Significance
The capsaicin receptor transient receptor potential cation channel vanilloid 1 (TRPV1) and anoctamin 1 (ANO1) work as receptors activated by noxious stimuli in sensory nerve endings. It is believed that activation of the two channels causes cation influx and anion efflux, respectively, both of which lead to depolarization. We show that ANO1 is activated by calcium ions entering neurons through TRPV1 activation based on their physical binding on the cell membrane. Indeed, both capsaicin-activated inward currents in sensory neurons and capsaicin-induced pain-related behaviors in mice were inhibited significantly by ANO1 blockade. To our knowledge, this is the first evidence for a mechanism by which interaction between TRPV1 and ANO1 functions as a pain-enhancing mechanism.
Keywords: pain perception, primary sensory neuron, anoctamin 1, TRPV1
Abstract
The capsaicin receptor transient receptor potential cation channel vanilloid 1 (TRPV1) is activated by various noxious stimuli, and the stimuli are converted into electrical signals in primary sensory neurons. It is believed that cation influx through TRPV1 causes depolarization, leading to the activation of voltage-gated sodium channels, followed by the generation of action potential. Here we report that the capsaicin-evoked action potential could be induced by two components: a cation influx-mediated depolarization caused by TRPV1 activation and a subsequent anion efflux-mediated depolarization via activation of anoctamin 1 (ANO1), a calcium-activated chloride channel, resulting from the entry of calcium through TRPV1. The interaction between TRPV1 and ANO1 is based on their physical binding. Capsaicin activated the chloride currents in an extracellular calcium-dependent manner in HEK293T cells expressing TRPV1 and ANO1. Similarly, in mouse dorsal root ganglion neurons, capsaicin-activated inward currents were inhibited significantly by a specific ANO1 antagonist, T16Ainh-A01 (A01), in the presence of a high concentration of EGTA but not in the presence of BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid]. The generation of a capsaicin-evoked action potential also was inhibited by A01. Furthermore, pain-related behaviors in mice treated with capsaicin, but not with αβ-methylene ATP, were reduced significantly by the concomitant administration of A01. These results indicate that TRPV1–ANO1 interaction is a significant pain-enhancing mechanism in the peripheral nervous system.
When calcium ions enter cells through ion channels or transporters, they can initiate a variety of reactions, either as free calcium ions or after their binding by specific calcium-binding proteins (1). One such important reaction is the activation of calcium-binding proteins by calcium nanodomains of the calcium pathways (2). In this regard, some transient receptor potential (TRP) channels have high calcium permeability (3), and it is likely that they activate calcium-binding proteins in the cytosol or plasma membrane. Indeed, TRP vanilloid 4 (TRPV4), a thermosensitive TRP channel (reportedly an osmo- or mechano-sensor) (4–8) and anoctamin 1 (ANO1; a calcium-activated chloride channel) (9–11) function as a complex in epithelial cells of the choroid plexus (12). Upon entering choroid plexus epithelial cells, calcium activates ANO1, leading to chloride efflux. Although the interaction between TRP channels and anoctamins could work in a variety of ways (12), the direction of chloride movement is determined simply by the relationship between chloride equilibrium potentials and membrane potentials, depending on the intracellular chloride concentrations (13). This concept prompted us to pursue other interactions between TRP channels and anoctamins. We focused on primary sensory neurons because activation of chloride channels in sensory neurons causes chloride efflux and depolarization, as the result of the high intracellular chloride concentrations (14, 15).
TRPV1 senses various noxious stimuli in primary sensory neurons, leading to pain perception through the generation of action potentials upon the activation of voltage-gated sodium channels. Mammalian TRPV1 is activated by noxious heat, acid, and many chemical compounds including capsaicin (16–18). The calcium permeability of TRPV1 is more than 10 times that of sodium, suggesting that TRPV1 could activate anoctamins readily, leading to further depolarization. ANO1 plays an important role in nociception in primary sensory neurons (19), and bradykinin-induced and neuropathic pain-related behaviors were reduced in ANO1 conditional-knockout mice (20, 21), suggesting that interaction between the two proteins could strongly enhance nociceptive signals.
Results
Coexpression of TRPV1 and ANO1 in Dorsal Root Ganglions and Their Interaction in HEK293T Cells.
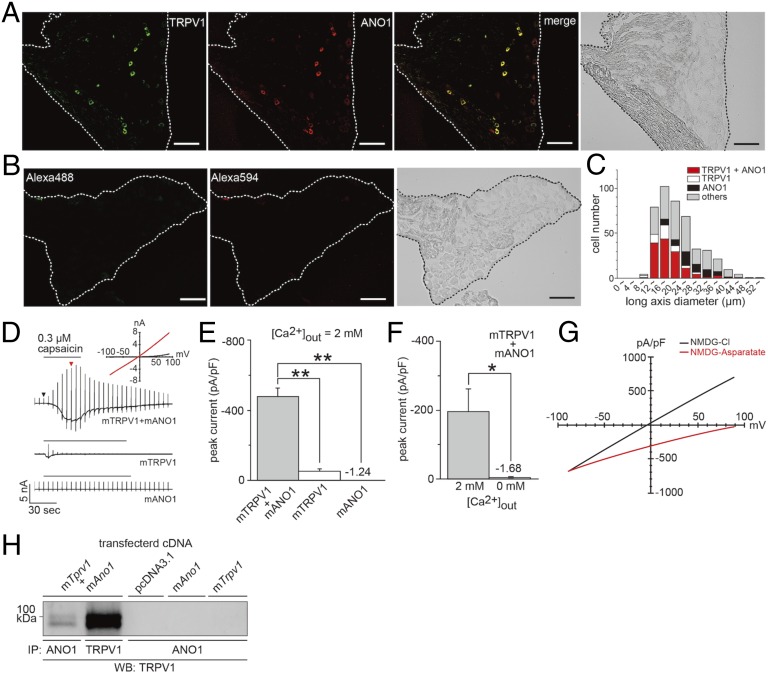
We first examined the coexpression of TRPV1 and ANO1 in mouse dorsal root ganglion (DRG) neurons and found that they indeed were coexpressed as previously reported (19). Specifically, 78.2% (136/174 TRPV1-expressing neurons) of anti-TRPV1 antibody-immunoreactive neurons also reacted with an anti-ANO1 antibody (Fig. 1 A–C). Next, we investigated the interaction between TRPV1 and ANO1. We used a whole-cell patch-clamp technique in HEK293T cells expressing mouse TRPV1 (mTRPV1) and mouse ANO1 (mANO1) or cells expressing either mTRPV1 or mANO1 alone. In these experiments, we used an N-methyl-d-glucamine chloride (NMDG-Cl) bath and pipette solutions that allowed us to observe chloride currents (Fig. 1 D–G). Capsaicin-activated chloride currents with a linear current–voltage relationship were significantly greater in cells expressing mTRPV1 and mANO1 than in cells expressing either mTRPV1 or mANO1 alone, depending on the extracellular calcium concentrations (Fig. 1 D–F). These results suggested that mANO1 is activated by calcium influx through mTRPV1 activation. The finding that there was no outward current activation with an extracellular NMDG-aspartate and an intracellular NMDG-Cl solution (Fig. 1G, red) further indicated that chloride was the charge carrier of the observed currents. Furthermore, the physical interaction of mTRPV1 with mANO1 was detected by immunoprecipitation of cells expressing mTRPV1 and mANO1 but not cells expressing either mTRPV1 or mANO1 alone (Fig. 1H). These results indicated that the functional interaction is based on physical binding.
Fig. 1.
Coexpression of TRPV1 and ANO1 in the DRG and TRPV1–ANO1 interaction in HEK293T cells. (A and B) Immunofluorescent images with an anti-TRPV1 antibody (green) and an anti-ANO1 antibody (red) in 10-µm DRG slices from mice. Images at the far right in A and B are bright-field images. (Scale bars, 50 µm.) (C) Cell size-dependent distribution of mouse DRG neurons expressing both TRPV1 and ANO1, TRPV1 alone, or ANO1 alone (n = 444 cells). (D) Representative traces of whole-cell chloride currents activated by capsaicin in HEK293T cells expressing mTRPV1 and mANO1 (Top), cells expressing mTRPV1 alone (Middle), and mock-transfected cells (Bottom). Holding potential was −60 mV, and ramp pulses were from −100 to +100 mV. (Inset) Current–voltage (I–V) curves at the time indicated by black and red arrows in D, Top. (E and F) Comparison of the peak currents in cells expressing mTRPV1 and mANO1, cells expressing mTRPV1 alone, and cells expressing mANO1 alone (E; n = 5 cells) and the effect of extracellular calcium (F; n = 5 cells). Data are shown as the mean ± SEM of five peaks. **P < 0.01; *P < 0.05. (G) I–V relationship of capsaicin-activated currents with NMDG-Cl (black; n = 4 cells) and NMDG-aspartate (red; n = 3 cells) extracellular solutions. (H) Physical interaction between TRPV1 and ANO1 in HEK293T cells. Samples were immunoprecipitated (IP) with an anti-ANO1 antibody and blotted with an anti-TRPV1 antibody.
TRP subfamily A, member 1 (TRPA1), another nociceptive TRP channel with high calcium permeability, and TRPV1 are coexpressed in some DRG neurons, and they reportedly interact with each other as a functional complex (22, 23). Therefore, we examined TRPA1–ANO1 interaction using a whole-cell patch-clamp technique in HEK293T cells expressing mTRPA1 and mANO1. We found their functional interaction depended on the extracellular calcium concentrations, observations similar to those made with mTRPV1 and mANO1 (Fig. S1 A–C). Although some reports have shown an interaction between TRPV1 and TRPA1 (22, 23), capsaicin-activated chloride currents did not differ between cells expressing mTRPV1 and mANO1 and cells expressing mTRPV1, mTRPA1, and mANO1, suggesting that interaction between mTRPV1 and mANO1 is not affected by the TRPV1–TRPA1 interaction (Fig. S1D).
TRPV1–ANO1 Interaction in DRG Neurons.
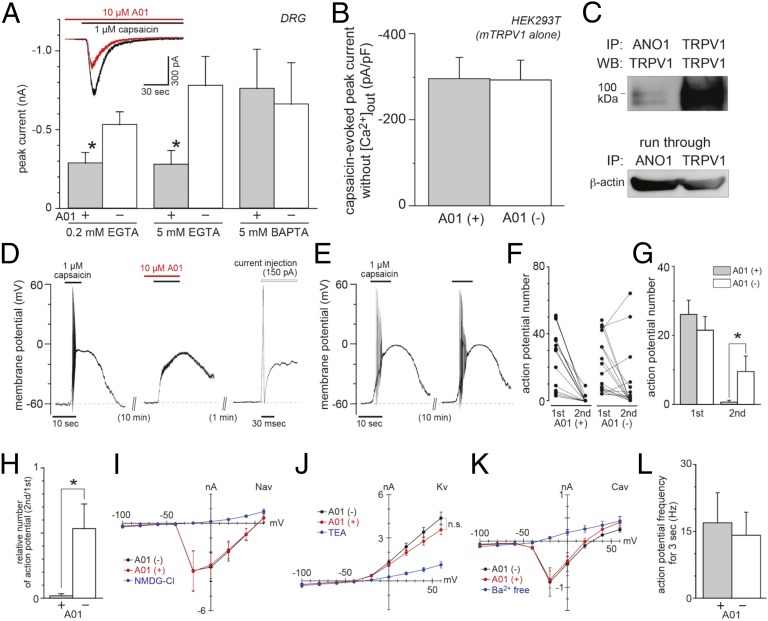
Next, we investigated the proteins’ interaction in small (<24 µm in diameter) DRG neurons by observing capsaicin-activated currents using an NaCl bath (containing 2 mM calcium) and an NMDG-Cl pipette solution in which sodium inward, calcium inward, and chloride outward movements were observed as inward currents. Thus, capsaicin-activated inward currents could include both TRPV1-mediated cation currents and ANO1-mediated anion currents. Approximately 80% of TRPV1-positive neurons expressed ANO1 (Fig. 1), indicating that the TRPV1–ANO1 interaction was strongly involved in the reactions in native neurons. As expected, capsaicin-activated currents were reduced by an ANO1 inhibitor, T16Ainh-A01 (A01), in small DRG neurons with 0.2 and 5 mM EGTA (Fig. 2A). However, current reductions were not observed using a faster chelator, 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA; 5 mM), suggesting that the TRPV1–ANO1 interaction occurred in a local calcium nanodomain (24). Furthermore, the observation that A01 did not affect the inward currents without extracellular calcium in HEK293T cells expressing mTRPV1 alone (Fig. 2B) argued against off-target effects of A01 in TRPV1 activation by capsaicin. The direct interaction between TRPV1 and ANO1 in DRGs (Fig. 2C) supported our idea that calcium ions entering the cells through TRPV1 immediately activated ANO1, consistent with the data obtained with calcium chelators. We also analyzed A01 effects in time courses of the normalized currents, but they were not changed significantly in DRG neurons regardless of chelator concentrations (Fig. S2). These results suggested that A01 affected only TRPV1-mediated events without modulating its kinetics in DRG neurons, although the capsaicin-activated current was prolonged in HEK293T cells expressing TRPV1 and ANO1 (Fig. 1D).
Fig. 2.
TRPV1–ANO1 interaction in DRG neurons. (A) Capsaicin-activated currents at −60 mV (including 0.2 mM EGTA, 5 mM EGTA, or 5 mM BAPTA) without (n = 24, 13, and 10 cells, respectively) or with (n = 20, 11, and 9 cells, respectively) an ANO1 blocker, T16Ainh-A01 (A01), in the presence of extracellular calcium in DRG neurons. (B) Capsaicin-activated currents in HEK293T cells expressing mTRPV1 alone in the absence of extracellular calcium (n = 5 cells). (C) Physical interaction between TRPV1 and ANO1 in DRG. (D) A representative trace of the capsaicin-evoked action potential of a DRG neuron. A depolarizing current was injected to check the action potential generation. (E) A representative trace of the capsaicin-evoked action potentials without A01. (F and G) Comparison of the action potential numbers in each cell before and after A01 application (n = 18 cells each). (H) Comparison of the relative number of action potentials (n = 18 cells each). (I –K) Current–voltage relationships of the voltage-gated channel currents with (red) or without (black) A01. Blue lines show the loss of Nav (I), Kv (J), and Cav (K) channel currents (n = 4–9 cells). The holding potential was −60 mV, and step pulses were applied. (L) Averaged frequency of action potentials for 3 s evoked by depolarizing current injection (+150 pA) with or without A01 (n = 8 cells each). Basal voltage was kept at −60 mV. Data are shown as mean ± SEM; *P < 0.05. n.s., not significant.
Thus, TRPV1 activation appeared to have dual effects on membrane potentials: a cation influx-evoked depolarization and a chloride efflux-evoked depolarization. Next, to investigate whether TRPV1–ANO1 interaction is involved in generating action potential, we observed capsaicin-evoked action potentials in isolated small DRG neurons with or without A01. The current-clamp recordings were performed under conditions in which the calculated equilibrium chloride potential was approximately −20 mV, which is within the physiological range (15). ATP (4 mM) was included in the potassium-base pipette solution to maintain the activation of the sodium–potassium pump and TRPV1 (25). The action potential generated by the second capsaicin application disappeared with 10 µM A01 in 16 of 18 neurons that responded to the first capsaicin application (Fig. 2 D, F, and G). We confirmed that the neurons still had the ability to show action potentials after the second capsaicin application with a depolarizing current injection (Fig. 2D, Far Right). In contrast, action potential generation was observed in 10 of 18 DRG neurons in the second capsaicin application without A01 (Fig. 2 E and F), and the difference in the action potential numbers was statistically significant (Fig. 2G). Moreover, the relative number of action potentials was reduced drastically in the presence of A01 (Fig. 2H). We also checked whether A01 affected the voltage-gated channels. The results showed that A01 did not change voltage-gated sodium (Nav), potassium (Kv), or calcium (Cav, with barium as a charge carrier) channel currents, but Nav, Kv, and Cav channel currents were reduced drastically in the NMDG-Cl bath solution, in the bath solution with tetraethylammonium (TEA), and in the barium-free bath solution, respectively (Fig. 2 I–K). Furthermore, the action potentials evoked by depolarizing current injection were not affected by A01 (Fig. 2L). These results indicated that TRPV1–ANO1 interaction could affect neural excitation in small DRG neurons.
Effects of an ANO1 Blocker in Capsaicin-Induced Pain-Related Behaviors.
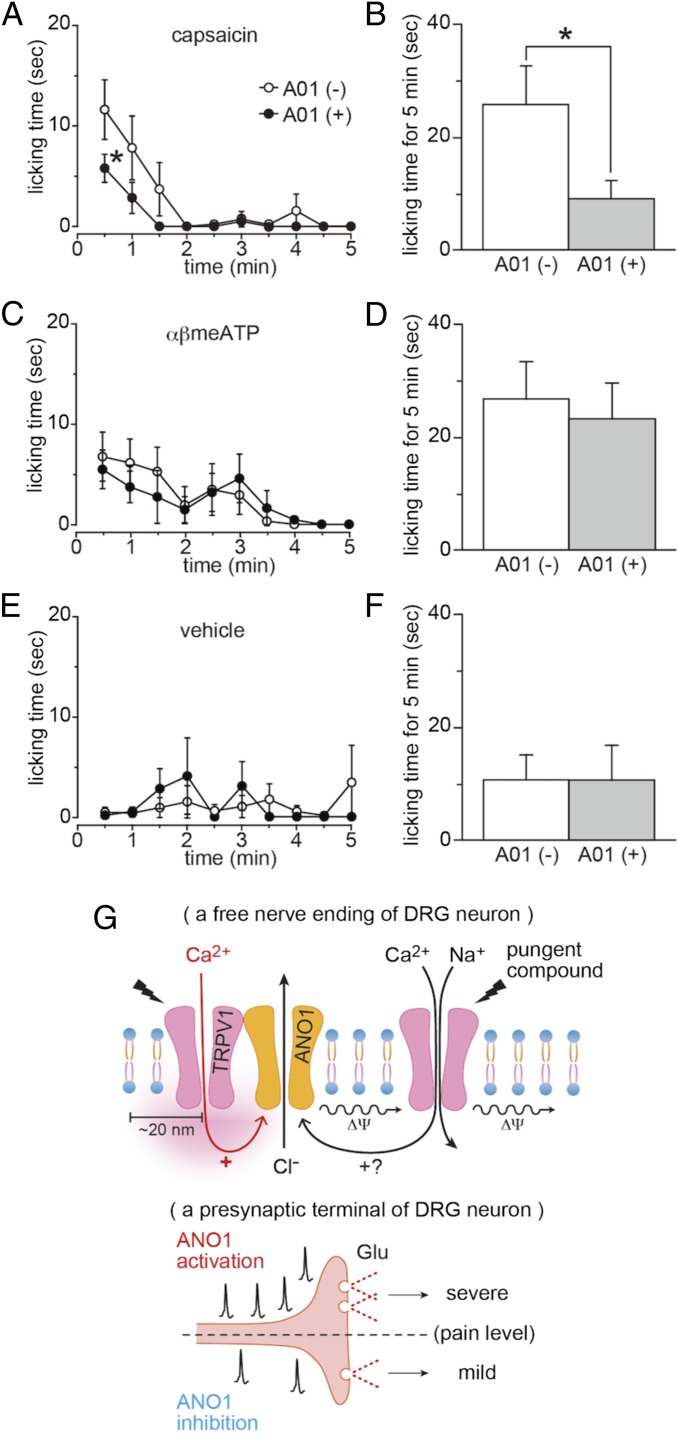
The data presented thus far suggested that inhibition of ANO1 might reduce capsaicin-induced pain-related behaviors. Therefore, we investigated the licking behaviors of mice whose hind paws were injected with 10 µL of capsaicin (300 µM) with or without A01 (300 µM). As expected, capsaicin-induced pain-related behaviors for the first 30 s and for a total of 5 min were reduced significantly by concomitant administration of A01, whereas licking behaviors induced by αβ-methylene ATP were not affected by A01, and A01 itself did not cause any pain-related behavior (Fig. 3 A–F). These data indicated that the effects of A01 were specific for the capsaicin-induced pain-related behaviors, supporting a specific interaction between TRPV1 and ANO1.
Fig. 3.
Reduction of capsaicin-evoked pain-related behaviors by A01 and a schematic model for TRPV1–ANO1 interaction in pain sensation. (A and B) Time courses of the capsaicin-induced pain-related licking behaviors with (n = 7 mice) or without (n = 8 mice) A01 every 30 s (A) and the summation for 5 min (B). Both capsaicin and A01 concentrations were 300 µM (10 µL). Data are shown as mean ± SEM; *P < 0.05. (C and D) Time courses of αβ-methylene ATP (αβmeATP)-induced pain-related licking behaviors with (n = 7 mice) or without (n = 8 mice) A01 every 30 s (C) and the summation over 5 min (D). αβmeATP and A01 concentrations were 10 mM and 300 µM, respectively (10 µL). Data are shown as mean ± SEM. (E and F) Time courses of licking behaviors with (n = 7 mice) or without (n = 7 mice) A01 every 30 s (E) and the summation over 5 min (F). A01 concentration was 300 µM (10 µL). Data are shown as mean ± SEM. (G) A schematic model based on the data obtained showing TRPV1–ANO1 interaction in pain sensation. Capsaicin-evoked depolarization (Δψ) contains two components: a cation influx-mediated depolarization through TRPV1 activation and a chloride efflux-mediated depolarization through ANO1 activation caused by calcium entering the cells through TRPV1. Summation could lead to the generation of an enhanced action potential (Upper), followed by increased glutamate release (Lower). Inhibition of ANO1 or the TRPV1-ANO1 interaction could reduce the glutamate release (Lower).
TRPV1–ANO1 Interaction in Presynaptic Terminals.
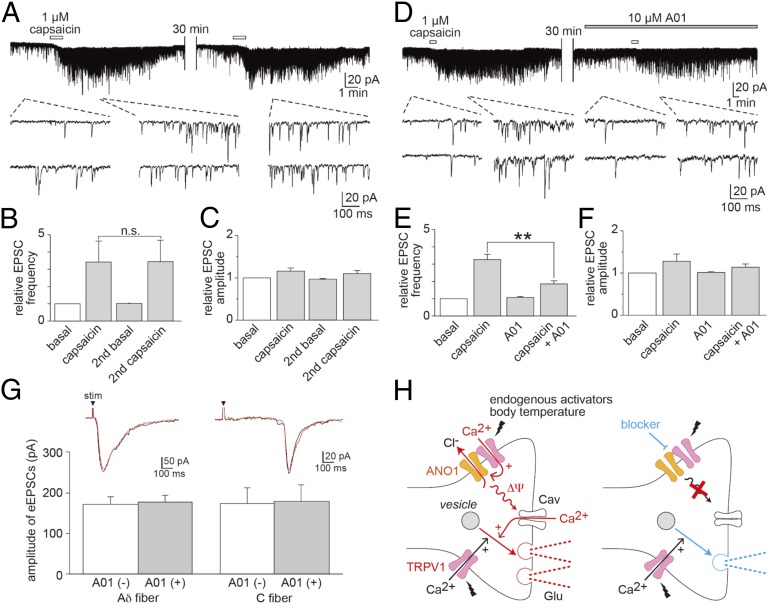
Proteins produced in DRG cell bodies are transported both to sensory nerve endings and to presynaptic termini of primary afferent neurons in the spinal cord (17), suggesting that TRPV1–ANO1 interaction in the presynaptic termini could be involved in synaptic transmission. Therefore, we compared the capsaicin-induced facilitation of spontaneous excitatory postsynaptic currents (sEPSCs) in substantia gelatinosa (SG) neurons of the superficial spinal dorsal horn of mice. Six of 19 neurons (31.5%) did not respond to capsaicin administration to the spinal cord preparation (Fig. S3), a ratio similar to a previous report (26). Among the responsive 13 neurons, we compared the facilitation of sEPSCs upon the second capsaicin application after a long interval that should have minimized the desensitization through calmodulin binding. Facilitation of sEPSC frequencies was observed in the first two capsaicin applications in the absence of A01 (Fig. 4 A–C). Such facilitation was reduced significantly, by approximately one-half, in the presence of A01, although the amplitudes were unchanged (Fig. 4 D–F). These results suggested that A01 inhibits glutamate release through the interaction between TRPV1 and ANO1 without postsynaptic effects.
Fig. 4.
The effects of A01 on sEPSCs and eEPSCs and a schematic model for TRPV1-ANO1 interaction in the spinal cord of mice. (A) A representative trace (Upper) with magnified traces (Lower) of sEPSCs obtained in an SG neuron without A01. The holding potential was −70 mV. (B and C) Comparison of the frequencies (B) and amplitudes (C) of sEPSCs before and after the first and second capsaicin applications (n = 8 cells). (D) A representative trace (Upper) with magnified traces (Lower) of sEPSCs with A01 (10 µM). (E and F) Comparison of the frequencies (E) and amplitudes (F) of sEPSCs before and after the first and second capsaicin applications (n = 5 cells). Data are shown as mean ± SEM; **P < 0.01; n.s., not significant. (G, Upper) Representative root eEPSCs in the SG neurons with monosynaptic Aδ- (Left) or C- (Right) fiber input in the absence (black) and presence (red) of A01 (10 µM). stim, electrical stimulation. (G, Lower) Comparison of the A01 effects with and without A01 in Aδ- (Left; n = 6 cells) and C-fiber (Right; n = 7 cells). (H) A schematic model showing that glutamate releases through the TRPV1–ANO1 interaction in a presynaptic terminus. (Left) Certain endogenous agonists and elevated body temperature during inflammation could activate TRPV1, and TRPV1–ANO1 interaction could facilitate depolarization, leading to further activation of the Cav channels, followed by increased glutamate release. (Right) Inhibition of the TRPV1–ANO1 interaction could lead to reduced glutamate release.
To prove further that the effects of ANO1 on sEPSCs are specific to TRPV1-mediated events without the involvement of other calcium-permeable channels, we examined the effects of A01 on the root evoked EPSCs (eEPSCs) by electrical stimulation. We did not observe any effect of A01 on the eEPSCs in the SG neurons with monosynaptic Aδ- or C-fiber input (Fig. 4G), suggesting specific ANO1 involvement upon TRPV1 activation.
Discussion
We found that the capsaicin-evoked depolarization was composed of two components: a cation influx-mediated depolarization caused by TRPV1 activation and a subsequent anion efflux-mediated depolarization via ANO1 activation caused by the entry of calcium through TRPV1. The physical binding of TRPV1 to ANO1 could facilitate their interaction, and calcium within the nanodomains of the TRPV1–ANO1 complex could participate in the interaction, as is consistent with the observation that EGTA is totally ineffective on free calcium within 20 nm of a channel (24). The generation of action potential through TRPV1 activation was inhibited by an ANO1 blocker, A01 (Fig. 2 D–H). The reduction in action potential generation also was observed in the absence of A01, although less frequently, partly because TRPV1 could be desensitized to some extent even in the presence of ATP and because TRPV1 activation induces the inactivation of Nav channels (27). Nevertheless, these results indicate that the TRPV1–ANO1 interaction could induce the generation of action potential in DRG neurons. Moreover, the facilitated action potentials could lead to enhanced glutamate release at the synapse in the spinal cord (Fig. 3G), followed by more pain-related behaviors (Fig. 3 A and B). In the presynaptic terminals, TRPV1 is not exposed to capsaicin or high temperatures but could be activated by reported endogenous activators (28) or body temperature because temperature thresholds for TRPV1 activation reportedly are reduced to the body-temperature range under inflammatory conditions (29). TRPV1–ANO1 interaction in the spinal cord could enhance synaptic transmission under conditions in which it could be inhibited by TRPV1–ANO1 interaction blockade (Fig. 4H). Moreover, A01 effects were not observed in root-evoked EPSCs (Fig. 4G) although intracellular calcium ions should be increased locally in the presynaptic terminals. Thus, TRPV1–ANO1 interaction should occur with local calcium increases within nanodomains, although it is possible that global calcium increases contribute to ANO1 activation to some extent.
TRPV1 and TRPA1 have high calcium permeability compared with other channels involved in nociception, e.g., P2X receptors (30). Influxes of calcium and sodium upon activation of TRPV1 cause depolarization, leading to the generation of the action potential. Furthermore, increased intracellular calcium leads to the release of several peptides, including CGRP and substance P, a phenomenon known as “neurogenic inflammation” (31). Thus, we propose an additional role for TRPV1’s high calcium permeability: That the activation of ANO1 bound to TRPV1 produces further depolarization through chloride efflux.
Our results indicate that a significant portion of capsaicin-evoked events involve TRPV1–ANO1 interaction. Many TRPV1 antagonists have been used to reduce pain in clinical trials. However, most have not shown efficacy, and patients have noted hyperthermia and abnormal thermal sensations as side effects (32, 33). Therefore, inhibiting the TRPV1–ANO1 interaction could be an alternative way to treat sensations of pain. In acute inflammatory pain, TRPV1 is sensitized by protein kinase C downstream from Gq protein-coupled receptors, including an ATP receptor, thereby reducing the temperature threshold for TRPV1 activation (29, 34). In neuropathic pain models, TRPV1 function and expression reportedly are increased (35). Therefore, it is expected that ANO1 activation caused by TRPV1 activity also should be enhanced under those conditions. Furthermore, it has been reported recently that ANO1 is involved in neuropathic pain in mice (21). All these facts clearly suggest that the inhibition of TRPV1–ANO1 interaction would be an intriguing way to develop novel analgesic agents.
Materials and Methods
Animals.
All mouse experiments were conducted with 6- to 8-wk-old C57BL/6NCr mice. All animal experimental procedures were performed according to the National Institute for Physiological Sciences guidelines.
Whole-Cell Voltage-Clamp Recordings in HEK293T Cells.
HEK293T cells were cultured in DMEM (high glucose) with l-glutamine and phenol red (Wako) containing 10% (vol/vol) FBS (lot no. S06537S1560; Biowest), penicillin/streptomycin (1:200; Life Technologies), and GlutaMAX (1:100; Life Technologies) at 37 °C in humidified air containing 5% CO2. The cells were transfected with cDNA carrying mouse Trpv1, mouse Trpa1, mouse Ano1 (a generous gift from U. Oh, Seoul National University, Seoul, Korea), or pcDNA3.1 using Lipofectamine (Invitrogen). The cells were used 24–36 h after transfection. The bath solution contained 140 mM NMDG-Cl or NMDG-aspartate, 1 mM MgCl2, 2 mM CaCl2, or 5 mM EGTA, 10 mM glucose, and 10 mM Hepes, pH 7.40. The pipette solution contained 140 mM NMDG-Cl or KCl, 1 mM MgCl2, 5 mM BAPTA, and 10 mM Hepes, pH 7.30. The free calcium concentration of the pipette solution was maintained at 100 nM calculated with the MAXC program (Stanford University). The holding potential was −60 mV, and ramp pulses from −100 to +100 mV were applied for 300 ms every 5 s. Currents were recorded using an Axopatch 200B amplifier (Molecular Devices), filtered at 5 kHz with a low-pass filter, and digitized with Digidata 1440A (Axon Instruments). pCLAMP 10 (Axon Instruments) data acquisition software, was used.
Whole-Cell Voltage-Clamp Recordings in DRG Neurons.
Data were collected from small (<24 µm) DRG neurons using the setup described above for whole-cell voltage-clamp recordings of HEK293T cells. The basic NaCl bath solution contained 130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM Hepes, pH 7.40. The pipette solution (pH 7.30) contained 140 mM NMDG-Cl, 1 mM MgCl2, 10 mM Hepes with 0.2 mM EGTA, 5 mM EGTA, or 5 mM BAPTA. When we recorded currents of Kv and Cav channels, we changed the composition of the bath solution from NaCl to NMDG-Cl. We replaced KCl with CsCl for the recordings of Nav channel currents and with NMDG-Cl for recordings of Cav channel currents. EGTA (5 mM) was added to all bath solutions for the recordings of Nav, Kv, and Cav channel currents. We used BaCl2 (the free concentration was 2 mM calculated using the MAXC program) instead of CaCl2 for recordings of Cav channel currents. The basic KCl pipette solution contained 140 mM KCl; 1 mM MgCl2; 5 mM EGTA or BAPTA or 0.2 mM EGTA; and 10 mM Hepes, pH 7.30. We changed the composition of the pipette solution from KCl to CsCl or NMDG-Cl for the recordings of Nav or Cav channel currents, respectively. BAPTA (5 mM) was added to the pipette solutions for the recordings of Cav channel currents, and 0.2 mM EGTA was added to the pipette solutions for the recordings of capsaicin-activated currents in DRG neurons. The free calcium concentration of the pipette solution was maintained at 100 nM except for the recordings of voltage-gated channel currents. The holding potential was −60 mV, and step pulses from −100 to +100 mV (∆20 mV) were applied for 100–500 ms every 3 s.
Whole-Cell Current-Clamp Recordings of DRG Neurons.
Whole-cell current-clamp recordings of DRG neurons were collected in the setup used for whole-cell voltage-clamp recordings. The bath solution contained 130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM Hepes, pH 7.40, with NaOH. The pipette solution contained 67 mM KCl, 65 mM K-gluconate, 1 mM MgCl2, 5 mM EGTA, 4 mM ATP-Mg, 1 mM GTP-Na2, and 10 mM Hepes, pH 7.30, with KOH. Free calcium was maintained at 100 nM. Basal voltages were maintained around −60 mV by current injection.
Whole-Cell Patch-Clamp Recordings from Dorsal Horn Neurons in the Spinal Cord.
The SG was easily discernible with transmitted illumination as a relatively translucent band across the dorsal horn in the transverse slice preparations. Blind whole-cell voltage-clamp recordings were made from SG neurons, as previously described (36). The pipette solution contained 135 mM K-gluconate, 5 mM KCl, 0.5 mM CaCl2, 2 mM MgCl2, 5 mM EGTA, 5 mM Hepes, and 5 mM ATP-Mg, pH 7.20, with KOH. The tip resistance of the patch pipettes was 6–12 MΩ. Series resistance was assessed from current in response to a 5-mV hyperpolarizing step. Series resistance was monitored during the recording session, and data were rejected if its value changed by >15%. Ionic currents were monitored with a patch-clamp amplifier, Axopatch 700B (Molecular Devices). The data were digitized with an analog-to-digital converter (Digidata 1440A; Molecular Devices), stored on a personal computer using a data acquisition program (Clampex version 10; Molecular Devices), and analyzed using the software package Mini Analysis version 6.0.3 (Synaptosoft). Recordings were made in voltage-clamp mode at a holding potential of −70 mV to isolate sEPSCs. Drugs were dissolved in the Krebs solution and applied by superfusion. The dorsal root was stimulated using a suction electrode at a frequency of 0.2 Hz to elicit EPSCs. The Aδ- or C-afferent–mediated responses were distinguished on the basis of the conduction velocity of the afferent fibers and stimulus thresholds. The conduction velocity was calculated from the latency of synaptic responses and the length of the dorsal root. The Aδ- and C-fiber–evoked responses were considered monosynaptic if the latency remained constant without failure when the root was stimulated at 20 Hz for the Aδ-fiber–evoked EPSCs and when stimulated at 2 Hz for the C-fiber–evoked EPSCs (37).
Pain-Related Behavior Test.
Mice were handled gently for 1 h every 48 h before the behavior test. Mice were injected with capsaicin (300 µM) or αβ-methylene ATP with or without A01 (total 10 µL) into the top of the hind paw using a fine (30-G) needle filled with saline (Otsuka Pharmaceutical) containing 0.3% ethanol and 3% (vol/vol) dimethyl sulfoxide [capsaicin and A01 were dissolved in 0.3% ethanol and 3% (vol/vol) dimethyl sulfoxide, respectively]. Mice were wrapped gently in the measurer’s hand and kept on their backs with their snouts toward the small finger of the measurer. Mice remained very quiet during injection in this position. Their behavior was recorded using a digital camera (P6000; Nikon).
Supplementary Material
Acknowledgments
We thank Dr. K. Shibasaki (Gunma University) for technical support, Dr. M. Kido (Kyushu University) for the generous gift of the anti-TRPV1 antibody, and Dr. U. Oh (Seoul National University) for the generous gift of the Ano1 cDNA. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (Brain Environment 26111732) (to M.T.) and by The Salt Science Research Foundation (M.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421507112/-/DCSupplemental.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Chad JE, Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010;2(10):a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103(3):525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2(10):695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe H, et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277(49):47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 7.Güler AD, et al. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22(15):6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134(6):1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 11.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 12.Takayama Y, Shibasaki K, Suzuki Y, Yamanaka A, Tominaga M. Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. FASEB J. 2014;28(5):2238–2248. doi: 10.1096/fj.13-243436. [DOI] [PubMed] [Google Scholar]
- 13.Tominaga M, Takayama Y. Interaction between TRP and Ca2+-activated chloride channels. Channels (Austin) 2014;8(3):178–179. doi: 10.4161/chan.29001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonin RP & De Koninck Y (2013) Restoring ionotropic inhibition as an analgesic strategy. Neurosci Lett 557(Pt A):43–51. [DOI] [PubMed]
- 15.Mao S, et al. Molecular and functional expression of cation-chloride cotransporters in dorsal root ganglion neurons during postnatal maturation. J Neurophysiol. 2012;108(3):834–852. doi: 10.1152/jn.00970.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 17.Tominaga M, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 18.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 19.Cho H, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15(7):1015–1021. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- 20.Jin X, et al. Activation of the Cl- channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal. 2013;6(290):ra73. doi: 10.1126/scisignal.2004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee B, et al. Anoctamin 1 contributes to inflammatory and nerve-injury induced hypersensitivity. Mol Pain. 2014;10:5. doi: 10.1186/1744-8069-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akopian AN. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr Pharm Biotechnol. 2011;12(1):89–94. doi: 10.2174/138920111793937952. [DOI] [PubMed] [Google Scholar]
- 23.Weng HJ, et al. Tmem100 Is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron. 2015;85(4):833–846. doi: 10.1016/j.neuron.2014.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neher E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron. 1998;20(3):389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 25.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54(6):905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Uta D, et al. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur J Neurosci. 2010;31(11):1960–1973. doi: 10.1111/j.1460-9568.2010.07255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Oortgiesen M, Li L, Simon SA. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. J Neurophysiol. 2001;85(2):745–758. doi: 10.1152/jn.2001.85.2.745. [DOI] [PubMed] [Google Scholar]
- 28.Szolcsányi J, Sándor Z. Multisteric TRPV1 nocisensor: A target for analgesics. Trends Pharmacol Sci. 2012;33(12):646–655. doi: 10.1016/j.tips.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98(12):6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virginio C, North RA, Surprenant A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurones. J Physiol. 1998;510(Pt 1):27–35. doi: 10.1111/j.1469-7793.1998.027bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci. 2014;17(2):164–174. doi: 10.1038/nn.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: A clinical perspective. Br J Pharmacol. 2014;171(10):2474–2507. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavva NR, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136(1-2):202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277(16):13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 35.Palazzo E, et al. Transient receptor potential vanilloid type 1 and pain development. Curr Opin Pharmacol. 2012;12(1):9–17. doi: 10.1016/j.coph.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Akimoto N, et al. CCL-1 in the spinal cord contributes to neuropathic pain induced by nerve injury. Cell Death Dis. 2013;4:e679. doi: 10.1038/cddis.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakatsuka T, Park JS, Kumamoto E, Tamaki T, Yoshimura M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain. 1999;82(1):39–47. doi: 10.1016/S0304-3959(99)00037-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.