C) Viewed from center of specimen visualizing approximately half the organism; (D and E) Viewed from outside the specimen showing both inner and outer spheres (D) or just the inner sphere (E), plus tubercle locations. (Scale bar, 10 μm.)
These data confirm the inner sphere wall was more solid (∼200 nm) and complete than the outer sphere wall (Fig. 1A). As with the original material (ref. 4, figure 8, images 5–8), we find that the inner sphere is cut by a distinctive linear “split,” causing localized invagination or overlap of the wall (Fig. 2c and white arrow in Fig. S4). These observations suggest that the inner sphere of Eosphaera was rather rigid and cyst-like. The outer sphere of Eosphaera was more membranous, degrading to form rough-edged openings, much as with walls of the tubercles. The presence of a tubercle beyond the outer sphere (Fig. 2D, blue) and adjacent to small openings in the latter (Fig. 2D, red patch) suggests outward expulsion facilitated by thinning of the outer sphere membrane.
The remarkable features of Eosphaera raise questions about the lack of comparable forms in the living microbial world. Comparisons to the extant chlorophyte Volvox (15) are problematic because that taxon does not display an inner sphere. This leaves three possible hypotheses. (i) Eosphaera was a member of an extant clade, such as cyanobacteria, for which the occupation of multicellular morphospace has changed with time. At 1.88 Ga, and in an ecosystem without even eukaryotic algae, the adaptive landscape for multicellularity would have been different; hence the theoretical morphospace may have been differently occupied. (ii) Eosphaera was a member of an extinct clade (e.g., as sister or stem to a modern clade) that occupied a previously unknown region of multicellular morphospace. (iii) Eosphaera uniquely preserves a symbiotic association between two different kinds of cell: a single large host cell, preserved as the outer sphere (cell wall) plus an inner sphere (i.e., an endocyst with excystment opening), and multiple small endosymbionts preserved as internal and external tubercles (cell membranes). For instance, Eosphaera could represent a phagocytic cell playing host to small cyanobacterial cells arranged around the inner margins of the host cell membrane. Such a hypothesis needs testing, but it could help explain why Eosphaera is not found much earlier, or indeed much later, in the fossil record.
Eosphaera tyleri therefore raises questions like those now arising elsewhere in the early macrofossil record, including Ediacaran fossils such as Charnia: bizarre forms unmatched by the modern biosphere. Once compared with extant cnidarian seapens, Charnia is now thought to be something wholly distinct, largely restricted to a specific time period, and forming part of an extinct (rangeomorph) group having a distinct pattern of growth (16). That said, such enigmatic forms must still have a closest living modern relative, even if they cannot be placed directly within a modern crown group. The extension of such arguments to the Precambrian microfossil record therefore demonstrates the extraordinary potential of the early fossil record to expand known biodiversity.
The Apex Chert: Testing for Biogenicity
The ∼3.46-Ga Apex Basalt, Warrawoona Group, Western Australia, contains some of the oldest and best-preserved silica-rich hydrothermal and fissure eruption systems (17–19) as well as the earliest pumice to contain putative biological signals (20). More famously, it is the source of 11 species of Apex chert microfossils, including morphotypes compared with (but not classified as) cyanobacteria (Fig. 3) (e.g., refs. 5 and 6).
Fig. 3.
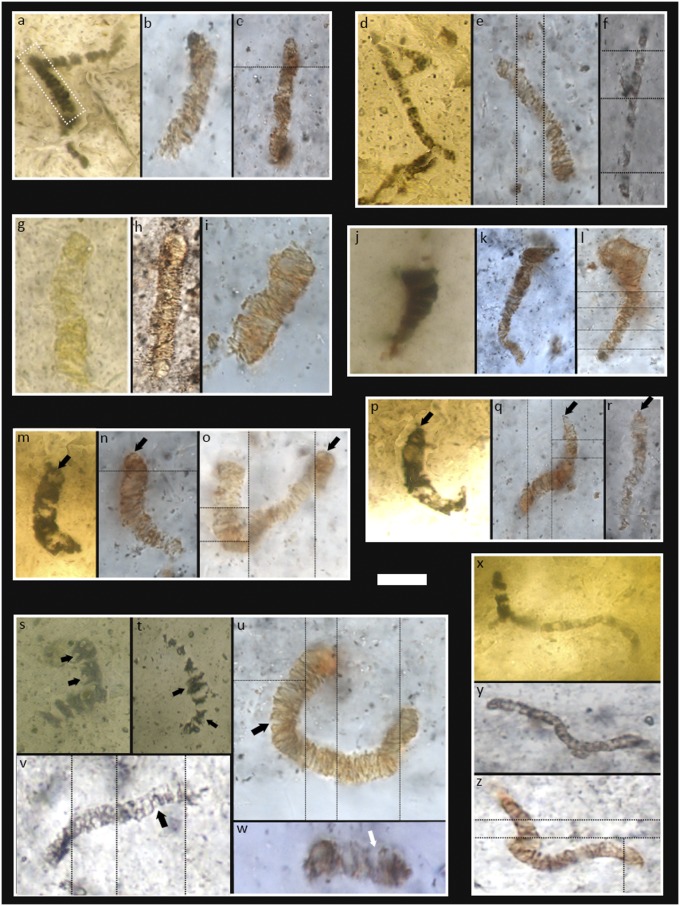
Apex chert pseudofossil holotypes and paratypes (6), reimaged from the type thin sections (cf. 19), plus newly discovered comparable microstructures from sample CHIN-03. Archaeoscillatoriopsis disciformis holotype (A) plus comparable examples from CHIN-03 (B and C). Dashed box in A is the holotype as presented in ref. 6, although it was later found to be part of a larger microstructure wrapped around the edge of a rhombic mineral crystal (17). Primaevifilum delicatulum holotype (D) plus comparable examples from CHIN-03 (E and F). Archaeoscillatoriopsis grandis paratype (G) plus comparable examples from CHIN-03 (H and I). Primaevifilum attenuatum holotype (J) plus comparable examples from CHIN-03 (K and L). Note attenuation of trichomes toward apices, a feature that was erected as a defining characteristic of this species (6) (Table S1). Primaevifilum laticellulosum holotype (M) plus comparable examples from CHIN-03 (N and O). Note pillow-shaped terminal cells (arrows), a feature that was erected as a defining characteristic of this species (6) (Table S1). Primaevifilum conicoterminatum holotype (P) plus comparable examples from CHIN-03 (Q and R). Note conical terminal cells (arrows), a feature that was erected as a defining characteristic of this species (Table S1). (S−W) Examples of bifurcated cells and cell pairs (arrows) in the type thin sections (S and T) and CHIN-03 (U−W). Primaevifilum amoenum holotype (X) plus comparable examples from CHIN-03 (Y and Z). Thin black lines separate images taken at different focal depths. (Scale bar: 7 μm in W; 8 μm in B and E; 9 μm in I; 10 μm in O, U, V, Y, and Z; 12 μm in C, F, and N; 13 μm in D and G; 14 μm in A, J, L, M, P−T, and X; 16 μm in K; and 22 μm in H.
Context is crucial to tests for biogenicity. We have therefore mapped the context for these candidate microfossils at kilometer to micrometer scales (17–19), and shown that they came not from a wave-washed beach or stream but from a hydrothermally influenced subsurface vein system, some 100 m below the stratiform Apex chert. Our initial mapping of the microfossils (17, 18) argued that they come from within a succession of carbonaceous and metalliferous hydrobreccia fabrics; this has been supported by further research (21, 22). The holotypes and paratypes were also examined and the populations mapped, leading to the conclusion that many of the microfossil-like structures are indistinguishable from associated mineral growths (17–19). Separate studies have reported further microfossil-like artifacts from the Apex chert (22, 23), but those objects are not carbonaceous in nature, and their direct relevance to the interpretation of the Apex holotypes has been questioned (24).
Here we return to original microfossil material, collected from the site in 1999. Analysis of previously published type specimens is seriously hampered by 150- to 380-μm-thick preparations (6, 18), making optical petrography difficult. There is also an understandable prohibition (by the Natural History Museum, London) on destructive or intrusive techniques. Our materials are therefore standard thin sections of ∼30 μm, making optical characterization more straightforward. Petrographic thin sections through sample CHIN-03 reveal a population of carbonaceous, filamentous, microfossil-like structures in an early generation of hydrobreccia, exactly like those in the type material (Fig. 3). This population shows size-frequency distributions closely comparable with the type material (Fig. S5) (cf. 6). It also demonstrates all of the diagnostic characters observed in the type material (Table S1): disk-shaped and quadrate medial cells (Fig. 3); attenuation of trichomes (Fig. 3 J–L); rounded, pillow-shaped and conical terminal cells (Fig. 3 A–C, M–O, P–R, and X–Z); and bifurcating cells and cell pairs (Fig. 3 S–W). Such data confirm these specimens are part of the same population used to erect the Apex chert microbiota (e.g., ref. 6) and then to defend it (e.g., refs. 24 and 25). Specifically, microstructures in CHIN-03 are directly comparable to type specimens of Archaeoscillatoriopsis spp. (Fig. 3 A–C and G–I) and Primaevifilum spp. (Fig. 3 D–F, J–R, and X–Z).
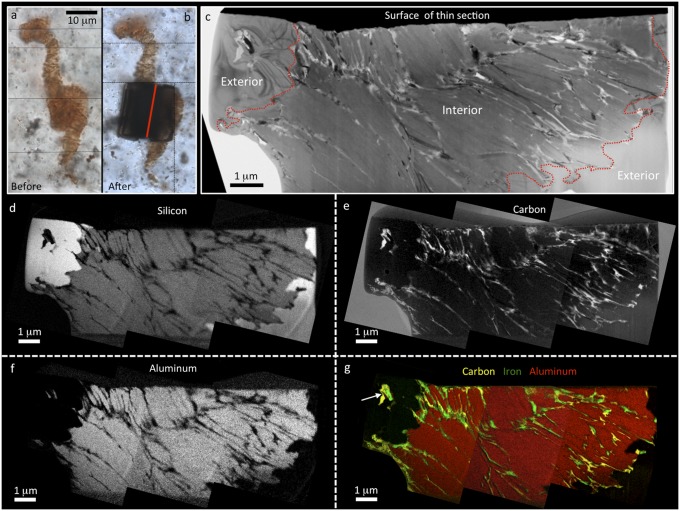
Critical to biogenicity of such Apex filaments is a suggested presence of cell lumina [i.e., “discernable walled compartments that typically are devoid of remnants of their originally water-rich cytoplasmic contents” (24)], having 3D wall compartments of carbonaceous (kerogenous) composition (e.g., refs. 24 and 25). TEM analyses of ultrathin wafers cut through these microfossil-like structures from CHIN-03 (Fig. 4 and Fig. S6) show, however, that they do not comprise hollow cell lumina, infilled with silica, and enclosed by carbonaceous cell walls, as previously suggested (24, 25). Instead, the microfossil-like structures comprise vermiform aggregates of plate-like aluminosilicate grains (Fig. 4), occasionally interspersed with silica (Fig. S6). The chemistry of this vermiform aluminosilicate is variable. Many grains are potassium rich, while others are barium rich, with magnesium and iron often as minor components. Electron diffraction in the TEM (Fig. S6) shows d spacings indicative of a 2:1 layered phyllosilicate crystal lattice structure found in both micas and clay minerals such as illite and vermiculite (26). The micromorphology of the phyllosilicate most closely resembles vermiculite, a common alteration product of mica (27). However, the range in chemical composition, especially the presence of barium, suggests a complex hydrothermal association of mica alteration products that are best termed vermiculite-like.
Fig. 4.
Nanoscale structure and chemistry of a pseudofossil comparable to Primaevifilum spp. from sample CHIN-03. Optical photomicrographs before (A) and after (B) extraction of an ultrathin wafer for analysis by TEM. Position of wafer indicated by red line in B. Thin black lines separate images taken at different focal depths. Bright-field TEM image (C) and corresponding energy-filtered TEM elemental maps of silicon (D), carbon (E), and aluminum (F) from the pseudofossil below the surface of the thin section. These show that the pseudofossil is almost entirely composed of platy aluminosilicate grains. Boundaries of the pseudofossil are indicated by dashed red lines in the TEM image and are marked by a clear transition from aluminosilicate to quartz (see Al and Si maps where brighter white colors equate to higher elemental concentrations). Carbon is abundant throughout the pseudofossil and is found in patches along quartz−aluminosilicate boundaries and interleaved between aluminosilicate grains within the pseudofossil. Carbon does not show any cell-like distribution. (G) False color three-element overlay showing carbon (yellow) and iron (green) interleaved between aluminosilicate (red). Patches of carbon and iron are also seen exterior to the pseudofossil at quartz grain boundaries (arrow).
Energy-filtered TEM mapping confirms that carbon is closely associated with the microfossil-like artifacts. Carbon Raman spectra are indistinguishable from previous analyses (17, 24, 25). Apex chert carbon has been extensively investigated in an attempt to determine biogenicity (e.g., refs. 17, 24, 25, and 28). It appears to be kerogenous with a geochemical maturity and isotopic composition consistent with a biological origin, although a source from abiotic synthesis within a hydrothermally influenced setting (17, 29) is still debated. More importantly, a biogenic carbon source can create microfossil-like artifacts when redistributed from either adjacent units or from biological communities within the system. Recent studies have shown that there are multiple populations of carbonaceous material within the Apex chert (29–31), each with different thermal alteration histories, suggesting repeated episodes of carbon introduction and/or redistribution within this unit. This has been interpreted to result from multiple pulses of hydrothermal and metamorphic activity, fluid flow, and weathering (30, 31), providing ideal conditions for the creation of carbonaceous microfossil-like artifacts. Of particular note is the discovery of a late-stage carbon population, associated with hematite at the original microfossil site (31), that exhibits a very similar Raman spectrum to those previously illustrated (24, 25) for the microfossil holotypes.
Our TEM nanoscale mapping confirms redistribution of carbon both within and around the Apex chert microfossil-like artifacts, in marked contrast to patterns found by us in bona fide fossil microbes from the Gunflint chert (Fig. 1). This Apex carbon is seen wrapped around the margins of the vermiform phyllosilicate–quartz grain boundaries, and is interleaved between vermiform phyllosilicate grains in pseudofossil interiors, along grain boundaries, and along triple junctions of quartz exterior to the pseudofossils (Figs. 1 and 4 and Fig. S6). These interleaved carbon intrusions only resemble cellular compartment walls when investigated with lower spatial resolution (for example, in optical or laser Raman work). Higher spatial resolution analysis of supposed cellular compartments reveals very inconsistent lengths (<50 nm up to ca. 1 μm; Fig. 4 and Fig. S6), with length/width ratios that match crystal growth patterns and are unlike any known microbial cells. We note that silica can also disrupt some phyllosilicate microstructures (Fig. S6), and this may give the appearance, in lower-resolution studies, that silica infills some pseudofossils.
Carbon distribution in “Apex microfossils” is, therefore, not comparable with true cellular morphology. Our nonbiological formation model, consistent with multiple fluid flow events (30, 31), is: (i) hydration of mica flakes (abundant in the country rock) during widespread hydrothermal activity resulting in vermiculite-like phyllosilicate formation; (ii) continued heating plus rapid expulsion of water from phyllosilicate crystal lattices, causing exfoliation (i.e., accordion-like expansion at right angles to the cleavage plane), and creating the initial vermiform morphological expression of microfossil-like artifacts; and (iii) adsorption of later hydrocarbons (and locally additional iron) onto the vermiculite, mimicking cell walls. We note that exfoliated vermiculite has high adsorption capacity resulting from the strong capillary action of slit-like pores between plate-like grains (27), encouraging its use for cleaning up oil spills (27, 32).
The Strelley Pool Arenite: New Environments and Taphonomic Windows
Why are few cellular fossils found in rocks before 2.5 Ga? For decades, the main search image has been cyanobacteria-like assemblages as silicified algal mats and stromatolites. Have we been looking for fossils in the wrong places?
The ∼3.43-Ga Strelley Pool Formation, Western Australia, shows that early siliclastic sandstones, a hitherto unrecognized resource, can contain remarkable signals. Quartz sands at the base of this formation were deposited during the earliest stage of a marine transgression across Earth’s oldest known unconformity surface (33). Associated grains of rounded detrital pyrite show no signs of oxidation, nor do localized coatings of pyrite around rounded quartz grains (7). Organic matter trapped between grains near the base of the sand body is closely associated with this pyrite. Quartz, pyrite, and organic matter were then rapidly coated and cemented by early diagenetic silica (chert) and overlain by a 2- to 8-m-thick unit of quartzite. These highly indurated rocks were less subject to deformation and fracturing than associated lavas, ashes, and bedded cherts, and so were able to provide remarkable protection to the enclosed organic contents.
Along the 60-km outcrop of the Strelley Pool arenite, remarkable preservation is found only near the base in placer deposits that accumulated detrital pyrite under tidal conditions on a beach. Even here, remarkable preservation is limited to small patches and intraclasts of black sandstone, where the quartz and pyrite grains were cemented by thin and multiple laminae of chert, which coated the grains as epitaxial and meniscus cements (Fig. 5 and Figs. S7 and S8). These features suggest silica cementation was episodic, with some open and air-filled pore space.
Fig. 5.
Indigenous microfossils preserved within beach-rock dripstone microfabric in chertified quartz arenites of the 3.43-Ga Strelley Pool Formation, Western Australia. (A−D) Optical light micrographs of petrographic thin sections. (A) Chain of globular cells, preserved by enclosure within a microstalactite (see also Fig. S9). (B) Chain-like cluster of globular cells, here preserved within a thin chert lamina draped around a quartz grain (see also Fig. S7). (C) Isolated elliptical cells, here preserved within a chert-filled void between quartz grains. (D) Sheath-like tube preserved within a chert filled void between quartz grains (see also Fig. S8). (E−G) The 3D reconstructions of two cells from the same sample, using FIB-SEM sequential slicing. (E) Five successive stages of reconstruction of cell 1 (arrowed in C) showing a thin, hollow, crumpled wall of carbonaceous matter (green), pierced by holes from decomposition and silica growth. (F) Reconstruction of the silica-filled interior of the same cell (pale blue), showing potential external fold (arrow; unlike botryoidal silica). (G) Three views (partial, exterior, interior) of cell 2 from the same thin section, showing a thin, hollow, crumpled and pierced carbonaceous wall (purple). (Scale bar, 10 μm.)
Small fingers of dripstone chert are draped around the candidates for carbonaceous microfossils (Fig. 5A and Fig. S9). These comprise hollow, rounded to elliptical bag-shaped bodies, loosely arranged in chains or clusters. Similar clusters also occur within epitaxial cements (Fig. 5B) alongside isolated coccoidal and tubular forms (Fig. 5 C and D). The carbonaceous walls of the latter contain nitrogen and predate the growth of microspherulitic silica cements (7, 8). While ultrastructural similarities in silicification are found with the 1.88-Ga Gunflint microbiota (Fig. 1) (8), an even closer textural fabric is seen within the early Devonian Rhynie chert (Fig. S10). Both show coccoidal and filamentous forms encased within cylinders and fingers of chert, similar to modern silica beach rock around hydrothermal springs in New Zealand (34).
At Strelley Pool, well-preserved detrital pyrite grains plus multiple sulfur isotope data suggest low levels of oxygen arresting organic decay. The association of early candidate microfossils (see Table S2 for full list of evidence for the biogenicity of these microfossils) with low levels of oxygen tends to suggest anaerobic metabolic pathways. Their association with meniscate silica films suggests they were clinging to moist surfaces around sand grains that often became air filled, perhaps during aerial emergence associated with tidal cycles. Given the shallow tidal setting, and the translucent nature of the quartz grains, these biofilms were likely exposed to photic conditions. Together with the presence of 34S-depleted pyrite grains closely associated with the microfossils (7), this points to the possibility of anoxygenic photosynthetic pathways involving sulfur, similar to the activities of modern purple sulfur bacteria. However, there are differences, too. The wall structures are thick (mostly 500–600 nm), more like those from sheaths and cysts of the Gunflint chert (Fig. 1) and much thicker than expected from most proteobacterial cell membranes. They could have been members of an extant clade, such as proteobacteria (e.g., cyanobacteria, sulfur bacteria) in which cells are provided with thick exopolymers, or they may have been members of an extinct clade, for which the cell wall morphospace was different from that of modern groups.
These unexpected findings within silica beach sands show how new and unexpected taphonomic windows are opening up on the early fossil record. Other promising rocks include microbially induced sedimentary structures in siliciclastic sands, pyritic nodules, lake deposits, tufas, calcretes, speleothems, and lava flows.
Conclusion
The fossil record is critical to forming a proper appreciation of both the diversity and disparity of life. Inspection of the modern biosphere conspicuously fails to anticipate the existence of morphologically curious groups such as fossil rangeomorphs, anomalocardids, graptolites, ammonites, or sauropods in the distant past. While we now take the strangeness of this long-lost biosphere for granted, its influence upon recent scientific thought has arguably been profound, ranging from the role of extinction to an understanding of the stem−crown group concept.
Only now, with the examination of appropriate taphonomic windows in targeted paleoenvironments, and with use of cutting-edge techniques, are we coming to a similar position with the evolution of ancestral prokaryotes and eukaryotes before the Cambrian Explosion. High-resolution morphology and microtaphonomy can now be extracted from ancient deposits such as the Gunflint chert, allowing us to posit forms novel to biology even among prokaryotes, much as is now being achieved for invertebrate and vertebrate fossils from the more recent past.
While the quality of the early fossil record is much better than Darwin (1) might ever have dared to imagine, we must still map out its limits, and push back the boundaries using new techniques, to test out ideas both old and new. Over the last decade, the criteria required for recognizing early life have been substantially refined, and many classic deposits have been reexamined. There are also new places to look in the fossil record, so that environments long assumed to be barren of life may yet prove to be teeming. Both the vast genetic diversity and habitat range found within modern prokaryotes have also liberated scientists from searching for early fossil cyanobacteria along ancient wave-lapped shores. As new techniques develop, and as we look in more places, provided with a better understanding of biogenicity criteria, the early fossil record has the potential to help to drive forward novel biological thinking on major evolutionary questions on Earth, and maybe beyond.
Materials and Methods
Samples for TEM were prepared using dual-beam FIB systems at the Electron Microscopy Unit (EMU), University of New South Wales, and at Adelaide Microscopy. TEM samples were analyzed using a JEOL 2100 LaB6 TEM in the Centre for Microscopy, Characterization and Analysis, The University of Western Australia. Sequential FIB milling and scanning electron imaging was performed on a Zeiss Auriga Crossbeam dual-beam instrument at EMU and a FEI Helios Nanolab instrument at Adelaide Microscopy. The 3D volume rendering was performed using the Serial Paleontological Image Editing and Rendering System (SPIERS) software suite. For detailed information on materials and methods, see SI Text.
Supplementary Material
Acknowledgments
Martin van Kranendonk, Owen Green, Cris Stoakes, the late John Lindsay, and the Geological Survey of Western Australia are thanked for fieldwork assistance, Charlie Kong is thanked for preparation of TEM wafers, and Russell Garwood is thanked for advice regarding SPIERS software. Two anonymous reviewers are thanked for their highly constructive and thoughtful reviews. We acknowledge the facilities and scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility. These facilities are funded by the universities and state and commonwealth governments. D.W. is funded by the Australian Research Council via a grant to the Centre of Excellence for Core to Crust Fluid Systems.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
1Deceased December 16, 2014.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1405338111/-/DCSupplemental.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection. 1st Ed John Murray; London: 1859. [Google Scholar]
- 2.Peach B, et al. 1907. The Geological Structure of the Northwest Highlands of Scotland, Memoirs of the Geological Survey of Great Britain (Her Majesty’s Stationary Office, Glasgow, Scotland)
- 3.Strother PK, Battison L, Brasier MD, Wellman CH. Earth’s earliest non-marine eukaryotes. Nature. 2011;473(7348):505–509. doi: 10.1038/nature09943. [DOI] [PubMed] [Google Scholar]
- 4.Barghoorn ES, Tyler SA. Microorganisms from the Gunflint Chert. Science. 1965;147(3658):563–577. doi: 10.1126/science.147.3658.563. [DOI] [PubMed] [Google Scholar]
- 5.Schopf JW, Packer BM. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science. 1987;237(4810):70–73. doi: 10.1126/science.11539686. [DOI] [PubMed] [Google Scholar]
- 6.Schopf JW. Microfossils of the Early Archean Apex chert: New evidence of the antiquity of life. Science. 1993;260(5108):640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- 7.Wacey D, Kilburn MR, Saunders M, Cliff J, Brasier MD. Microfossils of sulfur metabolizing cells in ∼3.4 billion year old rocks of Western Australia. Nat Geosci. 2011;4:698–702. [Google Scholar]
- 8.Wacey D, et al. Taphonomy of very ancient microfossils from the ∼3400 Ma Strelley Pool Formation and ∼1900 Ma Gunflint Formation: New insights using focused ion beam. Precambrian Res. 2012;220-221:234–250. [Google Scholar]
- 9.Wacey D, et al. Nanoscale analysis of pyritized microfossils reveals differential heterotrophic consumption in the ∼1.9-Ga Gunflint chert. Proc Natl Acad Sci USA. 2013;110(20):8020–8024. doi: 10.1073/pnas.1221965110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoll AH, Strother PK, Rossi S. Distribution and diagenesis of microfossils from the lower Proterozoic Duck Creek Dolomite, Western Australia. Precambrian Res. 1988;38:257–279. doi: 10.1016/0301-9268(88)90005-8. [DOI] [PubMed] [Google Scholar]
- 11.Lanier WP. Interstitial and peloid microfossils from the 2.0 Ga Gunflint formation: Implications for the paleoecology of the Gunflint stromatolites. Precambrian Res. 1989;45(4):291–318. [Google Scholar]
- 12.Sansom RS, Gabbott SE, Purnell MA. Non-random decay of chordate characters causes bias in fossil interpretation. Nature. 2010;463(7282):797–800. doi: 10.1038/nature08745. [DOI] [PubMed] [Google Scholar]
- 13.Strother PK, Tobin K. Observations on the genus Huroniospora Barghoorn: Implications for paleoecology of the Gunflint microbiota. Precambrian Res. 1987;36(3-4):323–333. [Google Scholar]
- 14.Kirchman DL, editor. Microbial Ecology of the Oceans. 2nd Ed. Wiley; New York: 2008. 593 pp. [Google Scholar]
- 15.Kazmierczak J. The eukaryotic nature of Eosphaera-like ferriferous structures from the Precambrian Gunflint Iron Formation, Canada: A comparative study. Precambrian Res. 1979;9:1–22. [Google Scholar]
- 16.Antcliffe JB, Brasier MD. Charnia and seapens are poles apart. J Geol Soc London. 2007;164:49–52. [Google Scholar]
- 17.Brasier MD, et al. Questioning the evidence for Earth’s oldest fossils. Nature. 2002;416(6876):76–81. doi: 10.1038/416076a. [DOI] [PubMed] [Google Scholar]
- 18.Brasier MD, et al. Critical testing of Earth's oldest putative fossil assemblage from the ∼3.5 Ga Apex Chert, Chinaman Creek, western Australia. Precambrian Res. 2005;140(1-2):55–102. [Google Scholar]
- 19.Brasier MD, et al. 2011. Geology and putative microfossil assemblage of the c. 3460 Ma ‘Apex chert’, Chinaman Creek, Western Australia—A field and petrographic guide (Geol Surv West Aust, East Perth, WA, Australia), Record 2011/7), 60 pp.
- 20.Brasier MD, Matthewman R, McMahon S, Kilburn M, Wacey D. Pumice from the ∼3460 Ma Apex Basalt, Western Australia: A natural laboratory for the early biosphere. Precambrian Res. 2013;224:1–10. [Google Scholar]
- 21.Van Kranendonk MJ. Volcanic degassing, hydrothermal circulation and the flourishing of early life on Earth: A review of the evidence from c. 3490-3240 Ma rocks of the Pilbara Supergroup, Pilbara Craton, Western Australia. Earth Sci Rev. 2006;74(3-4):197–240. [Google Scholar]
- 22.Pinti DL, Mineau R, Clement V. Hydrothermal alteration and microfossil artefacts of the 3,465-million-year-old Apex chert. Nat Geosci. 2009;2:640–643. [Google Scholar]
- 23.Marshall CP, Emry JR, Olcott Marshall A. Haematite pseudomicrofossils present in the 3.5-billion-year-old Apex Chert. Nat Geosci. 2011;4:240–243. [Google Scholar]
- 24.Schopf JW, Kudryavtsev AB. Biogenicity of Earth's earliest fossils: A resolution of the controversy. Gond Res. 2012;22(3-4):761–771. [Google Scholar]
- 25.Schopf JW, Kudryavtsev AB, Agresti DG, Wdowiak TJ, Czaja AD. Laser–Raman imagery of Earth’s earliest fossils. Nature. 2002;416(6876):73–76. doi: 10.1038/416073a. [DOI] [PubMed] [Google Scholar]
- 26.Downs RT, Bartelmehs KL, Gibbs GV, Boisen MB. Interactive software for calculating and displaying X-ray or neutron powder diffractometer patterns of crystalline materials. Am Mineral. 1993;78:1104–1107. [Google Scholar]
- 27.Medeiros MD, Sansiviero MTC, Araujo MH, Lago RM. Modification of vermiculite by polymerization and carbonization of glycerol to produce highly efficient materials for oil removal. Appl Clay Sci. 2009;45(4):213–219. [Google Scholar]
- 28.De Gregorio BT, Sharp TG. The structure and distribution of carbon in the 3.5 Ga Apex chert: Implications for the biogenicity of Earth’s oldest putative microfossils. Am Mineral. 2006;91:784–789. [Google Scholar]
- 29.Olcott Marshall A, Jehlicka J, Rouzaud J-N, Marshall CP. Multiple generations of carbonaceous material deposited in Apex chert by basin-scale pervasive hydrothermal fluid flow. Gond Res. 2014;25(1):284–289. [Google Scholar]
- 30.Marshall AO, Emry JR, Marshall CP. Multiple generations of carbon in the Apex chert and implications for preservation of microfossils. Astrobiology. 2012;12(2):160–166. doi: 10.1089/ast.2011.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sforna MC, van Zuilen MA, Philippot P. Structural characterization by Raman hyperspectral mapping of organic carbon in the 3.46 billion-year-old Apex chert, Western Australia. Geochim Cosmochim Acta. 2014;124(1):18–33. [Google Scholar]
- 32.Zhao M-Q, Huang J-Q, Zhang Q, Luo W-L, Wei F. Improvement of oil adsorption performance by a sponge-like natural vermiculite-carbon nanotube hybrid. Appl Clay Sci. 2011;53(1):1–7. [Google Scholar]
- 33.Buick R, et al. Record of emergent continental crust ∼3.5 billion years ago in the Pilbara craton of Australia. Nature. 1995;375:574–577. [Google Scholar]
- 34.Jones B, Rosen MR, Renaut RW. Silica-cemented beachrock from Lake Taupo, North Island, New Zealand. J Sediment Res. 1997;67(5):805–814. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.