Significance
Interleukin-32 (IL-32) is induced by IL-1β, Toll-like receptor agonists, and nucleotide oligomerization domain as well as by Mycobacterium tuberculosis (MTB). Expression of human IL-32γ in the lungs of mice reduced the burden of MTB in both the lungs but also in the spleen and was associated with increased survival. Mechanistically, increased numbers of host-protective innate and adaptive immune cells were present in the IL-32 transgenic mice. Alveolar macrophages from the transgenic mice were also better able to control MTB infection and had increased colocalization of MTB with lysosomes. IL-32 expression was increased in the surgically resected lungs of tuberculosis patients, particularly in macrophages, airway epithelial cells, B cells, and T cells. Thus, IL-32 enhances host immunity against MTB.
Keywords: cytokine, transgenic mouse, tuberculosis, host immunity, interleukin-32
Abstract
Silencing of interleukin-32 (IL-32) in a differentiated human promonocytic cell line impairs killing of Mycobacterium tuberculosis (MTB) but the role of IL-32 in vivo against MTB remains unknown. To study the effects of IL-32 in vivo, a transgenic mouse was generated in which the human IL-32γ gene is expressed using the surfactant protein C promoter (SPC-IL-32γTg). Wild-type and SPC-IL-32γTg mice were infected with a low-dose aerosol of a hypervirulent strain of MTB (W-Beijing HN878). At 30 and 60 d after infection, the transgenic mice had 66% and 85% fewer MTB in the lungs and 49% and 68% fewer MTB in the spleens, respectively; the transgenic mice also exhibited greater survival. Increased numbers of host-protective innate and adaptive immune cells were present in SPC-IL-32γTg mice, including tumor necrosis factor-alpha (TNFα) positive lung macrophages and dendritic cells, and IFN-gamma (IFNγ) and TNFα positive CD4+ and CD8+ T cells in the lungs and mediastinal lymph nodes. Alveolar macrophages from transgenic mice infected with MTB ex vivo had reduced bacterial burden and increased colocalization of green fluorescent protein-labeled MTB with lysosomes. Furthermore, mouse macrophages made to express IL-32γ but not the splice variant IL-32β were better able to limit MTB growth than macrophages capable of producing both. The lungs of patients with tuberculosis showed increased IL-32 expression, particularly in macrophages of granulomas and airway epithelial cells but also B cells and T cells. We conclude that IL-32γ enhances host immunity to MTB.
Tuberculosis (TB) is a leading cause of morbidity and mortality in the world with nearly 9 million new cases and over 1 million deaths per year. Mycobacterium tuberculosis (MTB) strains possessing high levels of drug resistance such as multidrug resistant or extensively drug resistant MTB threaten to make TB into an incurable disease (1). Whereas development of new classes of effective anti-TB antibiotics is important to treat existing cases (2), history teaches that MTB will eventually develop resistance. Thus, it is important to continue to seek previously undiscovered immune responses to MTB in hopes that new approaches may be applied to treat human TB.
First described in 2005, interleukin-32 (IL-32) is a pleiotropic cytokine that induces proinflammatory cytokines such as tumor necrosis factor-alpha (TNFα) and IL-1β (3). IL-32 protects against infections with HIV (4), influenza (5), and Mycobacterium avium (6). This protective effect is likely due to the proinflammatory nature of IL-32, which is also implicated in the pathogenesis of a number of inflammatory disorders (3, 7, 8). We previously demonstrated that infection of human macrophages or peripheral blood mononuclear cells (PBMCs) with MTB H37Rv induced IL-32 (9, 10). Silencing endogenous IL-32 by siRNA in THP-1 macrophages, differentiated from a human promonocytic cell line, increased the intracellular burden of MTB, indicating that IL-32 plays a host-protective role (9). However, the role of IL-32 in the response to TB in vivo remains unknown.
IL-32 is composed of six isoforms (α, β, γ, δ, ε, and ζ) due to alternatively spliced mRNA variants (3). IL-32γ is biologically the most active, likely due to the lack of exonic deletions (11). Because a full-length mouse homolog of IL-32 is not present in the databases, studying a role for endogenous IL-32 in mice is not possible. Because murine macrophages do respond to IL-32 as measured by TNFα production (3), we developed a transgenic (Tg) mouse strain in which human IL-32γ is expressed in type II alveolar epithelial cells under the control of the surfactant protein C (SPC) gene promoter (SPC-IL-32γTg). Wild-type (WT) C57BL/6 and SPC-IL-32γTg mice were infected with a low-dose aerosol of MTB W-Beijing HN878, a hypervirulent MTB strain isolated from a patient with TB (12). We hypothesized that expression of human IL-32 in the lungs of mice would protect against MTB infection. If borne out, IL-32 may represent a target for previously unidentified immunotherapy to treat TB.
Results
Integration of SPC-IL-32γ Transgene.
Microinjection of the SPC-IL-32γ transgene into C57BL/6 zygotes was performed (Fig. S1A). The 420-bp fragment of the SPC-IL-32γ transgene was detected by PCR (Fig. S1B) and Southern blot analysis confirmed that the SPC-IL-32γ transgene (4.4-kb DNA fragment) was successfully integrated into the genome of founder mice (Fig. S1C). As expected, there was no transgene signal in the WT mice (Fig. S1C). Inbreeding produced a uniform colony of SPC-IL-32γTg mice, confirmed by positive PCR for the SPC-IL-32γ transgene (Fig. S2A). Offspring of the SPC-IL-32γTg mice were healthy at birth and grew normally.
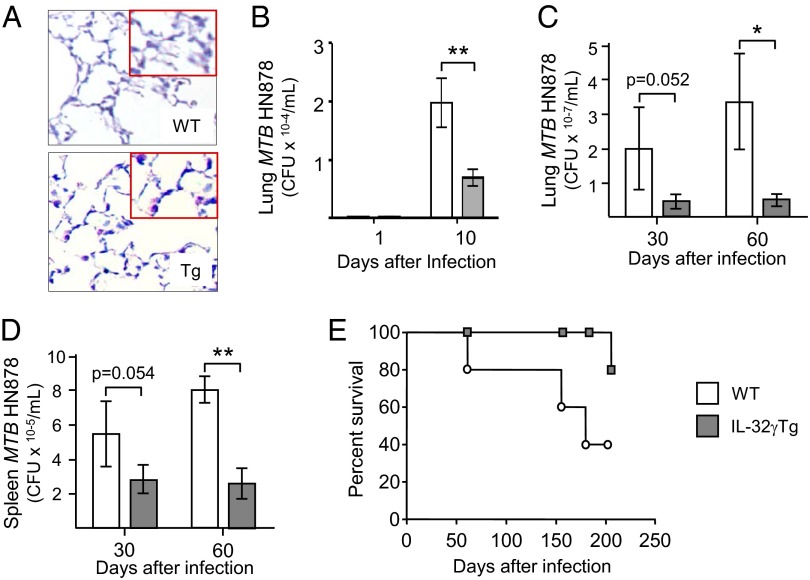
RT-PCR of homogenized lung tissues revealed that IL-32 mRNA was present in the lungs of SPC-IL-32γTg mice but absent from WT mice (Fig. S2B). IL-32γ protein levels were measured from the whole lungs of four WT and four SPC-IL-32γTg mice by ELISA and normalized for total protein. As shown in Fig. S2C, the mean lung level of IL-32γ in the Tg mice was significantly greater than the negligible nonspecific background signal in the lungs of the WT mice. Immunohistochemistry of representative WT and SPC-IL-32γTg mice showed that IL-32γ was expressed in type II alveolar cells of the Tg mice but not the WT mice (Fig. 1A). Analysis of the bronchoalveolar lavage fluid revealed there was a trend toward increased leukocyte count in the SPC-IL-32γTg mice (123 cells/µL) compared with WT mice (85 cells per microliter), and no difference in the cell differential with the large majority of the cells being alveolar macrophages (AMs) in both mouse strains.
Fig. 1.
SPC-IL-32γTg mice are more resistant to MTB infection than wild-type (WT) controls. (A) Immunohistochemistry (200×) for IL-32γ of uninfected WT and SPC-IL-32γTg mice (Inset, 400×). Data shown are representative of three WT and three SPC-IL-32γTg mice. (B) One and 10 d after infection, the mice were killed, and cfu in the homogenized lungs of the WT mice (open bars) and SPC-IL-32γTg mice (closed bars) were determined by culture; cfu for WT and SPC-IL-32γTg mice at day 1 = 170 ± 15/mL and 175 ± 18/mL, respectively. Thirty and 60 d after infection, the mice were killed, and cfu in the homogenized (C) lungs and (D) spleens of the WT mice (open bars) and SPC-IL-32γTg mice (closed bars) were determined by culture. For A–D, data are shown as mean ± SEM with n = 5 mice for each time point, *P < 0.05 and **P < 0.01. (E) WT (open circles) and SPC-IL-32γTg (closed squares) mice were infected with low-dose aerosol of MTB W-Beijing HN878 by the Glas-Col aerosol generator and folllowed for survival for up to 200 d (data shown for 10 WT and 10 SPC-IL-32γTg mice, P = 0.0525).
SPC-IL-32γTg Mice Are Protected Against MTB Infection.
SPC-IL-32γTg and WT mice were infected with aerosolized MTB W-Beijing HN878 and the bacterial burden in the lungs and spleens were determined. One day after infection, there was no significant difference in the number of MTB between the strains (Fig. 1B). However, by day 10, there was significantly less MTB isolated from the lungs of SPC-IL-32γTg mice compared with the WT mice (Fig. 1B). By 30 and 60 d after infection, there was 66% and 85% fewer MTB, respectively, in the lungs of the SPC-IL-32γTg mice compared with the WT mice (Fig. 1C). At 30 and 60 d after MTB infection, there were 49% and 68% fewer MTB, respectively, in the spleens of the SPC-IL-32γTg mice (Fig. 1D).
Survival of 10 WT and 10 SPC-IL-323γTg mice infected with MTB W-Beijing HN878 was determined. As shown in Fig. 1E, SPC-IL-32γTg mice survived longer than WT mice and the difference approached significance.
We quantified the severity of lung and splenic lesions in the WT and SPC-IL-32γTg mice using the area fraction fractionator method. There was no significant difference between the mouse strains in the number of lung lesions at 30 d after infection and a trend toward fewer TB lesions in the lungs of the SPC-IL-32γTg mice after 60 d of infection (Fig. S3A). There was no significant difference in the necrotic area of the lung lesions in the two mouse strains (Fig. S3B). Similarly, there was no significant difference in the number or severity of lesions in the spleens between the two mouse strains (Fig. S3 C and D).
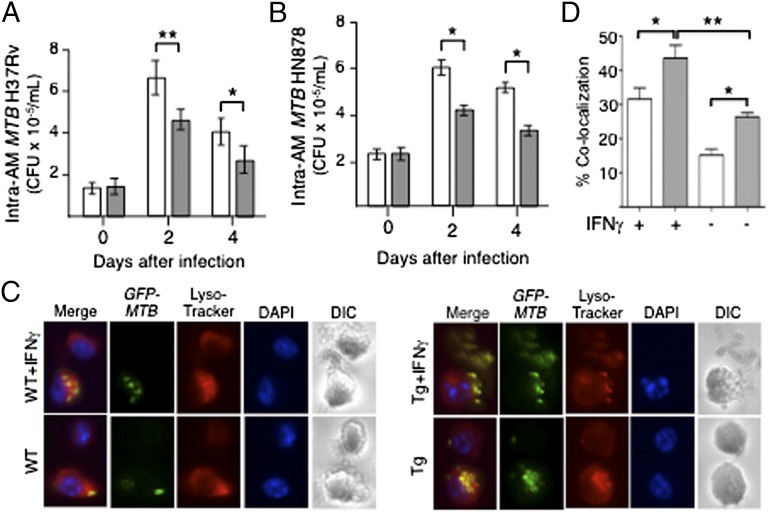
Because IL-32 is secreted and can stimulate other cells (13–15), we compared the ability of AMs from SPC-IL-32γTg and WT mice to control an MTB infection. AMs were isolated from the lungs of SPC-IL-32γTg and WT mice by bronchoalveolar lavage. Cultured AMs were infected with MTB H37Rv or W-Beijing HN878 and the number of intracellular MTB were determined (9). There was a modest but significant reduction in the number of intracellular H37Rv and W-Beijing HN878 recovered from the AMs of SPC-IL-32γTg mice at 2 and 4 d after infection compared with AMs of WT mice (Fig. 2 A and B, respectively).
Fig. 2.
Alveolar macrophages (AMs) of SPC-IL-32γTg mice are better able to control MTB infection than AMs from WT mice. (A) AMs of WT (open bars) and SPC-IL-32γTg (closed bars) mice were infected with MTB H37Rv at a multiplicity of infection (MOI) of 10 bacilli to one macrophage, and intracellular MTB quantified at the indicated time points. (B) AMs of WT (open bars) and SPC-IL-32γTg (closed bars) mice were infected with MTB W-Beijing HN878 at an MOI of 10 and intracellular bacterial burden was determined after the indicated times of infection. (C) AMs of WT (open bars) and SPC-IL-32γTg (closed bars) mice were infected with GFP-labeled MTB H37Rv with and without IFNγ (10 units/mL) stimulation, followed by staining for lysosomes and nuclei with LysoTracker Red and DAPI, respectively. (D) The percentage of cells with colocalization of GFP-MTB and lysosomes was calculated based on the number of cells with colocalization divided by the number of GFP-MTB infected cells. For A and B, data represent the mean ± SEM of six independent experiments. For C and D, data represent the mean ± SEM of three independent experiments, *P < 0.05, **P < 0.01.
Because colocalization of phagocytosed MTB with lysosomes is a central mechanism by which intracellular MTB are disposed (16), we quantified the colocalization of GFP-labeled MTB with lysosomes in AMs of WT and Tg mice. As shown in Fig. 2 C and D, stimulation of AMs from either mouse strain with IFNγ increased the percentage of cells in which GFP-MTB colocalized with lysosomes. Importantly, AMs from SPC-IL-32γTg mice displayed significantly greater colocalization than WT AMs, whether in the presence or absence of IFNγ (Fig. 2 C and D).
Increased Influx of TNFα-Positive Innate Immune Cells in the Lungs of SPC-IL-32γTg Mice.
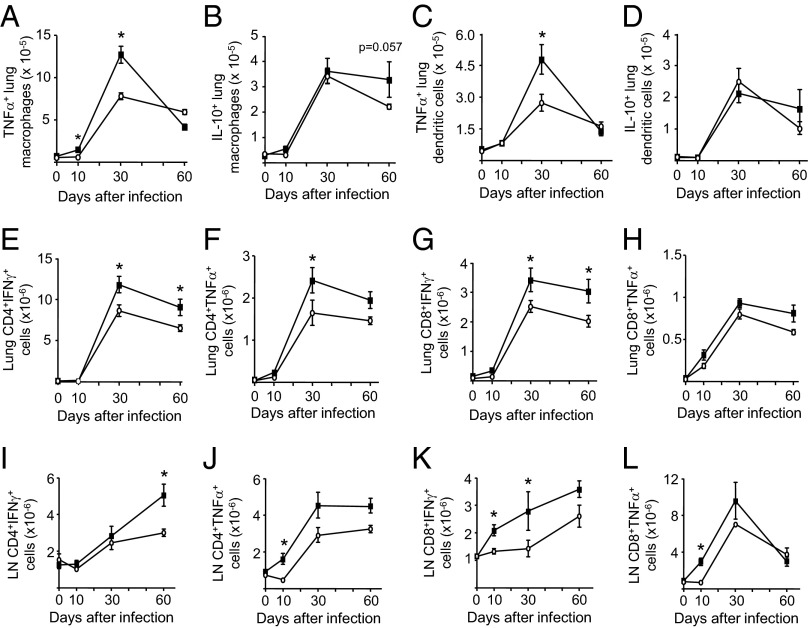
The number of AMs and lung dendritic cells expressing TNFα, IL-12p40, IL-10, and class II MHC molecules were determined in infected WT and SPC-IL-32γTg mice. At 30 d after infection, significantly greater numbers of TNFα-positive AMs (CD11bhiCD11cneg) were isolated from SPC-IL-32γTg mice than from WT mice but there was no difference by 60 d (Fig. 3A). There was a clear trend of increased IL-10-expressing macrophages after 60 d of infection in the SPC-IL-32γTg mice (Fig. 3B). However, there was no significant difference in the number of AMs expressing IL-12 or class II MHC between the WT and SPC-IL-32γTg mice.
Fig. 3.
Analysis of innate and adaptive immune cells in the lungs and mediastinal lymph nodes of MTB-infected WT and SPC-IL-32γTg mice. The number of lung macrophages (CD11bhiCD11cneg) producing (A) TNFα and (B) IL-10 and lung dendritic cells (DEC205posCD11bnegCD11chi) positive for (C) TNFα and (D) IL-10 in WT (open circles) and SPC-IL-32γTg (closed squares) mice are shown over the course of the infection. The number of lung CD4+ T cells producing (E) IFNγ and (F) TNFα in control (open circles) and SPC-IL-32γTg (closed squares) mice are shown over the course of the infection. The number of lung CD8+ T cells producing (G) IFNγ and (H) TNFα in control (open circles) and SPC-IL-32γTg (closed squares) mice are shown over the course of the infection. The number of nodal CD4+ T cells producing (I) IFNγ and (J) TNFa in control (open circles) and SPC-IL-32γTg (closed squares) mice are shown over the course of the infection. The number of nodal CD8+ T cells producing (K) IFNγ and (L) TNFα in control (open circles) and SPC-IL-32γTg (closed squares) mice are shown over the course of the infection. For all experiments, the results are expressed as the mean number of cells ± SEM with n = 5 mice for each time point, *P < 0.05.
There were also significantly greater numbers of TNFα-expressing dendritic cells (DEC205posCD11bnegCD11chi) in the lungs of the SPC-IL-32γTg mice at 30 d but this difference was lost by 60 d (Fig. 3C). There was no significant difference in the number of lung dendritic cells expressing IL-10 (Fig. 3D), IL-12, or class II MHC between the mouse strains.
Caspase-3–dependent apoptosis is one mechanism by which IL-32 reduces intracellular MTB in THP-1 cells (9). Thus, we analyzed the expression of caspase-3 and Bcl-2 (an antiapoptotic marker) but found no difference in the number of caspase-3+ or Bcl-2+ AMs and dendritic cells in the lungs or draining lymph nodes between the two mouse strains (Fig. S4).
Increased IFNγ- and TNFα-Expressing CD4+ and CD8+ T Cells in Infected SPC-IL-32γTg Mice.
CD4+ and CD8+ T effector cells are critically important to anti-TB immunity (17). We analyzed the populations of these cells expressing TNFα and IFNγ in the lungs and mediastinal lymph nodes of infected WT and Tg mice. SPC-IL-32γTg mice infected with MTB had significantly more IFNγ+CD4+ T cells in the lungs at 30 and 60 d postinfection (Fig. 3E) as well as increased TNFα+CD4+ T cells at 30 d after infection (Fig. 3F). Compared with WT mice, SPC-IL-32γTg mice also had more IFNγ+CD8+ T cells at 30 and 60 d and displayed a trend toward increased TNFα+CD8+ T cells at 60 d compared with infected control mice (Fig. 3 G and H). In the mediastinal lymph nodes, infected SPC-IL-32γTg mice had significantly more IFNγ+CD4+ T cells 60 d after infection (Fig. 3I). The Tg mice also had more TNFα+CD4+ T cells recovered from the lymph nodes at 10, 30, and 60 d after infection (Fig. 3J) as well as increased number of IFNγ- and TNFα-expressing CD8+ T cells after 10 and 30 d of infection (Fig. 3 K and L). We found no significant difference in the influx of TH17 cells into the lungs or mediastinal lymph nodes of the two mouse strains at all time points studied.
Increased NK1.1+ Cells in the Lungs of the SPC-IL-32γTg Mice.
NK1.1+ cells are increasingly recognized as important in lung immunity against MTB (18). In the SPC-IL-32γTg mice, the number of NK1.1+ were significantly increased in the lungs at 1, 10, and 60 d after infection but were decreased in the lymph nodes 10 d after infection (Fig. S5).
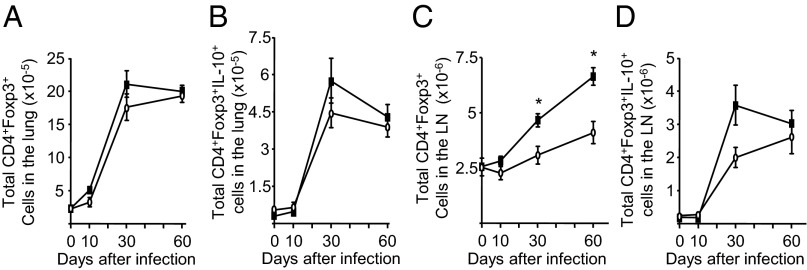
Increased CD4+ T Regulatory Cells in the Lymph Nodes of Infected SPC-IL-32γTg Mice.
CD4+Foxp3+ T regulatory cells (Tregs) inhibit the proinflammatory immune response to virulent MTB (12, 19). There was no difference in the influx of total Tregs or IL-10+ Tregs in the lungs of the WT and SPC-IL-32γTg mice over the course of the infection (Fig. 4 A and B). In the mediastinal lymph nodes of the SPC-IL-32γTg mice, there was a significant increase in the number of Tregs at later time points of infection (days 30 and 60) (Fig. 4C) as well as a trend of increased IL-10+ Tregs at day 30 (Fig. 4D).
Fig. 4.
Analysis of T regulatory cells in the lungs and mediastinal lymph nodes of MTB-infected WT and SPC-IL-32γTg mice. The (A) total number of CD4+Foxp3+ and (B) CD4+Foxp3+IL-10+ Treg cells in the lungs of control (open circles) and SPC-IL-32γTg (closed squares) mice are shown over the course of the infection. The (C) total number of CD4+Foxp3+ and (D) CD4+Foxp3+IL-10+ Treg cells in the lymph nodes of control (open circles) and SPC-IL-32γTg (closed squares) mice are shown over the course of the infection. Results are expressed as the mean number of cells ± SEM with n = 5 mice for each time point, *P < 0.05.
Mouse Macrophages Transfected with IL-32γ Mutated at the Splice Site (IL-32γM) Have Greater Capacity to Control MTB Infection than Cells Transfected with WT IL-32γ.
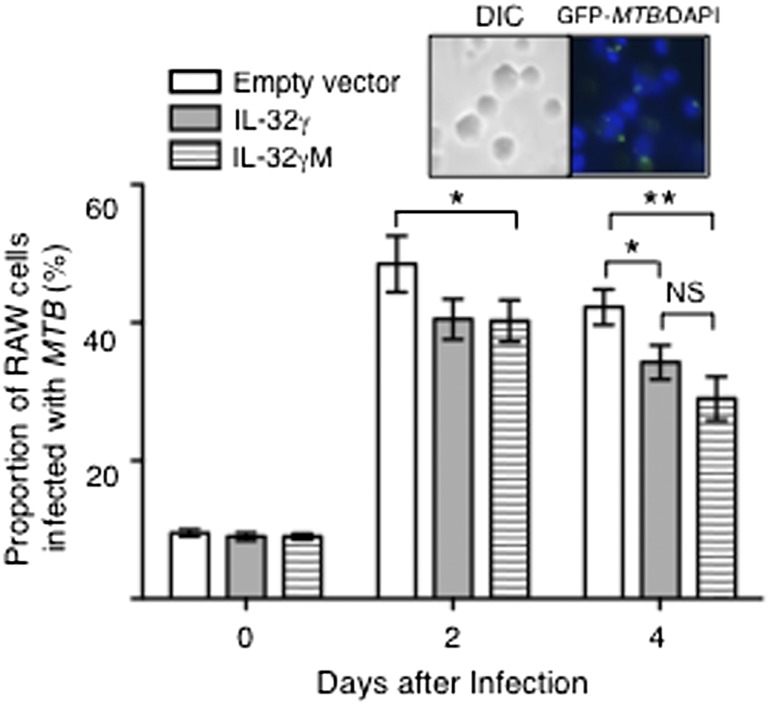
Wild-type IL-32γ is known to produce altenative mRNA splice variants, resulting in at least six isoforms of IL-32 (11). IL-32β is considered to be more immunosuppressive because it induces IL-10 (20). We have also found that the IL-32β transcript is expressed in the lungs of the SPC-IL-32γTg mice (Fig. S6). To determine if loss of splicing to produce IL-32β affects the anti-MTB capacity of macrophages, RAW 264.7 mouse macrophages were transfected with WT IL-32γ or IL-32γM, the latter having a mutation of the donor site (GU → AU)––such that splicing of the mRNA of IL-32γ to IL-32β is silenced (21). The cells were then infected with GFP-MTB H37Rv and the proportion of GFP-MTB–infected cells were quantified by fluorescent microscopy. As shown in Fig. S7, the transfected cells contained transcripts for IL-32γ. One hour after infection (day 0), there was no difference in the number of intracellular MTB (Fig. 5). Two days after infection, the percentage of IL-32γ or IL-32γM transfected cells infected with MTB was significantly lower than control cells. However, 4 d after infection, there was a trend toward further reduction in the percentage of cells infected with MTB in macrophages transfected with IL-32γM (Fig. 5).
Fig. 5.
Mouse macrophages transfected with IL-32γ mutated at the splice site (IL-32γM) possess greater anti-MTB activity than those transfected with WT IL-32γ. The proportion of GFP-MTB–infected RAW 264.7 cells transfected with empty vector or pCDNA-CMV containing the WT IL-32γ gene or the nonsplicable IL-32γ gene were determined after 1 h and 2 and 4 d after infection. Inset shows representative DIC and fluorescent microphotograph of GFP-MTB infected RAW 264.7 cells. Data shown are the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01.
IL-32 Is Induced in Primary Human Cells and Lung Tissues in Response to MTB Infection.
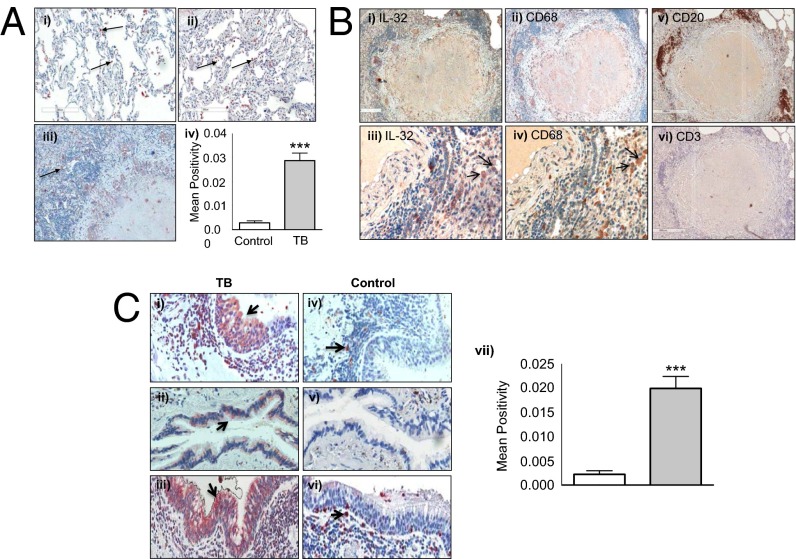
One key difference in the histopathologic lesions of murine and human TB is that infected mice do not develop necrotic granulomas that are seen in MTB-infected human tissues. To determine the extent and distribution of IL-32 expression in the lungs of patients with TB, we performed immunohistochemistry for IL-32 on surgically resected lung tissues from patients with active TB and without TB. Compared with lungs with normal histology (Fig. 6 A, i), IL-32 expression in the lung tissue samples obtained from patients with TB was significantly increased in both nongranulomatous pneumonitis (Fig. 6 A, ii) and necrotizing granulomatous pneumonitis (Fig. 6 A, iii). Morphometric quantitation revealed that IL-32 expression was significantly greater in TB lung tissues compared with control samples (Fig. 6 A, iv). In the inflammatory region of necrotizing granulomas, IL-32 was mostly expressed by macrophages and B cells (Fig. 6 B, i–v); interestingly, there were relatively few T cells in the walls of the granulomas (Fig. 6 B, vi). Examining serial lung tissue sections, we estimated by microscopy that of IL-32 positive cells, 48 ± 6% were macrophages, 22 ± 2% were airway epithelial cells, 21 ± 1% were B cells, and 9 ± 1% were T cells. Expression of IL-32 in the airway columnar epithelium is noticeably increased in TB-infected tissue specimens (Fig. 6 C, i–iii) compared with uninfected control tissue samples (Fig. 6 C, iv–vi).
Fig. 6.
IL-32 expression in histologically normal and TB human lung tissue samples. In representative (A, i) normal lung and (A, ii) TB lung showing alveolar macrophages (arrows) immunostained positive for IL-32 (original magnification, 200×). (A, iii) A region of a TB granuloma with central necrotic, surrounding inflammatory, and outer fibrotic zone (200×). (A, iv) The mean IL-32 positivity for the four control and seven TB lung samples. (B, i) A granuloma immunostained for IL-32 (original magnification, 40×). (B, ii) The same granuloma immunostained for CD68 (macrophages) (40×). A higher magnification of the granuloma wall showing IL-32 staining (arrows) (B, iii) is localized mainly to macrophages (arrows) (B, iv) (400×). (B, v and vi) A TB granuloma immunostained for CD20 and CD3, respectively (40×). (C, i–iii) Lung tissues of a patient with pulmonary TB stained for IL-32. (C, iv–vi) Histologically normal lung tissues stained for IL-32. Magnification in C, i, iii, iv, and vi is 400× and in C, ii and v, 200×. (C, vii) Quantification of IL-32 staining by morphometric analysis.
Given the increased IL-32 expression in the macrophages of TB-infected lung tissue, it was important to validate whether MTB can induce IL-32 expression in primary human cells. Thus, PBMCs were isolated from five healthy volunteers and either remained uninfected or infected with MTB H37Rv or W-Beijing HN878 at a multiplicity of infection (MOI) of 10 for 6 and 24 h. MTB significantly induced IL-32 expression with no difference in induction between the two MTB strains (Fig. S8).
Discussion
Cytokines and chemokines play essential roles in host defense against TB through various mechanisms including the influx and activation of immune cells, promoting phagosome maturation, inducing apoptosis or autophagy of infected phagocytic cells, and inducing the production of effector molecules such as nitric oxide, immunity-related GTPases, and antimicrobial peptides (16, 22, 23). IL-32 protects against MTB in THP-1 macrophages using some of these effector mechanisms (9). To elucidate the biological significance of IL-32 with MTB infection in vivo, we infected Tg mice that express human IL-32γ in the lungs with a hypervirulent, clinical strain of MTB (12). Whereas IL-32γ in this Tg strain is regulated by the SPC promoter and thus constitutively expressed by type II alveolar epithelial cells, IL-32 can be secreted and can act on other cell types (14, 15). There was reduced MTB burden in the lungs and spleens of SPC-IL-32γTg mice compared with WT mice. Whereas the relative reduction of MTB in the SPC-IL-32γTg mice was modest, the absolute reduction of bacilli is considerable. Furthermore, because C57BL/6 mice already have robust immunity, we did not necessarily expect that these mice made to additionally express IL-32 would have as significant a reduction in MTB compared with mice given effective anti-TB drugs. Although the severity and extent of the lung lesions at 2 mo after infection were not significantly different between the two mouse strains, mice expressing human IL-32γ had improved survival compared with WT mice.
In exploring the mechanisms by which human IL-32 exerts its protection, we observed a modest increase in the number of TNFα+ AMs and dendritic cells in the lungs of the SPC-IL-32γTg mice relatively early in the course of the infection but not at a later time point. There were consistent and sustained increases in IFNγ+CD4+, TNFα+CD4+, and IFNγ+CD8+ T cells as well as a modest increase of NK1.1+ cells in the lungs of the SPC-IL-32γTg mice. A similar pattern of increased TNFα- and IFNγ-positive CD4+ and CD8+ T cells was also seen in the mediastinal lymph nodes of the SPC-IL-32γTg mice although the effect was not as robust for TNFα+CD8+ T cells. These findings likely account for the protective effect of IL-32 and are also consistent with the ability of IL-32 to induce TNFα (3, 9) which, in turn, can induce IFNγ production. Schenk et al. (15) reported that with leprosy infection, IL-32 potently induced the differentiation of primary human monocytes to dendritic cells following stimulation with a NOD2 ligand. This observation is consistent with our finding of increased TNFα+ dendritic cells in the SPC-IL-32γTg mice. In ex vivo experiments, we also found greater colocalization of MTB with lysosomes by the AMs of Tg mice than WT AMs, indicating that enhanced disposal of MTB through the lysosomal pathway is one mechanism for the protective effect of IL-32γ; whether this effect is directly related to IL-32γ or other proinflammatory cytokines it induces such as TNFα remains to be determined.
What could account for the initial increase and then a fall in the number of host protective TNFα+ AMs and dendritic cells in the lungs of the infected SPC-IL-32γTg mice? Choi et al. studied dextran sodium sulfate (DSS)-induced colitis using another IL-32γTg strain in which the IL-32γ transgene was under the control of the β-actin promoter and thus expressed ubiquitously (20). They reported that IL-32 exacerbated DSS-induced colitis initially but at later time points, there was more rapid healing and less inflammation (20). This spontaneous recovery of IL-32–induced colitis was attributed to secondary induction of IL-10, a cytokine with antiinflammatory effects (20). The authors hypothesized that the increase in IL-10 was due to accumulation of IL-32β, a splice variant of IL-32γ that induces IL-10 (24), an antiinflammatory cytokine also known to predispose mice to MTB (25). Because the SPC-IL-32γTg mice also produce IL-32β, this may account for the trend toward increased IL-10-positive lung macrophages and dendritic cells in the SPC-IL-32γTg mice at day 60 of infection. Thus, the eventual accumulation of IL-32β and subsequent induction of IL-10 may have partially abrogated the protective, proinflammatory effect of IL-32γ in the Tg mice by day 60. This notion is supported by our finding that mouse macrophages transfected with IL-32γM in which mRNA splicing to produce IL-32β was silenced were better able to control MTB infection than macrophages transfected with WT IL-32γ.
Although excessive Treg influx has been shown to exacerbate TB infection (12), studies also show that in the presence of effective immunity, Tregs are required to curb the potentially damaging inflammatory response that is induced against MTB (26). Because we found increased Treg influx in the lymph nodes of the SPC-IL-32γTg mice, which may have been in response to the increased proinflammatory effects of IL-32γ, this increase in Tregs may have abrogated some of the protective effects of IL-32γ at the later time points.
Bao et al. reported elevated serum levels of IL-32 from patients with active pulmonary TB (27), supporting the evidence that IL-32 is also secreted. To help correlate the findings in the SPC-IL-32γTg mice with human tissues, we examined IL-32 expression in the lung tissue samples from patients with TB. Compared with histologically normal lungs, the resected TB lung samples showed significantly increased IL-32 expression. The predominant cell types that expressed IL-32 were macrophages and airway epithelial cells, similar to that found in the lung samples of patients with nontuberculous mycobacterial lung disease (6). The increased number of IL-32–positive cells in the TB lungs reflects the large number of epithelioid macrophages in the inflammatory and granulomatous responses. Because airway epithelial cells are innate immune cells capable of killing mycobacteria through phagocytosis and elaboration of antimicrobial peptides as well as stimulating IFNγ release by CD8+ T cells (28), it is plausible that increased expression of IL-32 in these cells also reflects a host-protective immune response against MTB. Montoya et al. recently showed in human macrophages that IL-32 promoted the production of both defensin-B and cathelicidin, antimicromial peptides with anti-MTB activity (29).
In summary, expression of human IL-32 in the lungs of mice is protective against MTB. This protection is likely due to increased influx of host-protective innate and adaptive immune cells. It is important to query whether Tg mice that express IL-32γ ubiquitously, such as the β-actin-IL-32γTg mice, would display even greater protection against MTB than the SPC-IL-32γTg mice or conversely, worse outcome due to excessive inflammation.
Materials and Methods
The SPC-IL-32γTg mice were generated at the Mouse Genetics Core facility at National Jewish Health (NJH). Aerosolized infection of the mice with MTB was performed in the Biosafety Level 3 facility at Colorado State University (CSU). All experimental protocols were approved by the animal care and use committees of NJH and CSU, and conform to National Institutes of Health guidelines. Details on the experimental methods and statistical analyses can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Stephan W. Glasser (University of Cincinnati) for providing the SPC expression vector pUC18SPC3.7, Drs. Carlyne Cool and Steve Groshong of National Jewish Health for advice on the use of the Aperio technology, and Drs. Su Young and Jida Choi for measuring the IL-32 levels in the lungs of the SPC-IL-32γTg mice. This study was funded by grants from the Department of Veterans Affairs Veterans Health Administration, Office of Research and Development 1 I01 BX001028-01A2 (to E.D.C.); National Institutes of Health (NIH) AI15614 (to C.A.D.); National Research Foundation of Korea (S.-H.K.); and NIH R21 AI081959, NIH Innovation Award 1DP2OD006450, and American Recovery and Reinvestment Act funds (to D.J.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424302112/-/DCSupplemental.
References
- 1.Gandhi NR, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 2.Diacon AH, et al. TMC207-C208 Study Group Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371(8):723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 3.Kim S-H, Han S-Y, Azam T, Yoon D-Y, Dinarello CA. Interleukin-32: A cytokine and inducer of TNFalpha. Immunity. 2005;22(1):131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Nold MF, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181(1):557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 5.Li W, et al. Negative feedback regulation of IL-32 production by iNOS activation in response to dsRNA or influenza virus infection. Eur J Immunol. 2009;39(4):1019–1024. doi: 10.1002/eji.200838885. [DOI] [PubMed] [Google Scholar]
- 6.Bai X, et al. IL-32 expression in the airway epithelial cells of patients with Mycobacterium avium complex lung disease. Int Immunol. 2011;23(11):679–691. doi: 10.1093/intimm/dxr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinhuis B, et al. Tumour necrosis factor alpha-driven IL-32 expression in rheumatoid arthritis synovial tissue amplifies an inflammatory cascade. Ann Rheum Dis. 2011;70(4):660–667. doi: 10.1136/ard.2010.139196. [DOI] [PubMed] [Google Scholar]
- 8.Joosten LA, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103(9):3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai X, et al. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J Immunol. 2010;184(7):3830–3840. doi: 10.4049/jimmunol.0901913. [DOI] [PubMed] [Google Scholar]
- 10.Netea MG, et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase- 1/IL-18/interferon-gamma-dependent mechanism. PLoS Med. 2006;3(8):e277. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JD, et al. Identification of the most active interleukin-32 isoform. Immunology. 2009;126(4):535–542. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ordway D, et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol. 2007;179(1):522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 13.Bae S, et al. Characterizing antiviral mechanism of interleukin-32 and a circulating soluble isoform in viral infection. Cytokine. 2012;58(1):79–86. doi: 10.1016/j.cyto.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Netea MG, et al. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105(9):3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk M, et al. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med. 2012;18(4):555–563. doi: 10.1038/nm.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salgame P, Bhatt K, Drage MG, Pecora ND, Harding CV. Mycobacterium tuberculosis interactions with dendritic cells and macrophages. In: Kaufmann SHE, Britton WJ, editors. Handbook of Tuberculosis: Immunology and Cell Biology. Wiley-VCH; Weinheim, Germany: 2008. pp. 45–59. [Google Scholar]
- 17.Grotzke JE, Lewinsohn DM. Role of CD8+ T lymphocytes in control of Mycobacterium tuberculosis infection. Microbes Infect. 2005;7(4):776–788. doi: 10.1016/j.micinf.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PF, Samten B, Shams H, Vankayalapatib R. Progress in understanding the human immune responses to Mycobacterium tuberculosis. Tuberculosis (Edinb) 2009;89(Suppl 1):S5–S9. doi: 10.1016/S1472-9792(09)70004-6. [DOI] [PubMed] [Google Scholar]
- 19.Shang S, et al. Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis (Edinb) 2011;91(5):378–385. doi: 10.1016/j.tube.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J, et al. Paradoxical effects of constitutive human IL-32gamma in transgenic mice during experimental colitis. Proc Natl Acad Sci USA. 2010;107(49):21082–21086. doi: 10.1073/pnas.1015418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinhuis B, et al. Inflammation-dependent secretion and splicing of IL-32gamma in rheumatoid arthritis. Proc Natl Acad Sci USA. 2011;108(12):4962–4967. doi: 10.1073/pnas.1016005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor GA. IRG proteins: Key mediators of interferon-regulated host resistance to intracellular pathogens. Cell Microbiol. 2007;9(5):1099–1107. doi: 10.1111/j.1462-5822.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 24.Kang JW, et al. A proinflammatory cytokine interleukin-32beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 2009;128(1) Suppl:e532–e540. doi: 10.1111/j.1365-2567.2008.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner J, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169(11):6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 26.Leepiyasakulchai C, Ignatowicz L, Pawlowski A, Källenius G, Sköld M. Failure to recruit anti-inflammatory CD103+ dendritic cells and a diminished CD4+ Foxp3+ regulatory T cell pool in mice that display excessive lung inflammation and increased susceptibility to Mycobacterium tuberculosis. Infect Immun. 2012;80(3):1128–1139. doi: 10.1128/IAI.05552-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao F, et al. Elevated levels of serum IL-32 in patients with active pulmonary tuberculosis. Afr J Microbiol Res. 2012;6:7292–7294. [Google Scholar]
- 28.Harriff MJ, et al. Human lung epithelial cells contain Mycobacteriumtuberculosis in a late endosomal vacuole and are efficiently recognized by CD8⁺ T cells. PLoS ONE. 2014;9(5):e97515. doi: 10.1371/journal.pone.0097515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montoya D, et al. 2014. IL-32 is a molecular marker of a host defense network in human tuberculosis. Sci Transl Med 6:250ra114.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.