Significance
Angelman syndrome (AS) is a human neurodevelopmental disorder caused by mutation of a specific gene, UBE3A. Studies of behavior in adult mouse models of AS reveal abnormalities similar to those observed in humans with AS. Because AS affects children, we hypothesized that it might be helpful to study this disorder using juvenile mice. We found that young AS mice display aberrant communication and motor behaviors and increased brain activity. Reducing the expression of the synaptic protein ARC reverses abnormal brain activity in AS mice, but has no effect on communication and motor behaviors in these mice. These findings suggest new approaches for identifying the neural circuits that are defective in AS, and for developing therapies for treating this disorder.
Keywords: Angelman syndrome, ARC, EPHEXIN5, ultrasonic vocalizations, EEG
Abstract
Angelman syndrome (AS) is a neurodevelopmental disorder arising from loss-of-function mutations in the maternally inherited copy of the UBE3A gene, and is characterized by an absence of speech, excessive laughter, cognitive delay, motor deficits, and seizures. Despite the fact that the symptoms of AS occur in early childhood, behavioral characterization of AS mouse models has focused primarily on adult phenotypes. In this report we describe juvenile behaviors in AS mice that are strain-independent and clinically relevant. We find that young AS mice, compared with their wild-type littermates, produce an increased number of ultrasonic vocalizations. In addition, young AS mice have defects in motor coordination, as well as abnormal brain activity that results in an enhanced seizure-like response to an audiogenic challenge. The enhanced seizure-like activity, but not the increased ultrasonic vocalizations or motor deficits, is rescued in juvenile AS mice by genetically reducing the expression level of the activity-regulated cytoskeleton-associated protein, Arc. These findings suggest that therapeutic interventions that reduce the level of Arc expression have the potential to reverse the seizures associated with AS. In addition, the identification of aberrant behaviors in young AS mice may provide clues regarding the neural circuit defects that occur in AS and ultimately allow new approaches for treating this disorder.
Angelman syndrome (AS) is a human neurodevelopmental disorder that occurs in the first few years of life and is characterized by severe developmental delay, an absence of purposeful speech, motor discoordination, an abnormal EEG, and unusual behavioral traits, such as easily provoked laughter and hand flapping (1, 2). Individuals with AS have mutations in the maternally inherited copy of the UBE3A gene, resulting in a loss of function of the UBE3A protein (also known as E6AP) (3). This gene resides within the genomic locus 15q11.2-q13 that is paternally imprinted selectively in neurons, such that the allele of UBE3A inherited from the father is silenced in neurons and the maternally inherited copy is expressed (4, 5). How loss of UBE3A results in the distinct clinical phenotype of AS is for the most part unknown.
The imprinting of UBE3A is evolutionarily conserved, and thus AS can be modeled in mice that lack a functional copy of maternally inherited Ube3a but have a wild-type copy of the paternally inherited Ube3a allele (AS mice) (6). AS mice have been useful for defining the cellular and molecular function of UBE3A in neurons.
The Ube3a gene encodes an E3 ubiquitin ligase that catalyzes the addition of ubiquitin to specific proteins, thereby modifying their function or targeting the protein for degradation by the proteasome (7). Recent studies have identified several neuronal proteins that are mis-regulated in AS neurons, including CAMKII, RhoA guanine nucleotide exchange factor 5 (EPHEXIN5), activity-regulated cytoskeleton-associated protein (ARC), GAT1, α1-NAKA, NAV1.6, and ANKYRIN-G (8–12). Some of these proteins are thought to be direct substrates of UBE3A. These proteins have increased expression in AS, possibly due to a failure to be targeted for degradation. However, it is possible that some of these proteins are not direct targets of the UBE3A ligase but rather, their mis-regulation could be an indirect consequence of the disruption of UBE3A function. It remains to be determined how proteins that are mis-regulated upon loss of UBE3A contribute to the etiology of AS.
The AS mouse model has been used extensively for testing potential drugs and gene therapies for treating AS (13, 14). One therapeutic approach has been to identify proteins that are up-regulated in the brains of AS mice and to then search for pharmacological agents that target the expression or activity of the up-regulated protein. An alternative approach has been to restore the expression of Ube3a in the brains of AS mice by de-repressing the paternal Ube3a allele (15, 16). With approaches for reversing the effects of Ube3a loss now in hand, a set of robust behavioral assays is needed to assess the efficacy with which various therapeutic agents reverse the phenotypes of AS.
AS mice have significant neural circuit defects, suggesting that in the absence of Ube3a there is a disruption of excitatory/inhibitory balance in the brain (6, 10, 12, 13, 17–19). In addition, AS mice display defects in learning and memory, motor coordination, locomotor activity (14, 17, 20, 21), and an increased number of seizures when exposed to an audiogenic challenge (6, 11). However, there have been conflicting reports regarding the robustness of the AS phenotypes observed (22). One possible explanation for the disparate findings is that these studies have been conducted using adult mice at a time when the aberrant AS behaviors may have begun to subside. Indeed, seizures are prominent in AS in early childhood (<3 y of age) but begin to vary considerably in frequency and severity as children with AS age (2, 23, 24). In addition, some core features of human AS that are seen in early childhood, such as abnormal communication, have not yet been characterized in the AS mouse. Finally, it remains a possibility that the behavioral abnormalities observed in adult AS mice might be a result of secondary rather than primary effects of mutating the UBE3A protein.
In this study we characterized behaviors in young AS mice to identify robust and reproducible abnormalities that are a direct consequence of the Ube3a mutation, and thus might provide useful assays for testing the efficacy of therapies for AS. Here, we report the identification of significant strain-independent behavioral phenotypes in juvenile AS mice. We find that previously described motor behaviors, including hypoactivity and the hindlimb clasp, have developed in AS mice by the time of weaning. Similarly, we observe an abnormal cortical EEG (25) in AS mice before adulthood. In addition, we report two new phenotypes in young AS mice: abnormal ultrasonic vocalizations and enhanced latency to recover from an audiogenic stimulus. Finally, we find that reducing the level of the activity-regulated cytoskeletal protein ARC in AS mice selectively attenuates the seizure-like and EEG deficits in these animals. We conclude that young AS mice recapitulate hallmark features of AS in children, and that the early-onset behaviors we have characterized in these mice may provide new approaches for evaluating the efficacy of therapies for treating AS in humans.
Results
Overview of Behavioral Analysis.
For the studies described below, we classified mice younger than postnatal day 42 (<PND 42) as juvenile, and mice older than postnatal day 42 (>PND 42) as adults. We assessed AS phenotypes using two different mouse strains, pure B6 (B6) and F1 hybrids of B6:129 (Hybrid), unless otherwise noted. In all cases the experiments were carried out using both male and female mice at an ∼1:1 ratio. The time window for behavioral characterization extends from PND 3 to PND 35.
AS Mice Display an Altered Developmental Time Course of Ultrasonic Vocalizations.
Ultrasonic vocalizations (USVs) produced by mouse pups when they are isolated from their mother have been suggested to be a means by which the pups seek parental care. Notably, this form of communication is disrupted in various mouse models of autism (26–28). To determine if there is a defect in USV production in AS mice we recorded and quantified USVs emitted by wild-type and AS littermates when isolated from their homecage for 4 min. We found that throughout early postnatal development, the frequency and amplitude of USVs are similar between wild-type and AS mice (Fig. S1 A and B). In addition, the intercall and interburst interval between USVs was unperturbed in AS mice (Fig. S1 C and D). These data suggest the robust structural and temporal features of USV production are largely intact in AS mice.
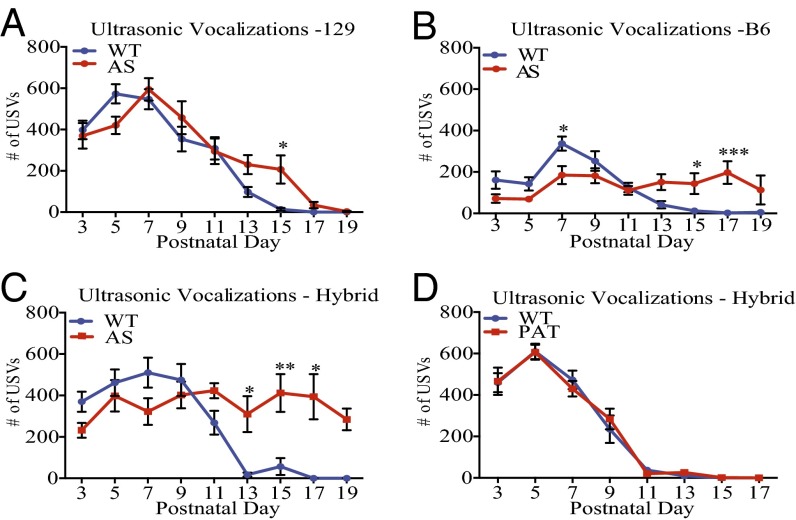
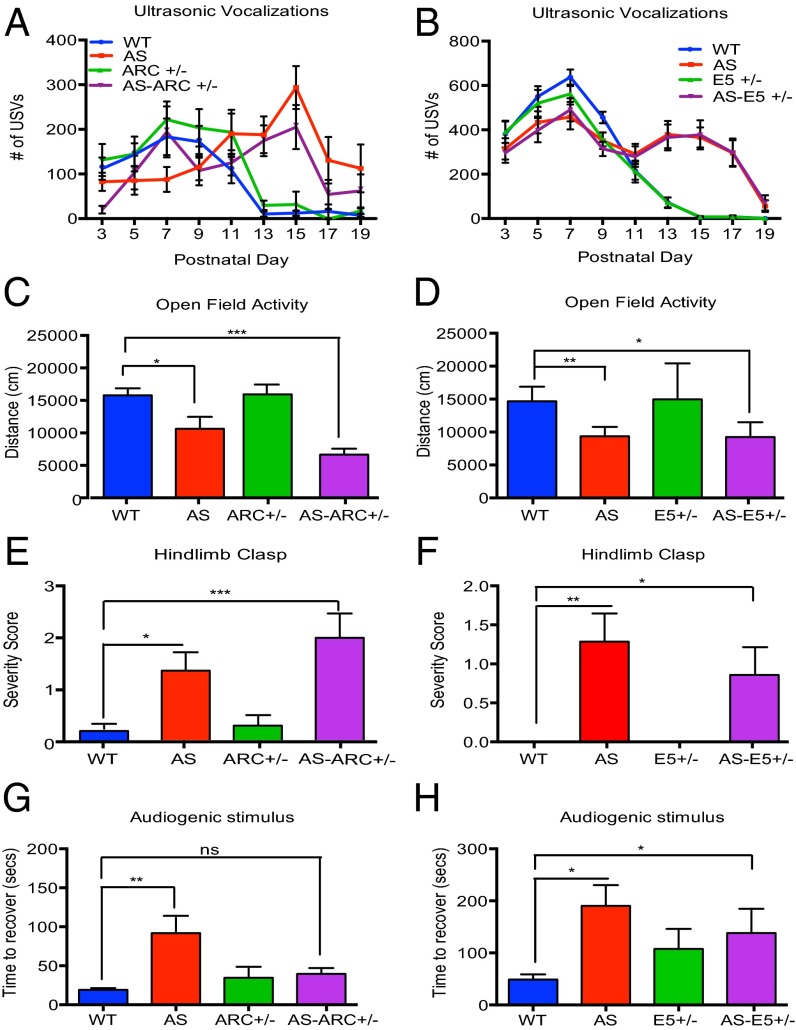
In contrast, we found a significant difference in the total number of USVs emitted by AS mice (Fig. 1 A–C). As in previous studies (29), we found that wild-type mice display a highly stereotyped time-course of USV production, with a peak in the total number of calls produced at PND 7 and a drastic reduction by PND 15. We found that AS mice produce a larger number of USVs relative to wild-type mice between PND 13 and PND 17. Similar findings were obtained when the AS mutation was crossed to three different background strains of mice.
Fig. 1.
Loss of the maternally inherited Ube3a gene leads to abnormal USV production. (A–D) The total number of USVs emitted from maternally isolated mouse pups throughout postnatal development. (A–C) Wild-type and AS littermates are compared on a 129 (n: WT = 12; AS = 15), B6 (n: WT = 15; AS = 18), and Hybrid (n: WT = 24; AS = 22) genetic background, respectively. (D) Mutation of the paternal copy of Ube3a (PAT mice) does not lead to changes in USV production (n: WT = 11; PAT = 13). Statistics for A–D: Two-way, nonrepeating-measures ANOVA; Bonferroni correction for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.0001. Error bars represent SEM.
To determine whether disruption of the maternally inherited, neuronally expressed Ube3a gene is the cause of the altered USVs, we performed the USV assay using mice in which the paternal Ube3a gene, rather than the maternally inherited gene, is mutated (termed PAT mice). Because the paternally derived Ube3a gene is not expressed in neurons, PAT mice might be predicted not to display a USV phenotype because neurons in these mice express the wild-type Ube3a gene inherited from their mother. We found that USV production in PAT mice is indistinguishable from that of their wild-type littermates (Fig. 1D).
Variables inherent in our experimental design could account for the increase in USV production in AS mice. One possibility is that the increase in USV production is caused by improper or insufficient care of AS mouse pups by their mother (30). To address this possibility, we weighed AS and wild-type pups immediately after USV testing on each day. We found that AS and wild-type littermates show no differences in weight gain throughout early development (Fig. S2 A–C), suggesting that the pups have access to maternal care, irrespective of genotype. Previous studies have shown that postnatal handling of mouse pups can modify emotional circuits, reducing anxiety-like behaviors and stress responses in adulthood (31, 32). However, when USV production was monitored on PND 16 with AS and wild-type mice that had experienced no prior investigator handling, we found that AS mice still produced significantly more USVs than their wild-type littermates (Fig. S2 D and E). This finding suggests that repetitive handling is not likely an explanation for the observed phenotype.
Early Postnatal Motor Deficits in AS Mice.
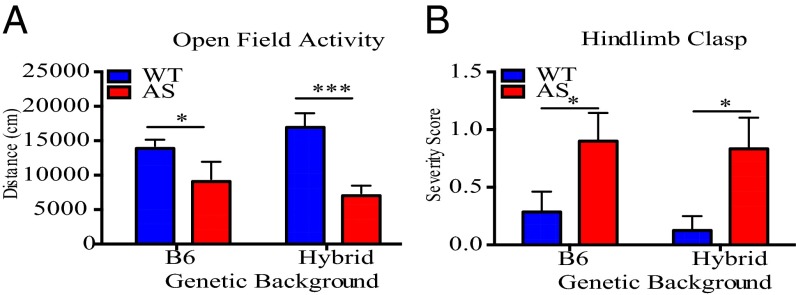
To assess motor coordination during early postnatal development, we tested locomotor activity of AS and wild-type mice at PND 21 using the open-field assay. In this assay, mice are allowed to freely explore an arena for 10 min while movement of the mice is recorded and then analyzed using motion-tracking software. This analysis revealed that AS mice travel significantly less than their wild-type littermates (Fig. 2A).
Fig. 2.
Young AS mice have abnormal motor activity compared with wild-type littermates in open-field arena and hindlimb-clasp assay. (A) Total distanced traveled in an open-field arena at PND 21 is compared between wild-type and AS littermates on a B6 (n: WT = 11; AS = 4) and Hybrid (n: WT = 9, AS = 10) genetic background. (B) Hindlimb-clasp behavior is compared between wild-type and AS littermates at PND 30 on a B6 (n: WT = 7; AS = 5) and Hybrid (n: WT = 7; AS = 6) genetic background. Severity score for hindlimb clasp was determined by the average time spent clasping during two 20-s trials (see Materials and Methods). Statistics for both panels: Wild-type and AS littermates on B6 or Hybrid backgrounds were analyzed individually with an unpaired, two-tailed t test before plotting on a single graph. *P < 0.05, ***P < 0.0001. Error bars represent SEM.
We next assayed wild-type and AS mice for the presence of the hindlimb clasp, a behavior that is considered to be a general indication of an impaired motor response circuit. At PND 30, AS mice have a pronounced hindlimb-clasp phenotype compared with wild-type mice (Fig. 2B). We next considered the possibility that the hindlimb-clasp response might manifest itself earlier than PND 30 because other deficits in AS mice occurred at earlier times during development than previously appreciated. We found that hindlimb-clasp behavior occurs in both wild-type and AS mice as early as PND 13; interestingly, the frequency of this behavior is dramatically increased within the population of AS mice at a similar time to the onset of USV abnormalities (Fig. S3).
Juvenile AS Mice Have an Enhanced Seizure-Like Response to Audiogenic Stimulus and Abnormal Cortical EEGs.
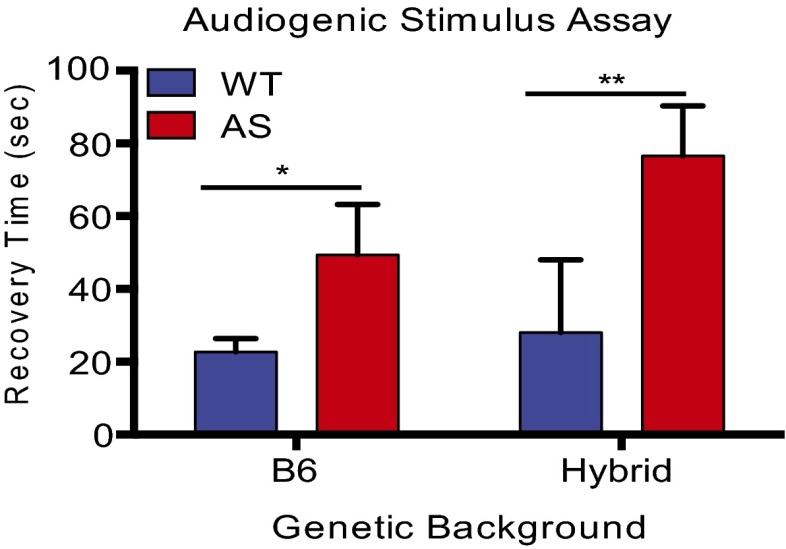
Seizures in AS occur as early as 6 months of age, and often precede clinical diagnosis of AS in humans. The susceptibility to seizures has been studied in adult AS mice (>PND 42) using an audiogenic stimulus assay (6, 11). Previous studies indicate that adult mice on a pure B6 background are more resistant to seizure induction than other strains, such as 129; however, juvenile mice have not been investigated (33). In our pilot experiments using the audiogenic stimulus assay at PND 25, we found that 60% of wild-type mice on a 129 background exhibited debilitating tonic-clonic seizures that led to death. The high frequency of fatal seizures observed in the 129 wild-type population may limit our ability to detect differences in seizure activity between wild-type and AS mice. Thus, we focused on mice in the B6 background, which are known to have less severe seizures. Pilot experiments using B6 mice revealed that none of our wild-type mice exhibited seizure-induced death and the mice ceased moving and displayed freezing behavior when the audiogenic stimulus was terminated (referred to in this paper as a “seizure-like” response). Thus, we were able to quantify the seizure-like response of these mice by measuring the latency with which these mice regain motor activity when the audiogenic stimulus is discontinued. This analysis revealed that young AS mice take a significantly longer time to recover following the cessation of the audiogenic stimulus (Fig. 3).
Fig. 3.
AS mice have an enhanced latency to recover from an audiogenic stimulus. Total time to recover motor activity following an audiogenic stimulus between AS mice and wild-type littermates on a B6 background (n: WT = 7; AS = 5) and Hybrid background (n: WT = 7; AS = 8). Statistics: Unpaired, two-tailed t test. *P < 0.05, **P < 0.01. Error bars represent SEM.
In humans with AS, seizure progression is quite variable with age. Although seizures are first observed in early childhood, they are typically less prevalent in older children but can reappear in adults (23). Therefore, we asked if the response of wild-type and AS mice to an audiogenic stimulus changes as the mice mature. Using the audiogenic stimulus protocol, we found that in contrast to our observations with young mice, during adulthood (PND 42–90) there is no significant difference between wild-type and AS mice in their latency to recover from an audiogenic stimulus (Fig. S4). The average wild-type latency to recover from the audiogenic stimulus does not change as wild-type mice mature, suggesting that as AS mice mature, their ability to recover from an audiogenic stimulus improves.
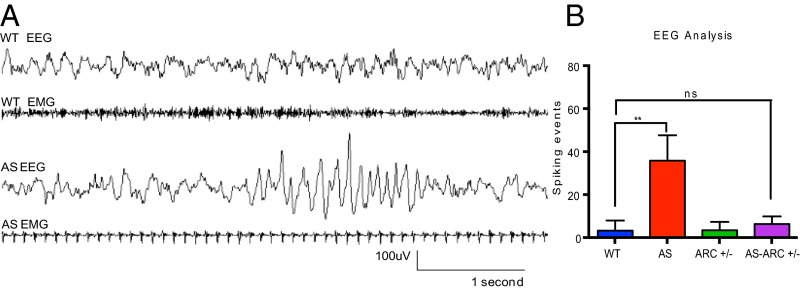
To investigate further the neurological basis of the enhanced seizure susceptibility in AS mice, we used subdural EEG recordings to measure basal cortical activity. Electrodes were implanted in mice in the posterior cortex on PND 25 and recordings were performed between ages PND 30 and PND 35. Basal brain activity in AS mice was indistinguishable from that of their wild-type littermates; however, AS mice possessed infrequent high-amplitude “spiking” events. These high amplitude (>200 μV) spikes, occurring within the 4- to 8-Hz range, have been observed in the hippocampus and cortex of adult AS mice and qualitatively described (20). We quantified spiking activity (SI Materials and Methods and Fig. S5) and found AS mice have significantly more spiking events compared with wild-type littermates (see, for example, Fig. 6). Thus, abnormal EEG activity can be observed in AS mice as early as PND 30.
Fig. 6.
AS mice have significantly more large amplitude spiking events compared with wild-type mice and this phenotype can be reversed by lowering levels of Arc. (A) Representative EEG from wild-type and AS mice at PND 30. High-amplitude (∼200 μV), 4- to 8-Hz spiking events are depicted in the AS EEG trace. Note: Simultaneous EMG recording shows immobility of AS mouse during spiking activity. (B) Quantification of spiking activity from progeny of Ube3a × Arc genetic interaction (n: WT = 9; AS = 9; ARC+/− = 13; AS-ARC+/− = 7). Statistics: Two-way ANOVA; Bonferroni correction for multiple comparisons. **P < 0.01; n.s., not significant. Error bars represent SEM.
Lowering the Level of Arc Rescues Abnormal Brain Activity in AS Mice.
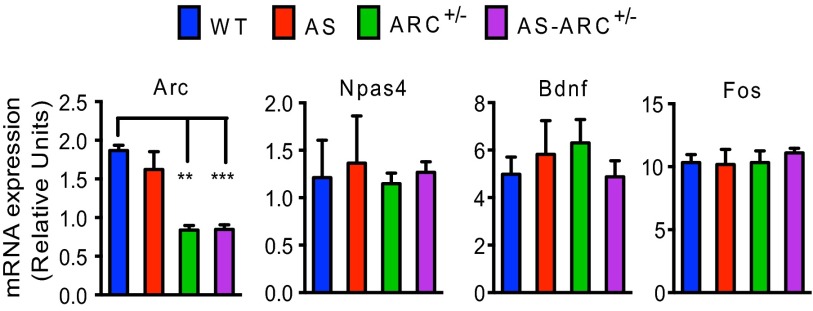
Having identified strain-independent and robust behavioral deficits, we next asked if it was possible to reverse these phenotypes by decreasing the expression of neuronal proteins that have been suggested to be up-regulated in the AS mouse. Toward this end, we focused our attention on ARC and EPHEXIN5 (E5), two proteins that regulate synapse number and function (8, 9, 34, 35). To reduce the level of ARC expression in AS mice, we mated Ube3am+/p− females with males that are heterozygous for a knockout allele for Arc (Arc+/−). This cross produces four genotypes: Ube3am−/p+ mice (AS mice), Ube3am−/p+ mice that also have the Arc+/− allele (AS-Arc+/− mice), as well as wild-type mice and Arc+/− mice for littermate comparison. To confirm that the expression of Arc is reduced using this genetic approach, we quantified Arc mRNA levels using quantitative PCR analysis on all four genotypes. Because Arc is an activity-regulated gene (36), we induced its expression by exposing mice to an enriched environment for 1 h before harvesting hippocampal mRNA. We found that Arc+/− mice, both in the wild-type and AS background, display ∼50% reduction in the level of Arc mRNA expression (Fig. 4, Far Left). In contrast, the expression of other activity-regulated genes (i.e., Fos, Bdnf, and Npas4) was not reduced in Arc+/− mice, suggesting that the decrease in Arc mRNA expression is a direct consequence of the mutation of Arc, and is not because of a global change in activity-regulated gene networks (Fig. 4).
Fig. 4.
Heterozygosity of Arc allele leads to 50% reduction in Arc mRNA levels. Following exposure to an enriched environment, ARC+/− and AS-ARC+/− mice have reduced Arc mRNA expression compared with wild-type littermates. Arc heterozygosity does not affect induction levels of other known immediate-early genes Npas4, Bdnf, and Fos. Relative units reflect Ct values normalized to Tubulin. (n: WT = 2; AS = 4; ARC+/− = 8; AS-ARC+/− = 4). Statistics: Two-way ANOVA; Bonferroni correction for multiple comparisons. **P < 0.01, ***P < 0.001. Error bars represent SEM.
We next asked if reducing the level of Arc or E5 expression reverses the defects in USV production, motor function, and seizure-related activity that we detect in AS mice. We found that reducing the level of Arc or E5 expression has no effect on the number of USVs in the AS mice (Fig. 5 A and B), does not modify the hypoactivity observed in the open-field assay (Fig. 5 C and D), and does not reverse the pronounced hindlimb clasp observed in AS mice (Fig. 5 E and F).
Fig. 5.
Lowering levels of Arc, but not Ephexin5, rescues audiogenic stimulus phenotype in AS mice, but not USV or motor deficits. (A–H) Behavioral analysis of progeny generated from Ube3a × Arc (Left) and Ube3a × E5 (Right) genetic interaction. (A and B) Total number of USVs emitted during maternal isolation throughout postnatal development (Ube3a × Arc n: WT = 16; AS = 18; ARC+/− = 13; AS-ARC+/− = 10 /Ube3a × E5 n: WT = 17; AS = 16; E5+/− = 19; AS-E5+/− = 17). (C and D) Total distance traveled in open field arena at PND21. (Ube3a × Arc n: WT = 13; AS = 6, ARC+/− = 5; AS-ARC+/− = 5 /Ube3a × E5 n: WT = 7; AS = 3; E5+/− = 4; AS-E5+/− = 5). (E and F) Hindlimb-clasp severity measured at PND 30 (Ube3a × Arc n: WT = 12; AS = 12; ARC+/− = 10; AS-ARC+/− = 7 /Ube3a × E5 n: WT = 9; AS = 7; E5+/− = 7; AS-E5+/− = 7). (G and H) Latency to recover motor activity following audiogenic stimulus at PND 30 (Ube3a × Arc n: WT = 12; AS = 13; ARC+/− = 10; AS-ARC+/− = 7 /Ube3a × E5 n: WT = 9; AS = 7; E5+/− = 7; AS-E5+/− = 7) Statistics for all panels: Two-way ANOVA; Bonferroni correction for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent SEM.
Given that ARC regulates surface AMPA receptor expression at excitatory synapses, and consequently influences neuronal excitability, and E5 has been shown to restrict the number of excitatory synapses, we considered the possibility that an increase in the level of ARC and E5 might underlie the seizure-related phenotypes in AS mice. To investigate seizure activity under conditions where Arc or E5 levels are reduced, we exposed mice to an audiogenic stimulus, as described above, and assessed the time it took for the mice to regain mobility after the stimulus was terminated. We found that reducing the level of Arc expression in AS mice completely reverses their enhanced response to the audiogenic stimulus. In contrast, reducing the level of E5 expression had no effect on the response of AS mice to the audiogenic stimulus (Fig. 5 G and H). To assess directly the effect of reducing Arc expression on the seizure-like activity observed in AS mice, EEG recordings were performed. This analysis revealed that reducing Arc mRNA expression completely reverses the increased spiking frequency observed in AS mice (Fig. 6).
Discussion
Mutations in Ube3a that lead to AS in humans have been successfully modeled in mice, thus providing a means to study the behavioral, neural circuit, synaptic, and molecular defects that underlie this complex disorder (37, 38). A key step toward understanding this disorder is to define in mice the behavioral abnormalities that parallel the deficits observed in humans, and then to attempt to understand the neural circuit and molecular underpinnings of these abnormalities. A feature of AS in humans is that this disorder is first manifested during early childhood, suggesting that analyzing behaviors in juvenile AS mice would be advantageous. In the present study we have used this approach to identify deficits in social communication, motor coordination, and neural activity in young AS mice. These findings should provide new avenues for studying the neural circuit and molecular basis of AS, and ultimately may allow the development of therapies for treating this disorder.
Our analysis of behavior in young AS mice revealed that, as in humans, behavioral deficits in AS mice emerge early in life. AS mice appear normal at birth and show no obvious defects in brain anatomy (6). However, as the brain begins to mature, in concert with input from the external environment, a significant number of behavioral abnormalities are detected in AS mice. Previous studies have suggested that visual experience modifies the development of neural circuits in young AS mice, and that in the absence of visual stimulation neural circuit defects fail to develop (18). In wild-type mouse pups there appears to be a critical period during development (PND 13–15) during which the suppression of USVs occurs. This period might involve the inhibition of motor circuits that facilitate USV production. Given that the defect in USV production in AS mice becomes apparent at PND 13 when wild-type mice are beginning to suppress USV production, we speculate that the persistence of USVs in AS mouse pups could reflect a defect in sensory-dependent neural circuit maturation. A similar defect in the development of inhibitory circuits could underlie the emergence of the hindlimb-clasp phenotype in AS mice, because this phenotype is first detected at the same time during postnatal development that USV production becomes abnormal. However, it is also possible that the increase in USVs produced by AS mice during PND 13–PND 17 results from a global developmental delay in USV production.
The USV production abnormality that we detect in young AS mice may be related to the excessive laughter observed in humans with AS. It has been suggested that pathological laughter is a manifestation of seizure activity in the brain (39). It is possible that the abnormal EEGs, the enhanced response to acoustic stimuli, and the failure to suppress USV production, reflect a common neural abnormality, possibly a defect in inhibition. Consistent with this idea, the temporal properties of the abnormal EEG and USVs in AS mice are strikingly similar.
In support of our findings with AS mice, a previous study reported the presence of abnormal USVs and cortical EEGs in a strain of mice (15q11-q13 deletion mice) that contain a 1.6-Mb deletion in chromosome 15q11 that is found in the majority of humans with AS (40). In addition to disrupting Ube3a function, this deletion knocks out expression of a large number of genes, including Cyfip1, Nipa1, Nipa2, Gcg5, Whcd1l1, Golga8E, and a Gaba receptor cluster. Given the nature of this deletion, it was not clear if the abnormal USVs and cortical EEGs observed in 15q11-q13 deletion mice are caused specifically by the loss of Ube3a or by disruption of additional genes in the 15q11-q13 locus. Our findings with AS mice provide strong support for the conclusion that the defects in USV production and cortical EEGs are specifically because of the disruption of Ube3a. By comparing the effect of deleting the maternally inherited versus the paternally inherited allele of Ube3a on USV production (Fig. 1D), we are able to conclude that the USV defect is likely because of the loss of Ube3a specifically in neurons. Identification of the particular neuronal subtypes that underlie these defects will require the selective disruption or replacement of Ube3a in defined neural subtypes.
In addition to abnormalities in communication, seizure activity is a consistent feature of AS. Seizures have been reported in 90% of children with AS that have a 15q11 deletion and greater than 50% of children that have mutations specifically within the UBE3A gene (2, 23, 41). In all cases, the EEGs of individuals with AS have been found to be abnormal (42). Although EEGs are not necessarily identical between different people, a hallmark of the EEG defect in humans with AS is the occurrence of abnormal bouts of high amplitude and low frequency (2–3 Hz) δ-waves early in life. Although certain features of the EEG abnormality that we detect in AS mice are specific to mice, the phenotype is observed in multiple strains of mice across multiple brain regions. This finding suggests that the EEG is a reliable way to monitor the abnormal brain activity that accompanies AS and therapies for treating AS might be tested for their efficacy in reversing the EEG phenotype.
One approach for treating AS might be to identify proteins that are up-regulated in the absence of UBE3A, and to then ask if reducing their expression reverses AS-associated deficits. In the present study we found that decreasing the level of Arc expression in AS mice abolishes the abnormal EEG phenotype and the enhanced response to an audiogenic stimulus seen in these mice. In contrast, reducing Arc expression had no effect on USV production, the hindlimb-clasp phenotype, or hypoactivity in AS mice. Decreasing the expression of E5, another protein that is mis-regulated in AS mice, did not ameliorate any of the deficits observed in AS, indicating that the effect of reducing Arc expression on neuronal excitability in AS mice is at least somewhat specific.
It remains to be determined how the reduction of Arc expression leads to an attenuation of excitability in AS mice. It has been shown previously that knockdown of Ube3A in neurons leads to increased AMPA receptors and this effect is reversed by reducing the expression of Arc with RNAi. Given that ARC functions at synapses to endocytose AMPA receptors, we would predict that lowering the level of Arc would increase AMPA receptor expression at synapses, thereby increasing neuronal excitability. When AMPA receptor expression at synapses increases in excitatory neurons, it would be predicted to lead to an enhancement of seizure activity. However, if AMPA receptor expression increases at excitatory synapses that form on inhibitory neurons, the increase in excitability would be predicted to inhibit neural circuit activity, possibly reducing seizure activity. It will be important in the future to determine the locus of Arc expression in AS mice in an effort to understand how the decrease in Arc expression attenuates seizure-like activity.
The molecular mechanism by which Ube3a and Arc interact to modify synaptic activity is not clear. One possibility that has been suggested is that ARC is a substrate of UBE3A, the expression of which is up-regulated in AS mice (8). However, in contrast to previous reports, through quantitative measurements we have been unable to detect a statistically significant difference in the level of Arc mRNA (Fig. 4, Far Left) or ARC protein in brains or neuronal extracts when wild-type and AS mice are compared (Fig. S6). Alternatively, it is possible that ARC is not a direct substrate of UBE3A (35) and that the reduction in ARC levels in AS-Arc+/− mice leads to an attenuation of seizure-like activity in these mice because UBE3A and ARC regulate a common cellular process, such as controlling the surface expression of AMPA receptors. It is also possible that a reduction in ARC levels in AS-Arc+/− mice might lead to an attenuation of seizure-like activity through noncell-autonomous, circuit level adaptations within the brain.
In conclusion, in this study we demonstrate that AS mice exhibit clinically relevant, strain-independent behavioral phenotypes early in life. The characterization of these behavioral defects in AS mice may facilitate identification of the neuronal circuits that are disrupted in AS, and provide a path toward the development of pharmacological or genetic therapies for treating this debilitating disorder.
Materials and Methods
Animals.
Mice harboring a null mutation in Ube3a were obtained from Jackson Laboratories and maintained on C57BL/6J (B6) or 129S2/SvPasCrl (129) background. For all behavioral experiments, B6 Ube3am+/p− females were used for mating, with the exception of experiments performed on a pure 129 background. To generate mice that have maternal deficiency of Ube3a (referred to in this report as AS mice), Ube3am+/p− females were crossed with either a B6 or 129 wild-type male. F1 progeny (Hybrid) represent a 50:50 contribution of B6 and 129. For genetic interaction experiments, male mice heterozygous for Arc (referred to as Arc+/−) or Ephexin5 (referred to as E5+/−) were crossed to Ube3am+/p− females. Arc+/− mice were a gift from the laboratory of Paul Worley, John Hopkins University, Baltimore, and maintained on a B6 background for at least 10 generations. E5+/− mice were generated in-house (9) and maintained on a 129 background for at least 10 generations. Animals were kept on a 12-h light/dark cycle and given food and water ad libitum. All experiments were performed and analyzed blind to genotype. Genotyping was conducted by PCR from tail tissue samples at least twice for validation. All procedures were conducted in strict compliance with the Institutional Animal Care and Use Committee at Harvard Medical School.
Maternal Isolation-Induced USV Assay (PND 3–19).
Individual pups were placed into a plastic bucket contained within a sound attenuated chamber (Med-Associates) for 4 min. USVs were sampled at 250 kHz using a broadband microphone (Avisoft) suspended 10 inches above the pup. Following testing on PND 3, pups were given unique identifiers on their paws using tattoo ink (Ketchum). See SI Materials and Methods and Fig. S7 for details regarding acoustical analysis.
Open-Field Assay (PND 21).
Activity was measured (weaning age) in a 20 × 20 × 20-inch Plexiglas box in 200 lx lighting. Movement was recorded using an overhead CCV camera for 10 min. Total distance traveled was analyzed offline using tracking analysis software (Noldus, Ethovision XT).
Hindlimb-Clasp Assay (PND 30).
Animals were suspended by their tail 10 cm above the laboratory bench (measured from the tip of the tail) for 20 s. Each animal received two trials, with 10-min intertrial intervals. Trials were recorded with a CCV camera and scored offline by an experimenter blind to genotype. Clasping behavior was defined by movement of the hindlimbs curling inward toward the belly of the animal. The total time spent clasping across two trials was averaged and severity score was determined as follows: 0.0 (0-s clasping), 0.5 (<1-s clasping), 1.0 (between 1- and 5-s clasping), 1.5 (between 5- and 10-s clasping), and 2.0 (>10-s clasping).
Audiogenic Stimulus Assay (PND 30).
Animals were habituated to testing environment (Plexiglas cylinder 10-inch diameter, 12-inch height) for 5 min. Audiogenic stimulus was performed by scraping the metal bars of a cage top ∼15 inches above the test subject. The stimulus lasted 45 seconds or until tonic-clonic seizure was observed visually. Following cessation of the stimulus, the latency to recover was recorded. Recovery was called when any of the four paws were lifted from the ground, including walking, grooming, or stretch attending behaviors.
EEG Recordings (PND 35).
Mice were acutely anesthetized in isoflourane to minimize anxiety during the process of connecting the headmount to the preamplifier. EEG/EMG recording started when recovery of movement was observed and data were collected continuously for 3 h (sampling rate 400 Hz). Data used for analysis started 1 h after recording start time. See SI Materials and Methods for details regarding surgery procedure and spiking analysis.
Supplementary Material
Acknowledgments
We thank Gary Yellen for sharing a custom MATLAB program for EEG analysis. This work was supported by National Institutes of Health R01 Grant NS045500 titled “Neurotrophic Factor Regulation of Gene Expression” (to M.E.G), a Quan fellowship (to C.M.-B), and an Albert J. Ryan fellowship (to C.M.-B).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504809112/-/DCSupplemental.
References
- 1.Angelman H. 'Puppet' children. A report on 3 cases. Dev Med Child Neurol. 1965;7:681–688. doi: 10.1111/j.1469-8749.2008.03035.x. [DOI] [PubMed] [Google Scholar]
- 2.Tan WH, et al. Angelman syndrome: Mutations influence features in early childhood. Am J Med Genet A. 2011;155A(1):81–90. doi: 10.1002/ajmg.a.33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15(1):70–73. doi: 10.1038/ng0197-70. ; erratum in Nat Genet 1997 15(4):411. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls RD, Knoll JH, Butler MG, Karam S, Lalande M. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature. 1989;342(6247):281–285. doi: 10.1038/342281a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton-Smith J, Laan L. Angelman syndrome: A review of the clinical and genetic aspects. J Med Genet. 2003;40(2):87–95. doi: 10.1136/jmg.40.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang YH, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21(4):799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 7.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10(13):4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greer PL, et al. The Angelman syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140(5):704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis SS, et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143(3):442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaphzan H, Buffington SA, Jung JI, Rasband MN, Klann E. Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of Angelman syndrome. J Neurosci. 2011;31(48):17637–17648. doi: 10.1523/JNEUROSCI.4162-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Woerden GM, et al. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10(3):280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- 12.Egawa K, et al. Decreased tonic inhibition in cerebellar granule cells causes motor dysfunction in a mouse model of Angelman syndrome. Sci Transl Med. 2012;4(163):ra157. doi: 10.1126/scitranslmed.3004655. [DOI] [PubMed] [Google Scholar]
- 13.Baudry M, et al. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol Dis. 2012;47(2):210–215. doi: 10.1016/j.nbd.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daily JL, et al. Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for Angelman syndrome. PLoS ONE. 2011;6(12):e27221. doi: 10.1371/journal.pone.0027221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng L, et al. Truncation of Ube3a-ATS unsilences paternal Ube3a and ameliorates behavioral defects in the Angelman syndrome mouse model. PLoS Genet. 2013;9(12):e1004039. doi: 10.1371/journal.pgen.1004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang HS, et al. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature. 2012;481(7380):185–189. doi: 10.1038/nature10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17(1):111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- 18.Yashiro K, et al. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009;12(6):777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riday TT, et al. Pathway-specific dopaminergic deficits in a mouse model of Angelman syndrome. J Clin Invest. 2012;122(12):4544–4554. doi: 10.1172/JCI61888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura K, et al. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol Dis. 2002;9(2):149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- 21.Heck DH, Zhao Y, Roy S, LeDoux MS, Reiter LT. Analysis of cerebellar function in Ube3a-deficient mice reveals novel genotype-specific behaviors. Hum Mol Genet. 2008;17(14):2181–2189. doi: 10.1093/hmg/ddn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang HS, et al. Behavioral deficits in an Angelman syndrome model: Effects of genetic background and age. Behav Brain Res. 2013;243:79–90. doi: 10.1016/j.bbr.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelc K, Boyd SG, Cheron G, Dan B. Epilepsy in Angelman syndrome. Seizure. 2008;17(3):211–217. doi: 10.1016/j.seizure.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Thibert RL, Larson AM, Hsieh DT, Raby AR, Thiele EA. Neurologic manifestations of Angelman syndrome. Pediatr Neurol. 2013;48(4):271–279. doi: 10.1016/j.pediatrneurol.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Colas D, Wagstaff J, Fort P, Salvert D, Sarda N. Sleep disturbances in Ube3a maternal-deficient mice modeling Angelman syndrome. Neurobiol Dis. 2005;20(2):471–478. doi: 10.1016/j.nbd.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Esposito G, et al. Infant calming responses during maternal carrying in humans and mice. Curr Biol. 2013;23(9):739–745. doi: 10.1016/j.cub.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguiz RM, Colvin JS, Wetsel WC. Neurophenotyping genetically modified mice for social behavior. Methods Mol Biol. 2011;768:343–363. doi: 10.1007/978-1-61779-204-5_19. [DOI] [PubMed] [Google Scholar]
- 28.Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33(4):508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn ME, et al. Genetic and developmental influences on infant mouse ultrasonic calling. II. Developmental patterns in the calls of mice 2-12 days of age. Behav Genet. 1998;28(4):315–325. doi: 10.1023/a:1021679615792. [DOI] [PubMed] [Google Scholar]
- 30.Hahn ME, Lavooy MJ. A review of the methods of studies on infant ultrasound production and maternal retrieval in small rodents. Behav Genet. 2005;35(1):31–52. doi: 10.1007/s10519-004-0854-7. [DOI] [PubMed] [Google Scholar]
- 31.Hennessy MB, Li J, Lowe EL, Levine S. Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev Psychobiol. 1980;13(6):573–584. doi: 10.1002/dev.420130603. [DOI] [PubMed] [Google Scholar]
- 32.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment. Science. 1999;284(5420):1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 33.Fuller JL, Sjursen FJ., Jr Audiogenic seizures in eleven mouse strains. J Hered. 1967;58(3):135–140. doi: 10.1093/oxfordjournals.jhered.a107565. [DOI] [PubMed] [Google Scholar]
- 34.Cao C, et al. Impairment of TrkB-PSD-95 signaling in Angelman syndrome. PLoS Biol. 2013;11(2):e1001478. doi: 10.1371/journal.pbio.1001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kühnle S, Mothes B, Matentzoglu K, Scheffner M. Role of the ubiquitin ligase E6AP/UBE3A in controlling levels of the synaptic protein Arc. Proc Natl Acad Sci USA. 2013;110(22):8888–8893. doi: 10.1073/pnas.1302792110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14(3):279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mabb AM, Judson MC, Zylka MJ, Philpot BD. Angelman syndrome: Insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci. 2011;34(6):293–303. doi: 10.1016/j.tins.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jana NR. Understanding the pathogenesis of Angelman syndrome through animal models. Neural Plast. 2012;2012:710943. doi: 10.1155/2012/710943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauterbach EC, Cummings JL, Kuppuswamy PS. Toward a more precise, clinically-informed pathophysiology of pathological laughing and crying. Neurosci Biobehav Rev. 2013;37(8):1893–1916. doi: 10.1016/j.neubiorev.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Jiang YH, et al. Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PLoS ONE. 2010;5(8):e12278. doi: 10.1371/journal.pone.0012278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yum MS, Lee EH, Kim JH, Ko TS, Yoo HW. Implications of slow waves and shifting epileptiform discharges in Angelman syndrome. Brain Dev. 2013;35(3):245–251. doi: 10.1016/j.braindev.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Vendrame M, et al. Analysis of EEG patterns and genotypes in patients with Angelman syndrome. Epilepsy Behav. 2012;23(3):261–265. doi: 10.1016/j.yebeh.2011.11.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.