Significance
The central nervous system monitors reduction in metabolic state and promotes feeding and foraging in response. While feeding behavior has been extensively studied, the regulation of foraging behavior remains largely unknown. In this study, we show that starvation-induced hyperactivity in adult fruit flies resembled foraging activity. We also found that octopamine, the insect counterpart of vertebrate norepinephrine, was crucial for starvation-induced foraging. We further showed that octopamine was not required for starvation-induced changes in feeding, suggesting independent regulations of energy intake behaviors upon starvation. Taken together, our results establish a quantitative foraging assay and a highly conserved neural substrate that regulates foraging, offering an entry point to further dissect the neural circuitry of this important behavior.
Keywords: Drosophila, starvation, foraging, octopamine
Abstract
Starved animals often exhibit elevated locomotion, which has been speculated to partly resemble foraging behavior and facilitate food acquisition and energy intake. Despite its importance, the neural mechanism underlying this behavior remains unknown in any species. In this study we confirmed and extended previous findings that starvation induced locomotor activity in adult fruit flies Drosophila melanogaster. We also showed that starvation-induced hyperactivity was directed toward the localization and acquisition of food sources, because it could be suppressed upon the detection of food cues via both central nutrient-sensing and peripheral sweet-sensing mechanisms, via induction of food ingestion. We further found that octopamine, the insect counterpart of vertebrate norepinephrine, as well as the neurons expressing octopamine, were both necessary and sufficient for starvation-induced hyperactivity. Octopamine was not required for starvation-induced changes in feeding behaviors, suggesting independent regulations of energy intake behaviors upon starvation. Taken together, our results establish a quantitative behavioral paradigm to investigate the regulation of energy homeostasis by the CNS and identify a conserved neural substrate that links organismal metabolic state to a specific behavioral output.
The CNS plays an essential role in energy homeostasis (1). It actively monitors changes in the internal energy state and modulates an array of physiological and behavioral responses to enable energy homeostasis. Foraging behavior is critical for the localization and acquisition of food supply and hence energy homeostasis. It has been extensively documented both in ethological settings (2, 3) and under well-controlled laboratory conditions (4). Laboratory rodents with limited food access exhibit stereotypic food anticipatory activity (FAA) several hours before the mealtime, which is characterized by a steady increase in locomotion and other appetitive behaviors (5). The neural substrate that drives FAA still remains elusive (5, 6). Notably, the regulation of FAA seems to be dissociable from that of feeding behavior (7, 8). These results hint at the presence of an independent and somewhat discrete regulatory mechanism of foraging behavior.
Foraging behavior has also been extensively studied in invertebrate species such as the roundworm Caenorhabditis elegans (9) and fruit flies Drosophila melanogaster (10). Roundworm populations exhibit two naturally emerged foraging patterns: “solitary” worms disperse across the bacterial lawn, and “social” worms aggregate along the food edge and form clumps (9). This behavioral dimorphism is controlled by natural variations of the npr-1 (neuropeptide receptor resemblance) gene that encodes a receptor homologous to the receptor family of orexigenic neuropeptide Y in mammals (9). A comparable scenario has also been identified in larval fruit flies (10), with two distinct forms of foraging present in nature: “rover” and “sitter.” On food sources, sitter but not rover reduces moving speed for feeding (11). Natural variations of a single gene named foraging that encodes a cGMP-dependent protein kinase are responsible for this behavioral dimorphism (10). It remains unclear, however, whether the foraging strategies outlined above are driven by animals’ metabolic state, and if so whether npr-1 and foraging are involved (4). It is worth noting that both the roundworm and fruit fly larvae are continuous feeders. It is therefore difficult to disassociate the effect of the internal energy state on foraging behavior from that of acute change in food availability.
In this present study, we sought to characterize foraging behavior in an intermittent feeder, the adult fruit fly. We confirmed that starved flies exhibited robust and sustained increase in their locomotor activity and provided evidence that it partly resembled foraging behavior. Furthermore, we found octopamine, a biological amine structurally related to vertebrate norepinephrine with similar physiological roles, both necessary and sufficient for starvation-induced hyperactivity. To summarize, our results reveal a highly conserved neural mechanism that promotes locomotion upon starvation, shedding important light on the regulation of foraging behavior by the CNS.
Results
Starvation Induces Hyperactivity of Adult Flies.
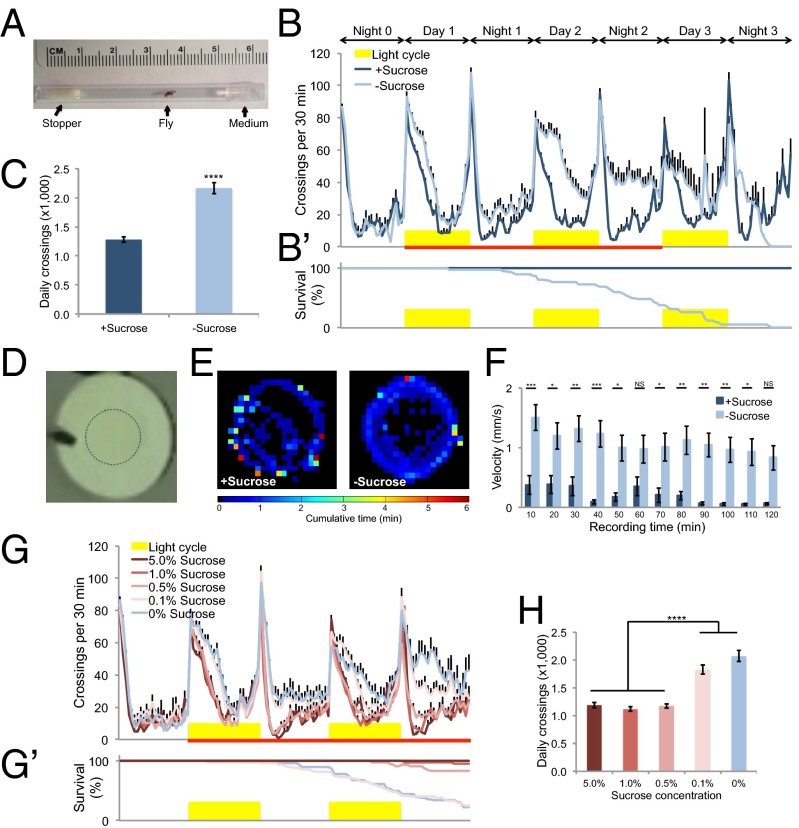
We first examined the locomotor effect of food deprivation in adult flies. To do so, we adapted an assay in which fly locomotion was indirectly measured by the flies’ frequency to cross the midline of tubes (Fig. 1A) (12). In the presence of sucrose, wild-type Canton-S flies exhibited a characteristic and relatively stable locomotor profile (Fig. 1B, dark blue). In contrast, flies showed incremental increase in locomotor activity following food deprivation (Fig. 1B, light blue and SI Appendix, Fig. S1). For a more quantitative measure of this behavioral effect, we compared the average locomotor activity of flies for a 2-d time window upon food deprivation (Fig. 1B, red line) and found that food-deprived flies showed a significant increase in locomotion compared with sucrose-fed flies (Fig. 1C). This observation was consistent with several previous reports (13–16). Moreover, this behavioral effect was independent of the light entrainment of circadian rhythm (SI Appendix, Fig. S2).
Fig. 1.
Starvation induces hyperactivity of adult flies. (A) Locomotion assay. One virgin female (“fly”) was introduced into a polycarbonate tube, with one end filled with 2% agar medium (“medium”) and the other plugged with a small piece of yarn (“stopper”). The tube was placed into the Drosophila Activity Monitor. Ruler above the tube illustrates size. (B and B′) Midline crossing activity in 30-min bins (B) and survival curve (B′) of wild-type Canton-S flies assayed in the presence and absence of 5% sucrose (n = 48). Yellow bars represent light-on period of 12 h (and in Figs. 2 and 3). Red line indicates a 2-d time window for quantifying average midline crossing activity (and in Figs. 2 and 3). (C) Average daily midline crossing activity of flies assayed in B. (D) Top view of a behavioral chamber [10 mm (D) × 4 mm (H)]. The dotted circle outlines agar patch ± 5% sucrose. (E) Spatial distribution of sucrose-fed Canton-S flies assayed in the presence of sucrose (Left) and starved Canton-S flies assayed in the absence of sucrose (Right) (n = 20). Color temperature represents average time spent on each pixel for the duration of the assay (2 h). (F) Walking velocity in 10-min bins of sucrose-fed Canton-S flies in the presence of sucrose and starved Canton-S flies in the absence of sucrose (n = 20). (G and G′) Midline crossing activity (G) and survival curve (G′) of Canton-S flies assayed on different sucrose concentrations (n = 41–50). (H) Average daily midline crossing activity of flies assayed in G and G′. Error bars represent SEM. Student’s t test (C) and one-way ANOVA (F and H) were applied for statistical analysis. NS, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We next sought to confirm this behavioral effect by using a more direct measure of the locomotor activity of fruit flies (17). We preincubated flies for ∼36 h either in the presence or absence of sucrose, respectively, and videorecorded and analyzed their activity in small behavioral chambers (Fig. 1D). We plotted the average spatial distribution of flies and found that fed flies tended to stay quiescent compared to food-deprived flies (Fig. 1E). In agreement with this observation, food-deprived flies exhibited consistently higher walking velocity than fed flies (Fig. 1F).
Hyperactivity upon food deprivation may be induced by starvation, or alternatively, by acute removal of food cues. The latter seemed unlikely because food deprivation did not induce hyperactivity immediately (Fig. 1B, night 0 and SI Appendix, Fig. S1). To further distinguish between these two alternatives, we assayed flies on different sucrose concentrations (Fig. 1 G and H). We found that flies showed comparable locomotor activity on sucrose mediums that could support their survival, but hyperactivity on media that could not support survival (Fig. 1 G and H, 5.0, 1.0, and 0.5% sucrose vs. 0.1 and 0% sucrose). Notably, all these sucrose concentrations fall within the dynamic range of the sweet-sensing gustatory neurons of fruit flies (18), and hence can likely be discriminated by flies. Taken together, these results suggest that hyperactivity induced by food deprivation is more likely a starvation response than a gustatory response (see next section).
Food Cues Suppress Starvation-Induced Hyperactivity.
Starvation-induced hyperactivity of adult flies has been thought to resemble foraging behavior in laboratory conditions (13–16). However, it can also be a nonforaging response, e.g., a stressful response to starvation. We therefore asked whether starvation-induced hyperactivity could be suppressed and even reversed upon the detection of food cues, which distinguished these two possibilities.
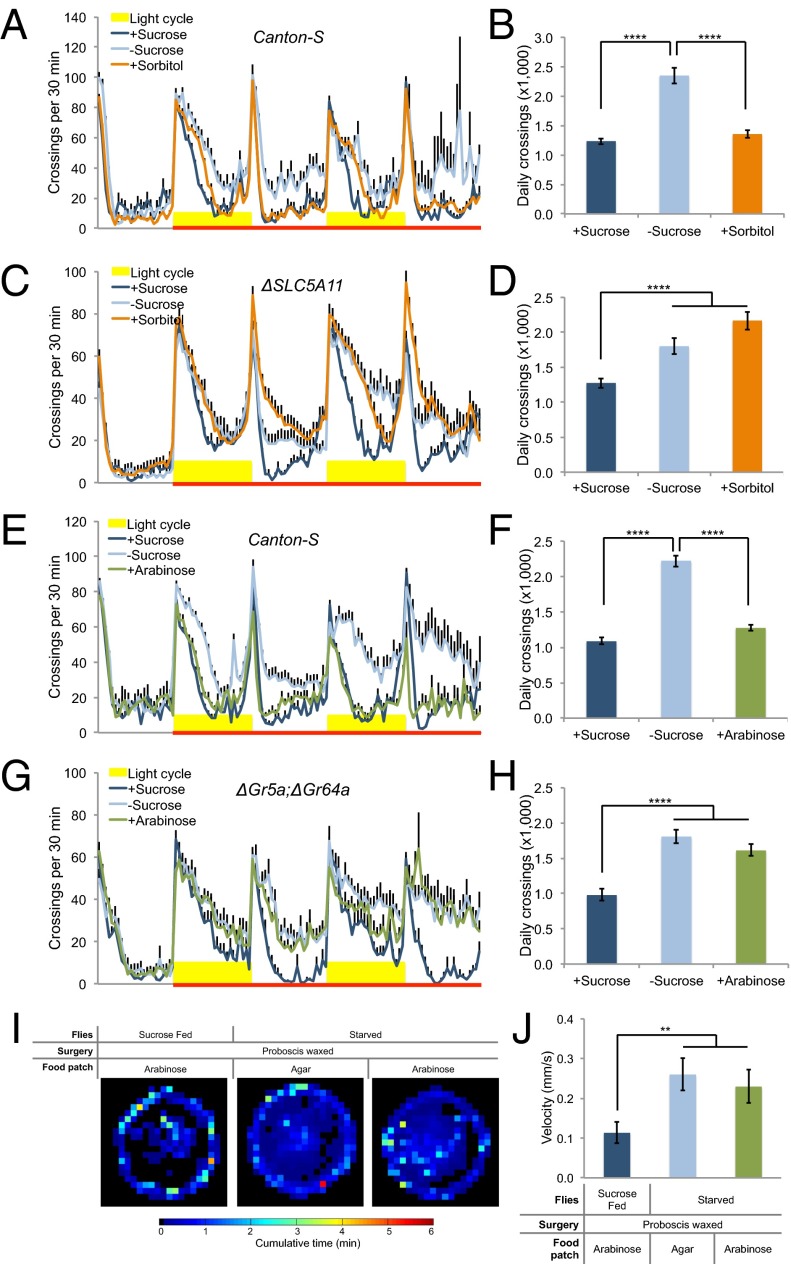
Animals are able to evaluate food quality based on both the nutrient content and the palatability (e.g., sweetness) (19). We first asked whether starvation-induced hyperactivity could be suppressed by nutrient supply in the absence of sweet taste. Sorbitol is a nutritious yet tasteless substrate to flies (20, 21). Wild-type flies exhibited comparable locomotor activity on sucrose vs. sorbitol (Fig. 2 A and B and SI Appendix, Figs. S3 and S4), suggesting that nutrient supply alone is sufficient to suppress starvation-induced hyperactivity. Taste-independent nutrient-sensing mechanisms have been reported in fruit flies (19, 22). Specifically, a recent report has shown that SLC5A11, a brain-specific sodium/solute cotransporter, mediates the evaluation of nutrient content by the CNS (23). We found that ΔSLC5A11 mutant flies exhibited comparable hyperactivity on sorbitol and on agar (Fig. 2 C and D and SI Appendix, Fig. S3), suggesting that sorbitol suppresses starvation-induced hyperactivity via a central nutrient-sensing mechanism that involves SLC5A11.
Fig. 2.
Food cues suppress starvation-induced hyperactivity. (A, C, E, and G) Midline crossing activity of indicated genotype assayed in the presence of 100 mM sucrose, 3 M sorbitol, 100 mM arabinose, and agar only (n = 32–64). (B, D, F, and H) Average daily midline crossing activity of flies assayed in A, C, E, and G. (I) Spatial distribution of sucrose-fed Canton-S flies assayed in the presence of 100 mM arabinose (Left), starved flies assayed in the presence of agar only (Middle), and 100 mM arabinose (Right) (n = 17–21). Color temperature represents average time spent on each pixel for the duration of the assay (2 h). Note that the proboscis of these flies was waxed to prevent fluid ingestion before the assay. (J) Average walking velocity of flies assayed in I. Error bars represent SEM. One-way ANOVA (B, D, F, H, and J) was applied for statistical analysis. NS, P > 0.05; **P < 0.01; ****P < 0.0001.
We next asked whether starvation-induced hyperactivity could also be suppressed by palatable food with no nutritional value. Arabinose tastes sweet to flies but nevertheless is nonnutritious and cannot support flies’ survival (20) (SI Appendix, Fig. S3). We found that starved flies did not exhibit hyperactivity in the presence of arabinose (Fig. 2 E and F and SI Appendix, Fig. S4), suggesting that the palatability of food is sufficient to suppress starvation-induced hyperactivity. Consistently, two other palatable sugars we tested, l-glucose (20) and sucralose (24), could also suppress starvation-induced hyperactivity (SI Appendix, Figs. S5 and S6). Flies mutated for gustatory receptors Gr5a and Gr64a are unresponsive to most sugars (18), including arabinose (SI Appendix, Fig. S7). We found that arabinose was unable to suppress starvation-induced hyperactivity in ΔGr5a; ΔGr64a double mutant flies (Fig. 2 G and H), suggesting that arabinose suppresses starvation-induced hyperactivity through peripheral sweet-sensing gustatory neurons. Consistent with these results, arabinose failed to suppress starvation-induced hyperactivity in taste-insensitive pox neuro mutants (22, 25) (SI Appendix, Fig. S8).
Both nutritious and palatable food cues can initiate feeding behavior and fluid ingestion. It has been shown that changes in osmolarity after food ingestion is an important satiety signal (26). We therefore asked whether fluid ingestion is a critical step for both types of food cues to suppress starvation-induced hyperactivity. Mutant flies with overfeeding and overingestion phenotypes exhibit normal starvation-induced hyperactivity (27). Consistently, we found that certain concentrations (e.g., 200 mM) of sorbitol failed to suppress starvation-induced hyperactivity, but could nevertheless support fly survival, which must be accompanied by adequate fluid ingestion (SI Appendix, Fig. S9). These results suggest that fluid ingestion is not sufficient to suppress locomotion. Meanwhile, we found that fluid ingestion was required for the suppression of starvation-induced hyperactivity. We waxed the tips of proboscises to prevent the flies from actual fluid intake, but not the engagement of the proboscises’ extension behavior, and found that after the surgery, arabinose could not suppress the hyperactivity of these flies under starvation conditions (Fig. 2 I and J). Taken together, these data suggest that fluid ingestion following the recognition of food cues may be an important step toward locomotor suppression, further supporting a role of elevated locomotion to facilitate the acquisition of food sources upon starvation. Fluid ingestion likely changes both hemolymph osmolality and mechanic tension of the gastrointestinal tract, both of which have been shown to modulate satiety and food intake (26, 28). It is therefore of interest to examine whether the same mechanisms also mediate the suppression of fly locomotion.
Octopamine Mediates Starvation-Induced Hyperactivity.
We next sought to investigate the neural mechanism of starvation-induced hyperactivity, which remained unclear in any animal species. Octopamine, the insect counterpart of vertebrate norepinephrine (29), serves as a plausible candidate. Studies in the cockroach Periplaneta americana showed that a subset of octopaminergic neurons can be activated by adipokinetic hormone, a starvation-induced, locomotion-stimulating hormone (30), implying a role of octopamine in starvation-induced changes in locomotion. Consistent with this observation, food deprivation promotes locomotor activity and synaptopod formation in fly larvae via octopamine signaling (31). It was unknown, however, whether octopamine was necessary or sufficient for starvation-induced hyperactivity.
We therefore tested whether octopamine signaling was required for starvation-induced hyperactivity in adult flies. We found that mutant flies carrying a null allele of tyramine beta-hydroxylase (TβH) (32), a key enzyme for the biosynthesis of octopamine, showed no increase in locomotor activity upon starvation, which was in sharp contrast to wild-type Canton-S flies (Fig. 3 A and B). This phenotype of TβHM18 mutant flies was unlikely a result of motor deficits, because TβHM18 mutant adults showed comparable locomotor activity to Canton-S controls under fed conditions (Fig. 3 A and B). This phenotype was also an unlikely result of enhanced starvation resistance, because TβHM18 mutant flies exhibited similar starvation-induced metabolic responses to Canton-S controls (SI Appendix, Fig. S10). Restoring TβH expression in octopaminergic/tyraminergic neurons in TβHM18 mutants partially but significantly rescued starvation-induced locomotion (SI Appendix, Fig. S11 A and B). In addition, panneuronal knockdown of TβH gene expression by RNA interference also eliminated starvation-induced hyperactivity (SI Appendix, Fig. S12). Both results further support the function of TβH in starvation-induced hyperactivity.
Fig. 3.
Octopamine mediates starvation-induced hyperactivity. (A and C) Midline crossing activity of indicated genotype assayed in the presence and absence of 5% sucrose (n = 29–49). (B and D) Average daily midline crossing activity of flies assayed in A and C. (E–G) Midline crossing activity of indicated genotype and environmental temperature, assayed in the presence and absence of 5% sucrose (n = 56–80). Note that for G, low environmental temperature caused a late onset of starvation-induced hyperactivity. (H) Average daily midline crossing activity of flies assayed in E–G. (I) Midline crossing activity of indicated genotype and environmental temperature, assayed in the presence of 5% sucrose (n = 63–76). Gray shade indicates nonpermissive temperature (27 °C). (J) Average daily midline crossing activity of flies assayed in I, before (red) and after (blue) the temperature shift. Error bars represent SEM. Two-way ANOVA (B, D, F, H, and J) was applied to test the effect of two independent variables (genotype vs. starvation/temperature) on fly locomotion, and statistical significance was identified for both variables (B, D, F, H, and J; P < 0.0001). Post hoc multiple comparisons were then performed. NS, P > 0.05; ****P < 0.0001.
TβH catalyzes the rate-limiting step of octopamine biosynthesis in which tyramine is converted to octopamine (32). It is therefore possible that the lack of starvation-induced hyperactivity in TβHM18 mutants may result from increased tyramine level rather than from lack of octopamine. To distinguish between these two possibilities, we tested mutant flies carrying a null allele of tyrosine decarboxylase 2 (Tdc2), an enzyme essential for the biosynthesis of tyramine in neurons (33). Tdc2RO54 mutants are therefore incapable of synthesizing both tyramine and octopamine in neurons. We found that like TβHM18 mutants, Tdc2RO54 mutant flies exhibited largely eliminated starvation-induced hyperactivity (Fig. 3 C and D) and that feeding Tdc2RO54 mutant flies with synthetic tyramine restored this behavioral response (SI Appendix, Fig. S11 C and D). These results argue that the lack of octopamine, but not excessive tyramine, is responsible for abolished starvation-induced hyperactivity in TβHM18 mutant flies. Notably, unlike TβHM18 mutants, Tdc2RO54 mutants showed reduced locomotor activity under fed conditions compared with Canton-S controls (Fig. 3 C and D), suggesting that tyramine biosynthesis is required for the general motor behavior of adult flies.
We next tested whether inhibition of neurons that synthesized octopamine eliminated starvation-induced hyperactivity. To do so, we ectopically expressed a potassium channel Kir2.1 (34) in octopaminergic/tyraminergic neurons (35) under the control of the TARGET system (36). Acute inhibition of these neurons by nonpermissive temperature blocked the effect of starvation to promote locomotor activity (Fig. 3 E and H), whereas the same genotype assayed at permissive temperature showed significantly increased locomotor activity upon starvation (Fig. 3 G and H). In addition, two control genotypes both showed significant starvation-induced hyperactivity at nonpermissive temperature (Fig. 3 F and H). Taken together, neuronal silencing of octopaminergic/tyraminergic neurons abolished starvation-induced hyperactivity.
To test whether octopaminergic neurons played a permissive or instructive role in starvation-induced hyperactivity, we examined the behavioral effect of acute activation of octopaminergic/tyraminergic neurons in fed flies in the presence of sucrose by ectopic expression of a temperature-sensitive cation channel Drosophila TRPA1 (37). These flies showed a significant increase in locomotion when the environmental temperature was ramped up from 20 °C to 27 °C, which activated TRPA1 and hence octopaminergic/tyraminergic neurons (Fig. 3 I and J, red). This observation is in agreement with the wakefulness-promoting effect of octopamine signaling (38). Control genotypes did not increase their locomotion upon temperature shift (Fig. 3 I and J, blue and green). Therefore, the results suggest that activation of octopaminergic/tyraminergic neurons is sufficient to drive locomotor increase in fed flies, resembling the effect of starvation on locomotion. Furthermore, neural activation of the same set of neurons in the absence of TβH gene expression did not induce hyperactivity (SI Appendix, Fig. S13), suggesting that octopaminergic neurons, but not tyraminergic neurons, are responsible for the hyperactivity phenotype.
Octopamine Is Not Required for Starvation-Induced Changes in Feeding Behavior.
Octopamine may be the master regulator of multiple starvation-induced behavioral responses. Alternatively, octopamine may be specifically involved in the regulation of locomotion by starvation, whereas distinct neural substrates mediate the regulation of different starvation-induced behaviors. We therefore examined whether octopamine signaling was required for starvation-induced changes in feeding behavior.
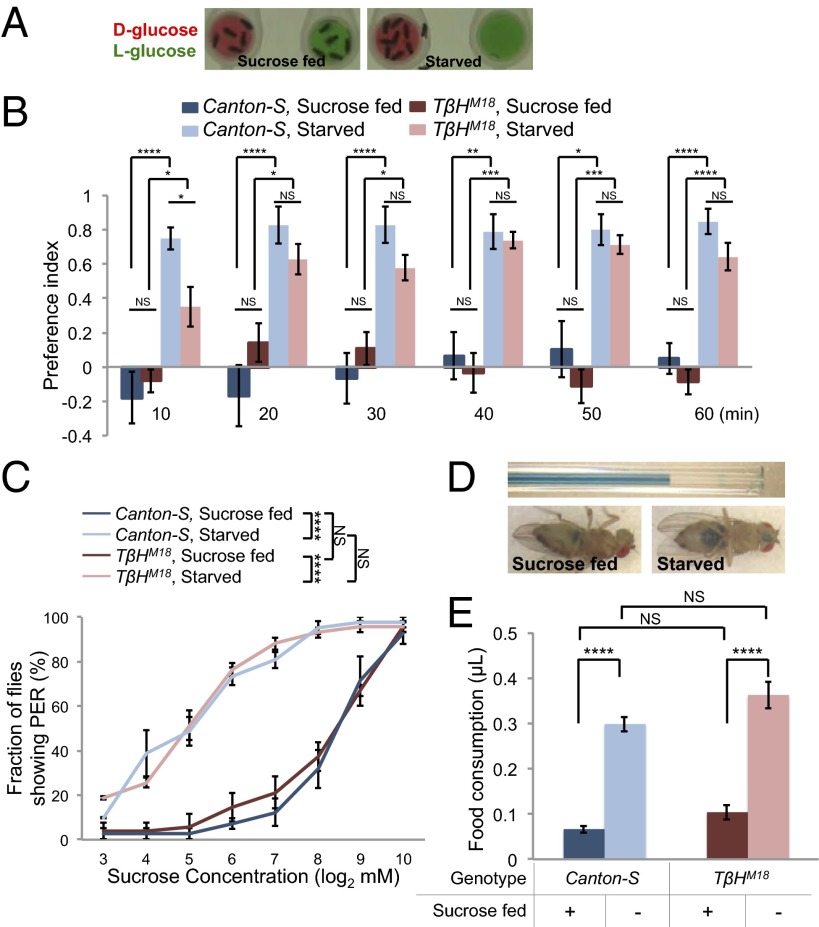
Fruit flies use both nutrient- and taste-sensing mechanisms to evaluate and choose among potential food sources (19, 22); and whereas fed flies mostly choose food sources based on sweetness, the preference of starved flies shifts toward nutritious food (19). We developed a simple and quantitative feeding preference assay, in which a group of flies was allowed to choose between two food sources containing either d-glucose or l-glucose (Fig. 4A). d-glucose and l-glucose evoke similar physiological responses in gustatory sensory neurons, whereas d-glucose but not l-glucose is nutritious and can support the survival of fruit flies (20). We found that fed Canton-S flies exhibited no preference between the two food sources, whereas they quickly established robust preference for d-glucose over l-glucose upon starvation (Fig. 4B). The data confirm that starvation induces a feeding preference toward a nutritious food source (19). TβHM18 mutant flies exhibited comparable responses as Canton-S flies under both fed and starved conditions (Fig. 4B), suggesting that octopamine is not required for elevated feeding preference toward nutritious food upon starvation. Notably, starved TβHM18 mutants seemed to take a longer time to establish such preference (Fig. 4B, 10 min). This delay was likely a consequence of the lack of starvation-induced hyperactivity of TβHM18 mutants (Fig. 3 A and B), because the mutants showed no deficit in locating and occupying food sources (SI Appendix, Fig. S14).
Fig. 4.
Octopamine is not required for starvation-induced changes in feeding behavior. For data shown in this figure, flies were preincubated in the presence (“sucrose fed”) or absence (“starved”) of 5% sucrose for ∼36 h before behavioral assays. (A) Feeding preference assay. Food sources contain either 100 mM d-glucose (red) or 100 mM l-glucose (green). Representative distributions of sucrose-fed flies (Left) and starved flies (Right) are shown. (B) Preference index of indicated genotype (n = 8 groups each of 10 flies) assayed in 10-min bins. (C) Fraction of flies showing PER response to different concentrations of sucrose (n = 4 groups each of ∼10 flies). (D) Food consumption assay using immobilized flies. (Upper) A graduated micropipette used to hand feed flies, filled with 800 mM sucrose (blue). (Lower) Abdomen of flies after hand feeding, showing the uptake of sucrose solution (blue). (E) Volume of food consumed by sucrose-fed and starved flies of indicated genotype (n = 27–30). Error bars represent SEM. Two-way ANOVA (B, C, and E) was applied to test the effect of two independent variables (genotype vs. starvation) on fly locomotion, and statistical significance was identified for both variables (B, C, and E; P < 0.01). Post hoc multiple comparisons were then performed. NS, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We next asked whether octopamine signaling played a role in starvation-induced food intake. In fruit flies, it has been shown that starvation increases the likelihood of proboscis extension reflex (PER) to sucrose, a behavioral component of feeding initiation (39). We found that TβHM18 mutant flies showed comparable PER responses to Canton-S controls under both fed and starved conditions (Fig. 4C). Starved flies have also been shown to consume more food than fed flies (40). We monitored food consumption of individual flies fed from a fluid-filled capillary. This feeding assay uses immobilized flies and minimizes the potential influence of altered locomotion by starvation, making it more suitable to measure the food consumption of TβHM18 mutant flies (Fig. 4D). We found that food consumption of TβHM18 mutant flies was comparable to controls under both fed and starved conditions (Fig. 4E). Taken together, these results suggest that octopamine signaling is not required for elevated likelihood to initiate feeding and increased food consumption upon starvation.
Discussion
Upon starvation, animals exhibit increased feeding and foraging behaviors, which in turn increases energy intake and restores energy homeostasis. The neural mechanism of feeding behavior, particularly how feeding is regulated by metabolic signals, has been extensively studied in both rodents and insect species (1, 41). In contrast how organismal metabolism influences foraging remains largely unclear (4).
In this present study, we aimed to establish a behavioral paradigm for foraging behavior and to study how it is regulated by starvation. It has been reported that starvation induces hyperactivity in both rodents and fruit flies (5, 13), but its relationship with foraging was unclear. We first confirmed that starvation induced a robust and sustained increase in locomotion in adult fruit flies (Fig. 1). Furthermore, we found that starvation-induced hyperactivity could be suppressed upon the detection of nutritious substrate via an internal energy sensor SLC5A11 (Fig. 2 A–D), as well as the detection of food palatability via sweet-sensing gustatory neurons expressing Gr5a and Gr64a (Fig. 2 E–H). We also showed that although food intake per se was not sufficient to drive the suppressive effect on locomotion, nutrient/sweetness-induced fluid ingestion was indeed required (Fig. 2 I and J). In addition, our data showed that mutant flies with a deficit in starvation-induced hyperactivity took longer time to locate and occupy desired food sources (Fig. 4B). Collectively, we conclude that starvation-induced hyperactivity in adult flies resembles foraging behavior in laboratory conditions, because it is directed toward and facilitates the localization and acquisition of food. This set of behavioral assays therefore offers a platform and an entry point to further dissect the neural basis of this evolutionarily conserved and critical behavior.
By using the foraging assay described above, we found octopamine, the insect counterpart of vertebrate norepinephrine (29), both necessary and sufficient for starvation-induced hyperactivity (Fig. 3). In fruit flies, octopamine is only synthesized and released in a small number of CNS neurons (∼100–150 neurons per fly) (35, 42). Therefore, the findings presented in this study offer a clear entry point to further dissect the neural circuitry that underlies foraging behavior in fruit flies. In addition, it is of obvious interest to examine whether norepinephrine is also involved in locomotor responses to starvation in rodents, such as FAA.
Tyramine is the precursor of octopamine synthesis and itself can also function as a neurotransmitter in insects (29). In the present study, we excluded the possibility that tyramine alone was either necessary or sufficient for starvation-induced hyperactivity (Fig. 3 and SI Appendix, Fig. S14). However, we have not excluded the possibility that octopamine and tyramine work in a synergistic way to regulate this behavior. Fruit flies express several receptors that are sensitive to both octopamine and tyramine in vitro (29, 43), which serves as a potential “hub” to integrate octopamine and tyramine signaling in vivo. Future research is needed to clarify the role of octopamine and tyramine in starvation-induced hyperactivity.
Octopamine plays an important role in the regulation of a variety of fly behaviors, such as sleep (38), learning (44), and aggression (45). It is of interest to investigate whether and how different subsets of octopaminergic neurons modulate different behaviors in flies (45, 46). Meanwhile, it is noteworthy that many of these behaviors regulated by octopamine signaling have a locomotor component. It is therefore plausible that the octopamine system may function as a general “arousal” center, modulating physical activity of flies in response to external/internal cues such as circadian rhythm, conspecific chemosensory stimuli, and metabolic signals.
Despite its importance in starvation-induced hyperactivity, we found that octopamine was not required for starvation-induced changes in feeding behavior (Fig. 4). These findings argue for independent regulations of multiple starvation-induced behavioral responses. Consistent with this idea, rodent studies have shown that several aspects of foraging behavior (e.g., FAA and food hoarding) do not require the genes (8) and brain regions (7) that are important for feeding control. In contrast, activating hypothalamic neurons expressing neuropeptide Y and agouti-related peptide promotes both feeding and locomotion (47), suggesting that feeding and foraging pathways converge at some point. It is therefore important to study whether there exists a common “hunger” center in the CNS that coordinates various starvation-induced behaviors or whether different neural mechanisms independently sense changes in the metabolic state and modulate energy homeostasis.
Materials and Methods
Flies.
Flies stocks were kept in vials containing standard fly medium made of yeast, corn, and agar at 25 °C and 60% humidity and on a 12-h light:12-h dark cycle if not otherwise indicated. For most experiments, virgin female flies were collected shortly after eclosion and kept in groups (10 flies per vial) for 3–5 d before experiments.
TβHM18 and Tdc2RO54 mutant strains were outcrossed to Canton-S background for several generations to minimize the effect of genetic background on behaviors. Wild-type white gene was also incorporated into the mutant strains to minimize the effect of white gene expression and eye color on fly behaviors.
Chemicals.
Sucrose (S7903), agar (A1296), d-sorbitol (S1876), d-(−)-arabinose (A3131), d-(+)-glucose (G8270), l-(−)-glucose (G5500), sucralose (69293), and tyramine hydrochloride (T2879) were purchased from Sigma-Aldrich. Assorted food dyes were purchased from McCormick. The working concentrations of these chemicals are specified in the main text and/or figure legends.
Locomotion Assays.
The locomotor activity of flies was assayed by two methods, one based on the Drosophila Activity Monitoring System (DAMS, Trikinetics) (12) and the other based on videorecording (17).
DAMS-based method.
Individual female flies were lightly anesthetized and introduced into polycarbonate tubes [5 mm (diameter, D) × 65 mm (length, L)]. One end of these tubes was filled with 2% (wt/vol) agar medium. Tubes were then inserted and secured in Drosophila activity monitors (DAM2). Midline crossing activity was sampled for every minute and pooled into 30-min bins for analysis. The average midline crossing activity was calculated based on a time window indicated by red and blue lines.
Videorecording-based method.
Sucrose-fed and starved flies were introduced by gentle aspiration into individual recording chambers [10 mm (D) × 4 mm (height, H)]. A small drop of 2% (wt/vol) agar medium ± 5% (wt/vol) sucrose [5 mm (D)] was placed in the center of each chamber before the assays (dotted circle in Fig. 1D). Flies were allowed to acclimate in recording chambers for 1 h, and then their activity was recorded for 2 h by a camcorder placed on top of recording chambers. Video clips were then analyzed by CADABRA software (17).
Other Behavioral Assays.
Feeding preference.
Sucrose-fed and starved flies were gently aspirated into fluon-coated behavioral chambers [50 mm (D) × 114 mm (H)] in groups of 10. Two elevated food cups [10 mm (D) × 7 mm (H)] containing either 100 mM d-glucose or 100 mM l-glucose were placed into the chamber before the assay. The chamber was videorecorded for 1 h immediately after the introduction of flies. The number of flies on each food cup was counted manually every minute and averaged into 10-min bins. Preference index = (no. of flies on d-glucose – no. of flies on l-glucose)/no. of flies combined.
PER.
PER was assayed as described previously (39). Briefly, sucrose-fed and starved flies were gently aspirated and mounted into Pipetman tips (200 μL). Flies were first fed with water until satiety and then subjected to stepwise increasing concentrations of sucrose. Each sucrose concentration was tested twice, and flies showing PER to at least one of the two trials were considered positive to that concentration.
Food consumption.
Flies were prepared and water was satiated as for PER assays. A graduated micropipette (VWR, 53432-604) filled with 800 mM of sucrose solution and 5% blue dye was presented to the proboscises of flies and the flies were allowed to drink until they became unresponsive to 10 serial sucrose stimuli. Food consumption was calculated based on volume change before vs. after feeding.
Statistical Analysis.
Data presented in this study were verified for normal distribution by D’Agostino–Pearson omnibus test. Student’s t test (for pairwise comparisons) and one-way ANOVA (for comparisons among more than two groups) were used. If one-way ANOVA detected a significant difference among groups, a post hoc test with Bonferroni correction was performed for multiple comparisons. Two-way ANOVA (and post hoc test if applicable) was applied for comparisons with more than one variant.
Supplementary Material
Acknowledgments
We thank Hubert Amrein, David Anderson, Anupama Dahanukar, Zhefeng Gong, Kristin Scott, Greg Suh, Yi Rao, and the Bloomington Drosophila Stock Center (Indiana University) for providing fly stock; Kristin Scott for her critical and insightful support along the course of our research; and Danping Chen for providing crucial scientific and administrative support in our laboratory. This study was funded by the Bowes Research Fellows Program at University of California, Berkeley and the Thousand Young Talents Plan of China.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417838112/-/DCSupplemental.
References
- 1.Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30(1):367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- 2.Stephens DW, Brown JS, Ydenberg RC. Foraging: Behavior and Ecology. Univ of Chicago Press; Chicago: 2007. [Google Scholar]
- 3.Stephens DW. Decision ecology: Foraging and the ecology of animal decision making. Cogn Affect Behav Neurosci. 2008;8(4):475–484. doi: 10.3758/CABN.8.4.475. [DOI] [PubMed] [Google Scholar]
- 4.Sokolowski MB. Social interactions in “simple” model systems. Neuron. 2010;65(6):780–794. doi: 10.1016/j.neuron.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104(4):535–545. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Mistlberger RE. Food-anticipatory circadian rhythms: Concepts and methods. Eur J Neurosci. 2009;30(9):1718–1729. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- 7.Davidson AJ. Lesion studies targeting food-anticipatory activity. Eur J Neurosci. 2009;30(9):1658–1664. doi: 10.1111/j.1460-9568.2009.06961.x. [DOI] [PubMed] [Google Scholar]
- 8.Gunapala KM, Gallardo CM, Hsu CT, Steele AD. Single gene deletions of orexin, leptin, neuropeptide Y, and ghrelin do not appreciably alter food anticipatory activity in mice. PLoS ONE. 2011;6(3):e18377. doi: 10.1371/journal.pone.0018377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94(5):679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 10.Osborne KA, et al. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277(5327):834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- 11.Kaun KR, Sokolowski MB. cGMP-dependent protein kinase: Linking foraging to energy homeostasis. Genome. 2009;52(1):1–7. doi: 10.1139/G08-090. [DOI] [PubMed] [Google Scholar]
- 12.Chiu JC, Low KH, Pike DH, Yildirim E, Edery I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J Vis Exp. 2010;(43):e2157. doi: 10.3791/2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167(1):311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meunier N, Belgacem YH, Martin J-R. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J Exp Biol. 2007;210(Pt 8):1424–1434. doi: 10.1242/jeb.02755. [DOI] [PubMed] [Google Scholar]
- 15.Johnson EC, et al. Altered metabolism and persistent starvation behaviors caused by reduced AMPK function in Drosophila. PLoS ONE. 2010;5(9):e12799. doi: 10.1371/journal.pone.0012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erion R, DiAngelo JR, Crocker A, Sehgal A. Interaction between sleep and metabolism in Drosophila with altered octopamine signaling. J Biol Chem. 2012;287(39):32406–32414. doi: 10.1074/jbc.M112.360875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6(4):297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahanukar A, Lei Y-T, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56(3):503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. J Neurosci. 2012;32(42):14767–14774. doi: 10.1523/JNEUROSCI.1887-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21(9):751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 21.Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21(9):746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dus M, Min S, Keene AC, Lee GY, Suh GSB. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci USA. 2011;108(28):11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dus M, Ai M, Suh GSB. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute co-transporter in Drosophila. Nat Neurosci. 2013;16(5):526–528. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keene AC, et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010;20(13):1209–1215. doi: 10.1016/j.cub.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nottebohm E, Dambly-Chaudière C, Ghysen A. Connectivity of chemosensory neurons is controlled by the gene poxn in Drosophila. Nature. 1992;359(6398):829–832. doi: 10.1038/359829a0. [DOI] [PubMed] [Google Scholar]
- 26.Gruber F, et al. Suppression of conditioned odor approach by feeding is independent of taste and nutritional value in Drosophila. Curr Biol. 2013;23(6):507–514. doi: 10.1016/j.cub.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Pool A-H, et al. Four GABAergic interneurons impose feeding restraint in Drosophila. Neuron. 2014;83(1):164–177. doi: 10.1016/j.neuron.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olds WH, Xu T. Regulation of food intake by mechanosensory ion channels in enteric neurons. eLife. 2014;3:e04402. doi: 10.7554/eLife.04402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roeder T. Tyramine and octopamine: Ruling behavior and metabolism. Annu Rev Entomol. 2005;50(1):447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 30.Wicher D. Metabolic regulation and behavior: How hunger produces arousal—an insect study. Endocr Metab Immune Disord Drug Targets. 2007;7(4):304–310. doi: 10.2174/187153007782794290. [DOI] [PubMed] [Google Scholar]
- 31.Koon AC, et al. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci. 2011;14(2):190–199. doi: 10.1038/nn.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monastirioti M, Linn CE, Jr, White K. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16(12):3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardie SL, Zhang JX, Hirsh J. Trace amines differentially regulate adult locomotor activity, cocaine sensitivity, and female fertility in Drosophila melanogaster. Dev Neurobiol. 2007;67(10):1396–1405. doi: 10.1002/dneu.20459. [DOI] [PubMed] [Google Scholar]
- 34.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21(5):1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole SH, et al. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280(15):14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 36.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004(220):pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 37.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454(7201):217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crocker A, Sehgal A. Octopamine regulates sleep in drosophila through protein kinase A-dependent mechanisms. J Neurosci. 2008;28(38):9377–9385. doi: 10.1523/JNEUROSCI.3072-08a.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inagaki HK, et al. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148(3):583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itskov PM, Ribeiro C. The dilemmas of the gourmet fly: The molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front Neurosci. 2013;7:12. doi: 10.3389/fnins.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dethier VG. The Hungry Fly: A Physiological Study of the Behavior Associated with Feeding. Harvard Univ Press; Cambridge, MA: 1976. [Google Scholar]
- 42.Busch S, Selcho M, Ito K, Tanimoto H. A map of octopaminergic neurons in the Drosophila brain. J Comp Neurol. 2009;513(6):643–667. doi: 10.1002/cne.21966. [DOI] [PubMed] [Google Scholar]
- 43.Bayliss A, Roselli G, Evans PD. A comparison of the signalling properties of two tyramine receptors from Drosophila. J Neurochem. 2013;125(1):37–48. doi: 10.1111/jnc.12158. [DOI] [PubMed] [Google Scholar]
- 44.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492(7429):433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11(9):1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 46.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65(5):670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.