Abstract
Background
The association between atopy and asthma is attenuated in non-affluent populations, an effect that may be explained by childhood infections such as geohelminths.
Objective
To investigate the association between atopy and wheeze in schoolchildren living in urban and rural areas of Ecuador and examine the effects of geohelminths on this association.
Methods
We performed nested case–control studies among comparable populations of schoolchildren living in rural communities and urban neighbourhoods in the Province of Esmeraldas, Ecuador. We detected geohelminths in stool samples, measured recent wheeze and environmental exposures by parental questionnaire, and atopy by specific IgE (sIgE) and skin prick test (SPT) reactivity to aeroallergens.
Results
Atopy, particularly sIgE to house dust mite (HDM), was more strongly associated with recent wheeze in urban than rural schoolchildren: (urban, adj. OR 5.19, 95% CI 3.37–8.00, P < 0.0001; rural, adj. OR 1.81, 95%CI 1.09–2.99, P = 0.02; interaction, P < 0.001). The population fractions of wheeze attributable to atopy were approximately two-fold greater in urban schoolchildren: SPT to any allergen (urban 23.5% vs. rural 10.1%), SPT to HDM (urban 18.5% vs. rural 9.6%), and anti-HDM IgE (urban 26.5% vs. rural 10.5%), while anti-Ascaris IgE was related to wheeze in a high proportion of rural (49.7%) and urban (35.4%) children. The association between atopy and recent wheeze was attenuated by markers of geohelminth infections.
Conclusions
Our data suggest that urban residence modifies the association between HDM atopy and recent wheeze, and this effect is explained partly by geohelminth infections.
Keywords: atopy, geohelminths, house dust mite, Latin America, rural, tropics, urban, wheeze
Introduction
Atopy is a consistently strong risk factor for asthma in industrialized countries [1, 2], but this association appears to be weaker [3–6] in non-industrialized countries. A predominance of non-atopic over atopic wheeze/asthma has been observed in several Latin American countries including Brazil [3, 4, 7], Peru [8], and Ecuador [9].
The association between atopy and allergic disease appears to be stronger in affluent compared to non-affluent [6] and in urban compared to rural populations [10, 11]. Urban–rural differences in the prevalence of environmental exposures that attenuate atopy could explain these observations. Examples of environmental exposures that may suppress atopy include household pets [12], farming exposures [13, 14], and infections during childhood [15]. Geohelminth infections, that are common chronic parasitic infections of predominantly low-income rural populations in the tropics, may have a role in attenuating the association between atopy and allergic symptoms [16] including wheeze [17, 18].
The aim of this study was to investigate if associations between atopy and recent wheeze are modified by rural vs. urban residence and to explore which environmental exposures including geohelminth infections might contribute to such an effect. To do this, we conducted nested case–control studies comparing schoolchildren living in rural communities, as reported previously [17], with urban neighbourhoods in the Province of Esmeraldas in Ecuador.
Methods
Study population and design
Cross-sectional surveys were carried out between March 2005 and January 2010 in schoolchildren living in rural and urban areas in the Province of Esmeraldas, the northernmost province on Ecuador's Pacific coast. The population of Esmeraldas Province in 2010 was 534 092 inhabitants (80% of African descent) of whom 189 504 were located in the Provincial capital, City of Esmeraldas (INEC, 2010). The Province of Esmeraldas is at sea level with an average annual temperature of 28 C and 80% relative humidity. In the City of Esmeraldas, the major sources of income are a port, commercial activities, and an oil refinery, while in the rural area, fishing, subsistence agriculture, logging, and palm oil extraction are the main economic activities. Study neighbourhoods in the City of Esmeraldas were located in the periphery of the city in areas where Afro-Ecuadorian migrants tended to have settled from the rural study Districts in Esmeraldas Province. Further details of the study population and study rationale are provided elsewhere [19].
Two case–control studies were conducted between November 2007 and February 2010, nested within the respective urban and rural cross-sectional surveys. Cases were defined as children with parentally reported recent wheeze (i.e. wheeze in the previous 12 months). Controls were a random sample of those without a history of wheeze ever. At the time of selection of cases and controls, a brief screening questionnaire was administered again to parents to confirm their child's case or control status. Four controls for each case were selected using computer generated random lists. All study measurements were carried out using the same standardized methodologies and materials in both case–control studies by the same field and laboratory observers.
Questionnaires
Data on risk factors were collected by a questionnaire that was administered in Spanish to the child's parent or guardian and which included the core allergy symptom questions of the ISAAC phase II study [20].
IgE measurements
Blood samples were collected, and plasma was stored at −20°C until testing. Total IgE and IgE antibodies specific for Dermatophagoides pteronyssinus, Periplaneta americana (American cockroach) and Ascaris lumbricoides were measured using the Pharmacia CAP system (Phadia AB, Uppsala, Sweden) according to the manufacturer's instructions.
Allergen skin prick testing
Skin prick testing were performed with seven allergen extracts (Greer laboratories, Lenoir, NC, USA): Dermatophagoides pteronyssinus/farinae mix, American cockroach (Periplaneta americana), Alternaria tenuis, cat, dog, ‘9 southern grass mix’ and ‘New stock fungi mix’, positive histamine, and negative saline controls. A positive reaction was defined as a mean wheal diameter at least 3 mm greater than the saline control 15 min after pricking the allergen onto the forearm.
Exercise-induced bronchospasm
Each child underwent a vigorous 6-min free running exercise with forced expiratory volume in 1 s (FEV1) measured before and 5 min after exercise as described previously [17]. The best of five efforts was used to measure Exercise-induced bronchospasm (EIB): [(Pre-exercise FEV1–Post-exercise FEV1)/(Pre-exercise FEV1)] × 100. EIB was defined as a fall of 10% in FEV1 after exercise.
Stool examinations
Single stool samples were collected and analysed for geohelminth eggs and larvae using the modified Kato Katz and formol-ether concentration methods [21].
Statistical analysis
In pilot studies, we estimated the prevalence of recent wheeze in the rural and urban areas to be 7.3% and 13.5%, respectively, and that a sample of 4000 children in the rural area and 2500 children in the urban area would be sufficient to yield 200 wheeze cases and 800 non-wheeze controls for the two case–control studies. Such a sample size would have 80% power at P < 0.05 to detect an effect on wheeze of OR<0.6 for common exposures (40–60% prevalence), and OR<0.4 for rare exposures (10%) [19]. Geohelminth infection was defined as the presence of at least one geohelminth parasite in a stool sample. Associations between risk factors and recent wheeze were evaluated by logistic regression, separately for urban and rural studies, with robust standard errors allowing for clustering by neighbourhood or community. Age, gender, and maternal education level were included as a priori confounders, and other covariates that were significant in univariate analyses were included also in multivariate models. The associations between atopic markers and recent wheeze were estimated after stratifying urban and rural studies by geohelminth infection status and other key variables. Interaction effects were assessed using Wald tests. Population-attributable fractions (PAFs) were calculated using the formula P x (OR-1)/OR where P is the prevalence of SPT or allergen-specific IgE among cases. Analyses were performed using Stata version 9.
Results
Selection of study populations
Case–control studies were nested in cross-sectional surveys of 3960 schoolchildren in rural communities and 2275 in urban neighbourhoods of the city of Esmeraldas. The age of study participants ranged from 6 to 19 years. For the present analysis, we included subjects for whom complete data were available for wheeze and allergen-specific IgE from both case–control studies: 149 cases and 227 controls in the rural and 104 cases and 120 controls in the urban case–control study. A flow diagram of selection of subjects for this analysis is provided in Fig.1.
Figure 1.
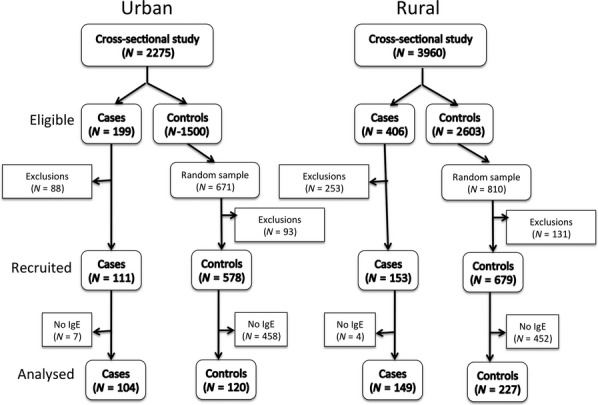
Flow diagram for selection of cases and controls into urban and rural case–controls studies. Eligible cases and controls were those identified as wheezers and non-wheezers in the respective cross-sectional studies who were then evaluated for inclusion in the case–control studies. A random sample of at least four controls was selected for each recruited case. Numbers of recruited cases and controls are shown (Recruited) and those included in the present analysis (Analysed) were those with IgE measurements. Reasons for exclusions from among those eligible were change of wheeze status between cross-sectional and case–control studies, change of address, or unable to contact.
Factors associated with wheeze
The distributions of risk factors for cases and controls in each of the rural and urban studies are shown in Tables1 and 2, and results of adjusted analyses are shown in Table3. After adjustment for confounders, maternal asthma remained strongly associated with recent wheeze in both study areas. Having a dog living inside the house was a significant risk factor for wheeze only in the urban study (P = 0.03). Recent anthelmintic treatment was associated with an increased risk of wheeze in both studies although this was only significant in the rural area (P = 0.01). The presence of anti-Ascaris IgE was a strong risk factor for wheeze in both urban (P < 0.0001) and rural (P < 0.0001) areas. SPT to any allergen was associated with recent wheeze in both areas although the association was stronger in the urban area (urban adj. OR 5.51 [95% CI 1.82–16.60, P = 0.003] vs. rural adj. OR 2.0 [1.05–3.82, P = 0.03]). Similarly, a stronger association was observed between recent wheeze and SPT to HDM in the urban (adj. OR 5.32, 95% CI 2.31–12.25, P < 0.0001) compared to rural (adj. OR 3.07, 95% CI 1.41–6.67, P = 0.005) areas, and there was also a trend of an association between SPT to cockroach and wheeze in the urban but not the rural area. The association between the presence of anti-HDM IgE and wheeze was much stronger in the urban (adj. OR 5.19, 95% CI 3.37–8.00, P < 0.0001) than rural areas (adj. OR 1.81, 95%CI 1.09–2.99, P = 0.02), and a significant interaction by area was observed (P = 0.001).
Table 1.
Distributions of sociodemographic, hygiene, and other relevant factors between wheeze cases and non-wheeze controls by area of residence. P values were calculated using the χ2 test adjusted for clustering. Monthly income represents monthly household income stratified according to the minimum wage of US$250. Recent anthelmintics – anthelmintic treatment in the previous 12 months. Missing values for urban/rural areas were as follows: age (0/2), monthly income (38/8), maternal educational level (5/4), current household smoker (3/3), breastfeeding (21/13), dog inside house (2/0), cat inside house (1/0), and farm animal contact (1/0)
| Variable | Rural |
P value | Urban |
P value | ||
|---|---|---|---|---|---|---|
| Non-wheeze controls N = 227 | Wheeze cases N = 149 | Non-wheeze controls N = 120 | Wheeze cases N = 104 | |||
| Sociodemographics | ||||||
| Age (years) | ||||||
| 6–10 | 56 (24.67) | 59 (39.6) | 90 (75) | 71 (69.61) | ||
| 11–19 | 171 (75.33) | 90 (60.4) | 0.002 | 30 (25) | 31 (30.39) | 0.37 |
| Gender | ||||||
| Female | 99 (43.61) | 66 (44.30) | 51 (42.50) | 49 (47.12) | ||
| Male | 128 (56.39) | 83 (55.70) | 0.85 | 69 (57.50) | 55 (52.88) | 0.48 |
| Maternal education level | ||||||
| Complete secondary | 16 (7.21) | 14 (9.40) | 34 (28.81) | 34 (33.33) | ||
| Complete primary | 94 (42.34) | 55 (36.91) | 60 (50.85) | 51 (50) | ||
| Illiterate/incomplete primary | 112 (50.45) | 80 (53.69) | 0.53 | 24 (20.34) | 17 (16.67) | 0.682 |
| Monthly income | ||||||
| ≤250 USD | 160 (76.92) | 94 (71.21) | 68 (58.62) | 56 (56) | ||
| >250 USD | 48 (23.08) | 38 (28.79) | 0.238 | 48 (41.38) | 44 (44) | 0.698 |
| Household electric appliances | ||||||
| 0–2 | 143 (63) | 108 (72.48) | 42 (35) | 52 (50) | ||
| 3–4 | 84 (37) | 41 (27.52) | 0.058 | 78 (65) | 52 (50) | 0.023 |
| Hygiene exposures | ||||||
| Birth order | ||||||
| 0–2 | 78 (34.4) | 59 (39.60) | 71 (59.17) | 55 (53.40) | ||
| 3–4 | 69 (30.4) | 51 (34.23) | 31 (25.83) | 27 (26.21) | ||
| >4 | 80 (35.2) | 39 (26.17) | 0.2 | 18 (15) | 21 (20.39) | 0.535 |
| Dog inside house | ||||||
| No | 112 (49.56) | 60 (40.54) | 91 (75.83) | 58 (55.77) | ||
| Yes | 114 (50.44) | 88 (59.46) | 0.08 | 29 (24.17) | 46 (44.23) | 0.002 |
| Cat inside house | ||||||
| No | 138 (60.79) | 100 (67.57) | 73 (60.83) | 62 (59.62) | ||
| Yes | 89 (39.21) | 48 (32.43) | 0.176 | 47 (39.17) | 42 (40.38) | 0.85 |
| Farm animal contact | ||||||
| No | 155 (68.28) | 104 (70.27) | 117 (97.50) | 99 (95.19) | ||
| Yes | 72 (31.72) | 44 (29.73) | 0.597 | 3 (2.50) | 5 (4.81) | 0.353 |
| Other relevant exposures | ||||||
| Breastfeeding | ||||||
| ≤6 months | 11 (5.12) | 6 (4.26) | 4 (3.54) | 11 (11.22) | ||
| >6 months | 204 (94.88) | 135 (95.74) | 0.717 | 109 (96.46) | 87 (88.78) | 0.03 |
| Current household smoker | ||||||
| No | 143 (63) | 94 (63.95) | 88 (73.95) | 73 (71.57) | ||
| Yes | 84 (37) | 53 (36.05) | 0.85 | 31 (26.05) | 29 (28.43) | 0.692 |
| Maternal asthma | ||||||
| No | 186 (86.51) | 79 (54.48) | 107 (90.68) | 56 (55.45) | ||
| Yes | 29 (13.49) | 66 (45.52) | <0.0001 | 11 (9.32) | 45 (44.55) | 0.0001 |
| Recent anthelmintics | ||||||
| No | 117 (52.70) | 52 (34.90) | 29 (24.37) | 14 (13.73) | ||
| Yes | 105 (47.30) | 97 (65.10) | 0.001 | 90 (75.63) | 88 (86.27) | 0.046 |
Table 2.
Distributions of helminthic infections, atopy markers, and exercise-induced bronchospasm between wheeze cases and non-wheeze controls by area of residence. P values were calculated using the χ2 test adjusted for clustering. A. lumbricoides – infections with Ascaris lumbricoides. T. trichiura – infections with Trichuris trichiura. SPT – allergen skin test reactivity. HDM – to D. pteronyssinus/D. farinae mix. Missing values for rural/urban areas were as follows: geohelminth infections (14/7), SPT to any allergen and HDM (3/3), SPT to cockroach (3/4), and exercise-induced bronchospasm (13/3)
| Rural | P value | Urban | P value | |||
|---|---|---|---|---|---|---|
| Variable | Non-wheeze controls N = 227 | Wheeze cases N = 149 | Non-wheeze controls N = 120 | Wheeze cases N = 104 | ||
| Helminth markers | ||||||
| Any geohelminth infection | ||||||
| Negative | 61 (27.60) | 39 (27.46) | 72 (61.54) | 56 (56) | ||
| Positive | 160 (72.40) | 103 (72.54) | 0.7 | 45 (38.46) | 44 (44) | 0.408 |
| A. lumbricoides | ||||||
| Negative | 116 (52.49) | 77 (54.23) | 94 (80.34) | 86 (86) | ||
| Positive | 105 (47.51) | 65 (45.77) | 0.8 | 23 (19.66) | 14 (14) | 0.269 |
| T. trichura | ||||||
| Negative | 92 (41.63) | 60 (41.96) | 86 (73.50) | 61 (61) | ||
| Positive | 129 (58.37) | 82 (57.75) | 0.95 | 31 (26.50) | 39 (39) | 0.05 |
| Anti-Ascaris IgE | ||||||
| <0.70 KU/L | 97 (42.73) | 33 (22.3) | 91 (75.83) | 51 (49.04) | ||
| ≥0.70 KU/L | 130 (57.27) | 115 (77.7) | < 0.0001 | 29 (24.17) | 53 (50.96) | 0.0001 |
| Atopy | ||||||
| SPT to any allergen | ||||||
| Negative | 194 (85.46) | 118 (79.73) | 110 (91.67) | 72 (71.29) | ||
| Positive | 33 (14.54) | 30 (20.27) | 0.147 | 10 (8.33) | 29 (28.71) | < 0.0001 |
| SPT to HDM | ||||||
| Negative | 209 (92.07) | 127 (85.81) | 112 (93.33) | 78 (77.23) | ||
| Positive | 18 (7.93) | 21 (14.19) | 0.05 | 8 (6.67) | 23 (22.77) | 0.001 |
| SPT to cockroach | ||||||
| Negative | 212 (93.39) | 138 (93.24) | 118 (98.33) | 93 (93) | ||
| Positive | 15 (6.61) | 10 (6.76) | 0.955 | 2 (1.67) | 7 (7) | 0.047 |
| Anti-HDM IgE | ||||||
| <0.70 KU/L | 191 (83.41) | 114 (76.51) | 107 (89.17) | 70 (67.31) | ||
| ≥0.70 KU/L | 38 (16.59) | 34 (22.49) | 0.13 | 13 (10.83) | 34 (32.69) | < 0.0001 |
| Anti-cockroach IgE | ||||||
| <0.70 KU/L | 197 (86.03) | 122 (81.88) | 104 (86.67) | 83 (79.81) | ||
| ≥0.70 KU/L | 31 (13.66) | 26 (17.57) | 0.30 | 16 (13.33) | 21 (20.19) | 0.168 |
| Exercise-induced bronchospasm | ||||||
| No | 213 (96.4) | 122 (85.9) | 91 (75.8) | 70 (69.3) | ||
| Yes | 8 (3.6) | 20 (14.1) | < 0.0001 | 29 (24.2) | 31 (30.7) | 0.28 |
Table 3.
Multivariable analysis of factors associated with recent wheeze. PAF% – population-attributable fraction. Interaction P value is for interaction by area of residence. A. lumbricoides – infections with Ascaris lumbricoides. T. trichiura – infections with Trichuris trichiura. Recent anthelmintics – anthelmintic treatment in the previous 12 months. SPT – allergen skin test reactivity. HDM – to D. pteronyssinus/D. farinae mix. Estimates of effect with P < 0.05 are shown in bold. ORs were estimated using logistic regression and were adjusted also for monthly income, number of domestic electric appliances, house construction materials, and frequency of physical exercise. Variables for atopy were excluded from the estimation of the associations between geohelminth infections and wheeze because of possible effect modification
| Variable | RURAL |
URBAN |
Interaction P value | ||||
|---|---|---|---|---|---|---|---|
| Adjusted OR | P value | PAF% | Adjusted OR | P value | PAF% | ||
| Sociodemographics | |||||||
| Age (years) | |||||||
| 6–10 | 1 | 1 | |||||
| 11–19 | 0.67 (0.43–1.04) | 0.08 | 1.13 (0.72–1.77) | 0.57 | 0.08 | ||
| Gender | |||||||
| Female | 1 | 1 | |||||
| Male | 0.79 (0.49–1.27) | 0.34 | 0.77 (0.46–1.28) | 0.32 | 0.94 | ||
| Hygiene exposures | |||||||
| Dog inside house | |||||||
| No | 1 | 1 | |||||
| Yes | 1.49 (0.89–2.49) | 0.13 | 2.37 (1.09–5.12) | 0.03 | 0.31 | ||
| Any geohelminth infection | |||||||
| No | |||||||
| Yes | 1.21 (0.68–2.18) | 0.52 | 1.22 (0.56–2.66) | 0.62 | 0.99 | ||
| A. lumbricoides | |||||||
| No | 1 | 1 | |||||
| Yes | 1.04 (0.64–1.69) | 0.85 | 0.83 (0.46–1.48) | 0.53 | 0.56 | ||
| T. trichura | |||||||
| No | 1 | 1 | |||||
| Yes | 1.09 (0.58–2.03) | 0.783 | 1.70 (0.78–3.69) | 0.177 | 0.36 | ||
| Anti-Ascaris IgE | |||||||
| <0.70 KU/L | 1 | 1 | |||||
| ≥0.70 KU/L | 2.76 (1.61–4.73) | <0.0001 | 49.7 | 3.33 (1.84–6.04) | < 0.0001 | 35.4 | 0.64 |
| Other relevant exposures | |||||||
| Maternal asthma | |||||||
| No | 1 | 1 | |||||
| Yes | 5.57 (3.25–9.53) | <0.0001 | 6.51 (2.66–15.9) | < 0.0001 | 0.78 | ||
| Recent anthelmintics | |||||||
| No | 1 | 1 | |||||
| Yes | 1.85 (1.14–3.0) | 0.01 | 1.73 (0.94–3.19) | 0.08 | 0.87 | ||
| Atopy | |||||||
| SPT to any allergen | |||||||
| No | 1 | 1 | |||||
| Yes | 2.0 (1.05–3.82) | 0.03 | 10.1 | 5.51 (1.82–16.6) | 0.003 | 23.5 | 0.11 |
| SPT to HDM | |||||||
| No | 1 | 1 | |||||
| Yes | 3.07 (1.41–6.67) | 0.005 | 9.6 | 5.32 (2.31–12.25) | < 0.0001 | 18.5 | 0.36 |
| SPT to cockroach | |||||||
| No | 1 | 1 | |||||
| Yes | 0.96 (0.32–2.85) | 0.95 | 0.3 | 4.73 (0.88–25.41) | 0.069 | 5.5 | 0.09 |
| Anti-HDM IgE | |||||||
| <0.70 KU/L | 1 | 1 | |||||
| ≥0.70 KU/L | 1.81 (1.09–2.99) | 0.02 | 10.5 | 5.19 (3.37–8.0) | < 0.0001 | 26.5 | 0.001 |
| Anti-cockroach IgE | |||||||
| <0.70 KU/L | 1 | 1 | |||||
| ≥0.70 KU/L | 1.41 (0.72–2.74) | 0.31 | 5.3 | 1.30 (0.68–2.45) | 0.416 | 4.7 | 0.86 |
Proportion of wheeze attributable to atopy
A high proportion of wheeze was attributable to the presence of anti-Ascaris IgE in both studies (rural 49.7% vs. urban 35.4%). A higher proportion of wheeze was explained by atopy in urban compared to rural studies: SPT to any allergen (urban 23.5% vs. rural 10.1%), SPT to HDM (urban 18.5% vs. rural 9.6%), anti-HDM IgE (urban 26.5% vs. rural 10.5%), and any allergen-specific IgE (urban 29.7% rural 13.1%).
Do environmental exposures explain the stronger association between anti-HDM IgE and recent wheeze among urban children?
Associations between atopy and wheeze were stronger in the urban compared to rural studies, and this effect was particularly marked for anti-HDM IgE for which a significant interaction effect by area was observed (Table3). To try to understand this better, we explored if environmental exposures such as geohelminth infections might be relevant. The findings are provided in Table4 in which associations between recent wheeze and the presence of anti-HDM IgE are shown for each level of environmental exposures of interest with interactions by area of residence. 1) Dogs inside the house – the association appeared to be weaker among children with household dogs but there was no evidence of interaction by area. 2) Recent anthelmintic treatment (i.e. within the previous 12 months) had a much stronger association, but only among urban children (interaction P < 0.001). 3) Monthly income – a stronger effect was seen among urban but not rural children living in households with a monthly income greater than the minimum wage (interaction P = 0.02). 4) Geohelminth infections – the association was stronger in children without active geohelminth infections, and this effect was stronger in urban than rural children (interaction P = 0.04). 5) Anti-Ascaris IgE – stronger associations were observed in urban and rural children without anti-Ascaris IgE, and among children with anti-Ascaris IgE, there was some evidence of a greater association in urban but not rural children (interaction P = 0.03). 6) Stratification into four groups by the presence or absence of the two markers for Ascaris infection showed a strong association between wheeze and anti-HDM IgE among children with neither infection marker although there was no statistical evidence of interaction by area.
Table 4.
Associations between recent wheeze and the presence of anti-HDM IgE stratified by key environmental exposures. Ascaris +/−, presence or absence of A. lumbricoides eggs in a stool sample. Anti-Ascaris IgE +/−, presence or absence of anti-Ascaris IgE ≥0.7 kU/L. ORs are adjusted for age, gender, maternal educational level, maternal asthma, monthly income (above vs. below the minimum wage) and recent anthelmintic treatment. P values are for interaction tests by area of residence. Individual ORs in bold have P < 0.05 – cells too small to allow estimation.
| Exposure | ORs for associations between anti-HDM IgE and recent wheeze by relevant exposures |
||
|---|---|---|---|
| Rural OR (95% CI) | Urban OR (95% CI) | Interaction P value | |
| Dog inside house | |||
| No | 2.60 (1.11–6.13) | 3.88 (1.94–7.75) | 0.39 |
| Yes | 1.22 (0.61–2.43) | – | – |
| Recent anthelmintic treatment | |||
| No | 2.01 (1.02–3.95) | 1.63 (0.21–12.7) | 0.71 |
| Yes | 1.46 (0.76–2.82) | 6.18 (4.34–8.79) | < 0.0001 |
| Monthly Income | |||
| ≤250 USD | 1.73 (0.9–3.31) | 3.24 (1.22–8.53) | 0.27 |
| >250 USD | 1.47 (0.48–4.44) | 6.12 (3.45–10.8) | 0.02 |
| Geohelminth exposures | |||
| Geohelminth infection | |||
| No | 2.62 (1.16–5.91) | 6.84 (4.72–9.9) | 0.04 |
| Yes | 1.38 (0.77–2.44) | 2.09 (0.38–11.5) | 0.75 |
| Anti-Ascaris IgE | |||
| <0.70 KU/L | 8.02 (1.37–46.9) | 3.59 (2.41–5.35) | 0.47 |
| ≥0.70 KU/L | 1.02 (0.6–1.75) | 2.86 (0.6–9.52) | 0.03 |
| Combinations of variables | |||
| Ascaris −/Anti-Ascaris IgE − | 9.33 (2.17–40.0) | 3.87 (2.18–6.88) | 0.25 |
| Ascaris +/Anti-Ascaris IgE − | 1.13 (0.18–6.90) | – | – |
| Ascaris −/Anti-Ascaris IgE + | 1.26 (0.49–3.20) | 2.92 (1.13–7.52) | 0.20 |
| Ascaris +/Anti-Ascaris IgE + | 0.79 (0.31–1.97) | 2.0 (0.33–11.9) | 0.38 |
Discussion
We have reported previously the findings of the rural case–control study [17] and now extend these observations to investigate the association between atopy and recent wheeze and the factors that modify this association by comparison with an urban case–control study. The case–control studies were conducted among comparable samples of schoolchildren using the same methodology in urban and rural areas within the same region in tropical Latin America. Our data show that the association between atopy, particularly when measured by the presence of specific IgE to house dust mite (HDM), and recent wheeze is significantly stronger in urban compared to rural children and that this effect may be explained, at least partly, by reduced exposures to or infections with geohelminth parasites in the urban population.
We explored the role of several possible exposures that might explain the apparent dissociation between atopy and wheeze in rural compared to urban populations. Exposures associated with socio-economic development have been suggested to have a role in strengthening the association between atopy and wheeze/asthma [6, 10], a factor that could underlie the increased prevalence of asthma in affluent compared to non-affluent regions [22]. A study of schools serving economically distinct populations in Kumasi, Ghana observed a higher frequency of specific IgE to HDM and also higher antibody titres among affluent cases compared to controls or cases from less-affluent schools [23]. In the present study, there was an association between recent wheeze and anti-HDM IgE among children from more affluent urban households in low-income urban neighbourhoods but not among their more affluent counterparts in the rural population. We also examined the effects on this association of hygiene-associated exposures that were significantly associated with recent wheeze in the adjusted analysis. We observed that the association between anti-HDM IgE and recent wheeze was strongest among: 1) urban children who had received recent anthelmintic treatments; 2) children who were free of active geohelminth particularly in the urban area; 3) children who were negative for anti-Ascaris IgE; and 4) children who were negative for both geohelminth infection markers. These data point to an absence of geohelminth infection or of a measurable immune response to geohelminth infections (i.e. anti-Ascaris IgE) as being an important determinant of the strength of the association between HDM atopy and wheeze. Conversely, geohelminth infections or infection markers may contribute to the attenuation of this association. Recent anthelmintic treatment and being free of active geohelminths, in the context of lower rates of exposure in the urban area, may be similar measures for a low risk of infection at any point in time. In contrast in the rural area, where rates of infection are high, recent anthelmintic treatment is generally not associated with geohelminth infection status. Consistent with our study findings, a case–control study of adults in Ethiopia showed a stronger association between SPT to D. pteronyssinus and wheeze in urban compared to rural populations despite a higher prevalence of SPT in the rural population, and attributed this effect to the presence of high-intensity parasite infection in the rural population particularly with hookworm [18]. Further, the presence of specific IgE to aeroallergens was associated with rhinitis in uninfected Sri Lankan children, while no association was observed in those with geohelminths [16]. Overall, our data provide further support for the hypothesis that geohelminth infections are responsible, at least partly, for the weaker association observed between atopy and wheeze in some non-affluent populations.
There are several possible explanations for the attenuating effects of geohelminth infections or immunological markers of infection on the association between anti-HDM IgE and recent wheeze. Firstly, geohelminth infections have been consistently associated with a reduced prevalence of SPT [24] although the prevalence of allergen-specific IgE may not be affected [25], indicating that geohelminths may affect the ability of effector cells such as mast cells to respond to an allergen stimulus rather than the process of allergen sensitization. A mechanism by which geohelminth infections might modulate effector cells of the immediate hypersensitivity response is through the induction of immune regulatory cytokines such as IL-10 that are associated with chronic infections with A. lumbricoides and T. trichiura [7, 26]. Alternatively, the strong attenuating effects of anti-Ascaris IgE observed in the rural population could be explained by immunologic cross-reactivity between Ascaris antigens and HDM. Extensive immunological cross-reactivity has been reported between aeroallergens and helminths including Ascaris [27, 28].
However, in a previous analysis of data from the rural case–control study [17], we surmised that cross-reactivity between HDM and Ascaris was unlikely explanation because the effect of anti-Ascaris IgE on wheeze was not altered by adjustment for anti-HDM. Similarly, adjustment for anti-HDM IgE in the urban case–control study did not materially affect the OR for the association between anti-Ascaris IgE and wheeze (data not shown).
Most wheeze was not associated with atopy to aeroallergens in rural and urban case–control studies. The fraction of wheeze attributable to SPT in the rural study was similar to a previous estimate of 11% from a different population in rural Ecuador [9]. However, PAFs observed in urban children were twice those estimated for rural children for all markers of atopy. PAFs for allergen-specific IgE were consistently greater than those for SPT. The PAF for allergen-specific IgE (both HDM and cockroach) in the urban study (23.5%) was similar to that reported (24%) in a study of younger children (range 4–11 years) in urban Brazil [3]. These values, although lower than those reported from affluent countries [24], are consistent with estimates from other non-affluent countries. For example, the ISAAC Phase II study that included 22 countries estimated the PAF for wheeze associated with SPT and allergen-specific IgE to be 40.7% and 45.6%, respectively, in affluent countries, but 20.3% and 18.3%, respectively, in non-affluent countries [9].
A high proportion of wheeze appeared to be explained by the presence of anti-Ascaris IgE in both rural (49.7%) and urban (35.4%) studies, proportions that were somewhat greater than the fractions explained by specific IgE to aeroallergens such as dust mite. A high proportion of wheeze in our study population may be explained by allergic-type responses to the presence of Ascaris larvae migrating through the lungs [24]. Such responses may be protective and limit the establishment of infections in the human host but may cause pulmonary inflammation and wheezing illness. It is possible that with declining exposures to geohelminths such as occurs in many urban populations, that allergic responses may become redirected to ubiquitous aeroallergens. A study of children aged 6–12 years in Venezuela [29] provided evidence that anti-Ascaris IgE was a risk factor for bronchial hyper-reactivity (BHR) in rural children where geohelminth prevalence was high, while among urban children with a low prevalence of geohelminths, anti-HDM IgE, but not anti-Ascaris IgE, was associated with BHR. Similarly, a study of Kenyan children aged 8–13 years showed that in a rural area, SPT to HDM was not associated with exercise induce bronchospasm (EIB), while among urban children it was a risk factor for EIB [30].
Strengths of the study include the measurement of allergen-specific IgE and SPT to the dominant allergens in the study population based on extensive previous studies in Ecuador [6, 9, 17, 31]. We can be confident, therefore, that we detected >90% of atopy by the measurement of specific IgE to house dust mite and cockroach. By repeating the parental questionnaire for inclusion in the case–control study, our wheeze cases were defined by the presence of persistent wheeze, perhaps a more specific marker for asthma illness. Sample sizes for the case–control studies were smaller than originally planned resulting in reduced power to detect associations, particularly for tests of interaction, and we cannot exclude type 2 statistical errors. However, we were able to detect significant associations between atopy and wheeze in urban and rural studies and interaction by study area, the primary study analyses, and hence had sufficient power at least for these. The use of questionnaire data to measure most study exposures may be subject to recall bias. The process of inclusion based on subjects with IgE data could have led to selection bias – however, we believe this unlikely because estimates for the associations between SPT and recent wheeze by study area for the whole case–control samples were not materially different from those obtained for the samples analysed in the present study (data not shown).
In summary, we have analysed data from case–control studies conducted in comparable populations of urban and rural schoolchildren in tropical Latin America and show that the association between IgE sensitization to house dust mite and wheeze is stronger in urban compared to rural populations, and that the attenuation of this association, particularly in rural populations, may be explained by a higher prevalence of geohelminths or IgE sensitization to Ascaris.
Acknowledgments
The Ecuadorian Elimination Programme for Onchocerciasis (Dr Eduardo Gomez, Lcda. Raquel Lovato, Lcda. Margarita Padilla, Lcda. Anabel Ponce, Ing. Sandra Barreno, Magdalena Cortez), CECOMET (Lcda. Monica Marquez), and the provincial offices of the Ministry of Public Health and the Sistema Nacional para la Erradicación de la Malaria (Dr César Díaz) are thanked for their support in visiting of rural communities and urban neighbourhoods. The health promoters, schoolteachers, parents, and children are thanked for their enthusiastic cooperation. The study forms part of the SCAALA (Social Changes, Asthma, and Allergies in Latin America) programme of research. The study was supported by: Wellcome Trust, UK, HCPC Latin America Centres of Excellence Programme (grant 072405/Z/03/Z); PJC is supported by the Wellcome Trust (grant 088862/Z/09/Z); and TAEPM is supported by NIH grant AI-20565. The funders had no role in the study design, and analysis of or decision to publish these data.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 2.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 3.Souza da Cunha S, Barreto ML, Fiaccone RL, et al. Asthma cases in childhood attributed to atopy in tropical area in Brazil. Rev Panam Salud Publica. 2010;28:405–11. doi: 10.1590/s1020-49892010001200001. [DOI] [PubMed] [Google Scholar]
- 4.Pereira MU, Sly PD, Pitrez PM, et al. Nonatopic asthma is associated with helminth infections and bronchiolitis in poor children. Eur Respir J. 2007;29:1154–60. doi: 10.1183/09031936.00127606. [DOI] [PubMed] [Google Scholar]
- 5.Nyan OA, Walraven GE, Banya WA, et al. Atopy, intestinal helminth infection and total serum IgE in rural and urban adult Gambian communities. Clin Exp Allergy. 2001;31:1672–8. doi: 10.1046/j.1365-2222.2001.00987.x. [DOI] [PubMed] [Google Scholar]
- 6.Weinmayr G, Weiland SK, Björkstén B, et al. Atopic sensitization and the international variation of asthma symptom prevalence in children. Am J Respir Crit Care Med. 2007;176:565–74. doi: 10.1164/rccm.200607-994OC. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva NB, Amorim LD, Alcantara-Neves NM. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010;78:3160–7. doi: 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penny ME, Murad S, Madrid SS, Herrera TS, Piñeiro A, Caceres DE, Lanata CF. Respiratory symptoms, asthma, exercise test spirometry, and atopy in schoolchildren from a Lima shanty town. Thorax. 2001;56:607–12. doi: 10.1136/thorax.56.8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moncayo AL, Vaca M, Oviedo G, et al. Risk factors for atopic and non-atopic asthma in a rural area of Ecuador. Thorax. 2010;65:409–16. doi: 10.1136/thx.2009.126490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obeng BB, Hartgers F, Boakye D, Yazdanbakhsh M. Out of Africa: what can be learned from the studies of allergic disorders in Africa and Africans? Curr Opin Allergy Clin Immunol. 2008;8:391–7. doi: 10.1097/ACI.0b013e32830ebb70. [DOI] [PubMed] [Google Scholar]
- 11.Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997;350:85–90. doi: 10.1016/S0140-6736(97)01151-3. [DOI] [PubMed] [Google Scholar]
- 12.Wegienka G, Johnson CC, Havstad S, Ownby DR, Nicholas C, Zoratti EM. Lifetime dog and cat exposure and dog- and cat-specific sensitization at age 18 years. Clin Exp Allergy. 2011;41:979–86. doi: 10.1111/j.1365-2222.2011.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun-Fahrländer C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 14.Illi S, Depner M, Genuneit J, et al. Protection from childhood asthma and allergy in Alpine farm environments-the GABRIEL Advanced Studies. J Allergy Clin Immunol. 2012;129 doi: 10.1016/j.jaci.2012.03.013. 1470-7 e6. [DOI] [PubMed] [Google Scholar]
- 15.Veiga RV, Cunha SS, Dattoli VC, et al. Chronic virus infections supress atopy but not asthma in a set of children from a large latin american city: a cross-section study. BMC Pulm Med. 2011;11:24. doi: 10.1186/1471-2466-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarasekera M, Gunawardena NK, de Silva NR, Douglass JA, O'Hehir RE, Weerasinghe A. Impact of helminth infection on childhood allergic diseases in an area in transition from high to low infection burden. Asia Pac Allergy. 2012;2:122–8. doi: 10.5415/apallergy.2012.2.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moncayo AL, Vaca M, Oviedo G, et al. Effects of geohelminth infection and age on the associations between allergen-specific IgE, skin test reactivity and wheeze: a case-control study. Clin Exp Allergy. 2013;43:60–72. doi: 10.1111/cea.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scrivener S, Yemaneberhan H, Zebenigus M, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493–9. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 19.Cooper PJ, Chico ME, Vaca MG, et al. Risk factors for asthma and allergy associated with urban migration: background and methodology of a cross-sectional study in Afro-Ecuadorian school children in Northeastern Ecuador (Esmeraldas-SCAALA Study) BMC Pulm Med. 2006;6:24. doi: 10.1186/1471-2466-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiland SK, Björkstén B, Brunekreef B, Cookson WO, von Mutius E, Strachan DP. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. Eur Respir J. 2004;24:406–12. doi: 10.1183/09031936.04.00090303. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Diagnostic techniques for intestinal parasitic infections (IPI) applicable to primary health care (PHC) services. Geneva: WHO; 1985. [Google Scholar]
- 22.Patel SP, Jarvelin MR, Little MP. Systematic review of worldwide variations of the prevalence of wheezing symptoms in children. Environ Health. 2008;7:57. doi: 10.1186/1476-069X-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens W, Addo-Yobo E, Roper J, Woodcock A, James H, Platts-Mills T, Custovic A. Differences in both prevalence and titre of specific immunoglobulin E among children with asthma in affluent and poor communities within a large town in Ghana. Clin Exp Allergy. 2011;41:1587–94. doi: 10.1111/j.1365-2222.2011.03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9:29–37. doi: 10.1097/ACI.0b013e32831f44a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alcantara-Neves NM, Veiga RV, Dattoli VC, et al. The effect of single and multiple infections on atopy and wheezing in children. J Allergy Clin Immunol. 2012;129:359–67. doi: 10.1016/j.jaci.2011.09.015. 367 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reina Ortiz M, Schreiber F, Benitez S, et al. Effects of chronic ascariasis and trichuriasis on cytokine production and gene expression in human blood: a cross-sectional study. PLoS Negl Trop Dis. 2011;5:e1157. doi: 10.1371/journal.pntd.0001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127:479–86. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acevedo N, Sánchez J, Erler A, et al. IgE cross-reactivity between Ascaris and domestic mite allergens: the role of tropomyosin and the nematode polyprotein ABA-1. Allergy. 2009;64:1635–43. doi: 10.1111/j.1398-9995.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 29.Hagel I, Cabrera M, Hurtado MA, Sanchez P, Puccio F, Di Prisco MC, Palenque M. Infection by Ascaris lumbricoides and bronchial hyper reactivity: an outstanding association in Venezuelan school children from endemic areas. Acta Trop. 2007;103:231–41. doi: 10.1016/j.actatropica.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Perzanowski MS, Ng'ang'a LW, Carter MC, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. 2002;140:582–8. doi: 10.1067/mpd.2002.122937. [DOI] [PubMed] [Google Scholar]
- 31.Cooper PJ, Chico ME, Bland M, Griffin GE, Nutman TB. Allergic symptoms, atopy, and geohelminth infections in a rural area of Ecuador. Am J Respir Crit Care Med. 2003;168:313–7. doi: 10.1164/rccm.200211-1320OC. [DOI] [PubMed] [Google Scholar]


