Abstract
Australopithecus fossils were regularly interpreted during the late 20th century in a framework that used living African apes, especially chimpanzees, as proxies for the immediate ancestors of the human clade. Such projection is now largely nullified by the discovery of Ardipithecus. In the context of accumulating evidence from genetics, developmental biology, anatomy, ecology, biogeography, and geology, Ardipithecus alters perspectives on how our earliest hominid ancestors—and our closest living relatives—evolved.
Keywords: human evolution, Australopithecus, hominid, Ethiopia
“...the stock whence two or more species have sprung, need in no respect be intermediate between those species.”
T. H. Huxley, 1860 (1)
Charles Darwin famously suggested that Africa was humanity’s most probable birth continent, but warned that without fossils, it was “…useless to speculate on this subject” (2). Nevertheless, Darwin and his less cautious contemporaries and intellectual descendants used humans and modern apes to triangulate ancestral anatomy and behaviors, which promulgated the erroneous metaphor of a hominid “missing link.” Even today, despite thousands of available fossils, this deeply embedded metaphor reinforces the misconceptions that extant apes—particularly chimpanzees—can be viewed as “living missing links,” or that that modern African apes combined can be used to represent the past “as time machines” (3).
The notion that modern great apes are little changed from the last common ancestors we shared with them promoted the assumption that hominid fossils anatomically intermediate between living apes and ourselves would eventually be found. Now, however, long sought and recently discovered African fossils provide escape from such persistent but inaccurate projection. These paleontological discoveries do not yet include the common ancestor we shared with chimpanzees (the CLCA). However, they substantially reveal the early evolution of the hominid clade (the term “hominid” denoting all species on the human side of the human/chimpanzee phylogenetic split). These fossils have begun to rectify the mistaken notion that contemporary apes, in particular common chimpanzees, can serve as adequate representations of the ancestral past.
Background
Darwin's human evolution scenario attempted to explain hominid tool use, bipedality, enlarged brains, and reduced canine teeth (2). It easily fit the fossil record of his day, when only a few Neanderthals were known. Homo erectus was found in the 1890s, and Australopithecus in the 1920s. Both were rejected as hominids by eminent authorities, but two grades of human evolution were eventually recognized. Australopithecus comprised different species of small-brained but bipedal Pliocene primates. Homo was its descendant.
In the 1960s molecular data challenged notions that species lineages of modern apes and humans could be traced directly to early and middle Miocene fossils. The data ultimately resolved the phylogenetic branching order among extant great apes and humans. However, without our current appreciation of the power of regulatory mechanisms, faith in simple DNA similarity also helped reify the notion that chimpanzees were appropriate primitive proxies for hominid ancestors (4).
During the 1970s Australopithecus afarensis discoveries pushed knowledge back to 3.7 million years ago (Ma), but even the iconic “Lucy” differed little from already known South African fossils. The preoccupation with chimpanzee comparisons led many to argue that Lucy and her conspecifics walked like apes, without human-like hip and knee extension. Thus, despite a host of unique specializations to committed terrestrial bipedality, many declared this species “...very close to what can be called a ‘missing link’” (5). Indeed, a widely used textbook still proclaims that, “Overall, Au. afarensis seems very much like a missing link between the living African apes and later hominins in its dental, cranial, and skeletal morphology” (6).
Australopithecus can no longer be legitimately viewed as a short-lived transition between apes and humans. Rather, it represents an adaptive plateau occupied for ∼3 Ma by up to four species lineages of small-brained African bipeds. Many assumed that when pre-afarensis fossils were eventually recovered they would increasingly resemble chimpanzees. Today “conventional wisdom” continues to reflect the deeply held assumption that Australopithecus is close to some imagined chimpanzee-like Miocene ape species. Furthermore, because Australopithecus is often found in open environments, hominid origins are frequently presented as the tale of a tropical forest ape forced to adapt to open savannas that expanded via global climate change. The new fossils disrupt such frameworks.
Conventional Wisdom Challenged
Ardipithecus is a primate that ruptures several deeply held perceptions, particularly those visualizing humans as “just a third species of chimpanzee” (7). Broader aspects of Australopithecus paleobiology emerged gradually during the 20th century. In contrast, the Ardipithecus niche was comprehensively revealed in ∼250 published pages of a single issue of Science in 2009 (8). Perhaps because Ardipithecus was so suddenly revealed in so many dimensions of context and anatomy—and is so different from Australopithecus—a form of “cognitive dissonance” settled over some practitioners of paleoanthropology. The condition’s symptoms range from post hoc cautionary advice (9) to speculation (10) or inexplicable omission (11). However, Ardipithecus was one of the few surviving lineages of the Miocene ape adaptive radiation: it preserved fundamental arboreal adaptations and it exclusively shares several independent character complexes with all later hominids. This primate therefore illuminates human and chimpanzee origins in ways that Australopithecus never could.
The Ardipithecus fossils fortuitously arrived at an auspicious time in the history of biology, just as revelations of developmental biology enhanced our understanding of how morphology evolves. Knock-outs and knock-ins, reporter alleles, and other revelations about the structural impact of transcription factors, such as homeoboxes and T-boxes (12), have entirely transformed how paleontologists can evaluate morphological change. Gould and Lewontin (13) predicted the impact of such advances even before they actually occurred. However, despite ubiquitous citation of their now famous “spandrels” paper (13), adaptationist interpretations of fossil morphology are still the norm in paleoanthropology. The new understanding of molecular and cellular processes and their roles in the transformation of structure (14) promises to profoundly affect the interpretation of hominid fossils. However, as Darwin appreciated, fossils are still the sine qua non in paleobiology.
Indeed, uniquely complete fossils such as Tiktaalik—recovered in accurate chronological, depositional, ecological, and populational contexts—constitute the most effective means of illuminating paleobiology. Completeness and context are critically important. Only special fossils allow such comprehensive, integrated biological analysis. Historically, when uniquely complete fossils have been discovered, their reception has often precipitated protracted debate. Initial denial is often followed by “adjustment” in a sort of Kuhnian pattern on a smaller scale. Such progress characterizes the historical sciences in general, with plate tectonics as a classic example. The partial Pliocene Ardipithecus ramidus skeleton ARA-VP-6/500 (“Ardi”) preserves so many anatomical parts—in such clear ecological context—that it transforms our understanding of early hominid evolution.
Ardi preserves crucial elements from a single adult female who died 4.4 Ma on a broad Ethiopian floodplain supporting grassy woodlands. Her hands and feet are extraordinarily preserved. Less well preserved, but nevertheless nearly complete elements of her teeth, skull, arms, legs, and pelvis provide further informative anatomy. This fossil and associated evidence allow assessment of locomotion, diet, habitat preference, and even social behavior.
The remains of well over 100 additional individuals from Ardi’s species confirm that her critical morphologies are not idiosyncratic but characteristic of the species. Furthermore, these additional individuals reveal normal variation for several body parts, such as the dentition (SI Text, Note 1; Tables S1–S4), allowing, for example, assessment of sexual dimorphism. These fossils belong to a stratigraphically associated, geologically contemporaneous, >7,000-specimen assemblage of vertebrate remains identified to genus or species and accompanied by a wealth of invertebrate, paleobotanical, and sedimentological data.
A Wooded Habitat
Scenarios about hominids arising in open savanna environments go back to Lamarck in 1809 (15). It was widely expected that pre-Australopithecus hominids would continue to be found associated with open African habitats. However, the uniquely high-resolution set of diverse contextual data surrounding the Ar. ramidus remains indicate that Ardipithecus preferred wooded habitats that were neither a closed tropical forest nor open grassland savanna.
The most direct lines of evidence for habitat—the “smoking guns” of habitat choice—are derived from the Ardipithecus fossils themselves: (i) craniofacial structure and masticatory apparatus, (ii) tooth anatomy and proportions, (iii) macro- and microscopic tooth wear, (iv) carbon isotopes in enamel, and (v) locomotor adaptations. This primate was adapted to chewing softer, less abrasive foods than any later Australopithecus, and at the same time was much better adapted to climbing trees than any other hominid yet found.
Inferences that early hominids preferred wooded habitats have been met with skepticism from savannaphils. Soil-based isotopic data (as well as phytoliths and relatively rare fossils of grazing ungulates) do demonstrate grass in the species’ regional environment. However, the abundant colobine monkeys and kudus found with Ardipithecus were not adapted to open savannas (as evidenced by their microwear, mesowear, isotopes, and postcranial ecomorphology). By analogous evidence, neither was Ardipithecus, which maintained a woodland-to-forest adaptation well into the Pliocene. Even the earliest Australopithecus species appears to have retained elements of woodland adaptation (16). Accordingly, despite valiant efforts at its resurrection (17), the hypothesis that opening grasslands led to hominid emergence and bipedality now stands effectively falsified.
Locomotion
Living primates display a wide range of locomotor abilities. Primatologists have for decades attempted to parse these into “modes,” such as “vertical clinging and leaping,” “quadrupedalism,” and “brachiation.” In characterizations of postural/locomotor adaptations of both living and fossil primates, such labels are combined with standard postural designations, such as “orthograde” (upright trunk posture) and “pronograde” (largely horizontal trunk posture). An even more detailed classification of primate positional and locomotor behaviors partitioned them into over 70 categories (18)! The difficulty that such pigeonhole terminologies have in encapsulating actual locomotor versatility and diversity among living primates is widely recognized.
African ape locomotion has been intensely studied and described. Napier and Napier (19) initially assigned chimpanzees (the most appropriate body mass analog for Ardipithecus) to the category “modified brachiation,” with the trunk usually “held in an upright position,” and with “true” brachiation used usually only for short distances. Chimpanzees are remarkably agile in the trees, even in high canopy, despite their large mass. Their semiorganized “hunts” for red colobus monkeys reveal how remarkably adapted they are to rapid ascent, descent, and navigation across even significant gaps between branches. In contrast, their terrestrial locomotion is by knuckle walking (KW) with occasional bent-hip-bent-knee (BHBK) bipedality.
Every primate species has a diverse positional and locomotor repertoire. However, the body plan and postcranial anatomy of each largely reflects its dominant and adaptively crucial locomotor behavior, and constrains its range of habitual positional/locomotor activities. It is impossible to observe such behavioral details for fossil primates, let alone apply highly parsed categorizations themselves founded upon the more limited array of extant species. Nowhere has this problem surfaced more explicitly than with Ar. ramidus, a species whose postcranium differed dramatically from that of any living primate. Indeed, relative to any living or known fossil ape, this versatile hominid had unique adaptations for both arboreal and terrestrial locomotion.
Hand.
Expectations influenced by the phylogenetic proximity of humans and chimpanzees led some to posit KW “traits” in early Australopithecus (20). Many predicted that pre-Australopithecus hands would be increasingly chimpanzee-like (21). Living African apes have evolved manual adaptations for sustained below-branch suspension and regular climbing on varied vertical supports, and for quadrupedal KW. Extant African ape ray two to five metacarpals are long, especially in chimpanzees. Their heads bear distinct articular expansion and grooving, the latter induced by a lifetime of KW. The primary thumb flexor is reduced and sometimes absent (instead, its tendon often joins that to the second ray) (22). The central bone (capitate) of the chimpanzee midcarpal joint demonstrates a smaller range of motion than in Ardipithecus. Chimpanzees have massive ligaments and structural enhancements of their articular surfaces that firmly and almost rigidly reinforce their palm and digits. Ardipithecus, Australopithecus, and modern humans lack these. The chimpanzee hand, unlike that of the especially mobile hand and wrist of Ardipithecus, is highly derived.
Ardipithecus metacarpals were almost as short as those of Australopithecus and Homo. The carpometacarpal joints were primitively less rigid, enabling some palmar flexibility (such as cupping) not seen in chimpanzees. Thumb musculature appears to have been robust, without modification of the tendon’s attachment to the base of the terminal pollical phalanx, but the pollex itself was shorter and more gracile than in humans. The Ardipithecus hand is not the anatomical intermediate between humans and modern apes that many had predicted.
Foot.
Early anatomists regularly referred to chimpanzees and gorillas as “quadrumanous,” citing their specialized grasping feet. This evolutionary outcome is very different from the highly derived anatomy of the human foot, which became a shock absorber, energy store/converter, and locomotor lever. As with the hand, Ardipithecus foot anatomy does not match expectations based on either chimpanzees or humans. It fits into no living primate pigeonhole.
Whereas the Ardipithecus great toe was widely divergent and opposable, its foot lacked key characters that imbue chimpanzee feet with their unique and powerful grasping ability. The modern great ape midfoot is foreshortened compared with those of other primates. The Ardipithecus foot shares shortened metatarsals with extant African apes, but not the especially shortened distal and mid tarsal bones. As part of this extreme shortening of their midfoot, living apes lack the os peroneum, a sesamoid bone always found in Old World monkeys (as well as living humans). Distinctively, Ardipithecus retained this sesamoid and its associated mechanisms. A wider comparison among the soft tissues of humans, chimpanzees, and monkeys reveals that humans also have retained many characters lost or modified in chimpanzees as the latter enhanced specializations for pedal grasping (22).
The Ar. ramidus foot also shows some newly evolved characters that indicate substantial adaptation to upright walking. Its great toe retained its primitive opposability, but its lateral rays took on the primary role of terrestrial propulsion, as shown by a tendency for a hypertrophied second ray and an Australopithecus-like dorsally developed lateral metatarsal head and associated morphologies. This unique combination indicates that Ardipithecus was able to use its lateral foot as an effective lever for “toe-off” during bipedal progression.
Lower Back and Pelvis.
Equally striking are differences in the lower backs and pelves of humans and modern African apes. The latter have very short lumbar regions. Whereas six to seven lumbar vertebrae are commonly found in Old World monkeys and known Miocene apes, living apes average only 3.5. Moreover, in the modern great ape pelvis, a strikingly narrowed sacrum combines with superiorly extending pelvic “wings” (iliac blades) to actually “entrap” and immobilize the lowest lumbar vertebra (sometimes two). This is the case even with inter- and intraspecies variation in degree of superior elongation of the ilium. Lumbar reduction couples with this entrapment “mechanism” to stiffen the backs in these large-bodied primates, providing injury prevention during arboreal acrobatics. At the same time, however, this stiffening makes modern ape trunks so inflexible that their bipedality requires a BHBK gait.
In contrast, humans, earlier Homo, and Australopithecus retained longer lower backs and evolved uniquely wide sacra. This combination allowed all of their lumbar vertebrae to participate in a specialized anterior bending called “lordosis,” in which the lower spine actually shifts forward to lie more anteriorly relative to the upper trunk. This uniquely derived condition places the center of mass over our feet when we stand up, thereby allowing us to avoid exhaustive BHBK walking.
Although most of her sacrum and lumbar vertebrae were not recovered, parts of both lateral halves of Ardi’s pelvis were. The left side, although damaged, has several relatively complete portions. Importantly, these preserve critical landmarks: the auricular surface (with which the sacrum articulated), the acetabulum (hip joint), and the pubic symphysis (where the two halves of the pelvis meet at its front). Their relative positions in Ar. ramidus are essentially the same as they are in Lucy and other Australopithecus and Homo; this reflects an upper pelvis reorganized for terrestrial bipedality, characterized by a superoinferiorly (craniocaudally) approximated upper sacrum (sacroiliac articulation) and hip joint, and a broad and more sagittally facing iliac blade (SI Text, Note 3).
Even as key upper parts of the Ar. ramidus pelvis were structurally hominid and clearly adapted to upright walking, the lower pelvis (ischium) that anchors powerful muscles used in arboreal climbing (adductors and hamstrings) was still primitively long, as in pronograde quadrupeds and in climbing apes. This anatomical montage of the pelvis uniquely defines Ar. ramidus and its locomotion. Ar. ramidus was less adept in the trees than are living chimpanzees, but was a more capable climber and clamberer than Australopithecus. Furthermore, its lower back and pelvis bore those fundamental features that provide balance and support during bipedal walking.
Limb Proportions.
In addition to their specialized hands, feet, and short rigid lower backs, modern great apes have long arms and short legs that contribute significantly to their remarkable arboreal agility. Human limb proportions are an extreme opposite. The completeness of the Ardi individual’s skeleton (and another individual’s associated arm bones) allows accurate determination of its limb proportions. Not only do its ratios differ from those of both humans and chimpanzees, they are actually closest to those of most known Miocene apes. The latter, with long, flexible vertebral columns and bilaterally narrow trunks, have a more primitive locomotor body plan than does Ardipithecus. However, like Ardi, most of these apes had arms and legs of about equal length. This finding further corroborates that Ar. ramidus was not dependent on, and perhaps only occasionally practiced, forelimb suspension as a mode of locomotion. Instead Ar. ramidus probably preferred slower, more deliberate climbing and clambering.
Arboreal “Multigrady.”
We began this section by noting the exceptional difficulty of “classifying” the locomotor and positional behaviors of living primates. Indeed, despite our best efforts to concisely apply the restrictive terms widely used in classifying movement and posture of living primates to the Ar. ramidus fossils, our efforts have engendered confusion. In hindsight, this is not surprising because the traditional terminology was never created to accommodate a novel creature such as Ar. ramidus, let alone its more distant ancestors.
Using conventional terminology, we wrote that Ar. ramidus “…combined arboreal palmigrade clambering and careful climbing with a form of terrestrial bipedality…” (8), and predicted that the CLCA “…[was] probably a palmigrade quadrupedal arboreal climber/clamberer that lacked specializations for suspension, vertical climbing, or knuckle-walking…” (23).
In the interest of clarity, we will attempt to more plainly spell out the unique aspects of the generalized and highly versatile postural/locomotor adaptation of Ar. ramidus. We did not intend to broadly describe Ardipithecus or the CLCA as quadrupedally monkey-like. Nor did we mean that it displayed an overall locomotor pattern or general postcranial morphology closely comparable to early Miocene apes, such as Proconsul (6). Ar. ramidus had a body plan that differed from these primates, species that were primarily adapted to pronograde quadrupedality. However, Ar. ramidus also exhibited no obvious specializations attributable to forelimb dominated suspension and vertical climbing, as seen in the living great apes. Despite retention of a primitively opposable great toe, its arboreal locomotion must have been only partially African ape-like.
Ar. ramidus was fairly large-bodied, so its movements must have been slow relative to acrobatic chimpanzees, probably usually with multiple simultaneous grasps-on-branches with both hands and feet. Sometimes arboreally orthograde, Ar. ramidus was undoubtedly at other times also effectively pronograde, depending upon each chosen arboreal route. Its feeding posture must have varied extensively. Arboreally, Ar. ramidus is best described as a relatively deliberate climber and clamberer, and not as dependent on suspensory-inclined locomotor repertoires as are living African apes.
In summary, Ar. ramidus shared with African apes and humans a trunk and appendicular skeleton more evolved than those of pronograde arboreal quadrupeds, but was not as adapted to orthograde vertical climbing or suspension as are extant great apes. Ar. ramidus was neither adapted for quadrupedal running and leaping typical of smaller pronograde monkeys, nor for the acrobatic gymnastics of quadrumanous and suspensory-oriented chimpanzees. On the ground, the lack of chimpanzee-like lumbar and pelvic specializations allowed Ardipithecus to move with much more effective bipedality than any living ape. In short, Ar. ramidus combined versatile but deliberate climbing involving body postures spanning both “pronogrady” and “orthogrady,” with a previously unknown form of bipedality.
Terrestrial Bipedality.
Despite having retained hallucial grasping and a primitively elongate ischium for climbing, the Ar. ramidus upper pelvis was dramatically shortened relative to those of all known apes, especially in the distance from its sacral articulation to the hip joint. Along with an inferred long lumbar region that allowed substantial lordosis, this would have enabled efficient balance control during upright walking with extended hip and knee, as in Australopithecus and Homo. Differences with these later hominids primarily relate to the foot, retention of larger posterior thigh muscles, and perhaps subtleties of knee and ankle joint kinematics. Aspects of currently available ankle morphology hint that the Ar. ramidus knee might have been positioned further from the midline than in later hominids, suggesting slightly greater mediolateral shifts of body weight during bipedal progression. Ar. ramidus also lacked a medial longitudinal pedal arch, necessitating a flat-footed stance phase with toe-off from rays two to five. Bipedal progression with extended knee and hip would not have been impeded per se, but compared with later Australopithecus and Homo, this musculoskeletal architecture would have compromised energy dissipation and loading efficiency in all lower limb joints during walking and running. The implications are that Ar. ramidus was not as well-adapted to longer, more strenuous bouts of terrestrial bipedality as were known Australopithecus species.
Craniodental Evolution
Conventional predictions about stem hominid craniodental anatomy varied widely before Ardipithecus. Superficially hominid-like Miocene ape fossils led many to conclude that Australopithecus evolved from a thick-enameled, robust-jawed Miocene ape (24). An alternative idea was that the derived Australopithecus condition (including thick enamel, enlarged back teeth, reduced canines, and an orthognathous face attached to a short cranial base) had evolved from a presumed primitive condition like that of living chimpanzees (4). Surprisingly, the Ardipithecus skull and dental batteries conform to such expectations only in limited ways, thereby revealing novel evolutionary trajectories for early hominid and modern ape craniodental anatomy.
Dentition and Diets.
Ar. ramidus lacks the specialized dental anatomy reflective of the dietary and feeding specializations of Pan or Gorilla. Both, but particularly chimpanzees, have broad spatulate incisors associated with frugivory. Common chimpanzees and bonobos have molar crowns endowed with peculiarly thin occlusal enamel in a wide occlusal basin suitable for crushing, coupled with peripheral crests for shearing. These traits accord with their dietary preference for ripe fruits and limited folivory. Gorilla molars are decidedly higher-cusped and more evenly thin-enameled, resulting in deeper occlusal shearing zones reflective of a more fibrous, herbivorous diet.
In these respects, the Ar. ramidus dentition differs from those of modern great apes. It combines somewhat thin molar enamel (surprisingly thin compared with most Australopithecus) with relatively unspecialized incisors and molars. The latter are broadly similar to a range of Miocene apes and to Australopithecus, strongly suggesting that gorilla and chimpanzee morphologies are each specialized and independently derived. Thus, the dietary niches of Ardipithecus and many Miocene apes were almost certainly broader and less specialized than those of extant African apes. Ar. ramidus probably included substantial components of opportunistic omnivory, further suggested by an enamel isotope signature slightly different from that of chimpanzees, and indistinguishable from those of earliest Australopithecus.
Cranial Structure.
The Ar. ramidus dentition and craniofacial morphology lack the mastication-intensive signals so well established in Australopithecus (e.g., facial size, zygomatic development, absolute and relative postcanine sizes, crown shape, cusp proportions, enamel thickness, wear pattern). Combined with the postcranial evidence, this aspect strongly suggests a niche considerably distinct from that of Australopithecus.
The Ar. ramidus cranium also differs substantially from those of the similarly sized chimpanzee and bonobo. By modern-to-modern species comparisons, the common chimpanzee condition is often considered less derived than that of the “paedomorphic” bonobo. However, a wider comparison including Ar. ramidus and gorillas shows that this is true only in a limited sense, and that both chimpanzees and bonobos each appear to exhibit some uniquely derived features. In particular, the common chimpanzee’s skull has an especially elongate anterior cranial base (nasopharyngeal region) associated with a more forwardly placed snout and face. This structural package results in exaggerated prognathism, a greater jaw gape for its large canines (25), and a distinctly larger skull relative to body size. This anatomical constellation is concordant with the species’ inter- and intragroup aggressive behavior, especially among males.
The anatomically tightly constrained Ar. ramidus female skull reconstruction reveals a cranial capacity as small as that of chimpanzees combined with an Australopithecus-like short cranial base with derived internal flexion. The latter is usually interpreted as indicative of bipedality (upright trunk and neck posture) and less often linked with altered brain structure. However, in features commonly related to posture, such as the position of the foramen magnum, the difference from a gracile-faced ape such as the bonobo is slight, casting significant doubt on that functional explanation. Rather, some subtle changes between internal (neural) and external (cervico-pharyngeal) developmental parameters may have altered cranial base proportions and flexion as a simple nonadaptive corollary of other craniofacial structural changes. Compared with Ar. ramidus and Sahelanthropus, early Australopithecus species had, on average, up to perhaps 25% larger cranial capacity. Perhaps this was linked with altered behavior, sociality, and cognition accompanying niche expansion (26) and transition to more fully committed terrestriality (e.g., ref. 27).
Sexual Dimorphism.
One of the strongest and most persistent misconceptions about early hominid paleobiology concerns the almost universal acceptance that Australopithecus (and in particular Au. afarensis) exhibits large, gorilla-like body size sexual dimorphism (in which linear skeletal dimensions differ by ∼30% between sexes, and males have ∼2× female body mass). To some observers, this has implied strong levels of male–male competition. However, because exaggerated body size dimorphism is usually coupled with strong canine dimorphism among higher primates (but not in Australopithecus), this has created a conundrum (28).
When the iconic Lucy was discovered, it was often contrasted with subsequently discovered, larger conspecifics. Despite greatly expanded sample size, even today the Lucy individual, with the most complete postcranium of Au. afarensis, is one of the smallest members of her species and in many ways does not represent the modal condition. This sampling bias underlies long-standing opposing views. Whereas many observers still argue for a gorilla-like, or even higher level of body size dimorphism, such studies are still highly influenced by Lucy, which contributes disproportionately to their estimates (29). However, when randomization techniques allowing independent and equal contribution of individuals are used, the results show that Au. afarensis postcranial dimorphism was at an intermediate level: larger than in the weakly dimorphic chimpanzees and broadly comparable to modern human levels (∼15% postcranial skeletal dimorphism). These results stem from a recently doubled available sample size now just reaching a statistically appropriate >15–24 individuals (30).
The Ar. ramidus fossils offer an additional, new, and unexpected perspective. Ardi’s limb bones are quite large, with measures exceeding those of Lucy by as much as 30%. Furthermore, the largest of eight known Ar. ramidus humeri is only slightly larger than Ardi’s estimated humeral size, and is comparable to those of the larger, presumably male specimens of Au. afarensis. Importantly, Ardi’s completeness allows a rarely possible craniodental sex assessment of her postcranium independent of inferences based on body size.
The relevant craniodental indicators of dimorphism in Ardi are thus her very small canines and gracile facial skeleton (especially thin supraorbital torus), suggesting a female assignment. A male Ar. ramidus might have looked more like the Sahelanthropus cranium that shows basicranial and facial structural patterns similar to Ardi, but is also endowed with a thick browridge, taller face, and developed nuchal crest. Nevertheless, we initially considered the possibility that Ardi might be a small-canined, gracile-headed male. We therefore paid particular attention to addressing the sex of Ardi on probabilistic grounds. By modeling canine dimorphism across the entire Aramis sample (represented by up to 21 individuals), we found Ar. ramidus canine size dimorphism to be only slightly greater (average canine dimensions ∼12% larger in males than in females) than the modern human condition. This small degree of dimorphism is nevertheless, in turn, sufficient to effectively preclude the possibility that the small-canined Ardi was male (see supplementary materials of ref. 31).
The Ardi skeleton, securely established as female, thus also suggests a small degree of body size dimorphism in the species, perhaps similar to that of chimpanzees or humans, as opposed to orangutans or gorillas. Apparently, both male and female Ar. ramidus were comparatively larger than many Au. afarensis females, particularly diminutive ones such as Lucy. This finding implies that female body size decreased during the emergence of Australopithecus, perhaps as a part of its adaptation to an expanding niche including less wooded habitats.
Social/Reproductive Behavior.
Homo sapiens is the only living primate committed to terrestrial bipedality. We are also the only living higher primate in which the canine plays no social role, and we are the only primate that engages in prolonged monogamous relationships within the context of a larger social group.
Ar. ramidus shares the first two of these characters with humans, which may elucidate the third. All of the earliest known hominids (Orrorin, Sahelanthropus, and Ardipithecus) had apparently already abandoned the primitive C/P3 “honing” complex in which a triangular, projecting upper canine is continuously sharpened by occlusion against the anterior lower premolar, especially in males. This phenomenon is ubiquitous among living and fossil apes and monkeys. However, even the male canines of Ar. ramidus are feminized: they are short and morphologically blunt, with tips wearing down to the level of the surrounding teeth.
It has been argued recently that because of “trade-offs” between canine projection (and resulting gape) and masticatory muscle function, male canine size reduction might have been associated with chewing function in early hominids (25). This argument, applied to emerging hominids, is dubious because Ar. ramidus shows dramatic male canine height reduction but no obvious signs of masticatory enhancement. It is therefore far more likely that reduction of male canine size and height, especially of the upper canine, signals a fundamental change in social behavior. Moreover, bipedality and male canine feminization appear to have been evolutionarily coupled. Just as its hands, feet, and pelvis indicate a unique locomotor behavior and ecological role for Ar. ramidus, so too do its face and teeth suggest that stem hominid social behavior was novel.
Placing Ardipithecus in Life’s Tree
Ar. ramidus obviously postdates the CLCA. It does not exhibit the derived features characteristic of either chimpanzees or gorillas. In contrast, Ar. ramidus shares four major, newly evolved, morphogenetically independent character complexes with later Australopithecus (as judged by comparison with fossil and modern outgroup apes): (i) a nonhoning C/P3 complex and feminized male canine (31), (ii) a short, broad cranial base (32), (iii) a broadened, shortened and “twisted” ilium (SI Text, Note 3), and (iv) tarsal and metatarsal/phalangeal specializations related to upright walking. No other fossil or modern ape shares such evolutionary derivations. It is therefore likely that Ardipithecus lies within the hominid clade (Fig. 1 and Fig. S1). At 4.4 Ma, it is obviously not an ancestral chimpanzee. Nor is it likely to be a relict ape that bears no special relationship to hominids (9, 10).
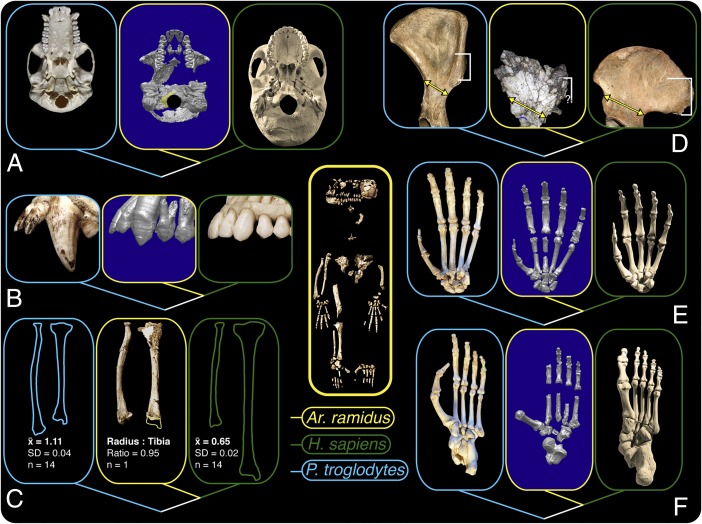
Fig. 1.
Evolution in different directions. Pan troglodytes (left boxes); Ar. ramidus (center boxes); H. sapiens (right boxes). Micro-CT renders on blue backgrounds. Ar. ramidus nests in the hominid clade based on uniquely derived character complexes shared exclusively with Australopithecus and Homo. In the skull (A), chimpanzees combine a primitively long posterior, and derived elongate anterior basicranium; hominids share derived shortened bases. In the dentition (B), hominids lost the primitive functional honing complex of fossil and modern great apes based on a projecting male canine (male Ar. ramidus dentition, ARA-VP-1/300, shown; there is no honing). In limb proportions (C), Ar. ramidus is primitive relative to the derived elongated arm of the chimpanzee and the lengthened leg of the human. Radius and tibia outlines adjusted to mean lengths; ARA-VP-6/500 tibia’s missing distal end is conservatively restored per SI Text, Note 2. In the pelvis (D), hominids share broader, lower iliac blades (yellow arrows; white brackets show the superior and inferior extent of the sacrum’s articular surface; short blue line on fossil indicates superior margin of hip joint). Note the low position of the sacral joint in hominids. The chimpanzee hand (E) bears elongate metacarpals and stiffening for climbing and suspension. Hominids primitively retain short metacarpals, whereas humans have shortened phalanges. The Ar. ramidus foot (F) shows a primitive midfoot that had not evolved into the shortened flexible structure of living apes; neither had it evolved into a modern human-like foot that functions both as a stiffened lever and a compliant shock absorber.
Continuity with Australopithecus
How is Ardipithecus related to later hominids? The temporally nearest Australopithecus is the little-known taxon Au. anamensis. Recent data from carbon isotopes of enamel (16), associated flora and fauna (33), and craniodental anatomy suggest that this species was related to Ardipithecus either as a close collateral relative, or in an ancestral-descendant relationship. The two taxa are superimposed in our study area’s single stratigraphic sequence, but an inadequate fossil record there and elsewhere makes it premature to choose among three different phylogenetic hypotheses (8), although the Burtele foot (34) suggests that some Ardipithecus species may have persisted past 3.5 Ma.
Despite dramatic morphological distinctions between Ar. ramidus and Australopithecus that signal niche differentiation, Ar. ramidus shares many finer details of structure and morphology with early Australopithecus. This aspect is particularly true of the more abundant dental remains representing many individuals of both taxa. These remains allow assessments of variation, as well as the discernment of temporally sequenced morphoclines between Ardipithecus and Australopithecus, and indeed among chronospecies of the latter.
For example, it is not just the itemized character state of the canine, as “small” (or “apically wearing”) that exclusively aligns the Ar. ramidus C/P3 complex with later hominids. Rather, the entirety of this morphological complex, comprising a multitude of largely (or partially) independent features, show obvious phenetic continuity and gradations along the series: Ar. kadabba—Ar. ramidus—Au. anamensis—Au. afarensis, and beyond. This is additional evidence that Australopithecus is the known taxon phylogenetically most closely related to Ar. ramidus. Although the cranium is represented by many fewer specimens than the dentition, it is nevertheless similarly revealing. As with the dentition, the cranial base shows that Ardipithecus and Australopithecus share so much structural detail that the likelihood of parallel acquisition is de facto negated (32, in contrast with ref. 9).
Such morphological sequences show that distinct adaptive complexes, such as those exemplified by Ar. ramidus and Australopithecus, probably arose through a complex sequence of evolutionary modifications. Some anatomical components (particularly in the dentition) probably emerged gradually in terms of geological time, whereas other morpho-functional complexes such as those of the foot and pelvis may have changed more abruptly under stronger selective pressures.
Ape and Human Evolution: Deciphering the Hominoid Bauplan
All higher vertebrates have individual overall body plans (or bauplans for higher taxa) that constitute a sort of fundamental structural “blueprint.” Evolutionary reorganization of a bauplan almost always involves deeply structured, concurrent changes in many characters. Albeit at a smaller scale, the bauplan metaphor helps to conceptualize evolution in the great ape and human clade. The Ardipithecus fossils reveal an unexpected primate with a unique bauplan.
Ardipithecus shares some key evolutionary novelties with all living apes and hominids (fossil and modern). Prominent among these is the anterior migration (“invagination”) of the vertebral column deeply into the thoracic space. This Miocene acquisition probably occurred independently multiple times (35, 36), transforming the primitive circular cross-section of the original ape trunk (today only retained in monkeys) into the flattened, elliptical one in our ancestors and ape relatives. This shift positioned the shoulder joint more posteriorly and laterally, allowing greater ability to circumduct the arm during arboreal climbing and clambering. Other forelimb structures associated with weight bearing during pronograde postures became modified.
This transformation was interpreted in the 19th and 20th centuries to be the result of natural selection favoring below-branch suspension and brachiation, or varied forms of climbing with the trunk in vertical or near-vertical postures (i.e., the suspensory orthograde bauplan). According to this perception, the spinal and thoracic reorganizations (see earlier) were adaptations for orthograde body posture and locomotion, as were enhanced forelimb length and shoulder mobility for vertical climbing and suspension. However, Ardipithecus, lacking forelimb elongation and other key suspension-linked features, shows that vertebral column “invagination” was not adaptively linked with either orthograde climbing or suspension per se. Rather, the derived thoraco-vertebral modifications are best considered a consequence of an adaptive response for enhanced forelimb circumduction. This bauplan shift must have occurred as a part of increasingly versatile climbing and clambering adaptations, but with much less emphasis on suspension and vertical climbing than previously thought.
The deeply embedded perception of the traditional “suspensory orthograde” bauplan has led many to project the anatomical packages exemplified by living apes onto hypothesized common ancestors (4, 11). However, Ardipithecus lacks the specializations of living African (and Asian) apes, such as the long forelimbs and short, stiff back. Its feet and hands do not indicate reliance on suspension or extant ape-like vertical climbing. A recent limited study on the proximal femur of the ∼6 Ma hominid Orrorin and a range of Miocene apes also concluded that the living Asian and African apes are probably independently derived from a more generalized ancestor (37). We predict that similar corroborative conclusions will follow detailed analyses of other anatomical regions.
Miocene Apes.
The markedly diverse fossil apes of the Miocene (currently ∼20 to >25 known genera) play no small role in understanding the evolutionary significance of Ar. ramidus. Some of these ∼23–7 Ma forms are known to have unique bauplans that vary from primitive to partially derived in terms of features discussed above.
For example, although tailless, Equatorius and Proconsul were primitive, exhibiting quadrupedal pronograde bauplans similar to those of some Old and New World monkeys. Nacholapithecus combined a largely Proconsul-like body plan with special climbing adaptations including proportionally large forelimbs (35). Pierolapithecus is reported to exhibit changes in its wrist similar to those we share with living great apes, but had only a partially invaginated vertebral column and short palms and dorsiflexing fingers (36). Dryopithecus exhibits suspensory features in its forelimb including long (but also dorsiflexing) fingers.
Another genus, Oreopithecus, was a highly specialized folivore exhibiting extreme adaptations to suspension, with forelimbs of uniquely exaggerated length among known Miocene apes. Its unique anatomy is so peculiar that it is now universally viewed as a specialized, extinct insular form. Oddly, however, over the decades (38) and continuing today (39), real and imagined elements of Oreopithecus anatomy have been mistaken as conferring hominid status and used as “cautionary tales” about the ability of parallelism to confound phylogenetic signal. For example, it was recently erroneously claimed (9) that Oreopithecus exhibits independently evolved specializations (“homoplasies”) with Ardipithecus. Fig. S1 illustrates the flaws of such claims.
Extensive parallelism is well-documented among higher primates. As the architects of the Modern Synthesis and their intellectual forbears recognized long before the genetic and developmental principles known today, identifying homology-based shared derived characters is a challenge in paleobiology. However, with multiple, independent character complexes exclusively linking Ar. ramidus to later hominids, cautionary tales about parallel evolution based on misinterpretations of exemplars such as Oreopithecus seem diversionary rather than constructive.
The Last Common Ancestor.
Despite a clear phylogenetic nesting of hominids with African apes, and despite a plethora of middle and late Miocene forms now known, the CLCA remains paleontologically elusive. Nevertheless, Ar. ramidus predicts much about its structure, and illuminates the emergence of evolutionary novelty in the human, chimpanzee, and gorilla clades. As inferred from Ardipithecus and other hominid fossils, the ancestral bauplan of the CLCA seems not to have involved a short and stiff lower spine, especially elongate forelimbs, or the many KW/suspensory adaptations of the wrist and hand that characterize today’s African (or Asian) apes. Indeed, whereas we hypothesize that the ancestor (CLCA) was not a quadrupedal primitive ape sensu any so-far known Miocene genus, neither was it as specialized as today’s highly derived African relicts of the Miocene ape radiation.
Although obviously not the common ancestor of humans and chimpanzees, Ardipithecus nevertheless provides strong evidence with which to infer that the CLCA was a generalized African ape. It was probably a relatively large-bodied primate with a unique, previously unknown bauplan combining invaginated vertebrae within a flattened thorax, enhanced shoulder mobility, elbow extension close to that in living apes, and ulnar withdrawal at the wrist. However, it otherwise lacked subsequently elongated forelimbs/forearms/hands and other specializations related to habitual suspension and enhanced vertical climbing. The CLCA foot maintained many primitive structures related to plantarflexive propulsion, but probably had also evolved plantigrade features, such as heel-strike. However, it still lacked the peculiar substrate-conforming, hand-like grasping foot of living African apes. As one of the CLCA’s descendants, Ardipithecus was an early biped that lacked the derivations that chimpanzees would evolve, yet had not yet acquired specializations for a greater commitment to terrestrial bipedality (such as hallucial adduction and shortened ischia).
Living Apes: The Pitfalls of Projection
The last half-century of field and laboratory research on our African ape relatives has revealed a great deal about their biology. Darwin was clear on the pitfalls of projecting living primates into the past as proxy ancestors, but without a fossil record these cautions proved difficult for many to heed. As Moore (40) has noted, using chimpanzee anatomy, physiology, behavior, and ecology to model early hominids is useful for carefully delimited modeling exercises, but simply projecting living forms into the past as proxies does not qualify as such: “...their greatest danger is that models are easily mistaken for that which they model; critically, by creating a mental prototype they can mask variation and so block use of the comparative method for testing hypotheses...This danger is especially serious in hominid modeling.”
Now that there is an emergent fossil record of hominid origins, to what extent do the living apes, particularly chimpanzees, help us understand our evolution …and theirs? Not surprisingly, our closest living relatives turn out to be important not just for their value in biodiversity, behavioral, and physiological studies, but also as models and case examples regarding specifics of evolutionary ecology and patterning. Chimpanzees are also highly informative hominid comparators precisely because they turn out to be so different from modern people and fossil hominids.
Ar. ramidus was a primate with no close analog among living monkeys or apes. Before its discovery, conventional wisdom held that chimpanzees were largely primitive, and that humans were derived from forms much like them. That meant that the degree of “primitiveness” of characteristics (including behavior) could be determined through simple comparison with the chimpanzee homolog. The fallacy of this logic is amply illustrated by Ardipithecus. For example, parsimony-based appeals to KW in contemporary African apes have been used to argue that this locomotor mode must have been the primitive condition for our last common ancestors with them (3, 4). However, despite intensive searching of African, European, and Asian deposits, no compelling Miocene evidence of KW has so far been found; Ar. ramidus strongly suggests that none will be, at least in any candidates for the last common ancestor.
Fossils: Evolutionary Pathways Uniquely Revealed
In focusing on human evolution, it is rarely mentioned that hominid fossils serve as informative outgroups for evaluating African apes. This is because Australopithecus (and even more so Homo) is derived in so many ways. However, with the chronological and evolutionary depth that Ar. ramidus now contributes, contrasting it with extant African apes reveals that major aspects of living African ape dietary, locomotor, and sociobehavioral adaptations must have evolved after their splits with the hominid clade. With the new hominid fossils, it is now possible to better appreciate chimpanzee specializations in anatomy, locomotion, diet, and aspects of social behavior. The latter include species characteristics of chimpanzees, such as territoriality and intergroup aggression, complex male alliances, strong intragroup competition and aggression linked to “advertised” female estrus, and unusually robust sperm competition resulting from extreme female promiscuity. Accompanying physiologic and genetic novelties support the idea that the chimpanzee adaptive complex is derived.
Rather than primitive “living missing links,” chimpanzees are thus disclosed as specialists that probably initially evolved in tropical forests, most likely when such habitats went through episodes of recurrent fragmentation and expansion. An earlier derivation from a similarly generalized African ape ancestor was the gorilla. In contrast to chimpanzees, gorillas characteristically occupy a larger-bodied herbivory-frugivory niche, with smaller home ranges that enable pronounced sexual dimorphism and one-male, multiple-female social groups.
Hominids appear to have emerged by developing a search-intensive terrestrial feeding niche, accompanied perhaps by food transport and sharing in less densely forested but still wooded areas. In this manner, Ardipithecus represents an evolutionary, adaptive, ecological, and anatomical bridge between Australopithecus and the yet to be found late Miocene African ape that was commonly ancestral to both chimpanzees and ourselves.
Unfortunately, the still sparse fossil record only exposes part of the locomotor diversity and bauplan transitions during the Miocene ape adaptive radiation. Additionally, Ar. ramidus provides only limited evidence about the nature and timing of crucial early events in hominid evolution. However, even this evidence is important in removing the confinements of the missing-link mentality that have distorted interpretations of human evolution for more than a century. In this manner, a distant Pliocene primate has again demonstrated paleontology's unique contribution to understanding the history of life on earth.
Supplementary Material
Acknowledgments
We thank the Ministry of Culture and Tourism, the Authority for Research and Conservation of Cultural Heritage, the National Museum of Ethiopia, and the Afar Regional Government, Ethiopia for permissions and facilitations; the Afar people of the Middle Awash and many other field and laboratory workers who contributed directly to the research efforts and results since 1981; and William Kimbel and three anonymous reviewers for helpful comments on the manuscript. This work was supported in part by the Institute of Geophysics and Planetary Physics of the University of California at Los Alamos National Laboratory, the Japan Society for the Promotion of Science, and the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403659111/-/DCSupplemental.
References
- 1.Huxley TH. The Origin of Species. Collected Essays Vol. 2 Darwiniana. Macmillan; London: 1860. pp. 71–79. [Google Scholar]
- 2.Darwin C. The Descent of Man. John Murray; London: 1871. [Google Scholar]
- 3.Wrangham R, Pilbeam D. African apes as time machines. In: Galdikas B, Briggs E, Shapiro S, Goodall J, editors. All Apes Great and Small Volume I: African Apes. Kluwer Academic/Plenum; New York: 2001. pp. 5–17. [Google Scholar]
- 4.Pilbeam D. Genetic and morphological records of the Hominoidea and hominid origins: A synthesis. Mol Phylogenet Evol. 1996;5(1):155–168. doi: 10.1006/mpev.1996.0010. [DOI] [PubMed] [Google Scholar]
- 5.Stern JT, Jr, Susman RL. The locomotor anatomy of Australopithecus afarensis. Am J Phys Anthropol. 1983;60(3):279–317. doi: 10.1002/ajpa.1330600302. [DOI] [PubMed] [Google Scholar]
- 6.Fleagle JG. Primate Evolution and Adaptation (3 Ed) Academic; San Diego: 2013. [Google Scholar]
- 7.Diamond J. The Rise and Fall of the Third Chimpanzee. Radius; London: 1991. [Google Scholar]
- 8.White TD, et al. Ardipithecus ramidus and the paleobiology of early hominids. Science. 2009;326(5949):75–86. [PubMed] [Google Scholar]
- 9.Wood B, Harrison T. The evolutionary context of the first hominins. Nature. 2011;470(7334):347–352. doi: 10.1038/nature09709. [DOI] [PubMed] [Google Scholar]
- 10.Harrison T. Anthropology. Apes among the tangled branches of human origins. Science. 2010;327(5965):532–534. doi: 10.1126/science.1184703. [DOI] [PubMed] [Google Scholar]
- 11.Thorpe SK, McClymont JM, Crompton RH. The arboreal origins of human bipedalism. Antiquity. 2014;88(341):906–914. [Google Scholar]
- 12.Carroll SB, Grenier JD, Weatherbee SD. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. 2nd Ed Blackwell; London: 2005. [Google Scholar]
- 13.Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme. Proc R Soc Lond B Biol Sci. 1979;205(1161):581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 14.Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457(7231):818–823. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- 15.Lamarck JB. Philosophie Zoologique. Dentu; Paris: 1809. [Google Scholar]
- 16.Cerling TE, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc Natl Acad Sci USA. 2013;110(26):10501–10506. doi: 10.1073/pnas.1222568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domínguez-Rodrigo M. Is the “savanna hypothesis” a dead concept for explaining the emergence of the earliest hominins? Curr Anthropol. 2014;55(1):59–81. [Google Scholar]
- 18.Hunt K, et al. Standardized descriptions of primate locomotor and postural modes. Primates. 1996;37(4):363–387. [Google Scholar]
- 19.Napier JR, Napier PH. A Handbook of Living Primates (2 Ed) Academic; San Diego: 1976. [Google Scholar]
- 20.Richmond BG, Strait DS. Evidence that humans evolved from a knuckle-walking ancestor. Nature. 2000;404(6776):382–385. doi: 10.1038/35006045. [DOI] [PubMed] [Google Scholar]
- 21.Tocheri MW, Orr CM, Jacofsky MC, Marzke MW. The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo. J Anat. 2008;212(4):544–562. doi: 10.1111/j.1469-7580.2008.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straus WL., Jr The riddle of man’s ancestry. Q Rev Biol. 1949;24(3):200–223. doi: 10.1086/397067. [DOI] [PubMed] [Google Scholar]
- 23.Lovejoy CO, Suwa G, Simpson SW, Matternes JH, White TD. The great divides: Ardipithecus ramidus reveals the postcrania of our last common ancestors with African apes. Science. 2009;326(5949):100–106. [PubMed] [Google Scholar]
- 24.Andrews P, Martin L. Hominoid dietary evolution. Philos Trans R Soc Lond B Biol Sci. 1991;334(1270):199–209, discussion 209. doi: 10.1098/rstb.1991.0109. [DOI] [PubMed] [Google Scholar]
- 25.Hylander WL. Functional links between canine height and jaw gape in catarrhines with special reference to early hominins. Am J Phys Anthropol. 2013;150(2):247–259. doi: 10.1002/ajpa.22195. [DOI] [PubMed] [Google Scholar]
- 26.White TD, et al. Asa Issie, Aramis and the origin of Australopithecus. Nature. 2006;440(7086):883–889. doi: 10.1038/nature04629. [DOI] [PubMed] [Google Scholar]
- 27.Ward CV, Kimbel WH, Johanson DC. Complete fourth metatarsal and arches in the foot of Australopithecus afarensis. Science. 2011;331(6018):750–753. doi: 10.1126/science.1201463. [DOI] [PubMed] [Google Scholar]
- 28.Plavcan JM, van Schaik CP. Interpreting hominid behavior on the basis of sexual dimorphism. J Hum Evol. 1997;32(4):345–374. doi: 10.1006/jhev.1996.0096. [DOI] [PubMed] [Google Scholar]
- 29.Gordon AD, Green DJ, Richmond BG. Strong postcranial size dimorphism in Australopithecus afarensis: Results from two new resampling methods for multivariate data sets with missing data. Am J Phys Anthropol. 2008;135(3):311–328. doi: 10.1002/ajpa.20745. [DOI] [PubMed] [Google Scholar]
- 30.Reno PL, McCollum MA, Meindl RS, Lovejoy CO. An enlarged postcranial sample confirms Australopithecus afarensis dimorphism was similar to modern humans. Philos Trans R Soc Lond B Biol Sci. 2010;365(1556):3355–3363. doi: 10.1098/rstb.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suwa G, et al. Paleobiological implications of the Ardipithecus ramidus dentition. Science. 2009;326(5949):94–99. [PubMed] [Google Scholar]
- 32.Kimbel WH, Suwa G, Asfaw B, Rak Y, White TD. Ardipithecus ramidus and the evolution of the human cranial base. Proc Natl Acad Sci USA. 2014;111(3):948–953. doi: 10.1073/pnas.1322639111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geraads B, Bobe R, Manthi FK. New ruminants (Mammalia) from the Pliocene of Kanapoi, Kenya, and a revision of previous collections, with a note on the Suidae. J Afr Earth Sci. 2013;85:53–61. [Google Scholar]
- 34.Haile-Selassie Y, et al. A new hominin foot from Ethiopia shows multiple Pliocene bipedal adaptations. Nature. 2012;483(7391):565–569. doi: 10.1038/nature10922. [DOI] [PubMed] [Google Scholar]
- 35.Nakatsukasa M, Kunimatsu Y. Nacholapithecus and its importance for understanding hominoid evolution. Evol Anthropol. 2009;18(3):103–119. [Google Scholar]
- 36.Moyà-Solà S, Köhler M, Alba DM, Casanovas-Vilar I, Galindo J. Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain. Science. 2004;306(5700):1339–1344. doi: 10.1126/science.1103094. [DOI] [PubMed] [Google Scholar]
- 37.Almécija S, et al. The femur of Orrorin tugenensis exhibits morphometric affinities with both Miocene apes and later hominins. Nat Commun. 2013;4:2888. doi: 10.1038/ncomms3888. [DOI] [PubMed] [Google Scholar]
- 38.Hürzeler J. Oreopithecus bambolii Gervais: A preliminary report. Verh Naturf Ges. Basel. 1958;69(1):1–48. [Google Scholar]
- 39.Köhler M, Moyà-Solà S. Ape-like or hominid-like? The positional behavior of Oreopithecus bambolii reconsidered. Proc Natl Acad Sci USA. 1997;94(21):11747–11750. doi: 10.1073/pnas.94.21.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore J. Savanna chimpanzees, referential models and the last common ancestor. In: McGrew WC, Marchant LF, Nishida T, editors. Great Ape Societies. Cambridge Univ Press; Cambridge: 1996. pp. 275–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.