Abstract
Aims
Anaemia and iron deficiency are constituents of the cardio-renal syndrome in chronic heart failure (CHF). We investigated the effects of i.v. iron in iron-deficient CHF patients on renal function, and the efficacy and safety of this therapy in patients with renal dysfunction.
Methods and results
The FAIR-HF trial randomized 459 CHF patients with iron deficiency (ferritin <100 µg/L, or between 100 and 299 µg/L if transferrin saturation was <20%): 304 to i.v. ferric carboxymaltose (FCM) and 155 to placebo, and followed-up for 24 weeks. Renal function was assessed at baseline and at weeks 4, 12, and 24, using the estimated glomerular filtration rate (eGFR, mL/min/1.73 m2), calculated from the Chronic Kidney Disease Epidemiology Collaboration (CKD–EPI) formula. At baseline, renal function was similar between groups (62.4 ± 20.6 vs. 62.9 ± 23.4 mL/min/1.73 m2, FCM vs. placebo). Compared with placebo, treatment with FCM was associated with an increase in eGFR [treatment effect: week 4, 2.11 ± 1.21 (P = 0.082); week 12, 2.41 ± 1.33 (P = 0.070); and week 24, 2.98 ± 1.44 mL/min/1.73 m2 (P = 0.039)]. This effect was seen in all pre-specified subgroups (P > 0.20 for interactions). No interaction between the favourable effects of FCM and baseline renal function was seen for the primary endpoints [improvement in Patient Global Assessment (P = 0.43) and NYHA class (P = 0.37) at 24 weeks]. Safety and adverse event profiles were similar in patients with baseline eGFR <60 and ≥60 mL/min/1.73 m2.
Conclusions
Treatment of iron deficiency in CHF patients with i.v. FCM was associated with an improvement in renal function. FCM therapy was effective and safe in CHF patients with renal dysfunction.
Keywords: Chronic heart failure, Iron deficiency, Renal function, Ferric carboxymaltose
Introduction
Renal dysfunction often complicates the natural course of chronic heart failure (CHF), and cardio-renal interactions are involved in the pathophysiology of CHF syndrome.1,2 However, there is no established evidence-based therapy for CHF patients with cardio-renal syndrome.3
Anaemia and iron deficiency (ID) constitute an important part of the pathophysiology of cardio-renal syndrome.4,5 In anaemic patients with chronic kidney disease (CKD), iron repletion is an established part of disease management, resulting in increased haemoglobin levels, decreased concomitant therapy with erythropoiesis-stimulating agents, and stabilization of renal function.6,7 It has also been demonstrated that CHF patients with anaemia and renal dysfunction may benefit from i.v. iron therapy with subsequent improvement in renal function.8
The importance of ID in CHF has only recently drawn clinical attention.9,10 ID is prevalent in CHF, may be present independently of anaemia, and predicts poor outcome, reduced exercise capacity, and impaired quality of life.11–15 I.v. iron supplementation in CHF patients with ID was proven to be well tolerated, resulting in favourable effects on symptoms, functional status, and quality of life.8,16–18
Iron is a metabolically active micronutrient involved in numerous biological processes, with a key role in oxygen transportation and storage, oxidative metabolism, and synthesis and degradation of lipids, carbohydrates, DNA, and RNA.19,20 Preserved iron metabolism is particularly important for cells and organs with high energy demand (e.g. skeletal muscle, kidney, myocardium).19–21 Thus, it can be hypothesized that iron repletion may favourably affect the function of these organs, in particular kidney function. On the other hand, there is concern that treatment with i.v. iron may promote oxidative stress and the proinflammatory response, leading to renal injury.22
To address these controversies, we assessed the effect of i.v. iron [with ferric carboxymaltose (FCM)] on renal function in CHF patients participating in the FAIR-HF (Ferinject® Assessment in patients with IRon deficiency and chronic Heart Failure) study.16 Additionally, we evaluated the efficacy and safety of i.v. iron in CHF patients with renal dysfunction.
Methods
Study design
The design, conduct, and principal results of the FAIR-HF study have been reported elsewhere.16,23 The study complied with the Declaration of Helsinki and the protocol was approved by local ethics committees in all centres. In summary, FAIR-HF was a multicentre, randomized, double-blind, placebo-controlled study which recruited 459 ambulatory patients with CHF in NYHA class II or III, with LVEF ≤40% (NYHA II) or ≤45% (NYHA III), a screening haemoglobin between 95 and 135 g/L, and ID defined on the basis of laboratory assessments when the screening serum ferritin level was <100 µg/L, or between 100 and 299 µg/L when transferrin saturation (TSAT) was <20%. The total iron dose required for iron repletion was calculated at baseline using the Ganzoni formula,24 and patients were randomized in a 2:1 ratio to receive FCM (Ferinject®, Vifor Pharma, Switzerland; 200 mg iron) or saline i.v. weekly until iron repletion (correction phase), then 4 weekly until week 24 (maintenance phase). Regular assessments were performed at weeks 4, 12, and 24, and a follow-up visit was completed at week 26 (or within 2 weeks after the last treatment).23
Renal function assessment
The estimated glomerular filtration rate (eGFR) was the primary index of renal function. The Chronic Kidney Disease Epidemiology Collaboration (CKD–EPI) formula25 was used to calculate the eGFR. Renal function was evaluated at baseline and at weeks 4, 12, and 24. Serum creatinine assessments used for eGFR calculation were performed at a central laboratory and was standardized using a Roche Alkaline picrate rate blanked and compensated methodology. The instrument platform used in the study was from Roche.
Patients were categorized on the basis of their baseline eGFR according to the cut-off values used by the National Kidney Foundation for the classification of chronic renal disease:26 preserved renal function (eGRF ≥60 mL/min/1.73 m2) and impaired renal function (<60 mL/min/1.73 m2).
There was no upper serum creatinine level considered as an exclusion criterion for the study. The primary outcome of this analysis was the change in eGFR (mL/min/1.73 m2) between baseline and weeks 4, 12, and 24. This was a prospectively planned safety endpoint of the trial.23 Originally, the Modification of Diet in Renal Disease (MDRD) formula27 was planned to be used for eGFR calculation. In this analysis, however, we applied the CKD–EPI formula to calculate eGFR as this formula is more robust and currently recommended, particularly in patients with systolic CHF.28,29 The MDRD formula was used for sensitivity analysis. Additionally, we analyzed three categorical changes in eGFR at week 24 defined as: no effect on renal function (change in eGFR from baseline between –3 and +3 mL/min/1.73 m2), improvement (increase in eGFR from baseline >3 mL/min/1.73 m2), and deterioration in renal function (decrease in eGFR from baseline >3 mL/min/1.73 m2). This outcome was defined retrospectively. For sensitivity analysis, we also used a relative cut-off level of 5% change from baseline eGFR
Statistical analysis
Continuous data were described using mean and standard deviation, or median with 25th and 75th percentiles, depending on the distribution of the data, and compared between those with or without impaired renal function using t-tests or Kruskal–Wallis tests, as appropriate. Categorical data are described using number (percentage) and are compared between the groups using χ2 tests or Fisher's exact test as appropriate. The primary outcome of interest as noted above was the change from baseline in eGFR at week 24. In addition, we were also interested in analyzing the original efficacy and safety endpoints of the trial according to the baseline renal function in the two eGFR subgroups (<60 and ≥60 mL/min/1.73 m2). The co-primary endpoints of the trial were the self-reported Patient Global Assessment (PGA) and NYHA class (adjusted for baseline class) at week 24.23 Secondary efficacy endpoints were the PGA and NYHA class at weeks 4 and 12, as well as the 6 min walk test distance, the overall Kansas City Cardiomyopathy Questionnaire (KCCQ) score,30 and the European Quality of Life–5 Dimensions (EQ-5D) visual analogue scale (VAS)31 at weeks 4, 12, and 24 (all adjusted for baseline values). For both the overall KCCQ and the EQ-5D VAS, the scores ranged from 0 to 100, with higher scores indicating better quality of life.30,31 Safety endpoints included any serious and non-serious adverse events, hospitalization, and death up to week 26.16,23
For the change in eGFR, comparisons of changes from baseline at 4, 12, and 24 weeks between the placebo and FCM group were evaluated by comparing the least square means at each visit from a repeated-measures model using an unstructured covariance structure and adjusting for the continuous baseline value and a visit × drug interaction. Interaction tests for subgroup analysis for the primary outcome were performed by adding an interaction term between the pre-specified subgroup and treatment.
For the other continuous endpoints, similar repeated-measures analyses were performed in subgroups of patients with impaired and preserved renal function, comparing the least square means between treatment groups at each visit and adjusting for the continuous baseline value as well as a visit × drug interaction. For categorical endpoints, including the original co-primary endpoints, differences in the distribution of responses to treatment at each of 4, 12, and 24 weeks in the two treatment groups were tested by ordered polytomous regression for impaired and preserved renal function patients separately.32 Odds ratios (ORs) for the treatment difference at each time point, 95% confidence intervals (CIs), and corresponding P-values are provided for each eGFR subgroup. Tests for interaction between impaired and preserved renal function patients were carried out in a joint model including all patients. For data on the NYHA class, the model was adjusted for the baseline value. Cox proportional hazard regression models for safety outcomes were used to compare the time to first event using the treatment received, estimating a hazard ratio (HR) for the FCM compared with placebo plus 95% CIs, and a corresponding P-value. Event rates using person–time ‘at risk’ denominators are also provided.
Differences between the percentages of patients withdrawing early from medication in each group were compared using Fisher's exact test. Deaths were analyzed separately. All analyses were conducted with SAS software, version 9.2 (SAS Institute, Cary, NC, USA).
Results
Characteristics of the study population by baseline renal function
Baseline characteristics of the FAIR-HF study population have been previously reported.16 In brief, 459 patients were recruited of which 304 were randomly assigned to FCM and 155 to placebo. Baseline clinical and laboratory characteristics and the use of cardiac medications at the time of enrolment were similar between the two treatment groups.16
At baseline, mean eGFR was 62.9 ± 23.4 and 62.4 ± 20.6 mL/min/1.73 m2 in the placebo and FCM groups, respectively; 130 (42.8%) patients in the FCM group and 73 (47.1%) in the placebo group, respectively, had impaired renal function, as evidenced by eGFR <60 mL/min/1.73 m2.
Table 1 presents the baseline demographic and clinical characteristics by baseline renal function. Patients with impaired renal function tended to be older, with more symptomatic CHF (as evidenced by higher NYHA class) and more frequent cardiovascular risk factors (dyslipidaemia and diabetes mellitus), as well as a medical history of myocardial infarction and coronary revascularization. These patients were more frequently prescribed diuretics, antithrombotics, lipid-lowering drugs, and antidiabetic treatment. This group also had lower haemoglobin, and higher serum ferritin and potassium levels. Importantly, there was no significant difference in the use of beta-blockers and drugs acting on the renin–angiotensin system.
Table 1.
Baseline demographics and clinical characteristics by renal function
| Patients with eGFR <60 mL/min/1.73 m2 (n = 203) | Patients with eGFR ≥60 mL/min/1.73 m2 (n = 256) | P-value | |
|---|---|---|---|
| Age (years) | 71.8 (9.17) | 64.4 (10.55) | <0.001 |
| Female sex, n (%) | 102 (50.25) | 142 (55.47) | 0.265 |
| Caucasian, n (%) | 202 (99.51) | 256 (100.0) | 0.261 |
| NYHA class, n (%) | |||
| II | 28 (13.79) | 54 (21.09) | 0.043 |
| III | 175 (86.21) | 202 (78.91) | |
| Left ventricular ejection fraction (%) | 33.0 [30.00, 35.00] | 33.0 [30.00, 35.00] | 0.297 |
| Body weight (kg) | 76.0 [65.20, 85.00] | 76.0 [67.50, 88.25] | 0.455 |
| Body mass index (kg/m2) | 27.2 [25.40, 31.02] | 27.5 [24.21, 31.25] | 0.924 |
| Blood pressure (mmHg) | |||
| Systolic | 125.3 (15.80) | 127.0 (13.80) | 0.230 |
| Diastolic | 75.04 (10.26) | 77.7 (8.75) | 0.004 |
| Pulse rate (b.p.m.) | 70.0 [62.00, 78.00] | 71.5 [64.00, 78.00] | 0.096 |
| Six-minute walk test distance (m) | 265.0 [184.00, 334.00] | 282.0 [204.00, 357.00] | 0.048 |
| Ischaemic cause of heart failure, n (%) | 170 (83.74) | 198 (77.34) | 0.088 |
| Cardiovascular risk factor | |||
| Hypertension (treated with drugs), n (%) | 168 (82.76) | 203 (79.30) | 0.349 |
| Dyslipidaemia (treated with drugs), n (%) | 104 (51.23) | 110 (42.97) | 0.078 |
| Diabetes mellitus, n (%) | 72 (35.47) | 58 (22.66) | 0.003 |
| History of atrial fibrillation, n (%) | 66 (32.51) | 72 (28.13) | 0.309 |
| Medical history | |||
| Myocardial infarction, n (%) | 131 (64.53) | 127 (49.61) | 0.001 |
| Angina pectoris, n (%) | 111 (54.68) | 149 (58.20) | 0.449 |
| Stroke, n (%) | 18 (8.87) | 15 (5.86) | 0.215 |
| Previous CABG, n (%) | 30 (14.78) | 17 (6.64) | 0.004 |
| Previous PTCA, n (%) | 38 (18.72) | 27 (10.55) | 0.013 |
| Previous coronary revascularization, n (%) | 59 (29.06) | 36 (14.06) | <0.001 |
| Laboratory measurements | |||
| Haemoglobin (g/L) | 117.8 (12.36) | 120.3 (13.38) | 0.040 |
| Mean corpuscular volume (fL) | 92.3 (7.19) | 91.1 (7.94) | 0.096 |
| Serum ferritin (µg/L) | 44.0 [24.00, 77.00] | 33.5 [16.00, 65.00] | <0.001 |
| Transferrin saturation (%) | 15.6 [11.29, 21.37] | 15.6 [9.93, 21.92] | 0.513 |
| C-reactive protein (mg/L) | 2.9 [2.90, 6.00] | 2.9 [2.90, 5.00] | 0.002 |
| Sodium (mmol/L) | 141.0 [139.00, 142.00] | 141.0 [139.00, 142.00] | 0.253 |
| Potassium (mmol/L) | 4.6 [4.30, 5.10] | 4.5 [4.20, 4.80] | <0.001 |
| Alanine aminotransferase (U/L) | 17.0 [13.00, 20.00] | 17.5 [14.00, 23.50] | 0.030 |
| Aspartate aminotransferase (U/L) | 20.0 [17.00, 25.00] | 21.0 [18.00, 25.00] | 0.296 |
| Creatinine (mg/dL) | 1.4 [1.15, 1.70] | 0.9 [0.79, 1.01] | <0.001 |
| eGFR (mL/min/1.73 m2) | 42.9 (12.35) | 78.1 (12.82) | - |
| Blood urea nitrogen (mg/dL) | 29.0 [22.00, 40.00] | 17.0 [14.00, 21.00] | <0.001 |
| Concomitant treatment | |||
| Diuretic, n (%) | 194 (95.57) | 226 (88.28) | 0.005 |
| Agents acting on the renin–angiotensin system, n (%) | 182 (89.66) | 240 (93.75) | 0.110 |
| Beta-blocker, n (%) | 170 (83.74) | 221 (86.33) | 0.439 |
| Cardiac glycosides, n (%) | 30 (14.78) | 41 (16.02) | 0.716 |
| Antithrombotic (including ASA), n (%) | 170 (83.74) | 187 (73.05) | 0.006 |
| Lipid-lowering, n (%) | 116 (57.14) | 98 (38.28) | <0.001 |
| Insulin, n (%) | 26 (12.81) | 10 (3.91) | <0.001 |
| Oral hypoglycaemic agents, n (%) | 39 (19.21) | 32 (12.50) | 0.048 |
Data reported are the number (percentage) of patients for categorical data and mean (SD) or median [lower quartile, upper quartile] for continuous variables depending on the distribution of the data.
P-values were obtained for continuous variables using either two-sample t-tests with Satterthwaite's assumed unequal variances or the Kruskal–Wallis test depending on the distribution of the data. For categorical variables, P-values were obtained using χ2 tests.
ASA, acetylsalicylic acid; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate; PTCA, percutaneous transluminal coronary angioplasty
The impact of ferric carboxymaltose on renal function
In patients receiving FCM, a modest increase in eGFR from baseline was detected throughout the whole study period, whereas patients in the placebo group demonstrated no significant changes in eGFR from baseline during the follow-up period (week 4, 2.61 ± 0.70 vs. 0.50 ± 0.99 mL/min/1.73 m2; week 12, 1.60 ± 0.77 vs. –0.81 ± 1.08 mL/min/1.73 m2; week 24, 2.64 ± 0.81 vs. –0.34 ± 1.1 mL/min/1.73 m2; FCM vs. placebo, respectively; least squares means ± SEs are shown). Treatment with FCM was associated with a strong trend towards an increase in eGFR at week 4 and 12, and a significant increase at week 24 with the following treatment effects: at week 4, 2.11 ± 1.21 (P = 0.082); at week 12, 2.41 ± 1.33 (P = 0.070); and at week 24, 2.98 ± 1.44 mL/min/1.73 m2 (P = 0.039) (Figure 1). This favourable effect was seen in all pre-specified subgroups (P > 0.20 for interaction including baseline renal function, age, sex, CHF severity and aetiology, LVEF, presence of anaemia, diabetes mellitus, baseline ferritin level, and body mass index) (Figure 2). In the sensitivity analysis using the MDRD formula to calculate eGFR, we obtained similar results. At week 4, treatment effect was 2.73 ± 1.444 (P = 0.059), at week 12, 2.82 ± 1.48 (P = 0.059), and at week 24, 3.91 ± 1.65 mL/min/1.73 m2 (P = 0.019).
Figure 1.
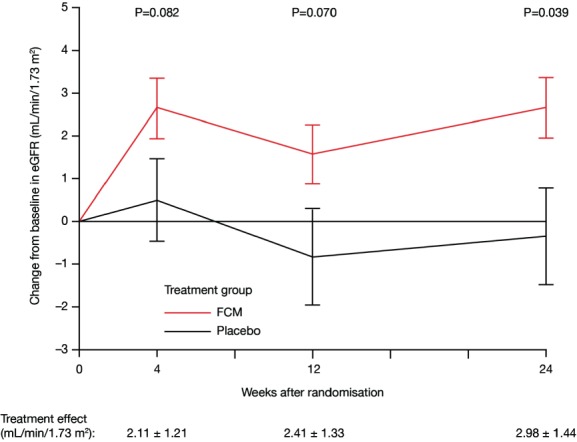
Impact of i.v. ferric carboxymaltose (FCM) treatment on renal function vs. placebo. Values are expressed as least squares means ± SE. eGFR, estimated glomerular filtration rate.
Figure 2.
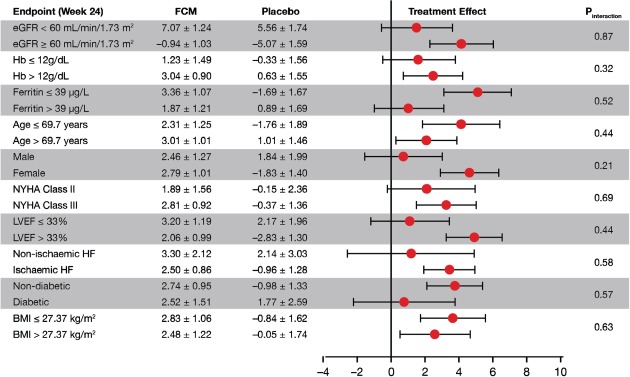
Treatment effect on renal function in pre-defined subgroups at week 24. Treatment effect was expressed as a continuous variable, being the least squares means change from baseline ± 1 SE. BMI, body mass index; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; HF, heart failure.
More patients in the FCM group demonstrated an improvement in renal function. An increase in eGFR >3 mL/min/1.73 m2 at the week 24 visit was 45% vs. 35%, FCM vs. placebo, respectively (P = 0.0012; Figure 3). In the sensitivity analysis using a relative cut-off level of 5% change from baseline eGFR value, at the week 24 visit deterioration in renal function (i.e. decline in eGFR >5% of baseline eGFR) was present in 27% vs. 43%, and improvement (i.e. increase in eGFR >5% of baseline eGFR) in 45% vs. 38%, whereas no change was present in 27% vs. 20% (FCM vs. placebo, respectively; P = 0.02).
Figure 3.
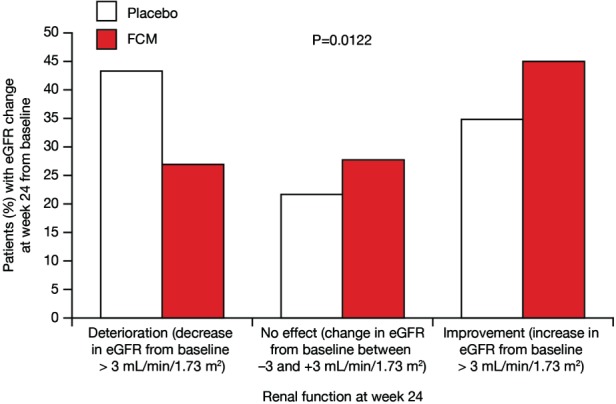
Impact of i.v. ferric carboxymaltose (FCM) on renal function categorized by change in estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) at week 24. Data were available for 347 patients with a change in eGFR at week 24 (106 patients in the placebo group and 241 patients in the FCM group).
Efficacy of ferric carboxymaltose in relation to renal function
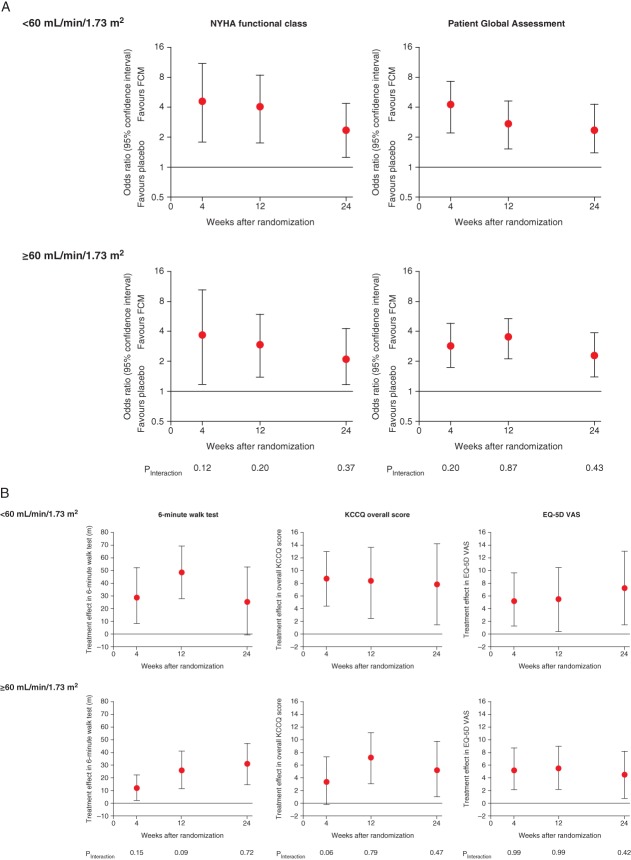
The original primary and secondary endpoints stratified by baseline eGFR (preserved and impaired renal function) are presented in Figure 4. There was no significant interaction between renal function and the effects of FCM on the primary and secondary endpoints.
Figure 4.
Primary (A) and secondary (B) endpoints of the FAIR-HF trials stratified by baseline estimated glomerular filtration rate (eGFR; mL/min/1.73 m2). FCM, ferric carboxymaltose.
Primary endpoints
At week 24, patients treated with FCM had improved NYHA class (adjusted for baseline) compared with those receiving placebo (OR 2.30, 95% CI 1.23–4.28, P = 0.009; and OR 2.43, 95% CI 1.30–4.55, P = 0.006, for patients with preserved and impaired renal function at baseline, respectively; Figure 4A). Self-reported PGA ranks were also better for patients receiving FCM vs. placebo (OR 2.41, 95% CI 1.48–3.95, P < 0.001; and OR 2.51, 95% CI 1.47–4.29, P < 0.001 for patients with preserved and impaired renal function at baseline, respectively; Figure 4A).
Secondary endpoints
Compared with placebo, FCM significantly improved self-reported PGA and NYHA class at weeks 4 and 12 in patients with preserved and impaired renal function (Figure 4A). Significant improvements were also seen in 6 min walk test distance and quality of life assessments by EQ-5D and KCCQ at weeks 4, 12, and 24 in patients with preserved and impaired renal function (Figure 4B).
Safety of ferric carboxymaltose in relation to renal function
Safety endpoints and main investigator-reported adverse events are listed in Table 2.
Table 2.
Safety endpoints and investigator-reported adverse events by baseline renal function
| Patients with eGFR <60 mL/min/1.73 m2 (n = 203) No. of patients with endpoint or event (incidence/100 patient-years at risk) | Patients with eGFR ≥60 mL/min/1.73 m2 (n = 256) No. of patients with endpoint or event (incidence/100 patient-years at risk) | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 73) | FCM (n = 130) | HR (95% CI) | P-value | Placebo (n = 81) | FCM (n = 175) | HR (95% CI) | P-value | |
| Safety endpoints | ||||||||
| Death | 3 (8.9) | 5 (8.1) | 0.91 (0.22–3.79) | 0.89 | 1 (2.5) | 0 (0.0) | – | – |
| Cardiovascular death | 3 (8.9) | 4 (6.5) | 0.72 (0.16–3.24) | 0.67 | 1 (2.5) | 0 (0.0) | – | – |
| Death due to worsening CHF | 3 (8.9) | 0 (0.0) | – | – | 0 (0.0) | 0 (0.0) | – | – |
| First hospitalization | 13 (42.9) | 15 (25.9) | 0.62 (0.30–1.31) | 0.21 | 4 (10.4) | 10 (12.0) | 1.14 (0.36–3.65) | 0.82 |
| Hospitalization for any cardiovascular reason | 12 (39.0) | 8 (13.4) | 0.36 (0.15–0.87) | 0.02 | 2 (5.1) | 7 (8.3) | 1.62 (0.34–7.79) | 0.55 |
| First hospitalization for worsening CHF | 6 (18.4) | 4 (6.6) | 0.36 (0.10–1.26) | 0.11 | 1 (2.5) | 2 (2.3) | 0.92 (0.08–10.09) | 0.94 |
| Any hospitalization or death | 14 (46.2) | 20 (34.5) | 0.76 (0.39–1.51) | 0.44 | 5 (13.1) | 10 (12.0) | 0.92 (0.31–2.68) | 0.87 |
| Hospitalization for any cardiovascular reason or death | 13 (42.2) | 13 (21.8) | 0.53 (0.25–1.14) | 0.11 | 3 (7.7) | 7 (8.3) | 1.08 (0.28–4.18) | 0.91 |
| Hospitalization for worsening CHF or death | 8 (24.6) | 9 (14.8) | 0.60 (0.23–1.56) | 0.29 | 2 (5.1) | 2 (2.3) | 0.46 (0.06–3.27) | 0.44 |
| Investigator-reported adverse events | ||||||||
| Cardiac disorders | 21 (72.8) | 18 (31.5) | 0.44 (0.24–0.83) | 0.01 | 12 (32.5) | 20 (24.9) | 0.77 (0.38–1.57) | 0.47 |
| Gastrointestinal disorders | 1 (3.0) | 11 (18.8) | 6.34 (0.82–49.14) | 0.08 | 4 (10.4) | 13 (15.6) | 1.49 (0.49–4.58) | 0.48 |
| General disorders and administration site conditions | 2 (6.0) | 15 (25.8) | 4.25 (0.97–18.58) | 0.06 | 4 (10.3) | 8 (9.5) | 0.93 (0.28–3.08) | 0.90 |
| Infections and infestations | 10 (32.0) | 19 (33.7) | 1.00 (0.46–2.16) | 1.00 | 14 (39.2) | 31 (39.4) | 1.00 (0.53–1.88) | 1.00 |
| Abnormal lab test, vital sign, or physical finding | 4 (12.1) | 15 (26.3) | 2.14 (0.71–6.44) | 0.18 | 6 (15.6) | 17 (20.7) | 1.33 (0.52–3.38) | 0.55 |
| Respiratory, thoracic, and mediastinal disorders | 5 (15.3) | 4 (6.6) | 0.43 (0.11–1.59) | 0.21 | 5 (13.2) | 5 (5.9) | 0.45 (0.13–1.55) | 0.21 |
| Vascular disorders | 2 (6.0) | 10 (16.9) | 3.06 (0.67–14.04) | 0.15 | 9 (24.6) | 10 (12.0) | 0.49 (0.20–1.20) | 0.12 |
P-value: comparison between placebo and FCM.
P-values were obtained using Wald tests via Cox proportional hazard models.
Patient-years for each person were calculated using the formula [min(event date, safety censor date) – start date]/365.25. Values were then summed for each group.
CHF, chronic heart failure; CI, confidence interval; FCM, ferric carboxymaltose; HR, hazard ratio.
During the study period, there were 8 (3.9%) deaths in patients with impaired renal function (3 in the placebo group and 5 in the FCM group, P = 1.00), whereas only 1 (0.4%) patient with preserved renal function died (in the placebo group).
In patients with impaired renal function, the FCM group compared with the placebo group experienced a trend toward lower incidence rate of first events for hospitalizations [HR 0.62, 95% CI 0.30–1.31, P = 0.21], which mainly resulted from a significantly lower incidence of first cardiovascular hospitalization (HR 0.36, 95% CI 0.15–0.87, P = 0.02). In these patients, the HR (FCM compared with placebo) for the time to first event for either any death or cardiovascular hospitalization was 0.53 (95% CI 0.25–1.14, P = 0.11).
In patients with preserved renal function, the incidence rates of both first all-cause hospitalizations and first cardiovascular hospitalizations were similar between the treatment groups, P = 0.82 and P = 0.55, respectively.
Patients with impaired renal function who were administered FCM experienced fewer cardiac disorders compared with those receiving placebo (HR 0.44, 95% CI 0.24–0.83, P = 0.01). In patients with preserved renal function, the rates of investigator-reported cardiac adverse events were similar in both treatment groups (P = 0.47).
During the study period, study treatment was stopped prematurely in 12 (5.9%) patients with impaired renal function [7 (5.4%) in the FCM group and 5 (6.8%) in the placebo group, P = 0.76] and in 18 (7%) patients with preserved renal function [9 (5.2%) in the FCM group and 9 (10.7%) in the placebo group; P = 0.12].
Discussion
There are two novel findings of this further analysis of the FAIR-HF study. Treatment with FCM for 24 weeks in ID patients with CHF is associated with modest improvement in renal function as evidenced by an increase in eGFR. This favourable effect is seen across the whole clinical spectrum of the disease. Additionally, there was no interaction between baseline renal function and the clinical benefits of FCM, which suggests that FCM is equally effective in patients with preserved and impaired renal function. Importantly, the overall safety and adverse event profiles of FCM were similar in these two groups. Interestingly, in patients with renal dysfunction, those treated with FCM had fewer hospitalizations for any cardiovascular reason and experienced cardiac disorders less frequently.
Renal dysfunction is a frequent co-morbidity in CHF that exerts an adverse effect on the cardiovascular system leading to a progression of the disease, and impaired renal function constitutes one of the strongest predictors of poor clinical outcomes in CHF.1,2,33 Interestingly, virtually none of the currently applied CHF therapies efficiently prevent deterioration in renal function.3,34 It seems that renoprotection may constitute a desirable feature of novel treatments in CHF potentially to improve clinical outcome; this, however, has not been yet tested/confirmed in clinical trials.
In chronic diseases, ID has been traditionally linked with anaemia.35 Only recently has it been recognized that patients with CHF are prone to develop ID irrespective of anaemia, and ID has multifaceted clinical consequences related to poor outcome, exercise intolerance, and impaired quality of life.11–15 It appears that i.v. iron supplementation in CHF patients with ID improves symptoms, functional capacity, and quality of life, with a favourable safety profile irrespective of co-existing anaemia.8,16–18,36 Taking into account the role iron plays in cellular energetics, it is prudent to state that the maintenance of iron homeostasis is particularly important not only for erythropoiesis but also for cells and organs characterized by intensive oxidative metabolism and high energy demand, including the kidney.20,21 Thus, it may be hypothesized that repletion of iron in CHF patients may ultimately result in long-term stabilization or even improvement of renal function. On the other hand, it needs to be remembered that iron may also induce oxidative stress, mainly via involvement in the production of reactive oxygen and nitrogen species, and its pro-oxidative properties potentially may have deleterious effects on renal structure and function.22,37–40 Thus, the safety of iron therapy needs to be clearly addressed in CHF patients, who potentially may also be prone to deterioration in renal function as part of the cardio-renal syndrome, particularly during long-term i.v. iron supplementation.
The results of our study confirm that 24 week therapy with i.v. FCM modestly improves renal function in addition to other clinical benefits. To the best of our knowledge, this is the largest study showing evidence that a drug with a positive effect on functional status and quality of life in CHF patients also exerts favourable effects on renal function. Overall, among therapies applied in CHF, only implantation of a ventricular assist device leads to improvement in renal function,41 whereas studies with other therapies (including digoxin and resynchronization therapy) provided conflicting data.42,43 In the context of i.v. iron supplementation, only Toblli et al.8 demonstrated that in anaemic CHF patients with ID and renal dysfunction, 5 week therapy with i.v. iron sucrose resulted in significant improvement in renal function as evidenced by an increase in creatinine clearance. However, this was a comparatively small study with only 40 patients, half of which received active treatment for a short period of time. Our study extends these preliminary observations to a much broader CHF population, as we have demonstrated improvement in renal function irrespective of baseline haemoglobin level and renal function.
The results of this analysis deserve broader interpretation in the context of the chosen renal endpoints. We have prospectively decided to use changes in eGFR as a safety endpoint in order to evaluate the effect of FCM therapy on renal function. Estimated GFR has already been widely accepted as a marker of renal function in clinical studies,29,44 and a decline in eGFR is closely linked to poor clinical outcomes.2,33 This concept is based on the assumption that the GFR indicates both the number of active nephrons in the kidney and the average filtration capacity of each nephron.45 Thus, changes in GFR may reflect either progression (theoretically also remission) of kidney disease or haemodynamic changes affecting the filtration rate.45 In some clinical studies, effects on GFR detected shortly after initiation of therapy were related to haemodynamic effects, whereas long-term changes were linked to direct effects on the kidney.45,46 In our study, an increase in eGFR was detectable after just 4 weeks of therapy, which seems to be due to an improvement in compromised renal haemodynamics rather than to any direct effects on the nephrons. As we have not evaluated haemodynamic indices during the study, this remains a speculative interpretation; on the other hand, there is evidence that i.v. iron may improve LVEF and decrease serum levels of natriuretic peptide, indicating potentially favourable haemodynamic effects in CHF patients.8 Subsequent steady levels of eGFR observed from week 12 to week 24 suggest stabilization of renal function with no worrying evidence of detrimental effects of long-term i.v. iron therapy (i.e. beyond 4 weeks). Previous studies in anaemic CKD patients show either no relevant changes or a small decline in GFR after i.v. iron supplementation.7,47,48 This is not necessarily contradictory to our results. In contrast to CKD, impaired GFR in CHF seems to be predominantly due to multiple haemodynamic changes (including impaired perfusion or/and venous congestion). Consequently, viable nephrons may be able to maintain their filtration capabilities, and therefore GFR has the potential to be improved. This interpretation, however, needs confirmation in clinical studies.
In this analysis, we used the CKD–EPI formula to calculate the eGFR as it is robust and accurate across a wide range of kidney function, being currently recommended particularly in patients with systolic CHF.27,29 This surrogate approach does not allow the measurement of ‘real’ renal function, and for precise quantitative assessment of GFR nuclear isotope estimates should be applied. As these methods are laborious and require special equipment, they are not suitable for large clinical trials. To overcome such difficulties, creatinine-based formulas to estimate GFR have been proposed and validated in different clinical populations.2,25,27,44
The evidence of equal clinical efficacy and safety of FCM therapy in CHF patients with renal impairment is of relevance. In clinical practice, these patients are often deprived of effective CHF treatment, being perceived as potentially more prone to side effects with less evidence of benefit. In fact, recent analyses from large clinical trials showed life-saving treatments with ACE inhibitors,49 beta-blockers,50,51 or resynchronization therapy42 to be effective regardless of baseline renal function. Our results confirm that this is also the case for i.v. iron supplementation in CHF patients with ID. Of interest, patients with renal impairment receiving FCM may have experienced a lower incidence rate of first cardiovascular hospitalization and fewer cardiac disorders. These intriguing results need to be confirmed in larger studies.
Limitations
The main limitation is the use of eGFR as a renal endpoint, which is only an indirect estimate of renal function with certain limitations in comparison with direct measures. We are aware that we have not evaluated the effects of FCM on creatinine production/excretion and non-renal clearance, all of which may potentially affect the eGFR calculation (being based on creatinine change). As originally planned, we have also performed the same analyses using the six-variable MDRD formula, finding no difference in the interpretation of the results. However, we are fully aware that additional studies with endpoints precisely evaluating multiple aspects of complex renal function are needed to confirm renoprotective effects of FCM therapy. Such studies are also required in order to understand the biological mechanisms underlying potential beneficial effects of iron replacement therapy in CHF, which are not addressed in this analysis. In particular, it would be clinically relevant to distinguish between haemodynamic and peripheral effects of i.v. iron therapy in CHF. In this context, it would be interesting to evaluate the effects of FCM on biomarkers reflecting tubular function, as they are true energy consumers in the kidneys.
In conclusion, correction of ID in CHF patients with i.v. FCM is associated with a modest, but significant improvement in renal function, seen across all the pre-specified subgroups. FCM therapy was effective in CHF patients with renal dysfunction and had a favourable safety profile.
Acknowledgments
Dr David Floyd provided editorial assistance in the development of the manuscript.
Funding
The FAIR-HF study and this analysis were supported by Vifor Pharma, Zurich, Switzerland.
Conflict of interest; S.D.A., P.P., J.C.C., G.F., R.W., K.D., and T.F.L. are members of the FAIR-HF steering committee. S.D.A., P.P., and R.W. are consultants and have received honoraria for speaking for Vifor Pharma and Amgen. S.D.A. has received honoraria for speaking for Roche Pharma and Teva. J.C.C., G.F., and T.L. have received honoraria for speaking for Vifor Pharma. C.M. and B.E.R. are employees of Vifor Pharma and hold stock in Galenica. G.G. is an employee of Vifor Pharma. I.F. and N.G. have received research funding from Vifor Pharma for the analysis of the FAIR-HF data set. I.M is a consultant and has received honoraria for speaking for Vifor Pharma, AMAG, Pharmacosmos, Affymax, Amgen, Ortho Biotech, Roche, and Takeda.
References
- 1.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P, Acute Dialysis Quality Initiative Consensus Group Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Candesartan in Heart Failure: Assessment of Reduction in Mortality Morbidity Investigators. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the Diagnosis, Treatment of Acute Chronic Heart Failure of the European Society of Cardiology, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, ESC Committee for Practice Guidelines ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg DS, Wexler D, Iaina A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur J Heart Fail. 2002;4:681–686. doi: 10.1016/s1388-9842(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 5.van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8:485–493. doi: 10.1038/nrcardio.2011.77. [DOI] [PubMed] [Google Scholar]
- 6.KDOQI; National Kidney Foundation. II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47(5 Suppl 3):S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant. 2006;21:120–124. doi: 10.1093/ndt/gfi087. [DOI] [PubMed] [Google Scholar]
- 8.Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Opasich C, Cazzola M, Scelsi L, De Feo S, Bosimini E, Lagioia R, Febo O, Ferrari R, Fucili A, Moratti R, Tramarin R, Tavazzi L. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J. 2005;26:2232–2237. doi: 10.1093/eurheartj/ehi388. [DOI] [PubMed] [Google Scholar]
- 10.Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou-Nana MI. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48:2485–2489. doi: 10.1016/j.jacc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 12.Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–582. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 14.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. doi: 10.1016/j.cardfail.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Comín-Colet J, Enjuanes C, González G, Torrens A, Cladellas M, Meroño O, Ribas N, Ruiz S, Gómez M, Verdú JM, Bruguera J. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail. 2013;15:1164–1172. doi: 10.1093/eurjhf/hft083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole-Wilson PA, Ponikowski P, for the FAIR-HF Trial Investigators Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 17.Bolger AP, Bartlett FR, Penston HS, O'Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48:1225–1227. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Cairo G, Bernuzzi F, Recalcati S. A precious metal: iron, an essential nutrient for all cells. Genes Nutr. 2006;1:25–39. doi: 10.1007/BF02829934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(Suppl 2):568S–579S. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R, Vasavada N, Sachs NG, Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 23.Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Mori C, von Eisenhart Rothe B, Pocock S, Poole-Wilson PA, Ponikowski P, FAIR-HF committees and investigators Rationale and design of Ferinject assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study: a randomized, placebo-controlled study of intravenous iron supplementation in patients with and without anaemia. Eur J Heart Fail. 2009;11:1084–1091. doi: 10.1093/eurjhf/hfp140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr. 1970;100:301–303. [in German] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Kidney Foundation. 2009. Kidney disease outcomes quality initiative—clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm.
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 28.Padala S, Tighiouart H, Inker LA, Contreras G, Beck GJ, Lewis J, Steffes M, Rodby RA, Schmid CH, Levey AS. Accuracy of a GFR estimating equation over time in people with a wide range of kidney function. Am J Kidney Dis. 2012;60:217–224. doi: 10.1053/j.ajkd.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valente MA, Hillege HL, Navis G, Voors AA, Dunselman PH, van Veldhuisen DJ, Damman K. The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the Modification of Diet in Renal Disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail. 2014;16:86–94. doi: 10.1093/eurjhf/hft128. [DOI] [PubMed] [Google Scholar]
- 30.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 31.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 32.McCullagh P. Regression models for ordinal data (with discussion) J R Stat Soc B. 1980;42:109–142. [Google Scholar]
- 33.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 34.Davenport A, Anker SD, Mebazaa A, Palazzuoli A, Vescovo G, Bellomo R, Ponikowski P, Anand I, Aspromonte N, Bagshaw S, Berl T, Bobek I, Cruz DN, Daliento L, Haapio M, Hillege H, House A, Katz N, Maisel A, Mankad S, McCullough P, Ronco F, Shaw A, Sheinfeld G, Soni S, Zamperetti N, Zanco P, Ronco C. Acute Dialysis Quality Initiative (ADQI) consensus group. ADQI 7: the clinical management of the Cardio-Renal syndromes: work group statements from the 7th ADQI consensus conference. Nephrol Dial Transplant. 2010;25:2077–2089. doi: 10.1093/ndt/gfq252. [DOI] [PubMed] [Google Scholar]
- 35.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790:682–693. doi: 10.1016/j.bbagen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Filippatos G, Farmakis D, Colet JC, Dickstein K, Lüscher TF, Willenheimer R, Parissis J, Gaudesius G, Mori C, von Eisenhart RB, Greenlaw N, Ford I, Ponikowski P, Anker SD. Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR-HF trial. Eur J Heart Fail. 2011;15:1267–1276. doi: 10.1093/eurjhf/hft099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker PD, Shah SV. Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest. 1988;81:334–341. doi: 10.1172/JCI113325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988;34:474–480. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- 39.Bishu K, Agarwal R. Acute injury with intravenous iron and concerns regarding long-term safety. Clin J Am Soc Nephrol. 2006;1(Suppl 1):S19–S23. doi: 10.2215/CJN.01420406. [DOI] [PubMed] [Google Scholar]
- 40.Hayat A. Safety issues with intravenous iron products in the management of anemia in chronic kidney disease. Clin Med Res. 2008;6:93–102. doi: 10.3121/cmr.2008.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Joyce L, Daly R, Park SJ, Kushwaha SS. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol. 2012;59:26–36. doi: 10.1016/j.jacc.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 42.Garg N, Thomas G, Jackson G, Rickard J, Nally JV, Jr, Tang WH, Navaneethan SD. Cardiac resynchronization therapy in CKD: a systematic review. Clin J Am Soc Nephrol. 2013;8:1293–1303. doi: 10.2215/CJN.00750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaduganathan M, Gheorghiade M. Return of digoxin and recovery of renal function. J Card Fail. 2013;19:303–305. doi: 10.1016/j.cardfail.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–1580. doi: 10.1161/CIRCULATIONAHA.105.610642. [DOI] [PubMed] [Google Scholar]
- 45.Stevens LA, Greene T, Levey AS. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol. 2006;1:874–884. doi: 10.2215/CJN.00600206. [DOI] [PubMed] [Google Scholar]
- 46.Wright JT, Bakris G, Greene T, Agodoa L, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 47.McMahon LP, Kent AB, Kerr PG, Healy H, Irish AB, Cooper B, Kark A, Roger SD. Maintenance of elevated versus physiological iron indices in non-anaemic patients with chronic kidney disease: a randomized controlled trial. Nephrol Dial Transplant. 2010;25:920–926. doi: 10.1093/ndt/gfp584. [DOI] [PubMed] [Google Scholar]
- 48.Van Wyck DB, Roppolo M, Martinez CO, Mazey RM, McMurray S, for the United States Iron Sucrose (Venofer) Clinical Trials Group A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68:2846–2856. doi: 10.1111/j.1523-1755.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 49.Tokmakova MP, Skali H, Kenchaiah S, Braunwald E, Rouleau JL, Packer M, Chertow GM, Moyé LA, Pfeffer MA, Solomon SD. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) study. Circulation. 2004;110:3667–3673. doi: 10.1161/01.CIR.0000149806.01354.BF. [DOI] [PubMed] [Google Scholar]
- 50.Ghali JK, Wikstrand J, Van Veldhuisen DJ, Fagerberg B, Goldstein S, Hjalmarson A, Johansson P, Kjekshus J, Ohlsson L, Samuelsson O, Waagstein F, Wedel H, MERIT-HF Study Group The influence of renal function on clinical outcome and response to beta-blockade in systolic heart failure: insights from Metoprolol CR/XL Randomized Intervention Trial in Chronic HF (MERIT-HF) J Card Fail. 2009;15:310–318. doi: 10.1016/j.cardfail.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Cohen-Solal A, Kotecha D, van Veldhuisen DJ, Babalis D, Böhm M, Coats AJ, Roughton M, Poole-Wilson P, Tavazzi L, Flather M, SENIORS Investigators Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail. 2009;11:872–880. doi: 10.1093/eurjhf/hfp104. [DOI] [PMC free article] [PubMed] [Google Scholar]