Abstract
Community ecology and paleoecology are both concerned with the composition and structure of biotic assemblages but are largely disconnected. Community ecology focuses on existing species assemblages and recently has begun to integrate history (phylogeny and continental or intercontinental dispersal) to constrain community processes. This division has left a “missing middle”: Ecological and environmental processes occurring on timescales from decades to millennia are not yet fully incorporated into community ecology. Quaternary paleoecology has a wealth of data documenting ecological dynamics at these timescales, and both fields can benefit from greater interaction and articulation. We discuss ecological insights revealed by Quaternary terrestrial records, suggest foundations for bridging between the disciplines, and identify topics where the disciplines can engage to mutual benefit.
Keywords: community ecology, Quaternary paleoecology, climate change
Community ecology and Quaternary paleoecology are both concerned with the composition and structure of biotic assemblages, including patterns of spatial variation and dynamics in changing environments. Community ecology focuses on the here and now of existing species assemblages, emphasizing mechanistic understanding of local species interactions and their consequences. Quaternary paleoecology uses geohistorical evidence (1) to infer properties of past communities and how they have changed, at local to regional scales, during the past few thousand to the past 2.6 million years. Both fields are under increasing demand to inform scientists, resource managers, and policymakers of what might be in store for biodiversity and ecosystems services under ongoing and future global change (2–5). Despite their shared concerns, however, community ecology and Quaternary paleoecology are fundamentally disconnected, with relatively little engagement.
Community ecology arose from population biology and natural history and has concentrated on local mechanisms, usually biotic, to explain such properties of local assemblages as diversity, dominance, and composition (6–8). Community ecology is rich in underlying theory and concept and in local empirical detail. Recent critiques have questioned whether local processes are sufficient to explain community properties and even whether the ecological community is a realistic construct in a dynamic world (2, 9–11). These issues remain controversial (12), but consensus is emerging that community properties cannot be understood without biogeographical and historical context (13, 14).
Repeated appeals have been made to integrate history into community ecology (10, 11, 15, 16). In community ecology, history often is treated as comprising only phylogeny and continental or intercontinental dispersal (8, 10, 11, 14, 17)—processes that are manifest mainly at timespans of 106–107 years. For example, in a recent discussion of processes influencing species distributions within a region, the primary example of historical processes was evolutionary diversification (11). Contemporary theory and concepts in community ecology are thus largely bimodal: The real-time processes of local communities set in the context of a regional species pool and the deep-time processes by which continental or subcontinental floras and faunas are envisioned to develop. Largely missing are the ecological and environmental dynamics of intermediate timescales, ranging from the past few centuries to the past million years. These dynamics shape ecological communities no less profoundly than real-time and deep-time processes.
Quaternary paleoecology focuses on ecological change at these intermediate timescales and in recent decades has emphasized climate change as the primary locus of explanation for ecological change (18–23). Compared with community ecology, paleoecology is somewhat depauperate in theory and concept and is often deficient in spatial, temporal, and taxonomic precision. The dearth of explicit explanatory theory is related to paleoecology’s strong empirical and inductive traditions, which derive in part from its roots in historical geology and paleontology (24). Shortcomings in precision derive largely from the nature of the science; whereas real-time ecologists have the luxury of observing and measuring in whatever detail is permitted by time and resources, paleoecological observations are restricted to whatever material evidence has been left in sediments, tree-rings, rodent middens, or other geohistorical archives (1, 24, 25). Paleoecologists can exercise some control over precision (via site selection, sampling density, type of fossil) but eventually run up against hard limits to detail (24–26). Despite its limitations, paleoecology offers something lacking in community ecology: records of species assemblages spanning many generations, embedded in patterns of regional colonization and extirpation, against a backdrop of environmental changes diverse in rate, duration, and nature.
Both community ecology and Quaternary paleoecology can benefit from greater engagement. Community ecology offers processes and theory that can be applied to understanding patterns in the Quaternary record. Paleoecology offers temporal context, filling the “missing middle” between the real-time concerns of community ecology and the deep-time patterns of phylogeny and biogeography (Fig. 1). Community ecology is grounded in natural history, which generates the fundamental observations, patterns, and questions to be explained by theory and tested by experiment or further observation (17). In this context, paleoecology can be viewed as a temporal extension of natural history; by extending the observational foundation of community ecology, it reveals fundamental patterns and dynamics to which ecologists are otherwise blind. Furthermore, Quaternary records provide a testing ground for theory and pattern in community ecology. Bridging between ecology and paleoecology can extend ecological theory and understanding to longer timescales, identify where existing theory needs to be modified or replaced to accommodate longer-term dynamics, and provide frameworks for understanding the complex ecological dynamics underlying paleoecological records. In this paper we provide an overview of what Quaternary terrestrial records reveal about environmental and community changes, suggest some foundations for bridging between community ecology and Quaternary paleoecology, and identify topics where the two fields can engage to mutual benefit. Our review is necessarily selective; in particular, we do not discuss paleoecology’s perspectives on the classic Clements/Gleason community-structure controversies, which are amply reviewed elsewhere (19, 22, 27–30).
Fig. 1.
The ecological community in space and time. Local communities aggregate (A) into spatial mosaics of communities, which in turn aggregate to form regional and then (sub)continental species pools. Each level constrains (C) processes occurring at finer spatial and temporal scales. For example, a spatial mosaic of communities in a region is determined by the composition, size, and spatial array of its individual elements (communities) and in turn constrains those elements by influencing propagule sources and dispersal, connectivity, adjacency, proximity, and other properties that influence community composition and dynamics. Similarly, a regional species pool is determined by composition of its various constituent communities and mosaics, aggregating the responses of countless individuals and populations to their physical environment and to each other. At the same time, the regional species pool constrains membership in regional mosaics and local communities and is subject to the constraints of the (sub)continental species pool, which is subject to phylogenetic and biogeographic processes operating across vast areas and large sweeps of time. Each level has a finite spatial extent and temporal duration. Filled rectangles indicate the most common or modal extent and duration of terrestrial communities, and dashed lines indicate the extremes. Climate influences each level, and climate change is a primary determinant of the composition of communities, spatial mosaics, and (sub)continental species pools. For example, paleoecological evidence indicates that terrestrial plant, vertebrate, and insect communities rarely persist without compositional change for more than a few thousand years (23, 48, 56, 57, 64). Regional species pools undergo change as species migrate, abandoning newly unsuitable habitats and colonizing newly favorable territory (18, 22, 50). Climate exerts influence at all levels, both directly and indirectly via aggregation of influences from below and constraint by influences from above.
Ecology and Time’s Environmental Texture
Much of ecological concept and theory has considered time as a simple, uniform dimension, wherein ecological processes unfold against a constant environmental backdrop. Time is treated as an independent variable, and ecological properties change as time-dependent ecological processes run their course. A classic example is the exponential model of population growth, together with its many derivative models incorporating resource competition, predation, multiple species, age structure, time lags, and other features. The environment itself may change, but only as a consequence of the ecological processes themselves (e.g., consumption of resources; autogenic successional processes such as soil development or lake infilling) or as a stochastic variable, fluctuating randomly about a constant mean. Long-term environmental change has long been acknowledged by ecologists, but only recently have environmental change and nonstationary variability been explicitly incorporated into ecological models, usually as discrete perturbations or at relatively short time intervals (seasons to decades) (31–34).
Time is richly textured by environmental change and variability. Paleoclimate records indicate a broad and apparently continuous spectrum of climate variability and change, from the years and decades experienced by individual organisms and populations to the tens of millions of years experienced by higher-order clades and biogeographic provinces. These climatic changes, their causes, and their ecological and evolutionary implications are reviewed and summarized elsewhere (19, 35–39).
We note here a few important features of Quaternary climate variability and change. First, climate variability is rarely, if ever, stationary in nature. Climate means evolve through time, as do variances, extremes, and modalities. For example, moisture records of the past two millennia from tree-rings and other sources indicate that there is no “normal” climate, revealing instead a continually evolving array of wet periods, dry periods, and intermediate periods, varying in duration, magnitude, frequency, and sequence (see figure 1 in ref. 35). Similar patterns of nonstationary variation in interannual to centennial variability are probably characteristic of the past million years (36, 40), although the details vary in space and time.
Second, although some climatic phenomena exhibit cyclic or quasicyclic behavior, environmental conditions do not necessarily repeat themselves. For example, individual El Niño events of the past century have differed substantially in magnitude, spatial extent, and duration (41, 42). Similarly, each of the six most recent interglacial periods has had a unique duration and spatial and temporal climatic patterns (43). Third, climate change often involves changing climatic realizations, whereby combinations of seasonal temperature, seasonal precipitation, and other factors change (19, 44, 45). Fourth, climate change can be gradual and directional, but it is often punctuated by episodic events and by rapid state transitions (40, 46, 47).
To summarize, no modal or normal climate state exists for the Quaternary. Climate has varied widely and changed both gradually and rapidly, across the planet. Changes have occurred at all timescales, from 100 to 106 years, although the nature, magnitude, and rate of change differ among scales and at different times. Changes have occurred in all parts of the globe, although the nature, magnitudes, and rates have differed among regions and locales. The changes have involved virtually all ecologically relevant manifestations of temperature and moisture, including means, extremes, variance, seasonal patterns, and persistence. The rich, complex, multiscale texture of the environment through time has had and will continue to have important consequences for individuals, populations, communities, and regional species pools.
Communities Come, Communities Go
In a recent review, Ricklefs (11) noted that an ecological community comprises a single place that happens to be occupied by an assemblage of species with overlapping distributions and environmental tolerances. He further argued that the community concept be “replaced by the spatial distributions of populations, which now become the primary focus for understanding biodiversity patterns” (p. 744 in ref. 11). Paleoecological records support a parallel conception involving time. An ecological community can be viewed as a single point in a spatial framework of species distributions superimposed on environmental gradients and patchworks and in a temporal framework of population and biogeographic responses to environmental change and variability. The space and time dimensions cannot be treated separately; spatial distributions evolve through time and are often contingent on temporal processes. Conversely, the temporal patterns of occurrence and abundance at an individual site are contingent on spatial distributions, patterns, and processes. Understanding of community properties is inevitably incomplete in the absence of both spatial and temporal context (Fig. 1).
The changing composition of forest communities at a single locale during the past 8,600 y is shown in Fig. 2. All the major and most of the minor upland forest species in the surrounding region (Central Upper Michigan) are represented in the Tower Lake record (48). Surrounding forests now are dominated by Fagus grandifolia, Tsuga canadensis, Acer saccharum, and Betula allegheniensis, with scattered Pinus strobus. This combination of species has existed at the site for the past 1,400 y, starting with the colonization and expansion of Fagus, which expanded eastward in the region during the past 3,000 y (49). Thus, the forest community at Tower Lake has existed for no more than 1,400 y, and it arose from an interaction between local ecological processes (dispersal, demography, competition) and regional biogeographic processes (range expansion) (18, 50). Those regional processes depended in turn on local ecological processes at countless other individual sites, were driven by regional climatic changes spanning centuries or more (18, 20, 51), and may have been modulated by annual- to centennial-scale climate variability (35, 48).
Fig. 2.
Pollen (silhouettes) and macrofossil abundances (horizontal bars) for selected tree taxa and macrofossil types at Tower Lake, MI. Pollen data are expressed as percentages, and macrofossil data represent numbers per 50 cm3 of sediment volume. Solid bars for Betula macrofossils denote B. allegheniensis, while open bars represent B. papyrifera. Horizontal lines delineate major transitions in forest composition. Reprinted with permission from ref. 48.
Not only has the modern forest community at Tower Lake persisted for only a short time, no forest community persisted there for longer than 1,500 y (Fig. 2). The 8,600-y sequence shows at least seven distinct forest phases. Within each, forest composition changed as decades and centuries passed, usually in more subtle ways than the major transitions (Fig. 2). The Tower Lake record spans only a few millennia, but set in the context of other records across the region (49, 52) and eastern North America (18, 50), it illustrates how an ecological community is one small, ephemeral point in a roiling, dynamic unfolding of environmental change, distribution dynamics, and spatially aggregated ecological processes.
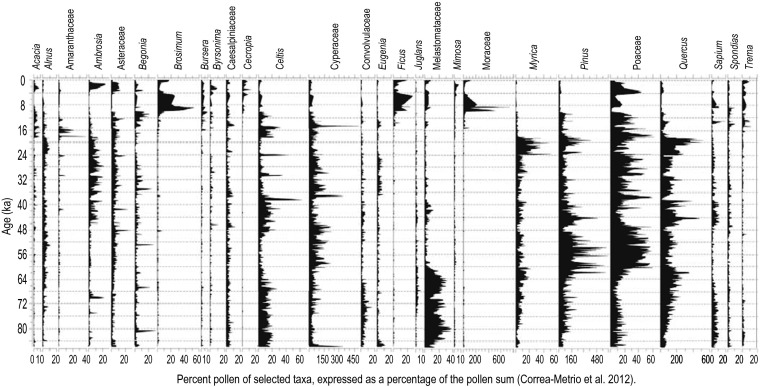
These dynamics are not restricted to temperate regions or to the postglacial period. An 86,000-y pollen record of upland vegetation from Lake Petén-Itzá in the Guatemalan Neotropics shows many similar features (Fig. 3). As at Tower Lake, the community observed at any single point in time is ephemeral, rarely persisting for more than a few millennia before being replaced by something new (53, 54). These changes were also driven largely by climate change, often accompanied by changes in fire regime (53–55).
Fig. 3.
Percent pollen of selected taxa, expressed as a percentage of the pollen sum from core PI-6, Lake Petén-Itzá, lowland Guatemala. Modified with permission from ref. 53.
The examples of Tower Lake and Lake Petén-Itzá are illustrative, not representative. No single modal pattern can be identified for the past 8,600 y, for the past 86,000 y, or for any other interval. During the past millennium, communities in different locales have variously persisted unchanged, undergone small shifts in species abundance, been colonized by new species, experienced population decline of dominants, lost incumbent species to extirpation, or even undergone nearly complete compositional turnover. Any other millennium in the past 20,000 y, and probably the past million years, would show similar diversity of patterns. Nor are these patterns restricted to plant communities. Similar patterns are recorded for terrestrial insects (56) and especially for vertebrates, for which the extensive Quaternary mammal record has recorded similar changes in species abundances (23, 57, 58), range shifts (22, 59), extirpations and colonizations (23, 60), and extinctions (61–63). Terrestrial communities are highly dynamic, and patterns vary widely in space and time. Some communities at some locales may persist over many millennia, but persistence for more than 10 millennia seems to be rare for the past 20,000 y (64) and possibly for the entire Quaternary. Communities are best understood not only as local realizations of overlapping species ranges and environmental gradients (11, 65) but also as passing manifestations of ecological and biogeographic processes in a world of ceaseless environmental change (19, 29).
What Governs Community Assembly and Disassembly?
Determinants of community structure and composition are a central focus of community ecology and tend to align with one of three modal concepts: interaction assembly, environment assembly, or neutral assembly (Fig. 4). Interaction assembly is deeply rooted in community ecology and emphasizes the Eltonian niche (66, 67), considering communities to be structured primarily by strong interactions among species (8, 33). Such interactions may include facilitation, mutualism, and trophic relationships (predation, parasitism, herbivory), but resource competition often is treated as first among equals (68, 69). Interaction-based communities have limited membership, governed by equilibrium population processes as determined by interspecific interactions (7, 8, 68). In contrast, neutral assembly considers communities to be structured entirely by random processes, particularly dispersal, recruitment, and mortality (70). Neutral communities have virtually unlimited membership, are nonequilibrium, and bear historical imprints; composition is influenced by legacies of past demographic and dispersal events, and hence community properties may drift as the result of singular events (70). Finally, communities under environment assembly are structured primarily by species’ physiological and demographic responses to the physical environment (19, 33). The environment-assembly concept is also niche-based but emphasizes the Grinnellian niche (6, 19, 67). It is predicated on all species having finite environmental requirements or tolerances, which impose strong filters on community membership. Community composition is governed by whether potential members’ fundamental (Grinnellian) niches overlap with the local environmental realization. These three concepts are not mutually exclusive, and some efforts have been made to reconcile interaction assembly and neutral assembly (71–73). Environment assembly has received less attention in community ecology than the other two, although it underlies the recent explosion of empirical models predicting species distribution and abundance and community composition under various climate-change scenarios (74).
Fig. 4.
Three primary loci of explanation for community properties: species interaction, environmental forcing, and neutral processes that cause ecological drift. Processes interact among these domains. Environmental change (e.g., climate change) can be a primary driver of changes in community properties, both directly (organismal and demographic responses) and indirectly (by altering interactions among species and setting neutral processes in motion). Climate change and variability continually perturb and redirect processes within all three loci to reshape community properties.
Paleoecological support for environment assembly is very strong, resting on both theoretical (19, 75) and empirical foundations (e.g., 20, 76–80). Paleoecologists generally look first to environmental change, particularly climate change, as a driver of the kinds of dynamics shown in Figs. 2 and 3, and they often look no further. They find implicit justification in three decades of broad-scale comparisons of paleoecological records with paleoenvironmental records (20, 77, 79) and with paleoclimate simulations from general circulation models (44, 51). By itself, ecological drift would produce a mosaic of community types independent of environmental mosaics. However, the temporal pattern at Tower Lake (Fig. 2) is similar to that at other sites across central Upper Michigan and regions to the south and east. The spatiotemporal coherence of faunal and floral assemblages over broad regions (18, 77, 81, 82) suggests overarching controls, and climate has no compelling competitor to explain regional coherence.
Although environment assembly seems necessary to explain the dynamics observed in paleoecological data, is it sufficient? Species and populations are not billiard balls knocked around the landscape by changing climate; climate change drives community change via processes that involve individuals, populations, and communities. Ecological interactions matter, and ultimately the changes observed in paleoecological time series represent outcomes of interactions among populations of different species, as influenced by a changing environment (Fig. 4). Although these interactions play out locally and often briefly, even the broadest geographic patterns are spatial aggregations of local interactions, and all temporal changes, regardless of scale, are ultimate outcomes of population processes and interactions. Paleoecological dynamics (Figs. 2 and 3) cannot be understood fully without considering population processes and species interactions.
Neutral assembly is formally embodied in a theoretical framework that assumes that niche properties are irrelevant to community structure and composition, which derive entirely from ecological drift (70). Drift processes are effectively random with respect to species niche properties (Eltonian or Grinnellian), yielding community realizations that cannot be predicted from either prevailing environment or species interactions. Ecological drift has a distinguished history in paleoecology; Davis (27, 28), for example, argued that the composition of Holocene forest communities was determined largely by effectively random processes of seed dispersal and proximity of glacial refugia. Although this view has been largely superseded by environment assembly in recent years, attention is being refocused on historical contingencies (35), which represent neutral assembly processes. Cross-scale interactions (83) involving climate variability and ecological responses at different temporal scales lead to contingent outcomes, with strong influences of historical processes (mortality, disturbance, dispersal, recruitment) (35). Those historical processes, although they involve both Eltonian and Grinnellian niche attributes, are effectively neutral because the ecological outcomes years, decades, or centuries later cannot be predicted from niche properties and environment alone. Recent paleoecological studies provide many examples (35, 84, 85).
The question of whether environment assembly is necessary to explain paleoecological records has been raised by a recent study that simulated pollen sequences based on neutral assumptions (86). The simulated profiles share features with observed diagrams (e.g., Figs. 2 and 3): successive dominance of different species, changing combinations of species, and turnover in species composition and abundance. The study provides a cautionary lesson, demonstrating how temporal structure can arise in the absence of a coherent structuring mechanism, and showing that environmental forcing need not be taken for granted. The Petén-Itzá pollen sequence (Fig. 3) has been used in an explicit test of whether vegetational changes are attributable to ecological drift or environmental change (55). That study goes beyond simple comparison to incorporate an explicit alternative hypothesis (neutral assembly) but concludes that environmental change explains most of the observed patterns. Similarly, McGill et al. (81) found that mammal assemblages were more coherent across space and time than predicted by neutral assembly, although patterns were not specifically attributed to environment or interaction assembly.
Just as neutral processes interact with environmental change (35), so too do species interactions (Fig. 4). A modest change in temperature or moisture may shift the competitive balance in favor of one species, leading to an increase in that species at the expense of others. Climate change may render circumstances more or less favorable for consumers, leading to changes in host or prey populations or to altered interactions between plants, herbivores, and climate (33, 87). Effects may be profound, with environment assembly contingent on species interactions as well as the reverse (88). For example, the late-glacial megaherbivore decline may have been a proximal driver of vegetation changes that ultimately were a response to climatic changes that occurred centuries or more before (89).
In the final analysis, asking whether communities are structured by interaction, neutral, or environment assembly processes is futile; communities are subject to diverse, interacting influences (90), and explanation may be more a matter of causal “thickets” than “chains” (91). Environmental change is a powerful driver of community change and should be incorporated more explicitly into community ecology (33). Species interactions govern the community outcomes of environmental change, often in subtle ways, and should be considered more explicitly in paleoecological explanation. Interactions of neutral processes with environmental change and species interactions introduce indeterminacies as well as historical legacies that may persist as local or even regional anachronisms. Paleoecological studies, informed by community ecology, can reveal the short- and long-term consequences of these interactions.
Spanning the Missing Middle
The missing middle of Quaternary ecological history provides abundant opportunity for collaboration between paleoecologists and community ecologists. Success will require mutual understanding and engagement. Paleoecologists must understand the ecological questions to which their data are applied, and ecologists must understand the nature of paleoecological data and inference. To avoid mismatches between data and applications, careful attention must be paid to scale and taphonomy (24–26, 48). We identify a nonexhaustive set of themes, topics, and questions upon which collaborations might be centered.
Community Assembly and Disassembly.
Paleoecological records show not only that communities come and go but also that community turnover occurs in many forms and at many rates. Some communities arise quickly, whereas others develop in slow transitions (Figs. 2 and 3). Conversely some communities disassemble gradually via decline and replacement of dominants, but others appear to undergo rapid collapse (Figs. 2 and 3). These and other records provide diverse case studies for understanding the various roles and interactions of environmental forcing, intrinsic community properties (e.g., resilience, hysteresis, keystone species), and ecological drift in community transitions, whether gradual or abrupt. Specific critical transitions can be targeted for intensive study, based on various criteria (e.g., knowledge of natural history of taxa, existence of independent paleoclimatic records, precision of paleoecological records, availability of multiple paleoecological sites for replication or pattern analysis, potential significance of observed patterns). The rapid increase or decline of a dominant species or the disappearance of a community, for example, is of obvious interest in conservation context and may be driven by rapid environmental change, cross-scale interactions, or both (23, 84, 92). Intensive, integrated study of a carefully selected array of paleoecological case studies would test fundamental theory and indicate the extent to which community turnover follows general rules. In addition, paleoecological data from individual sites or site arrays can be analyzed to test specific theoretical predictions concerning community turnover (e.g., refs. 55, 81, and 93–95).
Trait-Based Community Patterns.
Trait-based assembly and inference have become an important focus in community ecology (96, 97). Traits and related functional groups of species may govern whether and in what order species can join communities (97), and influence interactions of individuals with the environment and with each other. Paleontology has a rich tradition of connecting traits with ecological and evolutionary processes (98), and trait-based approaches to community assembly are ripe for paleoecological application (99). A critical challenge is to determine which traits extractable from the fossil record map directly or indirectly onto niche properties (Grinnellian or Eltonian), and how they are related to traits commonly measured by modern ecologists. An emerging agenda in paleoecology is to track specific traits through time with the goal of explicitly connecting the past with the present (99, 100). Merging trait-based paleoecology with community ecology can identify the stability of trait relationships through time. Much like temporal variation in species and communities, trait distributions within species are likely to vary through time and with changing environments. Paleoecological trait studies can assess conservatism of trait–environment relationships and trait properties within taxa. Furthermore, they can help identify the extent to which communities may display functional stasis while undergoing compositional turnover (60, 81).
Diversity Dynamics Through Time.
A growing body of work has demonstrated that environmental variability affects species diversity on short time scales (33, 101, 102), suggesting a species–time relationship (103) as an analog to the species–area relationship. However, key differences between spatial and temporal processes imply that they may have unique diversity-scaling relationships (103, 104). In addition, space and time constrain one another, motivating theoretical formulation and empirical testing of integrated species–time–area relationships (103, 105). White et al. (103) point to several fruitful areas to promote this integration. However, most of their case studies are modern, encompassing climatic variation over a few decades at most (e.g., refs. 101, 105), and their single fossil example starts with an interval length of >700,000 y (106). The missing middle remains unfilled. Brown et al. (102) examined how environmental variability may regulate diversity dynamics in several systems, including Holocene fossil pollen records at family resolution. They postulated that although environmental change maintains diversity by influencing colonization and extinction of different species, when sufficiently large it could alter diversity by changing species-level carrying capacity (102). These conjectures remain unexplored.
Ecological Rules in the Anthropocene.
Have ecological and evolutionary processes changed fundamentally during the Anthropocene (107)? Do anthropogenic activities override past controls on community dynamics? Addressing these questions requires accurate baselines to compare diversity trends and patterns, and the Quaternary provides critical linkages between deep time and the Anthropocene. For example, mammalian biodiversity patterns throughout the past 30 million years suggest that the Holocene is different from all time periods that came before (63), implying human impacts well before the Industrial Revolution. In some settings, beta diversity and functional diversity appear to have remained relatively stable despite compositional changes, providing context to evaluate whether ongoing processes override those of the past few million of years (108–110). Beyond issues of the Anthropocene, there are legitimate questions concerning whether the Quaternary itself is unique and whether ecological rules evolve with the global environmental setting (19, 111, 112). Existing paleoecological records provide an untapped source for investigation of diversity dynamics in diverse systems and at different timespans (113, 114).
Dynamics of Regional Species Pools.
Community ecologists are focusing attention on the biogeographic and phylogenetic contexts of species pools (e.g., refs. 115–117) and their implications for community properties (e.g., ref. 118). These studies tend to treat the regional species pool as static, subject to the same processes as (sub)continental pools (Fig. 1). Regional species pools, however, are subject to at least partial turnover over hundreds to thousands of years (refs. 18, 19, and 22, but see ref. 82) and are predicted to change rapidly under global change because of both extinction and range shifts (119, 120). Implications of species-pool dynamics are being explored from biogeographical and macroecological perspectives (82, 121), but the consequences for community assembly and structure represent open territory for collaboration between community ecologists and paleoecologists.
Clocking Time-Dependent Processes.
Many ecological processes are time-dependent, and paleoecological studies indicate that similar plant communities are established at different times in different places (64). Comparative studies of secondary communities (mycorrhizae, invertebrates, vertebrates), using antiquity of the plant community as an independent variable, can provide insights into processes of community development and evolution (122). Age may be one among many differences among sites, but other properties (e.g., physical environment, climate history, genetics) can be controlled in study design. Time-dependent processes may be sufficiently large to override other effects, many of which can be assessed directly from paleoecological and paleoenvironmental studies. For example, ancient DNA may provide information on local genetic changes (123, 124).
Meeting Cowles’ Challenge
More than a century ago Henry Chandler Cowles identified the central challenge of ecological dynamics, observing that vegetational response to the environment was “a variable approaching a variable rather than a constant” (125). The challenge has only grown since Cowles’ time: Ecological dynamics comprise a multitude of variables, with different response times, changing at different rates, and often interacting in subtle ways. The environment, too, is far more dynamic than envisioned even a decade ago, with nested climatic changes interacting across a range of temporal and spatial scales. Community ecology offers tools and theory to help understand ecological change; paleoecology offers diverse data and observations of actual ecological change. By working together, these disciplines can advance understanding of ecological dynamics in changing environments.
We live in a time of rapid environmental change and associated societal challenges. Novel communities and ecosystems are already widespread (126) and will only increase with global change in coming decades. Communities of the future will represent contingent outcomes of the complex dynamics of environmental change, species interactions, and ecological drift. Integrating the mechanisms of community ecology with the empirical richness of paleoecology will not only advance the science of ecology but will also increase its capacity to contribute to climate-change adaptation and minimize risks to biodiversity and ecological services.
Acknowledgments
Discussions and critiques were provided by D. Sax, M. King, C. Nolan, and anonymous reviewers. Funding for this work was provided by National Science Foundation Grants DEB-1257033 (to J.L.B.) and DEB-0345012 and EF-1065732 (to S.T.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.National Research Council . The Geological Record of Ecological Dynamics: Understanding the Biotic Effects of Future Environmental Change. National Academies; Washington, DC: 2005. [Google Scholar]
- 2.Lawton JH. Community Ecology in a Changing World. International Ecology Institute, Oldendorf/Luhe; Germany: 2000. [Google Scholar]
- 3.Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. Beyond predictions: Biodiversity conservation in a changing climate. Science. 2011;332(6025):53–58. doi: 10.1126/science.1200303. [DOI] [PubMed] [Google Scholar]
- 4.Loreau M. The Challenges of Biodiversity Science. International Ecology Institute, Oldendorf/Luhe; Germany: 2010. [Google Scholar]
- 5.Willis KJ, Bailey RM, Bhagwat SA, Birks HJB. Biodiversity baselines, thresholds and resilience: Testing predictions and assumptions using palaeoecological data. Trends Ecol Evol. 2010;25(10):583–591. doi: 10.1016/j.tree.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson GE. An Introduction to Population Ecology. Yale Univ Press; New Haven, CT: 1978. [Google Scholar]
- 7.Mittelbach GG. 2012. Community Ecology (Sinauer Associates Incorporated, Sunderland, MA)
- 8.Morin PJ. 2011. Community Ecology (John Wiley & Sons, Hoboken, NJ)
- 9.Lawton JH. Are there general laws in ecology? Oikos. 1999;84(2):177–192. [Google Scholar]
- 10.Ricklefs RE. Community diversity: Relative roles of local and regional processes. Science. 1987;235(4785):167–171. doi: 10.1126/science.235.4785.167. [DOI] [PubMed] [Google Scholar]
- 11.Ricklefs RE. Disintegration of the ecological community. Am Nat. 2008;172(6):741–750. doi: 10.1086/593002. [DOI] [PubMed] [Google Scholar]
- 12.Simberloff D. Community ecology: Is it time to move on? (An American Society of Naturalists presidential address) Am Nat. 2004;163(6):787–799. doi: 10.1086/420777. [DOI] [PubMed] [Google Scholar]
- 13.Ricklefs RE, Jenkins DG. Biogeography and ecology: Towards the integration of two disciplines. Philos Trans R Soc Lond B Biol Sci. 2011;366(1576):2438–2448. doi: 10.1098/rstb.2011.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vellend M. Conceptual synthesis in community ecology. Q Rev Biol. 2010;85(2):183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- 15.Ricklefs RE, Schluter D. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs RE, Schluter D, editors. Univ of Chicago Press; Chicago: 1993. pp. 350–363. [Google Scholar]
- 16.Wiens JJ. The niche, biogeography and species interactions. Philos Trans R Soc Lond B Biol Sci. 2011;366(1576):2336–2350. doi: 10.1098/rstb.2011.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricklefs RE. Naturalists, natural history, and the nature of biological diversity. Am Nat. 2012;179(4):423–435. doi: 10.1086/664622. [DOI] [PubMed] [Google Scholar]
- 18.Williams JW, Shuman BN, Webb T, III, Bartlein P, Leduc PL. Late-Quaternary vegetation dynamics in North America: Scaling from taxa to biomes. Ecol Monogr. 2004;74(2):309–334. [Google Scholar]
- 19.Jackson ST, Overpeck JT. Responses of plant populations and communities to environmental changes of the late Quaternary. Paleobiology. 2000;26(Supplement):194–220. [Google Scholar]
- 20.Webb T, III, Shuman BN, Williams JW. In: The Quaternary Period in the United States. Gillespie AR, Porter SC, Atwater BF, editors. Elsevier; New York: 2003. pp. 459–478. [Google Scholar]
- 21.Bush M, Flenley J. Tropical Rainforest Responses to Climatic Change. Springer; New York: 2007. [Google Scholar]
- 22.Graham RW, et al. Spatial response of mammals to Late Quaternary environmental fluctuations. Science. 1996;272(5268):1601–1606. doi: 10.1126/science.272.5268.1601. [DOI] [PubMed] [Google Scholar]
- 23.Blois JL, McGuire JL, Hadly EA. Small mammal diversity loss in response to late-Pleistocene climatic change. Nature. 2010;465(7299):771–774. doi: 10.1038/nature09077. [DOI] [PubMed] [Google Scholar]
- 24.Jackson ST. Representation of flora and vegetation in Quaternary fossil assemblages: Known and unknown knowns and unknowns. Quat Sci Rev. 2012;49:1–16. [Google Scholar]
- 25.Kidwell SM, Flessa KW. The quality of the fossil record: Populations, species, and communities. Annu Rev Earth Planet Sci. 1996;24:433–464. [Google Scholar]
- 26.Behrensmeyer AK, Kidwell S, Gastaldo R. Taphonomy and paleobiology. Paleobiology. 2000;26(SP4):103–147. [Google Scholar]
- 27.Davis MB. Pleistocene biogeography of temperate deciduous forests. Geosci Man. 1976;13:13–26. [Google Scholar]
- 28.Davis MB. Forest Succession: Concepts and Application. Springer; New York: 1981. pp. 132–153. [Google Scholar]
- 29.Webb T., III The appearance and disappearance of major vegetational assemblages: Long-term vegetational dynamics in eastern North America. Vegetatio. 1987;69(1-3):177–187. [Google Scholar]
- 30.Huntley B, Webb T., III . Vegetation History. Kluwer; Dordrecht, The Netherlands: 1988. [Google Scholar]
- 31.Chesson P, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am Nat. 1997;150(5):519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- 32.Forchhammer MC, Stenseth NC, Post E, Langvatn R. Population dynamics of Norwegian red deer: Density-dependence and climatic variation. Proc Biol Sci. 1998;265(1393):341–350. doi: 10.1098/rspb.1998.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Post E. Ecology of Climate Change. Princeton Univ Press; Princeton, NJ: 2013. [Google Scholar]
- 34.Stenseth NC, et al. Ecological effects of climate fluctuations. Science. 2002;297(5585):1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 35.Jackson ST, Betancourt JL, Booth RK, Gray ST. Ecology and the ratchet of events: Climate variability, niche dimensions, and species distributions. Proc Natl Acad Sci USA. 2009;106(Suppl 2):19685–19692. doi: 10.1073/pnas.0901644106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark PU, et al. Global climate evolution during the last deglaciation. Proc Natl Acad Sci USA. 2012;109(19):E1134–E1142. doi: 10.1073/pnas.1116619109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcott SA, Shakun JD, Clark PU, Mix AC. A reconstruction of regional and global temperature for the past 11,300 years. Science. 2013;339(6124):1198–1201. doi: 10.1126/science.1228026. [DOI] [PubMed] [Google Scholar]
- 38. PAGES 2k Consortium (2013) Continental-scale temperature variability during the past two millennia. Nature Geoscience 6(5):339–346.
- 39.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292(5517):686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 40.Harrison SP, Sanchez Goñi MF. Global patterns of vegetation response to millennial-scale variability and rapid climate change during the last glacial period. Quat Sci Rev. 2010;29(21-22):2957–2980. [Google Scholar]
- 41.Ashok K, Behera SK, Rao SA, Weng H, Yamagata T. El Niño Modoki and its possible teleconnection. J Geophys Res: Oceans (1978–2012) 2007;112:C11007. [Google Scholar]
- 42.Larkin NK, Harrison DE. Global seasonal temperature and precipitation anomalies during El Niño autumn and winter. Geophys Res Lett. 2005;32:L16705. [Google Scholar]
- 43.Lang N, Wolff EW. Interglacial and glacial variability from the last 800 ka in marine, ice and terrestrial archives. Climate of the Past. 2011;7(2):361–380. [Google Scholar]
- 44.Jackson ST, Williams JW. Modern analogs in Quaternary paleoecology: Here today, gone yesterday, gone tomorrow? Annu Rev Earth Planet Sci. 2004;32:495–537. [Google Scholar]
- 45.Williams JW, Jackson ST. Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ. 2007;5(9):475–482. [Google Scholar]
- 46.Shuman B. Patterns, processes, and impacts of abrupt climate change in a warm world: The past 11,700 years. WIREs Clim Change. 2012;3:19–43. [Google Scholar]
- 47.National Research Council . Abrupt Climate Change. National Academies; Washington, D.C: 2002. [Google Scholar]
- 48.Jackson ST, et al. Inferring local to regional changes in forest composition from Holocene macrofossils and pollen of a small lake in central Upper Michigan, USA. Quat Sci Rev. 2014;98:60–73. [Google Scholar]
- 49.Woods KD, Davis MB. Paleoecology of range limits: Beech in the Upper Peninsula of Michigan. Ecology. 1989;70(3):681–696. [Google Scholar]
- 50.Jackson S, Overpeck J, Webb T, III, Keattch SE, Anderson KH. Mapped plant-macrofossil and pollen records of late Quaternary vegetation change in eastern North America. Quat Sci Rev. 1997;16:1–70. [Google Scholar]
- 51.Webb T, III, Anderson K, Bartlein P, Webb R. Late Quaternary climate change in eastern North America: A comparison of pollen-derived estimates with climate model results. Quat Sci Rev. 1998;17:587–606. [Google Scholar]
- 52.Davis MB, Woods KD, Webb SL, Futyma RP. Dispersal versus climate: Expansion of Fagus and Tsuga into the Upper Great Lakes region. Vegetatio. 1986;67(2):93–103. [Google Scholar]
- 53.Correa-Metrio A, et al. Rapid climate change and no-analog vegetation in lowland Central America during the last 86,000 years. Quat Sci Rev. 2012;38:63–75. [Google Scholar]
- 54.Correa-Metrio A, et al. The influence of abrupt climate change on the ice-age vegetation of the Central American lowlands. J Biogeogr. 2012;39(3):497–509. [Google Scholar]
- 55.Correa-Metrio A, Meave JA, Lozano-García S, Bush MB. Environmental determinism and neutrality in vegetation at millennial time scales. J Veg Sci. 2014;25(3):627–635. [Google Scholar]
- 56.Elias SA. Quaternary Insects and Their Environments. Smithsonian Institution; Washington, D.C: 1994. [Google Scholar]
- 57.Grayson D. Mammalian responses to Middle Holocene climatic change in the Great Basin of the western United States. J Biogeogr. 2000;27(1):181–192. [Google Scholar]
- 58.Hadly EA. Influence of late-Holocene climate on northern Rocky Mountain mammals. Quat Res. 1996;46(3):298–310. [Google Scholar]
- 59.Lyons S. A quantitative assessment of the range shifts of Pleistocene mammals. J Mammal. 2003;84(2):385–402. [Google Scholar]
- 60.Barnosky AD, et al. Exceptional record of mid-Pleistocene vertebrates helps differentiate climatic from anthropogenic ecosystem perturbations. Proc Natl Acad Sci USA. 2004;101(25):9297–9302. doi: 10.1073/pnas.0402592101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyons SK, Smith FA, Brown J-H. Of mice, mastodons and men: Human-mediated extinctions on four continents. Evol Ecol Res. 2004;6(3):339–358. [Google Scholar]
- 62.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. Assessing the causes of late Pleistocene extinctions on the continents. Science. 2004;306(5693):70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 63.Carrasco MA, Barnosky AD, Graham RW. Quantifying the extent of North American mammal extinction relative to the pre-anthropogenic baseline. PLoS ONE. 2009;4(12):e8331. doi: 10.1371/journal.pone.0008331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackson ST. Vegetation, environment, and time: The origination and termination of ecosystems. J Veg Sci. 2006;17(5):549–557. [Google Scholar]
- 65.Whittaker R. Vegetation of the Great Smoky Mountains. Ecol Monogr. 1956;26(1):1–80. [Google Scholar]
- 66.Hutchinson GE. Concluding Remarks. Cold Spring Harb Symp Quant Biol. 1957;22:415–427. [Google Scholar]
- 67.Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10(12):1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 68.Diamond J. In: Ecology and Evolution of Communities. Cody ML, Diamond J, editors. Belknap, Cambridge, MA; 1975. pp. 342–444. [Google Scholar]
- 69.Weiher E, Keddy P. Ecological Assembly Rules. Cambridge Univ Press; New York: 1999. [Google Scholar]
- 70.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton Univ Press; Princeton, NJ: 2001. [DOI] [PubMed] [Google Scholar]
- 71.Adler PB, Hillerislambers J, Levine JM. A niche for neutrality. Ecol Lett. 2007;10(2):95–104. doi: 10.1111/j.1461-0248.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 72.Chase JM, Myers JA. Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B Biol Sci. 2011;366(1576):2351–2363. doi: 10.1098/rstb.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukami T. In: Community Ecology: Processes, Models, and Applications. Verhoef HA, Morin PJ, editors. Oxford Univ Press; New York: 2010. pp. 45–54. [Google Scholar]
- 74.Elith J, Leathwick JR. Species distribution models: Ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst. 2009;40:677–697. [Google Scholar]
- 75.Webb T. Is vegetation in equilibrium with climate? How to interpret late-Quaternary pollen data. Vegetatio. 1986;67(2):75–91. [Google Scholar]
- 76.Yu Z. Rapid response of forested vegetation to multiple climatic oscillations during the last deglaciation in the northeastern United States. Quat Res. 2007;67(2):297–303. [Google Scholar]
- 77.Shuman BN, Newby P, Huang Y, Webb T., III Evidence for the close climatic control of New England vegetation history. Ecology. 2004;85(5):1297–1310. [Google Scholar]
- 78.Weppner KN, Pierce JL, Betancourt JL. Holocene fire occurrence and alluvial responses at the leading edge of pinyon–juniper migration in the Northern Great Basin, USA. Quat Res. 2013;80(2):143–157. [Google Scholar]
- 79.Booth RK, et al. Multi-decadal drought and amplified moisture variability drove rapid forest community change in a humid region. Ecology. 2012;93(2):219–226. doi: 10.1890/11-1068.1. [DOI] [PubMed] [Google Scholar]
- 80.Williams JW, Post DM, Cwynar LC, Lotter AF, Levesque AJ. Rapid and widespread vegetation responses to past climate change in the North Atlantic region. Geology. 2002;30(11):971–974. [Google Scholar]
- 81.McGill BJ, Hadly EA, Maurer BA. Community inertia of Quaternary small mammal assemblages in North America. Proc Natl Acad Sci USA. 2005;102(46):16701–16706. doi: 10.1073/pnas.0504225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riddle B. The historical assembly of continental biotas: Late Quaternary range-shifting, areas of endemism, and biogeographic structure in the North American mammal fauna. Ecography. 1998;21(4):437–446. [Google Scholar]
- 83.Peters DPC, et al. Cross-scale interactions, nonlinearities, and forecasting catastrophic events. Proc Natl Acad Sci USA. 2004;101(42):15130–15135. doi: 10.1073/pnas.0403822101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Booth RK, Brewer S, Blaauw M, Minckley TA, Jackson ST. Decomposing the mid-Holocene Tsuga decline in eastern North America. Ecology. 2012;93(8):1841–1852. doi: 10.1890/11-2062.1. [DOI] [PubMed] [Google Scholar]
- 85.Pederson N, et al. The legacy of episodic climatic events in shaping temperate, broadleaf forests. Ecol Monogr. 84(4):599–620. [Google Scholar]
- 86.Blaauw M, Bennett KD, Christen JA. Random walk simulations of fossil proxy data. Holocene. 2010;20(4):645–649. [Google Scholar]
- 87.Post E, Forchhammer MC. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philos Trans R Soc Lond B Biol Sci. 2008;363(1501):2369–2375. doi: 10.1098/rstb.2007.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zarnetske PL, Skelly DK, Urban MC. Ecology. Biotic multipliers of climate change. Science. 2012;336(6088):1516–1518. doi: 10.1126/science.1222732. [DOI] [PubMed] [Google Scholar]
- 89.Gill JL, Williams JW, Jackson ST, Donnelly JP, Schellinger GC. Climatic and megaherbivory controls on late-glacial vegetation dynamics: A new, high-resolution, multi-proxy record from Silver Lake, Ohio. Quat Sci Rev. 2012;34:66–80. [Google Scholar]
- 90.Levins R, Lewontin R. Dialectics and reductionism in ecology. Synthese. 1980;43(1):47–78. [Google Scholar]
- 91.Wimsatt WC. Re-engineering Philosophy for Limited Beings. Harvard Univ Press; Cambridge, MA: 2007. [Google Scholar]
- 92.Williams JW, Blois JL, Shuman BN. Extrinsic and intrinsic forcing of abrupt ecological change: Case studies from the late Quaternary. J Ecol. 2011;99(3):664–677. [Google Scholar]
- 93.Blois JL, et al. A framework for evaluating the influence of climate, dispersal limitation, and biotic interactions using fossil pollen associations across the late Quaternary. Ecography. 37(11):1095–1108. [Google Scholar]
- 94.Mergeay J, De Meester L, Eggermont H, Verschuren D. Priority effects and species sorting in a long paleoecological record of repeated community assembly through time. Ecology. 2011;92(12):2267–2275. doi: 10.1890/10-1645.1. [DOI] [PubMed] [Google Scholar]
- 95.Jackson ST, Sax DF. Balancing biodiversity in a changing environment: Extinction debt, immigration credit and species turnover. Trends Ecol Evol. 2010;25(3):153–160. doi: 10.1016/j.tree.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 96.Kraft NJB, Cornwell WK, Webb CO, Ackerly DD. Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am Nat. 2007;170(2):271–283. doi: 10.1086/519400. [DOI] [PubMed] [Google Scholar]
- 97.Weiher E, et al. Advances, challenges and a developing synthesis of ecological community assembly theory. Philos Trans R Soc Lond B Biol Sci. 2011;366(1576):2403–2413. doi: 10.1098/rstb.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Foote M. The evolution of morphological diversity. Annu Rev Ecol Syst. 1997;28:129–152. [Google Scholar]
- 99.Eronen JT, et al. Ecometrics: The traits that bind the past and present together. Integr Zool. 2010;5(2):88–101. doi: 10.1111/j.1749-4877.2010.00192.x. [DOI] [PubMed] [Google Scholar]
- 100.Lawing AM, Head JJ, Polly PD. In: Palaeontology in Ecology and Conservation. Louys J, editor. Springer; Berlin: 2012. pp. 117–146. [Google Scholar]
- 101.Adler PB, Lauenroth WK. The power of time: Spatiotemporal scaling of species diversity. Ecol Lett. 2003;6(8):749–756. [Google Scholar]
- 102.Brown J, Ernest S, Parody J, Haskell J. Regulation of diversity: Maintenance of species richness in changing environments. Oecologia. 2001;126(3):321–332. doi: 10.1007/s004420000536. [DOI] [PubMed] [Google Scholar]
- 103.White EP, Ernest SKM, Adler PB, Hurlbert AH, Lyons SK. Integrating spatial and temporal approaches to understanding species richness. Philos Trans R Soc Lond B Biol Sci. 2010;365(1558):3633–3643. doi: 10.1098/rstb.2010.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blois JL, Williams JW, Fitzpatrick MC, Jackson ST, Ferrier S. Space can substitute for time in predicting climate-change effects on biodiversity. Proc Natl Acad Sci USA. 2013;110(23):9374–9379. doi: 10.1073/pnas.1220228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adler PB, et al. Evidence for a general species-time-area relationship. Ecology. 2005;86(8):2032–2039. [Google Scholar]
- 106.Raia P, Carotenuto F, Meloro C, Piras P, Barbera C. Species accumulation over space and time in European Plio-Holocene mammals. Evol Ecol. 2011;25(1):171–188. [Google Scholar]
- 107.Zalasiewicz J, Williams M, Haywood A, Ellis M. The Anthropocene: A new epoch of geological time? Philos Transact A Math Phys Eng Sci. 2011;369(1938):835–841. doi: 10.1098/rsta.2010.0339. [DOI] [PubMed] [Google Scholar]
- 108.Davis EB. Mammalian beta diversity in the Great Basin, western USA: Palaeontological data suggest deep origin of modern macroecological structure. Glob Ecol Biogeogr. 2005;14(5):479–490. [Google Scholar]
- 109.Stegner MA, Holmes M. Using palaeontological data to assess mammalian community structure: Potential aid in conservation planning. Palaeogeogr Palaeocl. 2013;372:138–146. [Google Scholar]
- 110.Atwater AL, Davis EB. Topographic and climate change differentially drive Pliocene and Pleistocene mammalian beta diversity of the Great Basin and Great Plains provinces of North America. Evol Ecol Res. 2011;13(8):833–850. [Google Scholar]
- 111.Wing SL, et al. Transient floral change and rapid global warming at the Paleocene-Eocene boundary. Science. 2005;310(5750):993–996. doi: 10.1126/science.1116913. [DOI] [PubMed] [Google Scholar]
- 112.Jackson JBC, Erwin DH. What can we learn about ecology and evolution from the fossil record? Trends Ecol Evol. 2006;21(6):322–328. doi: 10.1016/j.tree.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 113.Uhen MD, et al. From card catalogs to computers: Databases in vertebrate paleontology. J Vert Paleont. 2013;33(1):13–28. [Google Scholar]
- 114.Brewer S, Jackson ST, Williams JW. Paleoecoinformatics: Applying geohistorical data to ecological questions. Trends Ecol Evol. 2012;27(2):104–112. doi: 10.1016/j.tree.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 115.Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW. The merging of community ecology and phylogenetic biology. Ecol Lett. 2009;12(7):693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 116.Johnson MTJ, Stinchcombe JR. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol Evol. 2007;22(5):250–257. doi: 10.1016/j.tree.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 117.Losos JB. Phylogenetic perspectives on community ecology. Ecology. 1996;77(5):1344. [Google Scholar]
- 118.Lessard J-P, et al. Strong influence of regional species pools on continent-wide structuring of local communities. Proc Biol Sci. 2012;279(1727):266–274. doi: 10.1098/rspb.2011.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 120.Stralberg D, et al. Re-shuffling of species with climate disruption: A no-analog future for California birds? PLoS ONE. 2009;4(9):e6825. doi: 10.1371/journal.pone.0006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Svenning J-C, Fløjgaard C, Baselga A. Climate, history and neutrality as drivers of mammal beta diversity in Europe: Insights from multiscale deconstruction. J Anim Ecol. 2011;80(2):393–402. doi: 10.1111/j.1365-2656.2010.01771.x. [DOI] [PubMed] [Google Scholar]
- 122.Brown JH, Whitham TG, Morgan Ernest SK, Gehring CA. Complex species interactions and the dynamics of ecological systems: Long-term experiments. Science. 2001;293(5530):643–650. doi: 10.1126/science.293.5530.643. [DOI] [PubMed] [Google Scholar]
- 123.Hadly EA, et al. Genetic response to climatic change: Insights from ancient DNA and phylochronology. PLoS Biol. 2004;2(10):e290. doi: 10.1371/journal.pbio.0020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Magyari EK, et al. Population dynamics and genetic changes of Picea abies in the South Carpathians revealed by pollen and ancient DNA analyses. BMC Evol Biol. 2011;11:66. doi: 10.1186/1471-2148-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cowles HC. The physiographic ecology of Chicago and vicinity. Bot Gaz. 1901;31(2):73–108, 145–182. [Google Scholar]
- 126.Hobbs RJ, Higgs ES, Hall C. Novel Ecosystems. John Wiley & Sons; Hoboken, NJ: 2013. [Google Scholar]