Abstract
Wear debris-induced inflammation is considered to be the main cause for periprosthetic osteolysis in total hip replacements (THR). The objective of this retrieval study was to examine the tissue reactions and exposure to metal ions and wear particles in periprosthetic tissues and blood samples from patients with titanium (Ti)-based hip prostheses that were revised due to wear, osteolysis, and/or aseptic loosening. Semiquantitative, histological tissue evaluations in 30 THR-patients revealed numerous wear debris-loaded macrophages, inflammatory cells, and necrosis in both groups. Particle load was highest in tissues adjacent to loosened cemented Ti stems that contained mainly submicron zirconium (Zr) dioxide particles. Particles containing pure Ti and Ti alloy elements were most abundant in tissues near retrieved uncemented cups. Polyethylene particles were also detected, but accounted only for a small portion of the total particle number. The blood concentrations of Ti and Zr were highly elevated in cases with high abrasive wear and osteolysis. Our findings indicate that wear particles of different chemical composition induced similar inflammatory responses, which suggests that particle size and load might be more important than the wear particle composition in periprosthetic inflammation and osteolysis. © 2014 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials Published by Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 103B:709–717, 2015.
Keywords: osteolysis, hip prostheses, wear debris, metal ions, titanium
INTRODUCTION
Total hip replacements (THR) generally have a good clinical outcome in the majority of patients. However, some patients require revision surgery at some stage. Aseptic loosening due to periprosthetic osteolysis is still the major cause for revision.1,2 Debris generated by wear of the bearing surfaces and loosened components are thought to be the main cause for periprosthetic inflammation leading to osteolysis, which is affected by particle characteristics, such as particle size, load, shape, and chemical reactivity.1–3 It has been found that micrometer and nanometer-sized particulate wear debris is co-localized with inflammatory responses in tissues around loosened implants.4,5 Adverse local tissue reactions to metal debris, such as lymphocyte infiltration, proinflammatory cytokine release from macrophages, multinuclear giant cell formation and necrosis have mainly been associated with metal-on-metal hip prostheses.6 In addition to local responses, metal debris may spread over the whole body via systemic circulation (i.e., lymph and blood). They have been identified in the macrophages of various distinct organs such as liver and spleen.7 However, the impact of wear debris on these organs is not fully determined.
Degradation products released from metallic implants cannot only occur in the form of particulate wear debris, but also as inorganic metal salts or free metal ions. Metal ions are generated due to physicochemical processes, such as crevice8 and galvanic corrosion, or cellular digestion mechanisms.9 Increased ion release was an early concern with cementless hip prostheses because of the increased surface area of metal in the porous coated designs.10–12 Moreover, measurement of zirconium (Zr) in blood has been proposed as an indicator for bone cement debris degradation and loosening of cemented hip implants, when Zr is present in the polymethylmethacrylate matrix.13 Other implant-derived metal ions such as cobalt (Co), chromium (Cr), and titanium (Ti) have been shown to increase bone resorption and osteoclast differentiation in vitro,14–16 which suggests a contribution of metal ions in the mechanism of osteolysis. Titanium and its alloys are still the most used material for bone fixation with successful osseointegration17 and the level of Ti-ion release is low under normal circumstances.
This retrieval study was conducted to grade the inflammatory tissue reactions and assess the exposure to wear debris and metal ions in tissues surrounding Ti-based hip prostheses. These findings were correlated to systemic and local ion levels recorded in these patients.
MATERIAL AND METHODS
Patients
From our retrieval biobank, we selected blood and tissue samples from 30 patients with Ti-based hip implants that had been revised due to wear, osteolysis, and/or aseptic loosening (Table I). The retrievals had been consecutively collected in the period of 2008 to 2012 from four hospitals in Norway. We found it convenient to divide the patients in two groups by fixation status of the prosthesis: Fifteen patients had loose cemented stems, but well-fixed cemented cups. The Ti alloy stems (Titan®) were cemented using Palacos bone cement [Palacos G (Schering-Plough), Palacos Refobacin (Merck), Palacos R + G (Heraeus)], containing radio-opaque ZrO2 particles. The cemented cups showed no sign of failure and were not revised.
TABLE I.
Patient Data, Implant Duration, and Reasons for Revision
| Case No. | Gender, Age at Retrieval (yr) | Duration (months) | Reason for Revision |
|---|---|---|---|
| Uncementeda | |||
| 1 | M, 51 | 214 | Cup loosening |
| 2 | F, 70 | 196 | PE wear, pain |
| 3 | M, 65 | 167 | Acetabular osteolysis, loosening |
| 4 | F, 68 | 160 | Cup loosening |
| 5 | F, 78 | 173 | Acetabular osteolysis, cup loosening |
| 6 | F, 72 | 185 | Acetabular osteolysis, PE wear |
| 7 | F, bNA | 228 | Acetabular osteolysis, PE wear |
| 8 | M, 44 | 200 | PE wear |
| 9 | F, 54 | 174 | Acetabular osteolysis, PE wear |
| 10 | F, 77 | 87 | PE wear |
| 11 | M, 53 | 167 | Acetabular osteolysis, PE wear, pain |
| 12 | F, 76 | 232 | Acetabular osteolysis, PE wear |
| 13 | M, 70 | 222 | Cup loosening |
| 14 | M, 76 | 220 | PE wear, acetabular osteolysis |
| 15 | F, 56 | 200 | PE wear, acetabular osteolysis |
| Cementedc | |||
| 16 | M, 78 | 69 | Femoral osteolysis, stem loosening |
| 17 | F, 71 | 52 | Femoral osteolysis, stem loosening |
| 18 | F, 50 | 99 | Femoral osteolysis, stem loosening |
| 19 | M, 79 | 70 | Femoral osteolysis, stem loosening |
| 20 | M, 64 | 72 | Femoral osteolysis, stem loosening |
| 21 | M, 73 | 24 | Femoral osteolysis, stem loosening |
| 22 | M, 78 | 97 | Femoral osteolysis, stem loosening |
| 23 | M, 77 | 74 | Femoral osteolysis, stem loosening |
| 24 | M, 69 | 18 | Femoral osteolysis, stem loosening |
| 25 | M, 80 | 105 | Femoral osteolysis, stem loosening |
| 26 | F, 67 | 90 | Femoral osteolysis, stem loosening |
| 27 | F, 76 | 98 | Femoral osteolysis, stem loosening |
| 28 | F, 62 | 141 | Femoral osteolysis, stem loosening |
| 29 | M, 71 | 89 | Femoral osteolysis, stem loosening |
| 30 | bNA, 70 | 75 | Femoral osteolysis, stem loosening |
Uncemented TiAlV femoral stems (Profile, DePuy) and uncemented metal-backed cups with conventional UHMWPE liners. The head material in these cases was CoCr, except for case nr. 1 (alumina).
NA: not available.
Cemented femoral stems and PE-liners (TiAlV, Titan®, DePuy). The head materials were mainly CoCr exept for cases: 17, 20, and 28 (alumina).
The other fifteen patients had worn and loose uncemented cups, but well-fixed stems. The uncemented cups that were revised had metal-backing made of Ti alloy either coated with a porous commercially pure Ti [Tri-Lock Plus and Gemini (DePuy)] or with a sintered Ti fiber mesh at the bone-implant interface [Harris/Galante I/II and Trilogy (Zimmer)]. One patient had a metal-backing that was threaded and blasted with hydroxyapatite (Tropic, DePuy); the others were hemispherical and intended for press-fit and/or screw fixation. The liners were conventional ultra-high molecular weight polyethylene (UHMWPE) sterilized by gamma irradiation in air or in an inert atmosphere. The uncemented Ti alloy stem (Profile, DePuy) was in all cases well-fixed and not revised. Additional information about the patients and prosthesis materials was provided by the Norwegian Arthroplasty Register (Table I).
Histologic evaluation
Periprosthetic tissue samples from the joint capsule, the acetabular membrane, osteolytic lesions, or sites with evidence of metallosis were collected at revision surgery and fixed in 4% buffered formalin. Paraffin-embedded specimens were sectioned to a thickness of 5 µm and stained with haematoxylin and eosin (H&E). Tissue slides from all patients were semi-quantitatively evaluated by two senior pathologists (HKH, PKL) in a blinded fashion using a modified Mirra classification18 described by Doorn et al.19 The intensity was graded as absent (0), low (1+), moderate (2+), or high (3+) (Table II). Using light microscopy, three high power fields (HPF, 40×) per tissue section with high cellular concentration were counted and graded for acute and chronic inflammatory cells, histiocytes, foreign body giant cells, necrosis, and the amount of metal particles.
TABLE II.
Modified Mirra Classification Used to Assess Tissue Reactions and Wear Particle Load in Periprosthetic Tissue Samples
| Histology | 0 | 1+ | 2+ | 3+ |
|---|---|---|---|---|
| Acute inflammatory cells (neutrophils) | 0 cells/HPFa | 1–5 cells/HPF | 6–49 cells/HPF | 50 or more/HPF |
| Mononuclear histiocytes | 0 cells/HPF | 1–5 cells/HPF | 6–49 cells/HPF | 50 or more/HPF |
| Chronic inflammatory cells (lymphocytes, plasma cells, lymphoid follicles) | 0 cells/HPF | 1–9 cells/HPF | 10–49 cells/HPF | 50 or more/HPF |
| Giant cells (multinucleated histiocytes) | 0 cells/HPF | 1 cell/HPF | 2–4 cells/HPF | 5 or more cells/HPF |
| Metal particles | Normal colored histiocytes (no visible, black metal particles per histiocyte) | Slate blue histiocytes (<10 visible, black metal particles per histiocyte) | Dusty black histiocytes (10 to 100 visible, black Metal particles per histiocyte) | Jet black histiocytes (>100 visible, black metal particles per histiocyte) |
| Necrosis | 0 mm of necrosis/slide | 1–2 mm of necrosis/slide | 3–9 mm of necrosis/slide | >1 cm of necrosis/slide |
HPF: high power field (×40).
Particle characteristics were measured in the same sections with High-Resolution Optical Darkfield Microscopy (HR-ODM; Auburn, Al) as described by Flatebø et al.20 Using photographs (40×), the total amount of foreign body particles, particle density, equivalent diameter, and circularity (0–1) were determined with image analysis software (NIS-Elements 2.30, Nikon, Japan). Birefringent PE particles were identified by polarization microscopy (40×) and counted in a specified rectangular area with defined steps using a MicroStepperTM stepping stage with the associated software (PetrogLite, Conwy Valley Systems).
Particle isolation for analysis of morphology and chemical composition
Particles were isolated from the tissue samples using a modified enzymatic digestion method.21,22 All solutions/buffers were sterile-filtered before use. Approximately 0.2 g tissue (wet weight) was sliced into small pieces and washed 4 times with phosphate-buffered saline (PBS; Gibco, 659457), pH 6.8. PBS containing 5 mM l-Cystine (Sigma, C8755) was added and the sample was sonicated at 70% amplitude for 1 min using a VibraCell probe sonicator (Sonics, VCX 130). The tissue was incubated with papain (Sigma, P3125) in a 65°C shaking water bath for 24 h. Following centrifugation (Eppendorf 5810R) at 3220g for 30 min, the pellet was resuspended in an enzyme cocktail containing Proteinase K (MB-1120100), Pronase (Roche, 10165921001), and Collagenase (Roche, 10103586001) and incubated at 37°C for 24 h. After centrifugation, Tris-HCl buffer (Sigma, T5941) containing 20 mg Proteinase K was added to the pellet and the tissue suspension incubated at 55°C for 24 h. Following sonication at 70% amplitude for 1 min, Proteinase K was replenished and the sample was allowed to incubate in a 55°C shaking water bath for 24 h. The enzyme suspension was aspirated, collected in a second tube, and centrifuged at 16.000g for 30 min. The particle suspension was washed with 100% ethanol, diluted 1:20 in water, and sequentially filtered through membrane filters (Whatman, Ø 47 mm) with the pore sizes of 10, 1, 0.1, and 0.02 µm. The isolated particles were observed using a field emission scanning electron microscope (FE-SEM; Supra-55VP, Carl Zeiss AG, Germany) and their chemical composition was determined by energy dispersive X-ray spectroscopy (Thermo Fisher Scientific, MA). The supernatant from the last centrifugation step was ultra-centrifuged (110.000g) to remove any residual particulates and analyzed for ionic Ti and Zr concentration in the tissue samples.
Quantification of metal ion levels in tissue and blood
Whole blood samples (5–10 mL) were taken just prior to revision surgery from the patient's forearm through an intravenous catheter, 1.3 × 4.5 mm (Becton Dickinson Venflon TM Pro, Helsingborg, Sweden) into metal-free, polypropylene tubes (VWR, Oslo, Norway) and stored at −20°C. The concentration of Ti and Zr in blood and enzymatically digested tissue samples were determined by High-Resolution-Inductively Coupled Plasma-Mass Spectrometry (HR-ICP-MS; Element 2 Thermo Finnigan, Bremen, Germany). Prior to metal analysis, an aliquot of ∼1.5 g whole blood or 0.5 mL tissue supernatant was mixed with 3 mL 60% HNO3 (Merck, Darmstadt, Germany) and 2 mL 30% H2O2 (Sigma-Aldrich, Germany) and digested in a microwave-assisted system (Milestone 1200 Mega, Sorisole, Italy). A blank and Seronorm reference blood sample (Sero AS, Oslo, Norway) were treated the same way as the test samples. After digestion, deionized water (MilliQ, Millipore) was added to the samples to a total dilution of 60–70.
Statistical analysis
Statistical analysis was done using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). Data is presented as medians accompanied by an interquartile range (IQR) when the data was skewed, tested with D'Agostino and Pearson omnibus normality test. Otherwise, data is given as means with ranges. The Mann-Whitney U test was used to determine statistical significant differences between the studied groups. Relationships between different parameters were analyzed using Spearman's rho correlation. Results were considered statistically significant when p < 0.05.
Ethics
The project protocol and the biobank have been approved by the Regional Committee for Medical Research Ethics–Western Norway (REK number 2010/2817). The study was performed on coded samples, with written informed consent from every patient prior to blood and tissue sampling.
RESULTS
The mean age at retrieval of the 15 patients in the cemented group was 71 years (50–80 years) and the mean time until revision was 6.5 years (1.5–11.8 years) (Table I). In the uncemented group, the mean age at retrieval was 65 years (44–78 years) and the mean time until revision was 15.7 years (7–19.3 years). Radiographs illustrating the typical appearance of osteolysis and loosening in each patient group are shown in Figure 1(A,B). Acetabular osteolysis is apparent in Figure 1(A) and osteolysis around the femoral stem is shown in Figure 1(B).
FIGURE 1.
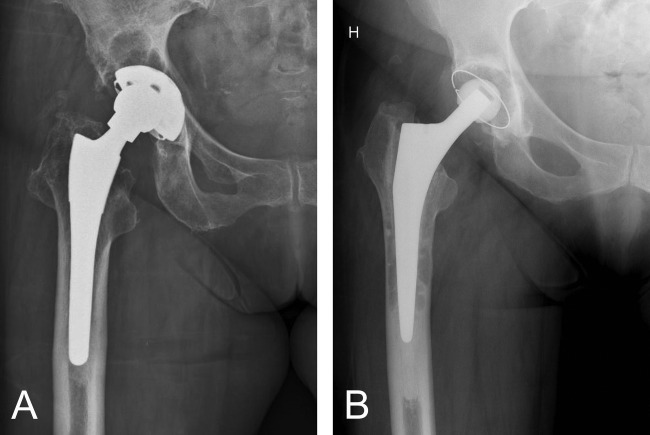
Radiological images illustrating the reason for revision in both studied patient groups. A: Wear and acetabular osteolysis and/or aseptic loosening of the cup were the reasons for revision in the uncemented group. B: Femoral osteolysis and stem loosening were the reasons for revision in the cemented group.
Histology
Histological tissue examination revealed numerous mononuclear histiocytes, lymphocytes, a few neutrophils and multinucleated giant cells in all patients. These inflammatory cell types were slightly more abundant in tissues associated with the uncemented cups (Table III). Necrosis, or more specifically coagulative necrosis, which was observed as lighter stained tissue areas, was found to be more prominent in tissues surrounding the cemented stem. Forty seven percent of these cases were graded as 3+, which is the highest grade in the Mirra classification. Diffusely distributed mononuclear histiocytes were the most prominent cell type in the periprosthetic tissues. A histiocytic grade of 3+ was given in 76% of the cases (Table III). Most histiocytes had ingested or were associated with wear particles, seen as black spots inside/on the cells. Chronic inflammatory cells, that is, lymphocytes, were randomly distributed between the histiocytes and not as abundant as these cells. In some cases (e.g., no. 5 and 15), lymphocytes were found in clusters [ Figure 2(A)]. A few, mainly perivascular located neutrophils were found. Multinucleated giant cells with ingested foreign material were observed in 57% of the cases (grade 1+ and 2+) [ Figures 2(A,B) and 3].
TABLE III.
Periprosthetic Tissue Reactions in Patients with Uncemented Metal-Backed Cups and Cemented Titanium Stems
| Case No. | Mononuclear Histiocytes | Chronic Inflammatory Cells | Multinucleated Giant Cells | Neutrophils | Metal Particles/Histiocytes | Necrosis |
|---|---|---|---|---|---|---|
| Uncemented | ||||||
| 1 | 2+ | 3+ | 0 | 2+ | 1+ | 0 |
| 2 | 3+ | 2+ | 2+ | 1+ | 2+ | 2+ |
| 3 | 3+ | 2+ | 2+ | 2+ | 1+ | 2+ |
| 4 | 0 | 0 | 0 | 0 | 0 | 3+ |
| 5 | 3+ | 3+ | 0 | 2+ | 1+ | 2+ |
| 6 | 3+ | 2+ | 0 | 1+ | 1+ | 2+ |
| 7 | 3+ | 2+ | 1+ | 2+ | 1+ | 3+ |
| 8 | 3+ | 2+ | 2+ | 2+ | 1+ | 2+ |
| 9 | 3+ | 2+ | 1+ | 1+ | 3+ | 2+ |
| 10 | 3+ | 2+ | 2+ | 2+ | 2+ | 0 |
| 11 | 3+ | 2+ | 0 | 2+ | 0 | 3+ |
| 12 | 3+ | 2+ | 0 | 1+ | 1+ | 1+ |
| 13 | 3+ | 2+ | 2+ | 2+ | 1+ | 2+ |
| 14 | 3+ | 1+ | 1+ | 1+ | 3+ | 2+ |
| 15 | 3+ | 3+ | 2+ | 2+ | 1+ | 1+ |
| Cemented | ||||||
| 16 | 3+ | 2+ | 1+ | 1+ | 2+ | 2+ |
| 17 | 0 | 0 | 0 | 0 | 0 | 3+ |
| 18 | 0 | 0 | 0 | 0 | 0 | 3+ |
| 19 | 0 | 0 | 0 | 0 | 0 | 3+ |
| 20 | 3+ | 2+ | 1+ | 1+ | 2+ | 1+ |
| 21 | 3+ | 2+ | 1+ | 1+ | 2+ | 2+ |
| 22 | 3+ | 2+ | 1+ | 2+ | 2+ | 3+ |
| 23 | 3+ | 2+ | 1+ | 2+ | 2+ | 2+ |
| 24 | 2+ | 1+ | 0 | 1+ | 1+ | 2+ |
| 25 | 3+ | 2+ | 0 | 1+ | 2+ | 3+ |
| 26 | 3+ | 2+ | 0 | 1+ | 2+ | 1+ |
| 27 | 3+ | 2+ | 1+ | 1+ | 2+ | 2+ |
| 28 | 3+ | 2+ | 1+ | 1+ | 1+ | 2+ |
| 29 | 2+ | 1+ | 0 | 1+ | 1+ | 3+ |
| 30 | 3+ | 2+ | 1+ | 1+ | 2+ | 3+ |
Uncemented cups: tissue samples were taken from the joint capsule and/or the acetabular membrane; cemented stems: tissue samples were taken from the proximal femur, distal femur, or the femur channel.
FIGURE 2.
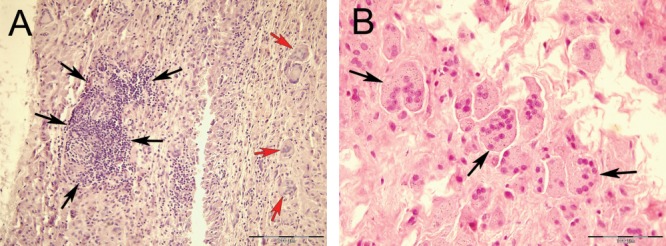
Light microscopy images of periprosthetic tissues. A: Lymphocyte infiltration (black arrows pointing to dark violet cell cluster on the left side) and multinucleated giant cells (red arrows; case no. 15 with uncemented metal-backed cup); H&E, 20×. B: Multinucleated giant cells (black arrows) containing metal particles (Ti and ZrO2; case no. 20 with cemented Ti stem); H&E, 40×.
FIGURE 3.
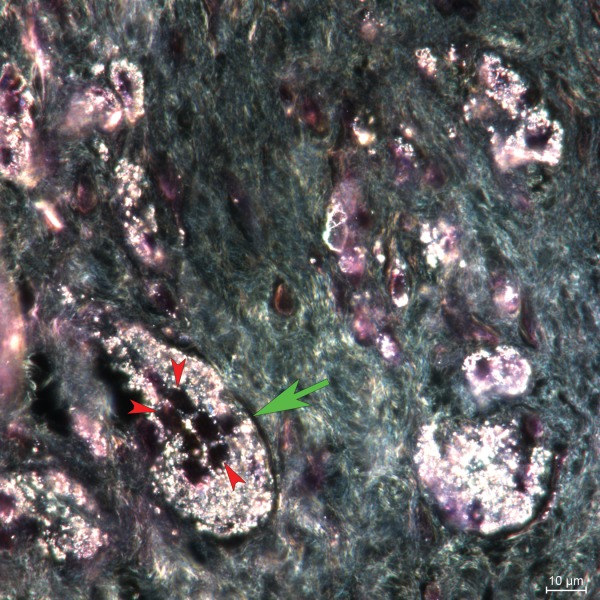
Darkfield microscopy image (H&E, 100×) showing a multinucleated foreign body giant cell (green arrow) containing metal particles (case no. 14). Metal particles inside the cells appear white; nuclei appear black (red arrows).
Particle analysis
Different microscopy techniques and energy-dispersive X-ray analysis ( Figure 4) indicated that implant wear products originated from different sources. Tissues around cemented stems contained mainly submicron ZrO2 particles, originating from bone cement degradation, but also some Ti alloy particles worn off from the stem. Particles containing pure Ti and Ti alloy elements were most abundant in tissues adjacent to the uncemented cups. A higher particle load (median cemented = 14,727 particles/mm2, IQR = 17,019 vs. median uncemented = 1519 particles/mm2, IQR = 3776; p < 0.001) was found in tissues adjacent to cemented stems (Table IV). Image analysis revealed particle equivalent diameters in the range of 0.12–6.46 µm in the uncemented group, with >50% of the particles being ≤ 0.4 µm ( Figure 5). The median equivalent diameter of particles (0.42 µm and IQR = 0.1) was smaller than in the cemented group (0.55 µm, IQR = 0.185, and p = 0.034). The observed ZrO2 particles were more uniform in their round shape than the Ti and Ti alloy particles, which had a more polygonal shape.
FIGURE 4.
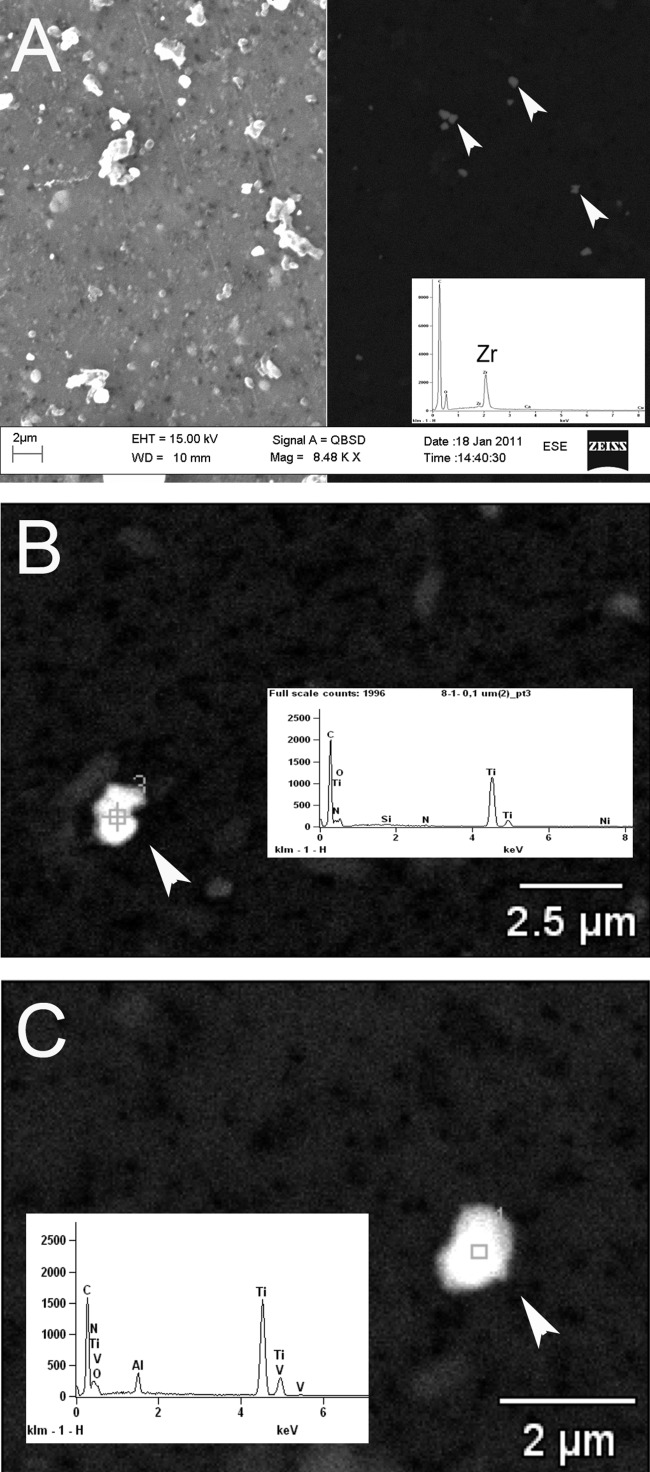
Scanning electron microscopy (SEM) images with EDXA spectra of isolated particles (white arrows). A: SEM image, left side: Secondary emission: showing all particles on the filter. SEM image, right side: backscattered, highlighting dense ZrO2 particles typically found in tissues near cemented stems (case no. 25). B: Ti particles, such as particle 3 in backscattered image, were most frequent in tissues around the uncemented cups. C: Ti alloy particle from an uncemented case (no. 5). These particles were also found around the cemented stems.
TABLE IV.
Particle Characterization Using Digital Images (40× Magnification) of Periprosthetic Tissues from Patients with Uncemented Metal-Backed Cups and Cemented Titanium Stems
| Case No. | Particle Density (per mm2) | Median Equivalent Diameter (µm) | Median Circularity | PE density (per mm2) |
|---|---|---|---|---|
| Uncemented | ||||
| 1 | 1413 | 0.57 | 0.85 | 1.7 |
| 2 | 14,719 | 0.36 | N/A | 1.9 |
| 3 | 1626 | 0.42 | 0.95 | 27.8 |
| 4 | 8114 | 0.42 | 1 | 11.5 |
| 5 | 2641 | 0.36 | 1 | 0.6 |
| 6 | 117 | 0.47 | 0.82 | 0 |
| 7 | 1214 | 1.05 | 0.92 | 7.3 |
| 8 | 5357 | 0.36 | 1 | 3.0 |
| 9 | N/A | N/A | N/A | 23.6 |
| 10 | 1338 | 0.42 | 1 | 4.4 |
| 11 | 69 | 2.6 | N/A | 0.2 |
| 12 | 3827 | 0.52 | 0.98 | 24.9 |
| 13 | 185 | 0.56 | N/A | 1.7 |
| 14 | 8937 | 0.42 | N/A | 1.4 |
| 15 | 1193 | 0.45 | 1 | 2.8 |
| Cemented | ||||
| 16 | 6897 | 0.59 | N/A | 0.6 |
| 17 | 14,922 | 0.47 | N/A | 3.6 |
| 18 | 13,041 | 0.51 | N/A | 2.8 |
| 19 | 14,727 | 0.51 | N/A | 3.0 |
| 20 | 9878 | 0.51 | N/A | 2.4 |
| 21 | 32,428 | 0.51 | N/A | 2.1 |
| 22 | 3664 | 0.55 | N/A | 0.2 |
| 23 | 21,098 | 1.05 | 0.86 | 4.9 |
| 24 | 14,164 | 0.7 | 1 | 0.8 |
| 25 | 9610 | 0.7 | 0.83 | 0 |
| 26 | 33,619 | 0.51 | N/A | 0 |
| 27 | 42,675 | 0.69 | N/A | 0.2 |
| 28 | 7902 | 0.42 | 1 | 0.3 |
| 29 | 35,592 | 0.73 | 0.95 | 0.9 |
| 30 | 14,987 | 0.6 | 0.9 | 0.3 |
N/A: Particle characteristic could not be measured appropriately.
Images were taken with High-Resolution Optical Darkfield Microscopy (HR-ODM). Total number of particles, particle density, median equivalent diameter, and median circularity were measured using NIS Elements image analysis software. Polyethylene particles (PE) were counted (total number and particle density) using a polarization microscope with a motorized table.
FIGURE 5.
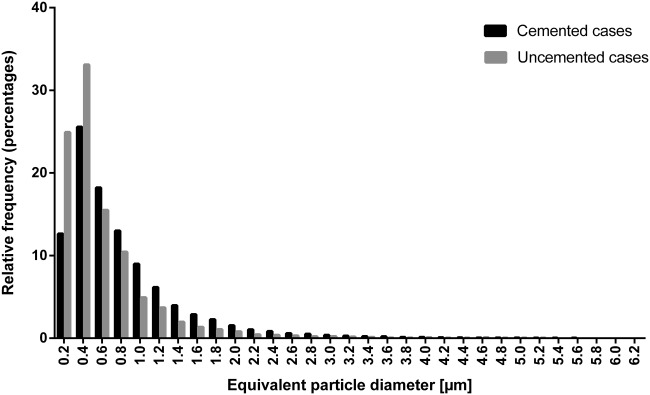
Frequency distribution of the equivalent diameter (µm) of the particles counted in the uncemented and cemented cases.
Polyethylene particles (PE) ( Figure 6) were found in almost all tissue samples and were generally more abundant in the tissues adjacent to the uncemented cups (median uncemented = 2.8 PE/mm2, IQR = 8.1 vs. median cemented = 0.8 PE/mm2, IQR = 2.4, and p = 0.053). The PE particles accounted only for a small portion of total number of particles in all samples (8% around uncemented cups, 0.24% around cemented stems). The size of PE particles ranged from submicron to over 100 µm along one dimension. Large PE particles (>100 µm) were in some cases associated with giant cells (p = 0.291).
FIGURE 6.
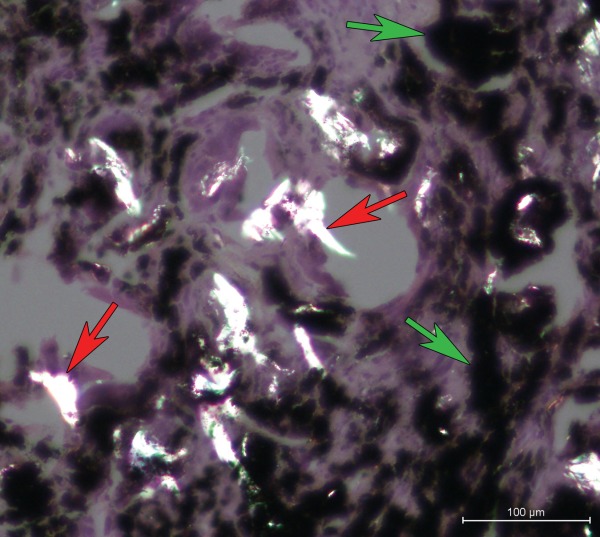
Polarization microscopy image (H&E, 20×) showing birefringent PE particles (red arrows) in the tissue (case no. 9). Metal degradation products appear in black (green arrows).
In tissues around cemented stems, the total number of particles positively correlated with the amount of mononuclear histiocytes (r = 0.92, p < 0.001), chronic inflammatory cells (r = 0.92, p < 0.001), multinucleated giant cells (r = 0.63, p = 0.036), and neutrophils (r = 1.00, p<0.001). A similar trend was also observed in the tissues surrounding the uncemented cups (ns).
Metal ion analysis
The median Ti concentrations in whole blood samples in patients with loose cemented stems (n = 10) and loose uncemented cups (n = 13) were 10.5 µg/L (IQR = 6.1) and 21.1 µg/L (IQR = 71.2), respectively (p = 0.73) [ Figure 7(A)]. In uncemented cases with high abrasive wear and metallosis, Ti concentrations over 100 µg/L were detected. The median Zr concentrations in blood in patients with loose cemented stems (n = 10) and loose uncemented cups (n = 13) were 1.87 µg/L (IQR = 0.64) and 0.37 µg/L (IQR = 0.56), respectively, (p = 0.002) [ Figure 7(B)]. The concentrations of Ti (r = 0.77 and p = 0.024) and Zr (r = 0.71 and p = 0.044) in whole blood were proportional to the number of wear particles/mm2 in the tissue sections. The ionic Ti and Zr concentrations in the enzymatically digested periprosthetic tissues were 10 to 100 times higher than in the blood samples: The two samples from the cemented group showed, as expected, the highest Zr concentration (82.6 and 166.6 µg/L), compared with the sample from the uncemented group (11.6 µg/L). Ti concentrations were 1284 and 1405.8 µg/L in the cemented cases and 329.2 µg/L in the uncemented case.
FIGURE 7.
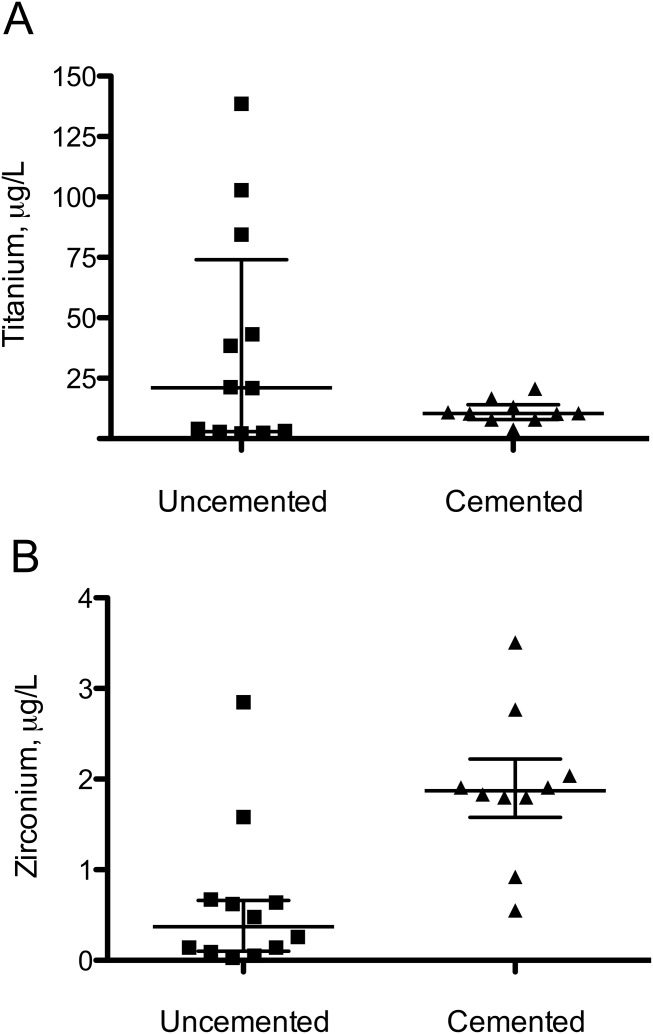
Metal concentration in blood samples, µg/L (medians and quartiles). A: Titanium. B: Zr.
DISCUSSION
Implant wear and associated immune reactions to wear debris are considered to be an important factor in the onset of osteolysis and aseptic loosening of hip implants. Previous studies have shown that the accumulation of submicron and micron-sized wear debris initiates a foreign body inflammatory reaction, which includes the activation of histiocytes (tissue macrophages) and the influx of other immune cells like lymphocytes and neutrophils.23,24 The presence of neutrophils in our analyzed tissue samples suggests that newly generated wear debris induced acute inflammatory responses in the soft tissue adjacent to the implant. The apparent, relatively strong pronounced coagulative necrosis (>80% of the tissue samples had a grade of 2+ and more) could be a sign of hypersensitivity.25
The continuous production of wear debris, activation of macrophages, ingestion of wear debris, and release of proinflammatory cytokines and other mediators can cause inflammatory-mediated morphological changes in the tissue, which contribute to osteolysis and implant loosening. In this study, we have shown that the periprosthetic tissues of patients with revised Ti-based hip implants contain various types of metallic degradation products, bone cement debris and PE particles, which all might have contributed to the inflammatory tissue responses we have observed.
It has been proposed that local periprosthetic tissue inflammation is affected by particle characteristics, like particle size,26 load, shape and chemical reactivity.1 Hallab et al. suggested that the local inflammatory response is proportional to the particle load in the tissue.1 We found a positive correlation between the histological grade of the tissue responses and the total number of particles. All tissue samples had a high particle load. Tissues around the cemented stems contained 10 fold more particles than the tissues around the cups, but both patient cohorts were sufficiently exposed to wear debris to induce foreign body reactions.
The ZrO2 particles, which originated from bone cement degradation, are extremely hard ceramic particles that are embedded as agglomerates in the bone cement matrix. The primary particles retain their uniform size and round or ovoid shape during wear processes. We found these particles relatively dispersed in the tissue, mostly internalized by histiocytes. Particles found in tissues from the uncemented cases were often polygonal and varied more in size, which may be attributed to different wear processes. Larger, pure Ti fragments were possibly broken off from the metal-backing moving against the bone, while smaller particles were probably generated when the femoral head wore through the PE-liner into the Ti alloy cup. Both ZrO2 and Ti-based wear debris were of submicron size. Cellular uptake and thus cellular responses to wear debris is affected by particle size. Nanometer- and submicron-sized particles are generally taken up by phagocytic cells through various endocytic pathways. The uptake of metal particles <150 nm was found to be mediated through receptor-mediated endocytosis and pinocytosis.27 Larger particles (>100 µm) are not as easily ingested as smaller particles by phagocytic cells. In response, tissue macrophages may fuse to form multinucleated giant cells. These multinucleated giant cells may not only have formed in the attempt to ingest larger particles, but also to cope with the enormous amount of smaller particles observed in the tissue samples.
In addition to ZrO2 and Ti-based particles, we also identified PE particles in the tissue samples using polarized light microscopy. These particles had an irregular shape and ranged from micron (≥1 µm) to larger, spindle-shaped particles (>100 µm) that were partially associated with multinucleated giant cells. In vitro studies have shown that exposure to PE particles within the phagocytic size range (0.1–10 µm) results in activation of macrophages,28,29 leading to release of cytokines that modulate osteoclast activation and osteolysis. In this study large PE particles (>100 µm) were in some cases observed within giant cells, but the total amount of PE particles did not correlate with the number of giant cells, which is in contrast to the findings by Ito et al.30 However, in our study the observed number of PE particles accounted unexpectedly only for a small portion of the total number of particles. This might be due to detection limitations with the polarized microscopy technique and most probably not due to sampling bias, since the tissue samples were taken from the joint capsule or the fibrous tissue behind the cup or osteolytic lesions. In the cemented cases, the PE cups were intact and the wear minor. In contrast, all liners from the uncemented cups were worn, although it must be noted that these cups failed quite late (Table I). In a post mortem retrieval study, Urban et al. characterized some well functioning uncemented acetabular components (Harris-Galante I) after a mean of eleven years in situ, but with particle induced granulomas and progression of osteolysis.17
Implant-derived metal ions have been shown to contribute to the mechanism of osteolysis by increasing bone resorption and osteoclast differentiation in vitro.14,15 Elevated concentrations of metal ions were measured in human body fluids and tissue samples from THR patients.11,12 Dorr et al.11 found Ti-concentrations ranging from 38 to 602 µg/L in the blood from subjects with osteolysis around Ti alloy implants, which is in line with our findings. It is expected that the metal ion concentrations in the periprosthetic tissues were 100–1000 fold higher than in the blood stream. Compared with previous findings11,31 our estimates show slightly lower metal ion concentrations in the tissue samples from related retrievals. This might be due to methodological differences between the studies. A method for separation and quantification of particle debris and ionic forms in tissue samples was developed in this study. We suggest that our method better separates between particulate metal debris and soluble forms. However, only a limited number of samples were analyzed, so further validation is necessary.
To our knowledge, there are only a few methods available in the literature to precisely quantify and speciate particulate debris and ionic forms.32,33 Thus, the precise metal ion concentration in tissue samples surrounding hip implants still remains unclear. There is evidence that the osteolytic effect of metal ions might have been underestimated in previous studies. It is therefore necessary to further study the exact role of metal ions in the mechanism of osteolysis around Ti-based hip implants. In summary, we conclude that different types of wear debris induced a similar inflammatory response in tissues adjacent to Ti-based hip implants revised for wear, osteolysis and/or loosening. Particle size, load, and metal ions might be more important factors than particle composition in the onset of inflammatory tissue responses eventually leading to osteolysis.
Acknowledgments
The authors thank Irene Ohlen Moldestad (retrieval handling and analysis), Anne Aarsand (histological tissue preparation at Dept. of Clinical Medicine, UiB), Egil S. Ericksen (SEM-analysis at Laboratory for Electron Microscopy, UiB), and Siv Hjorth Dundas (ICP-MS-analysis at Dept. of Earth Science, UiB) for their excellent technical support. Further, they thank the surgeons that contributed with retrieval samples.
REFERENCES
- 1.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67:182–188. [PubMed] [Google Scholar]
- 2.Ollivere B, Wimhurst JA, Clark IM, Donell ST. Current concepts in osteolysis. J Bone Joint Surg Br. 2012;94B:10–15. doi: 10.1302/0301-620X.94B1.28047. [DOI] [PubMed] [Google Scholar]
- 3.Noordin S, Masri B. Periprosthetic osteolysis: Genetics, mechanisms and potential therapeutic interventions. Can J Surg. 2012;55:408–417. doi: 10.1503/cjs.003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Domarus C, Rosenberg JP, Ruther W, Zustin J. Necrobiosis and T-lymphocyte infiltration in retrieved aseptically loosened metal-on-polyethylene arthroplasties. Acta Orthop. 2011;82:596–601. doi: 10.3109/17453674.2011.625534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber M, Reinisch G, Trettenhahn G, Zweymüller K, Lintner F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172–180. doi: 10.1016/j.actbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–2327. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban RM, Tomlinson MJ, Hall DJ, Jacobs JJ. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty. J Arthroplasty. 2004;19(8 Suppl 3):94–101. doi: 10.1016/j.arth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Willert HG, Broback LG, Buchhorn GH, Jensen PH, Koster G, Lang I, Ochsner P, Schenk R. Crevice corrosion of cemented titanium alloy stems in total hip replacements. Clin Orthop Relat Res. 1996:51–75. [PubMed] [Google Scholar]
- 9.Cadosch D, Chan E, Gautschi OP, Filgueira L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening-current concepts. J Biomed Mater Res A. 2009;91:1252–1262. doi: 10.1002/jbm.a.32625. [DOI] [PubMed] [Google Scholar]
- 10.Agins HJ, Alcock NW, Bansal M, Salvati EA, Wilson PD, Jr, Pellicci PM, Bullough PG. Metallic wear in failed titanium-alloy total hip replacements. A histological and quantitative analysis. J Bone Joint Surg Am. 1988;70:347–356. [PubMed] [Google Scholar]
- 11.Dorr LD, Bloebaum R, Emmanual J, Meldrum R. Histologic, biochemical, and ion analysis of tissue and fluids retrieved during total hip arthroplasty. Clin Orthop Relat Res. 1990:82–95. [PubMed] [Google Scholar]
- 12.Jacobs JJ, Skipor AK, Patterson LM, Hallab NJ, Paprosky WG, Black J, Galante JO. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447–1458. doi: 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kunze J, Koelling S, Reich M, Wimmer MA. Use of ultrasonic nebulizer with desolvator membrane for the determination of titanium and zirconium in human serum by means of inductively coupled plasma-mass spectroscopy. Fresenius J Anal Chem. 2000;366:165–166. doi: 10.1007/s002160050031. [DOI] [PubMed] [Google Scholar]
- 14.Cadosch D, Chan E, Gautschi OP, Meagher J, Zellweger R, Filgueira L. Titanium IV ions induced human osteoclast differentiation and enhanced bone resorption in vitro. J Biomed Mater Res A. 2009;91:29–36. doi: 10.1002/jbm.a.32183. [DOI] [PubMed] [Google Scholar]
- 15.Niki Y, Matsumoto H, Suda Y, Otani T, Fujikawa K, Toyama Y, Hisamori N, Nozue A. Metal ions induce bone-resorbing cytokine production through the redox pathway in synoviocytes and bone marrow macrophages. Biomaterials. 2003;24:1447–1457. doi: 10.1016/s0142-9612(02)00531-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang JY, Wicklund BH, Gustilo RB, Tsukayama DT. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomaterials. 1996;17:2233–2240. doi: 10.1016/0142-9612(96)00072-5. [DOI] [PubMed] [Google Scholar]
- 17.Urban RM, Hall DJ, Della Valle C, Wimmer MA, Jacobs JJ, Galante JO. Successful long-term fixation and progression of osteolysis associated with first-generation cementless acetabular components retrieved post mortem. J Bone Joint Surg Am. 2012;94:1877–1885. doi: 10.2106/JBJS.J.01507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976:221–240. [PubMed] [Google Scholar]
- 19.Doorn PF, Mirra JM, Campbell PA, Amstutz HC. Tissue reaction to metal on metal total hip prostheses. Clin Orthop Relat Res. 1996:S187–205. doi: 10.1097/00003086-199608001-00017. (329 Suppl) [DOI] [PubMed] [Google Scholar]
- 20.Flatebø RS, Høl PJ, Leknes KN, Kosler J, Lie SA, Gjerdet NR. Mapping of titanium particles in peri-implant oral mucosa by laser ablation inductively coupled plasma mass spectrometry and high-resolution optical darkfield microscopy. J Oral Pathol Med. 2011;40:412–420. doi: 10.1111/j.1600-0714.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- 21.Maloney WJ, Smith RL, Schmalzried TP, Chiba J, Huene D, Rubash H. Isolation and characterization of wear particles generated in patients who have had failure of a hip arthroplasty without cement. J Bone Joint Surg Am. 1995;77:1301–1310. doi: 10.2106/00004623-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Wirth MA, Agrawal CM, Mabrey JD, Dean DD, Blanchard CR, Miller MA, Rockwood CA., Jr Isolation and characterization of polyethylene wear debris associated with osteolysis following total shoulder arthroplasty. J Bone Joint Surg Am. 1999;81:29–37. doi: 10.2106/00004623-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19:213–214. doi: 10.1177/1753425912451779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day JS, Baxter RM, Ramsey ML, Morrey BF, Connor PM, Kurtz SM, Steinbeck MJ. Characterization of wear debris in total elbow arthroplasty. J Shoulder Elbow Surg. 2013;22:924–931. doi: 10.1016/j.jse.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints - A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87A:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JB, Besong AA, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res. 2000;52:296–307. doi: 10.1002/1097-4636(200011)52:2<296::aid-jbm8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Keegan GM, Learmonth ID, Case CP. Orthopaedic metals and their potential toxicity in the arthroplasty patient: A review of current knowledge and future strategies. J Bone Joint Surg Br. 2007;89:567–573. doi: 10.1302/0301-620X.89B5.18903. [DOI] [PubMed] [Google Scholar]
- 28.Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Comparison of the response of primary human peripheral blood mononuclear phagocytes from different donors to challenge with model polyethylene particles of known size and dose. Biomaterials. 2000;21:2033–2044. doi: 10.1016/s0142-9612(00)00089-2. [DOI] [PubMed] [Google Scholar]
- 29.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Ito S, Matsumoto T, Enomoto H, Shindo H. Histological analysis and biological effects of granulation tissue around loosened hip prostheses in the development of osteolysis. J Orthop Sci. 2004;9:478–487. doi: 10.1007/s00776-004-0808-1. [DOI] [PubMed] [Google Scholar]
- 31.Hallab NJ, Mikecz K, Vermes C, Skipor A, Jacobs JJ. Orthopaedic implant related metal toxicity in terms of human lymphocyte reactivity to metal-protein complexes produced from cobalt-base and titanium-base implant alloy degradation. Mol Cell Biochem. 2001;222:127–136. [PubMed] [Google Scholar]
- 32.Hart AJ, Quinn PD, Sampson B, Sandison A, Atkinson KD, Skinner JA, Powell JJ, Mosselmans JF. The chemical form of metallic debris in tissues surrounding metal-on-metal hips with unexplained failure. Acta Biomater. 2010;6:4439–4446. doi: 10.1016/j.actbio.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Goode AE, Perkins JM, Sandison A, Karunakaran C, Cheng H, Wall D, Skinner JA, Hart AJ, Porter AE, McComb DW, et al. Chemical speciation of nanoparticles surrounding metal-on-metal hips. Chem Commun (Camb) 2012;48:8335–8337. doi: 10.1039/c2cc33016d. [DOI] [PubMed] [Google Scholar]


