Significance
Acute kidney injury (AKI) is a common clinical condition caused by loss of kidney function. Lack of therapeutic options has contributed to high mortality rates in AKI patients. Drug-induced AKI, as observed during cisplatin-based anticancer therapy, is responsible for about 20% of renal failure cases. The initial injury triggers a proliferative response in renal tubular cells, which in the presence of cellular damage can further accelerate renal injury. Our study provides evidence that the small-molecule cell-cycle inhibitors palbociclib and LEE011 can prevent cisplatin-induced AKI by inhibiting two relevant targets: renal cell-cycle progression and organic cation transporter 2, a renal uptake transporter of cisplatin. The future development of cyclin-dependent kinase 4/6 inhibitors as renal protective agents could have significant clinical benefits.
Keywords: organic cation transporters, acute kidney injury, cisplatin, CDK4/6, cell cycle
Abstract
Acute kidney injury (AKI) is a potentially fatal syndrome characterized by a rapid decline in kidney function caused by ischemic or toxic injury to renal tubular cells. The widely used chemotherapy drug cisplatin accumulates preferentially in the renal tubular cells and is a frequent cause of drug-induced AKI. During the development of AKI the quiescent tubular cells reenter the cell cycle. Strategies that block cell-cycle progression ameliorate kidney injury, possibly by averting cell division in the presence of extensive DNA damage. However, the early signaling events that lead to cell-cycle activation during AKI are not known. In the current study, using mouse models of cisplatin nephrotoxicity, we show that the G1/S-regulating cyclin-dependent kinase 4/6 (CDK4/6) pathway is activated in parallel with renal cell-cycle entry but before the development of AKI. Targeted inhibition of CDK4/6 pathway by small-molecule inhibitors palbociclib (PD-0332991) and ribociclib (LEE011) resulted in inhibition of cell-cycle progression, amelioration of kidney injury, and improved overall survival. Of additional significance, these compounds were found to be potent inhibitors of organic cation transporter 2 (OCT2), which contributes to the cellular accumulation of cisplatin and subsequent kidney injury. The unique cell-cycle and OCT2-targeting activities of palbociclib and LEE011, combined with their potential for clinical translation, support their further exploration as therapeutic candidates for prevention of AKI.
Cell division is a fundamental biological process that is tightly regulated by evolutionarily conserved signaling pathways (1, 2). The initial decision to start cell division, the fidelity of subsequent DNA replication, and the final formation of daughter cells is monitored and regulated by these essential pathways (2–6). The cyclin-dependent kinases (CDKs) are the central players that orchestrate this orderly progression through the cell cycle (1, 2, 6, 7). The enzymatic activity of CDKs is regulated by complex mechanisms that include posttranslational modifications and expression of activating and inhibitory proteins (1, 2, 6, 7). The spatial and temporal changes in the activity of these CDK complexes are thought to generate the distinct substrate specificities that lead to sequential and unidirectional progression of the cell cycle (1, 8, 9).
Cell-cycle deregulation is a universal feature of human cancer and a long-sought-after target for anticancer therapy (1, 10–13). Frequent genetic or epigenetic changes in mitogenic pathways, CDKs, cyclins, or CDK inhibitors are observed in various human cancers (1, 4, 11). In particular, the G1/S-regulating CDK4/6–cyclin D–inhibitors of CDK4 (INK4)–retinoblastoma (Rb) protein pathway frequently is disrupted in cancer cells (11, 14). These observations provided an impetus to develop CDK inhibitors as anticancer drugs. However, the earlier class of CDK inhibitors had limited specificity, inadequate clinical activity, poor pharmacokinetic properties, and unacceptable toxicity profiles (10, 11, 14, 15). These disappointing initial efforts now have been followed by the development of the specific CDK4/6 inhibitors palbociclib (PD0332991), ribociclib (LEE011), and abemaciclib (LY2835219), which have demonstrated manageable toxicities, improved pharmacokinetic properties, and impressive antitumor activity, especially in certain forms of breast cancer (14, 16). Successful early clinical trials with these three CDK4/6 inhibitors have generated cautious enthusiasm that these drugs may emerge as a new class of anticancer agents (14, 17). Palbociclib recently was approved by Food and Drug Administration for the treatment of metastatic breast cancer and became the first CDK4/6 inhibitor approved for anticancer therapy (18).
In addition to its potential as an anticancer strategy, CDK4/6 inhibition in normal tissues could be exploited therapeutically for wide-ranging clinical conditions. For example, radiation-induced myelosuppression, caused by cell death of proliferating hematopoietic stem/progenitor cells, can be rescued by palbociclib (19, 20). Furthermore, cytotoxic anticancer agents cause significant toxicities to normal proliferating cells, which possibly could be mitigated by the concomitant use of CDK4/6 inhibitors (20, 21). More broadly, cell-cycle inhibition could have beneficial effects in disorders in which maladaptive proliferation of normal cells contributes to the disease pathology, as observed in vascular proliferative diseases, hyperproliferative skin diseases, and autoimmune disorders (22, 23). In support of this possibility, palbociclib treatment recently was reported to ameliorate disease progression in animal models of rheumatoid arthritis through cell-cycle inhibition of synovial fibroblasts (24).
Abnormal cellular proliferation also is a hallmark of various kidney diseases (25), and cell-cycle inhibition has been shown to ameliorate significantly the pathogenesis of polycystic kidney disease (26), nephritis (27), and acute kidney injury (AKI) (28). Remarkably, during AKI, the normally quiescent renal tubular cells reenter the cell cycle (29–34), and blocking cell-cycle progression can reduce renal injury (28). Here, we provide evidence that the CDK4/6 pathway is activated early during AKI and demonstrate significant protective effects of CDK4/6 inhibitors in animal models of cisplatin-induced AKI. In addition, we found that the CDK4/6 inhibitors palbociclib and LEE011 are potent inhibitors of organic cation transporter 2 (OCT2), a cisplatin uptake transporter highly expressed in renal tubular cells (35–37). Our findings provide a rationale for the clinical development of palbociclib and LEE011 for the prevention and treatment of AKI.
Results and Discussion
CDK4/6 Activation in Renal Tubular Cells During AKI.
Multiple cellular and physiological factors contribute to the development of AKI (38–43). However, the major etiology of AKI is renal tubular cell death caused by ischemia, infections, or drug-induced toxicities (44–47). During AKI, along with cell death, cell-cycle activation is initiated in the normally quiescent renal tubular cells (29–34). It is believed that this proliferative response may contribute to tissue regeneration (28, 32, 33, 38), although this notion has been challenged recently (48). These issues notwithstanding, it is clear that a significant fraction of renal tubular cells enter the cell cycle during AKI (32, 34) and that blocking or delaying cell-cycle entry has protective effects (28). Studies with both genetic mouse models and pharmacological inhibitors have shown that inducing cell-cycle arrest by overexpression of p21 or treatment with CDK2 inhibitors prevents AKI, whereas knockdown of p21 increases renal tissue damage (28, 29). Although CDK2-inhibiting strategies ameliorate AKI, CDK2-specific inhibitors that can be used clinically are unavailable. On the other hand, three CDK4/6 inhibitors currently are in the late stages of clinical development as anticancer therapeutics and have demonstrated manageable toxicity profiles (14). However, whether the CDK4/6 pathway is activated in renal cells during AKI is unknown.
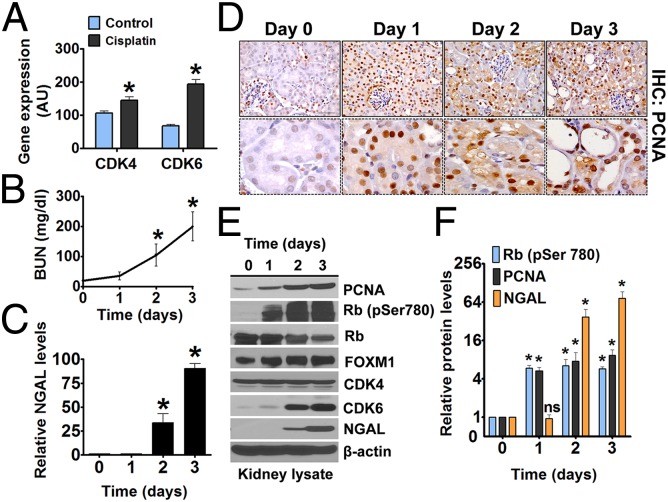
In our recent work (36) and as reported previously (29), expression of the cell-cycle regulatory protein p21 was highly elevated during cisplatin-induced AKI. Interestingly, we also observed increased mRNA expression of CDK4 and CDK6, which are required for G1/S cell-cycle transition (Fig. 1A). These changes were observed on day 3 after cisplatin administration, when the kidneys already were severely injured, so first we examined whether CDK4/6 expression and activation occur early during cisplatin nephrotoxicity. In our in vivo model, a single i.p. injection of 15 mg/kg cisplatin leads to severe renal injury on day 3 as shown by increases in the levels of blood urea nitrogen (BUN) and of renal neutrophil gelatinase-associated lipocalin (NGAL) (49), a more sensitive AKI biomarker (Fig. 1 B and C).
Fig. 1.
CDK4/6 activation during AKI. (A) Wild-type mice were treated with either vehicle or cisplatin (15 mg/kg), and renal tissues isolated on day 3 were used for microarray analysis of select genes, including CDK4 and CDK6. (B) Wild-type mice were treated with cisplatin (15 mg/kg), and BUN levels were measured after the mice were killed on the indicated days. (C) Wild-type mice were treated with cisplatin (15 mg/kg), and renal tissues were used for Western blot analysis of NGAL. The graph represents relative renal NGAL levels from three independent experiments. Densitometry of Western blots was followed by calculation of relative NGAL levels compared with control group after normalization to actin levels. (D, Upper) Renal tissues from cisplatin-treated mice were stained to detect PCNA levels before and after cisplatin treatment (400× magnification). (Lower) Staining in renal tubules. (E and F) Wild-type mice were treated with cisplatin (15 mg/kg). (E) renal tissues were used for Western blot analysis of indicated proteins. (F) The graph represents relative protein levels from three independent experiments. Densitometry of Western blots was followed by calculation of relative protein levels compared with the control group after normalization to actin levels. Data in the graphs are presented as mean ± SE; n ≥ 4; asterisks indicate a statistically significant difference compared with untreated group.
To determine the time point at which renal tubular cells enter the cell cycle, we examined the protein expression of proliferating cell nuclear antigen (PCNA) and Ki-67, established indicators of renal cell-cycle progression (29, 34). Interestingly, renal PCNA (Fig. 1D) and Ki-67 protein levels (Fig. S1) already were increased on day 1, indicating that cell-cycle activation occurs before the development of kidney injury. We also found by Western blot analysis that the protein expression of CDK6 increases in the kidneys after cisplatin treatment, confirming the results of mRNA expression (Fig. 1E). To determine the activation of CDK4/6 pathway, we examined the levels of phosphorylated Rb protein (phospho-Rb) in renal tissues. We found that Rb phosphorylation was very low in the kidneys of control animals but was increased substantially on day 1 after cisplatin treatment (Fig. 1 E and F). Hypophosphorylated Rb blocks G1/S cell-cycle progression, and inactivation of Rb by CDK4/6-mediated phosphorylation is essential for cell-cycle entry (50). Because phospho-Rb is a well-established indicator of CDK4/6 activation (14, 50), our results suggested that the CDK4/6 pathway is activated during cisplatin nephrotoxicity. For further confirmation, we evaluated the expression of forkhead box M1 (FOXM1) transcription factor, which is a key cell-cycle regulator that is stabilized and activated by CDK4/6-dependent phosphorylation (51). We found significant stabilization of FOXM1 protein expression during cisplatin nephrotoxicity (Fig. 1 E and F), further confirming CDK4/6 activation in renal tissues during AKI.
Overall, these initial studies showed that there is a robust activation of the CDK4/6–Rb pathway during cisplatin-induced AKI that coincides with cell-cycle entry. More significantly, both CDK4/6 activation and cell-cycle entry preceded the development of AKI. This early cell-cycle activation maybe deleterious in the presence of cisplatin-induced DNA damage. This reasoning led us to hypothesize that transiently blocking CDK4/6 activation during the early phase of AKI could reduce renal cell death and that this strategy most likely would not interfere with the later regenerative processes.
CDK4/6 Small-Molecule Inhibitors with OCT2 Inhibition Properties.
The activation of the CDK4/6–Rb pathway during cisplatin-induced AKI provided us with a rationale to test if the three currently available CDK4/6 inhibitors—palbociclib, LEE011, and LY2835219—can reduce renal injury. Before we conducted these experiments, we fortuitously noticed that one of these compounds, palbociclib, exists as a cation protonated on the nitrogen atom of the pyridine ring (52) and has a hydrophobic-aromatic site separated by a short distance from the positive charge, as well as multiple double-bonded oxygen moieties. These three structural motifs previously were found to be overrepresented in potent inhibitors of the organic cation transporter OCT2 (53–55). The presence of these motifs is of potential interest because OCT2 is responsible for accumulation of cisplatin in renal cells (35, 37, 56). In mice, two closely related organic cation transporters, Oct1 and Oct2, together fulfill a role equivalent to that of a single organic cation transporter, OCT2, in human kidneys. These transporters are highly expressed on the basolateral membrane of renal tubular cells, and their pharmacological or genetic inhibition reduces cisplatin nephrotoxicity (57).
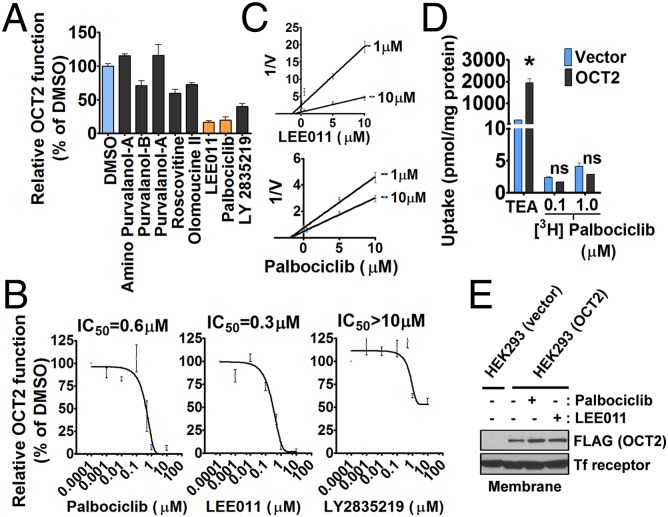
Based on their aforementioned structural features, we hypothesized that palbociclib and structurally related CDK inhibitors also might inhibit OCT2 function. In a targeted screen using tetraethylammonium (TEA) as an OCT2 substrate, we found that palbociclib and LEE011 can inhibit OCT2 function significantly, but other cell-cycle inhibitors had very limited effects (Fig. 2A). To confirm these findings, we performed dose–response experiments and found that palbociclib and LEE011 inhibit OCT2 function with IC50 values of about 0.5 µM, whereas LY2835219 had an IC50 value >10 µM (Fig. 2B). The differences in OCT2 inhibition might be attributed to the chemical structure of LY2835219, which is dissimilar from the almost identical structures of palbociclib and LEE011. Additional kinetic studies showed that these compounds inhibit OCT2 by a noncompetitive mechanism (Fig. 2C). This finding was supported further by uptake assays in which we found that palbociclib was not itself a transported OCT2 substrate (Fig. 2D). Moreover, palbociclib and LEE011 did not influence the expression and/or membrane localization of OCT2 (Fig. 2E). The OCT2-inhibitory properties of palbociclib and LEE011 were not restricted to TEA, because they also blocked the OCT2-mediated uptake of other relevant substrates such as cisplatin, 4-[4-(dimethylamino)styryl]-N-methylpyridinium-iodide (ASP+), metformin, and dopamine (Fig. S2). Moreover, the inhibitory effect of CDK4/6 inhibitors on OCT2 function was reversible (Fig. S3) and also was observed for the related transporters OCT1 and OCT3 and for Oct1 and Oct2, the two murine orthologs of human OCT2 (Fig. S4).
Fig. 2.
Identification of CDK4/6 inhibitors that also inhibit OCT2 function. (A) HeLa cells overexpressing OCT2 were coincubated with the OCT2 substrate TEA and the indicated cell-cycle inhibitors for 10 min followed by determination of TEA uptake. The TEA uptake after protein normalization and relative to the DMSO group was used as indicator of OCT2 function. (B) Dose–response curves of indicated CDK4/6 inhibitors in HeLa-OCT2 cells. (C) Dixon plots of LEE011 and palbociclib at two indicated concentrations using TEA as the OCT2 substrate. (D) Vector- or OCT2-transfected HeLa cells were incubated with palbociclib or TEA, and the relative uptake levels were measured after 10 min. (E) Vector- or OCT2-transfected HeLa cells were treated with the indicated compounds. After a 10-min incubation, membrane proteins were isolated by a standard biotinylation method, and the membrane expression of OCT2 was analyzed by Western blot analysis.
Palbociclib and LEE011 Inhibit OCT2 Function in Vivo.
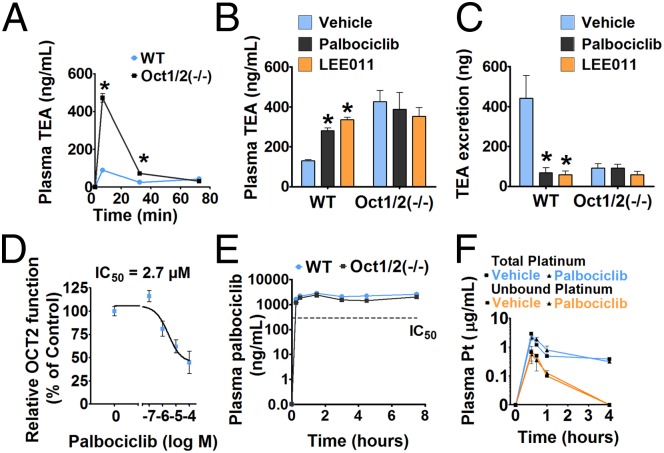
Our results suggested that palbociclib and LEE011, in addition to targeting CDK4/6 activity, have unique and potentially beneficial OCT2-inhibitory activity. Therefore, we next evaluated whether palbociclib and LEE011 can inhibit OCT2 function in vivo, using an experimental model in which TEA excretion is used as readout of in vivo OCT2 function (58). This method is based on the principle that the urinary excretion of TEA is dependent on OCT2, and genetic or pharmacological inhibition of OCT2 activity will lead to reduced TEA excretion. TEA is an excellent OCT2 substrate that is not metabolized extensively, and its excretion is reduced significantly in mice deficient for Oct1 and Oct2 (Oct1/2−/− mice), resulting in increased accumulation of TEA in plasma (59). To establish the experimental conditions, we initially performed a time-course experiment (Fig. 3A) and confirmed previous findings (59) that TEA excretion was reduced significantly in Oct1/2−/− mice. We thus had an experimental model to test whether OCT2 function is inhibited in vivo by palbociclib and LEE011. In the next series of experiments, wild-type or Oct1/2−/− mice were administered palbociclib or LEE011 [150 mg/kg, by mouth (p.o.)], followed 30 min later by i.v. TEA administration. These studies showed that palbociclib and LEE011 can increase plasma levels of TEA significantly and at the same time decrease its urinary excretion in wild-type mice but not in Oct1/2−/− mice (Fig. 3 B and C). These findings provide direct evidence that palbociclib and LEE011 can inhibit OCT2 function in vivo at a dose used in the initial preclinical development of these drugs (60) and equivalent to doses used in humans.
Fig. 3.
Palbociclib and LEE011 are potent OCT2 inhibitors in vivo. (A) Wild-type or Oct1/2−/− mice were injected i.v. with a 0.2-mg/kg dose of TEA, and plasma levels of TEA were measured at the indicated time points. (B) Wild-type or Oct1/2−/− mice were administered vehicle, palbociclib, or LEE011 (150 mg/kg each) by oral gavage. After 30 min, all mice were injected i.v. with a 0.2-mg/kg dose of TEA, and plasma levels of TEA were measured at 5 min. (C) Urinary TEA levels measured at 5 min under the same conditions. (D) Isolated renal tubules were incubated with palbociclib in the presence of the OCT2 substrate ASP+, and relative uptake was measured compared with control group. (E) Wild-type and Oct1/2−/− mice received palbociclib (150 mg/kg p.o.), and plasma levels of palbociclib were determined at the indicated time points. (F) Wild-type or Oct1/2−/− mice were administered either vehicle or palbociclib (150 mg/kg) by oral gavage. After 30 min, all mice were injected i.p. with a 15-mg/kg dose of cisplatin, and plasma levels of total platinum (total and protein-unbound) were measured at the indicated time points. Data in the graphs are presented as mean ± SE, n ≥ 4; asterisks indicate a statistically significant difference compared with the untreated group.
To address whether the observed inhibitory effects are caused by direct inhibition of OCT2 in renal tubules, we used an ex vivo uptake method (61) in which proximal tubules are isolated from mouse kidneys and OCT2 function is determined by measuring the levels of ASP+ uptake. These experiments further confirmed palbociclib-mediated inhibition of OCT2 function in tubular cells (Fig. 3D) and raised the possibility that certain CDK4/6 inhibitors could have significant renoprotective effects by blocking both CDK4/6 activation and OCT2 activity.
Before proceeding to test if these inhibitors can prevent cisplatin nephrotoxicity, we carried out detailed pharmacokinetic analyses to gain further insights into the pharmacological properties of these compounds. Pharmacokinetic studies in wild-type and Oct1/2−/− mice showed that the plasma levels of palbociclib are not altered by OCT2 deficiency, further supporting the notion that palbociclib is not an OCT2 substrate (Fig. 3E). Moreover, the observed plasma levels of palbociclib are sufficiently high to inhibit OCT2 activity for prolonged durations in vivo without substantially affecting the systemic disposition of cisplatin (Fig. 3F). These studies supported further exploration of CDK4/6 inhibitors in animal models of cisplatin nephrotoxicity.
Protective Effects of CDK4/6 Inhibitors in Mouse Models of AKI.
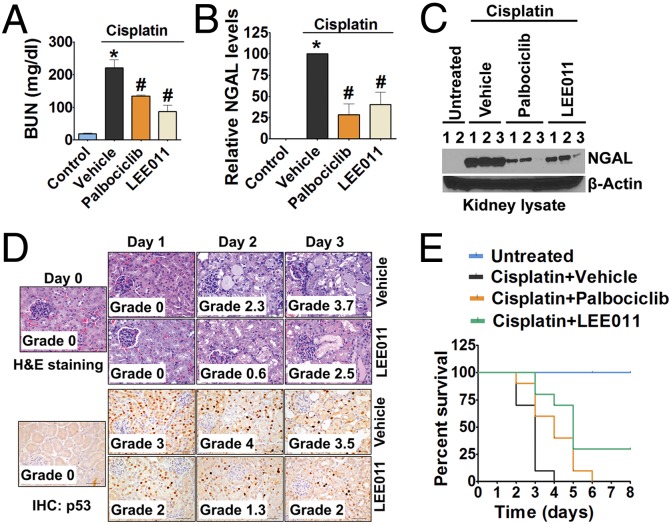
To evaluate whether CDK4/6 inhibitors can prevent AKI, we used a model of cisplatin nephrotoxicity in which a single i.p. injection of cisplatin (15 mg/kg) results in the development of severe AKI 3 d later (35, 36, 62). Vehicle, palbociclib, or LEE011 (150 mg/kg) was administered to mice by oral gavage, followed by i.p. injection of cisplatin (15 mg/kg) 30 min later. As shown in Fig. 4 A–C, cisplatin-induced increases in BUN and renal NGAL levels were suppressed significantly in mice treated with palbociclib or LEE011 compared with the vehicle-treated mice. Tissue histology further corroborated that these compounds prevented cisplatin-induced renal injury (Fig. 4D) as well as renal activation of p53, which is a critical mediator of AKI (63). Furthermore, survival experiments also showed significant overall protection by CDK4/6 inhibitors, especially LEE011 (Fig. 4E). Because this is a very severe model of AKI, with almost all animals dying on day 4, a survival advantage provided by CDK4/6 inhibitors was not anticipated and thus could be considered very significant. Interestingly, in a recent study (64), palbociclib was found to prevent ischemic AKI, suggesting that cell-cycle inhibition could have significant and wide-ranging therapeutic potential in the treatment of AKI. In the study cited, CDK4/6 activation during ischemic AKI was not determined; however, because cell-cycle activation is observed in multiple models of AKI, CDK4/6 activation in renal cells is likely to be a common phenomenon, irrespective of the underlying cause of AKI.
Fig. 4.
CDK4/6 inhibitors mitigate cisplatin-induced AKI. (A) Wild-type mice were administered either vehicle or 150 mg/kg CDK4/6 inhibitors p.o. followed by an i.p. injection of cisplatin (15 mg/kg), and BUN levels were measured on day 3. (B and C) Day 3 renal tissues were used for Western blot analysis of NGAL. (B) The graph represents relative renal NGAL levels from three independent experiments. (C) Densitometry of Western blots was followed by calculation of relative NGAL levels compared with the control group after normalization to actin levels. (D) Wild-type mice were treated with vehicle or LEE011, followed by cisplatin (15 mg/kg) injection, and tissues collected on the indicated days were analyzed to determine the level of tissue damage. (E) Wild-type mice were administered either vehicle or 150 mg/kg CDK4/6 inhibitors p.o. followed by i.p. injection of cisplatin (15 mg/kg), and overall survival was determined up to 2 wk after cisplatin administration. Three of the 10 mice treated with LEE011 survived for the duration of experiment. Data in the graphs are presented as mean ± SE; n ≥ 4; asterisks indicate a statistically significant difference compared with untreated group; hash marks (#) indicate statistically significant difference compared with vehicle group.
Renal Cell-Cycle Entry During AKI Is Inhibited by CDK4/6 Inhibitors.
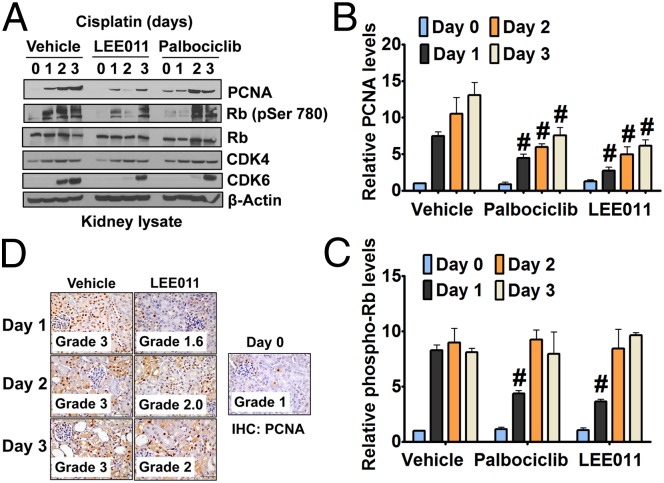
To examine if cisplatin-induced CDK4/6 activation is reduced by palbociclib or LEE011 in vivo, we performed Western blot analysis of renal tissues. As shown in Fig. 5 A–C, palbociclib or LEE011 treatment reduced both the phospho-Rb levels in kidneys and the robust induction of PCNA seen after cisplatin treatment, indicating that cell-cycle progression was blocked and/or delayed. This observation was corroborated further by renal Ki-67 levels (Fig. S5). Furthermore, immunohistochemistry confirmed that the increase in PCNA expression in renal tubular cells after cisplatin treatment was diminished significantly by LEE011 (Fig. 5D).
Fig. 5.
CDK4/6 inhibitors suppress cell-cycle activation. (A) Wild-type mice were administered either vehicle or 150 mg/kg CDK4/6 inhibitors (p.o.) followed by i.p. injection of cisplatin (15 mg/kg). Renal tissues were collected on days 0–3, and Western blot analysis was done for indicated proteins. (B and C) Graphical representation of relative renal PCNA (B) and phospho-Rb (C) levels from three independent experiments. Densitometry of Western blots was followed by calculation of relative protein levels compared with the vehicle-treated control group after normalization to actin levels. (D) Wild-type mice either were injected with vehicle or were administered 150 mg/kg LEE011 p.o. followed by the i.p. injection of cisplatin (15 mg/kg), and renal PCNA levels were determined on days 0–3. Data in the graphs are presented as mean ± SE; n ≥ 4; hash marks (#) indicate a statistically significant difference compared with the cisplatin + vehicle group.
OCT2-Independent Effects of CDK4/6 Inhibitors.
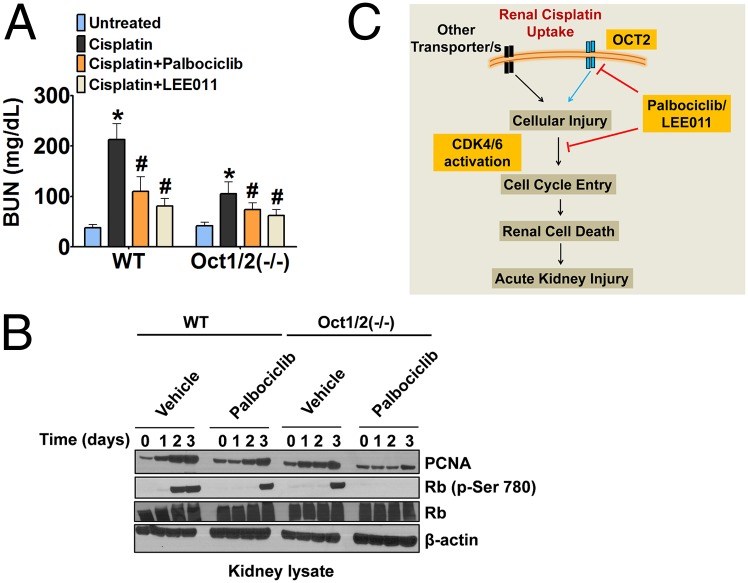
To address whether the renal protective effect of these inhibitors is caused by OCT2 and/or CDK4/6 inhibition, we initially used a cell-culture model of cisplatin-induced cell death. siRNA-mediated knockdown of CDK4/6 and OCT2 in renal tubular cells resulted in protection from cisplatin-induced cell death (Fig. S6 A and B). Moreover, palbociclib treatment provided further protection in cells with either CDK4/6 or OCT2 knockdown (Fig. S6C). These results combined with the previous data showing that palbociclib and LEE011 have dual CDK4/6 and OCT2 inhibition activity suggest that the renal-protective effects of palbociclib most likely are mediated through the inhibition of both CDK4/6 and OCT2. Because cultured cells generally represent a poor model of cell-cycle changes that occur in vivo, we used the Oct1/2−/− mice, as previously used by our group and others (35, 37). As reported earlier (35), OCT2-deficient mice were significantly protected against cisplatin nephrotoxicity (Fig. 6A); however, palbociclib and LEE011 were able to reduce renal injury further in these mice. Western analysis showed that, compared with wild-type mice, kidneys of Oct1/2−/− mice had significantly reduced induction of the CDK4/6 pathway and PCNA protein levels. Palbociclib strongly inhibited both the phosphorylation of Rb and the induction of PCNA in Oct1/2−/− mice (Fig. 6B). Based on these results, we propose that the mitigation of cisplatin-induced AKI associated with palbociclib and LEE011 most likely is related to the dual inhibition of CDK4/6 and OCT2 (Fig. 6C).
Fig. 6.
CDK4/6 inhibitors suppress cell-cycle activation in OCT2-deficient mice. (A) Wild-type mice and Oct1/2−/− mice either were injected with vehicle or were administered CDK4/6 inhibitors (150 mg/kg) p.o. followed by i.p. injection of cisplatin (15 mg/kg). BUN levels were measured on day 3. (B) Wild-type mice and Oct1/2−/− mice either were injected with vehicle or were administered LEE011 (150 mg/kg) p.o. followed by i.p. injection of cisplatin (15 mg/kg). Renal tissues were collected on days 0–3, and Western blot analysis was done for the indicated proteins. (C) Proposed model for the renal-protective effects of CDK4/6 inhibitors.
CDK4/6 Inhibitors as Adjunct Therapy During Cisplatin Treatment.
Combining cisplatin and CDK4/6 inhibitors could reduce nephrotoxicity during anticancer therapy. However, it is important to establish that the anticancer efficacy of cisplatin is not reduced by CDK4/6 inhibitors (11, 20). The success of such a combination therapy most likely would depend on two crucial factors, namely dosing strategy and the genetic status of the CDK4/6–cyclin D–INK4–Rb pathway in the cancer cells. Extensive in vivo experiments would be required to establish that these combinations not only lead to reduced toxicities but also do not result in antagonistic anticancer effects. To gain preliminary insights, we performed in vitro experiments in which cancer cells were treated with CDK4/6 inhibitors 30 min before cisplatin treatment, followed by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assays at 48 h. Our results in Rb-proficient ovarian cancer cell lines (A2780 and IGROV-1) and a testicular cancer cell line (NCCIT) showed that the CDK4/6 inhibitors do not antagonize the cytotoxic effects of cisplatin (cooperative index values, <0.8 for all cell lines) under these conditions (Fig. S7). Similar results have been reported previously for cotreatment experiments with carboplatin and palbociclib (65). Of note, the dosing regimen is expected to have a critical role in avoiding potential antagonistic effects. In our in vivo experiments, we pretreated mice with CDK4/6 inhibitors and injected cisplatin 30 min later. Under these short-pretreatment or cotreatment dosing conditions, cancer cells would not have time to synchronize in G1 phase, so the anticancer effects of cisplatin most likely would not be affected. Importantly, although the possibility remains, there is no clear evidence that blocking the cells in G1 phase would lead to reduced cisplatin toxicity in cancer cells (66). Another important consideration is that the cell-cycle effects of CDK4/6 inhibitors are completely dependent on functional Rb and the CDK4/6 pathway (14, 67). Rb is inactivated in multiple cancers, including in about 90% small-cell lung cancers (11, 20, 50), which are routinely treated with cisplatin. Combining cisplatin and CDK4/6 inhibitors during the treatment of these malignancies has the potential to reduce renal toxicities significantly without having cell-cycle–dependent antagonistic effects in cancer cells (20, 68).
Overall, our studies indicate that (i) the CDK4/6 pathway is strongly activated during AKI; (ii) pharmacological inhibition of CDK4/6 can prevent cell-cycle progression and CDK4/6 activation and can provide protection from cisplatin-induced AKI; and (iii) palbociclib and LEE011 have unique OCT2-inhibitory activity that could be exploited pharmacologically to prevent OCT2-mediated toxicities associated with multiple prescription drugs, including platinum-based chemotherapeutics (69). The favorable pharmacological properties of CDK4/6 inhibitors observed in clinical trials make them ideal candidates for the prevention of AKI. Notably, despite its high prevalence and mortality rates, no therapeutic interventions are available to prevent AKI (41). The current study provides a strong rationale and in vivo proof-of-concept experiments that support further exploration of CDK4/6 inhibitors in preventing and/or treating AKI.
Methods
Animal Experiments and Histological Analysis.
Wild-type and Oct1/2−/− mice on an FVB strain (Taconic) were bred in house and were housed and handled in accordance with the Institutional Animal Care and Use Committee of St. Jude Children's Research Hospital or as approved by a governmental committee overseeing animal welfare at the University of Münster and in accordance with national animal protection laws. In all experiments, 8- to 12-wk-old male mice were used for in vivo experiments. For histological analysis, a pathologist blinded to sample identity performed the staining and graded the results according to following scores: <10% = grade 0 (minimal); 11–25% = grade 1 (mild); 26–50% = grade 2 (moderate); 51–75% = grade 3 (marked); and >75% = grade 4 (severe). See SI Methods for detailed descriptions of animal experiments.
Tissue Lysate Preparation and Western Blot.
After collection and storage at −80 °C, frozen renal cortical tissue lysates were prepared in RIPA buffer supplemented with 1% SDS and protease and phosphatase inhibitors. Western blot analysis was done by standard procedures. See SI Methods for detailed descriptions.
Measurement of Palbociclib.
Concentrations of palbociclib in plasma of mice were determined by a validated method based on LC-MS/MS as described in detail in SI Methods.
Cell Culture and Cellular Accumulation Assays.
HeLa or HEK293 cells expressing various transporters were cultured in DMEM with 10% FBS. Detailed methods for uptake assays are described in SI Methods.
Statistical Analysis.
Data are presented as mean with SE. Statistical calculations were performed using GraphPad Prism software. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Drs. Anne Nies and Mathias Schwab of the Dr. Margarete Fischer-Bosch Institute of Clinical Pharmacology for providing cells stably expressing OCT1 and OCT3 and Dr. Ming Chang Hu of the University of Texas Southwestern Medical Center for providing the NRK cells. This work was supported in part by the American Lebanese Syrian Associated Charities, National Cancer Institute Grant R01CA151633, US Public Health Service Grant P30CA021765, and Grant Cia2/013/13 from the Interdisciplinary Center for Clinical Research Münster.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424313112/-/DCSupplemental.
References
- 1.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 2.Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100(1):71–78. doi: 10.1016/s0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhou BB, Elledge SJ. The DNA damage response: Putting checkpoints in perspective. Nature. 2000;408(6811):433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 4.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432(7015):316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 5.Bartek J, Lukas J. DNA damage checkpoints: From initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19(2):238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18(22):2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 7.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: Several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28(33):2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 8.Coudreuse D, Nurse P. Driving the cell cycle with a minimal CDK control network. Nature. 2010;468(7327):1074–1079. doi: 10.1038/nature09543. [DOI] [PubMed] [Google Scholar]
- 9.Merrick KA, Fisher RP. Why minimal is not optimal: Driving the mammalian cell cycle—and drug discovery—with a physiologic CDK control network. Cell Cycle. 2012;11(14):2600–2605. doi: 10.4161/cc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz GK, Shah MA. Targeting the cell cycle: A new approach to cancer therapy. J Clin Oncol. 2005;23(36):9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24(11):1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 12.Collins I, Garrett MD. Targeting the cell division cycle in cancer: CDK and cell cycle checkpoint kinase inhibitors. Curr Opin Pharmacol. 2005;5(4):366–373. doi: 10.1016/j.coph.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res. 2014;20(13):3379–3383. doi: 10.1158/1078-0432.CCR-13-1551. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro GI. Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin Cancer Res. 2004;10(12 Pt 2):4270s–4275s. doi: 10.1158/1078-0432.CCR-040020. [DOI] [PubMed] [Google Scholar]
- 16.DeMichele A, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: Phase II activity, safety and predictive biomarker assessment. Clin Cancer Res. 2015;21(6):995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- 17.Garber K. The cancer drug that almost wasn’t. Science. 2014;345(6199):865–867. doi: 10.1126/science.345.6199.865. [DOI] [PubMed] [Google Scholar]
- 18. (2015) First CDK 4/6 inhibitor heads to market. Cancer Disc 5(4):339–340. [DOI] [PubMed]
- 19.Johnson SM, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120(7):2528–2536. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts PJ, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104(6):476–487. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gudkov AV, Komarova EA. Radioprotection: Smart games with death. J Clin Invest. 2010;120(7):2270–2273. doi: 10.1172/JCI43794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabel EG. CDKs and CKIs: Molecular targets for tissue remodelling. Nat Rev Drug Discov. 2002;1(8):587–598. doi: 10.1038/nrd869. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz-Espín D, Serrano M. Cellular senescence: From physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 24.Hosoya T, et al. Cell cycle regulation therapy combined with cytokine blockade enhances antiarthritic effects without increasing immune suppression. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205566. [DOI] [PubMed] [Google Scholar]
- 25.Shankland SJ. Cell-cycle control and renal disease. Kidney Int. 1997;52(2):294–308. doi: 10.1038/ki.1997.335. [DOI] [PubMed] [Google Scholar]
- 26.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444(7121):949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 27.Pippin JW, Qu Q, Meijer L, Shankland SJ. Direct in vivo inhibition of the nuclear cell cycle cascade in experimental mesangial proliferative glomerulonephritis with Roscovitine, a novel cyclin-dependent kinase antagonist. J Clin Invest. 1997;100(10):2512–2520. doi: 10.1172/JCI119793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int. 2009;76(6):604–613. doi: 10.1038/ki.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price PM, Megyesi J, Safirstein RL. Cell cycle regulation: Repair and regeneration in acute renal failure. Semin Nephrol. 2003;23(5):449–459. doi: 10.1016/s0270-9295(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 30.Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1998;101(4):777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megyesi J, Andrade L, Vieira JM, Jr, Safirstein RL, Price PM. Coordination of the cell cycle is an important determinant of the syndrome of acute renal failure. Am J Physiol Renal Physiol. 2002;283(4):F810–F816. doi: 10.1152/ajprenal.00078.2002. [DOI] [PubMed] [Google Scholar]
- 32.Humphreys BD, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA. 2011;108(22):9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA. 2014;111(4):1527–1532. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543, 1p, 143. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86(4):396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprowl JA, et al. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res. 2014;20(15):4026–4035. doi: 10.1158/1078-0432.CCR-14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciarimboli G, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176(3):1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 39.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 40.Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl. 2004;66(S91):S56–S61. doi: 10.1111/j.1523-1755.2004.09109.x. [DOI] [PubMed] [Google Scholar]
- 41.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 42.Mehta RL, et al. Acute Kidney Injury Network Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14(8):2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 44.Linkermann A, et al. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25(12):2689–2701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 46.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22(6):999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 47.Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol. 2003;23(6):511–521. doi: 10.1053/s0270-9295(03)00130-x. [DOI] [PubMed] [Google Scholar]
- 48.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: An evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9(3):137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 49.Mishra J, et al. Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24(3):307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 50.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8(9):671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anders L, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20(5):620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alkorta I, Elguero J. A theoretical study of the structure and protonation of Palbociclib (PD 0332991) J Mol Struct. 2014;1056–1057(6):209–215. [Google Scholar]
- 53.Kido Y, Matsson P, Giacomini KM. Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem. 2011;54(13):4548–4558. doi: 10.1021/jm2001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suhre WM, Ekins S, Chang C, Swaan PW, Wright SH. Molecular determinants of substrate/inhibitor binding to the human and rabbit renal organic cation transporters hOCT2 and rbOCT2. Mol Pharmacol. 2005;67(4):1067–1077. doi: 10.1124/mol.104.004713. [DOI] [PubMed] [Google Scholar]
- 55.Zolk O, Fromm MF. Transporter-mediated drug uptake and efflux: Important determinants of adverse drug reactions. Clin Pharmacol Ther. 2011;89(6):798–805. doi: 10.1038/clpt.2010.354. [DOI] [PubMed] [Google Scholar]
- 56.Filipski KK, Loos WJ, Verweij J, Sparreboom A. Interaction of Cisplatin with the human organic cation transporter 2. Clin Cancer Res. 2008;14(12):3875–3880. doi: 10.1158/1078-0432.CCR-07-4793. [DOI] [PubMed] [Google Scholar]
- 57.Sprowl JA, Sparreboom A. Uptake carriers and oncology drug safety. Drug Metab Dispos. 2014;42(4):611–622. doi: 10.1124/dmd.113.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panesso MC, et al. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int. 2014;85(4):855–870. doi: 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23(21):7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fry DW, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–1438. [PubMed] [Google Scholar]
- 61.Guckel D, Ciarimboli G, Pavenstädt H, Schlatter E. Regulation of organic cation transport in isolated mouse proximal tubules involves complex changes in protein trafficking and substrate affinity. Cell Physiol Biochem. 2012;30(1):269–281. doi: 10.1159/000339063. [DOI] [PubMed] [Google Scholar]
- 62.Franke RM, et al. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-D-glucosaminidase. Clin Cancer Res. 2010;16(16):4198–4206. doi: 10.1158/1078-0432.CCR-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molitoris BA, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20(8):1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiRocco DP, et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am J Physiol Renal Physiol. 2014;306(4):F379–F388. doi: 10.1152/ajprenal.00475.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Konecny GE, et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res. 2011;17(6):1591–1602. doi: 10.1158/1078-0432.CCR-10-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: An emerging concept in cancer therapy. Clin Cancer Res. 2001;7(8):2168–2181. [PubMed] [Google Scholar]
- 67.Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene. 2010;29(28):4018–4032. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 68.Johnson N, Shapiro GI. Cyclin-dependent kinases (cdks) and the DNA damage response: Rationale for cdk inhibitor-chemotherapy combinations as an anticancer strategy for solid tumors. Expert Opin Ther Targets. 2010;14(11):1199–1212. doi: 10.1517/14728222.2010.525221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sprowl JA, Ness RA, Sparreboom A. Polymorphic transporters and platinum pharmacodynamics. Drug Metab Pharmacokinet. 2013;28(1):19–27. doi: 10.2133/dmpk.dmpk-12-rv-073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.