Abstract
A growing number of studies in multicellular organisms highlight low or moderate frequencies of paternal transmission of cytoplasmic organelles, including both mitochondria and chloroplasts. It is well established that strict maternal inheritance is selectively blind to cytoplasmic elements that are deleterious to males – ’mother's curse’. But it is not known how sensitive this conclusion is to slight levels of paternal cytoplasmic leakage. We assess the scope for polymorphism when individuals bear multiple cytoplasmic alleles in the presence of paternal leakage, bottlenecks and recurrent mutation. When fitness interactions among cytoplasmic elements within an individual are additive, we find that sexually antagonistic polymorphism is restricted to cases of strong selection on males. However, when fitness interactions among cytoplasmic elements are nonlinear, much more extensive polymorphism can be supported in the cytoplasm. In particular, mitochondrial mutants that have strong beneficial fitness effects in males and weak deleterious fitness effects in females when rare (i.e. ’reverse dominance’) are strongly favoured under paternal leakage. We discuss how such epistasis could arise through preferential segregation of mitochondria in sex-specific somatic tissues. Our analysis shows how paternal leakage can dampen the evolution of deleterious male effects associated with predominant maternal inheritance of cytoplasm, potentially explaining why ’mother's curse’ is less pervasive than predicted by earlier work.
Keywords: chloroplast, heteroplasmy, intralocus sexual conflict, maternal inheritance, mitochondria, mtDNA, organelle, sexual dimorphism
Introduction
Strict maternal inheritance of the cytoplasm is considered the norm in most higher eukaryotes. However, a growing number of studies in plants and animals report detectable levels of paternal transmission of cytoplasmic elements (e.g. Gyllensten et al., 1991; Schwartz & Vissing, 2002; Wolff et al., 2008; Nunes et al., 2013; Wolff et al., 2013; reviewed in Mogensen, 1996; McCauley, 2013). In some taxa, the rate reported is very low, for example 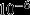 offspring in mice carry paternally inherited mitochondria (Gyllensten et al., 1991). But considerably higher levels of leakage occur in others: for example, 3–6% of seeds in Silene vulgaris bear paternal mtDNA (McCauley, 2013), and Drosophila melanogaster has recently been inferred to have levels of leakage of up to 6% (Nunes et al., 2013). Moreover, extreme examples of paternal mtDNA transmission in higher eukaryotes are found in sequoias (Sequoia sempervirens) and cucumber (Cucumis sempervirens), in which mitochondria are predominantly inherited through pollen, instead of ova (Neale et al., 1989; Havey, 1997; Crosby & Smith, 2012).
offspring in mice carry paternally inherited mitochondria (Gyllensten et al., 1991). But considerably higher levels of leakage occur in others: for example, 3–6% of seeds in Silene vulgaris bear paternal mtDNA (McCauley, 2013), and Drosophila melanogaster has recently been inferred to have levels of leakage of up to 6% (Nunes et al., 2013). Moreover, extreme examples of paternal mtDNA transmission in higher eukaryotes are found in sequoias (Sequoia sempervirens) and cucumber (Cucumis sempervirens), in which mitochondria are predominantly inherited through pollen, instead of ova (Neale et al., 1989; Havey, 1997; Crosby & Smith, 2012).
The findings above question whether conventional predictions about the evolutionary dynamics of cytoplasmic elements are robust to observed levels of paternal leakage (Wolff et al., 2013). One well-established idea is that cytoplasmic elements such as mitochondria, chloroplasts or endosymbionts invariably accumulate mutations that are detrimental to males (’mother's curse’, Frank & Hurst, 1996; Gemmell et al., 2004). This is because strict maternal inheritance masks any selection on male fitness effects, leading to the spread of sexually antagonistic alleles that are favoured in females but detrimental to males. Indeed, indirect evidence for mother's curse comes from a recent study by Innocenti et al. (2011), which shows that the introgression of a novel mitochondrial variant substantially affects male-specific gene expression, whereas female-specific gene expression remains unaffected. Moreover, a substantial number of mitochondrial mutations seem to exclusively affect male fitness, particularly in the context of male fertility (e.g. Nakada et al., 2006; Wai et al., 2010; Innocenti et al., 2011). One prominent example is Leber's disease, in which a mitochondrial mutation is responsible for loss of vision, which predominantly affects young males (Wallace et al., 1988). On the other hand, recent evaluations of mitochondrial disease in humans show that the majority of mtDNA mutations lack sex-specific effects (e.g. Park & Larsson, 2011; Wallace, 2013), begging the question whether other mechanisms exist that mitigate the extent of mother's curse (e.g. Wade & Brandvain, 2009).
Here, we ask whether small amounts of leakage affect cytoplasmically linked detrimental male fitness effects. To assess sexually antagonistic variation in the cytoplasm, we use a two-fold approach. First, we incorporate leakage in the population genetics model of Frank & Hurst (1996). This model implicitly assumes a rather simplistic form of mitochondrial inheritance, where all cytoplasmically linked alleles within an individual are identical. By contrast, studies of the dynamics of mitochondrial transmission (Birky, 1995, 2001; Stewart et al., 2008a) emphasize that bottlenecks, sampling effects and vegetative segregation are important, which potentially allow for heteroplasmy, in which individuals carry more than one cytoplasmically linked allele (Hadjivasiliou et al., 2012, 2013). At present, no predictions exist about how the interactions between multiple cytoplasmic alleles affect fitness and sexually antagonistic polymorphism. These interactions are likely to be important as epistatic and dominance interactions clearly affect the scope for sexually antagonistic variation on autosomes (Kidwell et al., 1977; Rice, 1984; Fry, 2010; Connallon & Clark, 2011; Mullon et al., 2012). We therefore assess the consequences of different types of epistatic interactions within the cytoplasm for sexually antagonistic variation in the presence of leakage.
The main goal of the current model was to investigate whether slight levels of leakage could provide an additional explanation for the limited prevalence of mother's curse in nature. In this context, a previous model on mitochondrial effects on male fertility (Wade & Brandvain, 2009) verbally alludes to the potential of leakage to reduce mother's curse. A systematic analysis of the effects of leakage on mother's curse is lacking for the broad range of leakage values that have been found. Another goal was to quantify how leakage affects heteroplasmy (McCauley, 2013), particularly when the fitness consequences of cytoplasmic genes diverge between the sexes. Although classical studies on the maintenance of genetic variation in the cytoplasm have considered leakage (e.g. Ohta, 1980; Takahata & Maruyama, 1981; Takahata & Slatkin, 1983), the role of sex-specific fitness effects and interactions between multiple cytoplasmic alleles within an individual have not been considered previously.
Model 1: haploid population genetics
In the remainder of this article, we refer to mitochondria as the cytoplasmic element in question, as they are the most common and ancient cytoplasmic element in nature (Lane & Martin, 2010; Martin, 2011). However, we emphasize that similar principles hold for other cytoplasmic elements (e.g. chloroplasts and endosymbionts such as Wolbachia). We first focus on paternal leakage in the population genetics model studied by Frank & Hurst (1996), in which all mitochondrial alleles are identical within an individual. We consider the fate of two mitochondrial alleles, 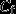 being a female-benefit, male-detriment allele, whereas
being a female-benefit, male-detriment allele, whereas 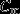 is a male-benefit, female-detriment allele. We track the frequency of the
is a male-benefit, female-detriment allele. We track the frequency of the 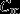 allele in females (frequency
allele in females (frequency 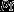 ) and males (frequency
) and males (frequency 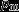 ). Similar to Frank & Hurst (1996), females bearing a
). Similar to Frank & Hurst (1996), females bearing a 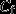 or a
or a 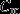 allele have survival probabilities of 1 and
allele have survival probabilities of 1 and  , respectively, whereas males have corresponding survival probabilities
, respectively, whereas males have corresponding survival probabilities  (allele
(allele 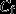 ) and 1 (allele
) and 1 (allele 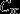 ) (see Table 1). Frequencies of the
) (see Table 1). Frequencies of the 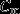 allele in females and males in the next generation are then given by
allele in females and males in the next generation are then given by
Table 1.
Sex-specific fitness functions used in the haploid model and the model involving multiple mitochondria. 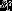 and
and 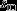 reflect the baseline mortality cost of bearing
reflect the baseline mortality cost of bearing 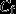 and
and 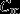 alleles. M reflects the total number of mitochondria present in an individual, m of which are of type
alleles. M reflects the total number of mitochondria present in an individual, m of which are of type 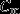 and M − m are of type
and M − m are of type 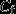 . k reflects how rapidly fitness increases (or decreases) with an increasing number of
. k reflects how rapidly fitness increases (or decreases) with an increasing number of 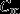 mitochondria
mitochondria
| Model | Context | Female fitness | Male fitness |
|---|---|---|---|
| Haploid model | Female-benefit cytoplasm 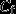
|
1 |  |
Male-benefit cytoplasm 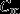
|
 |
1 | |
| Multiple mitochondria | Constant dominance | 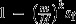 |
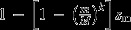 |
| Reverse dominance | 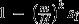 |
 |
|
| Sigmoidal fitness | 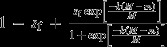 |
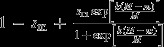 |
| (1a) |
| (1b) |
For 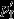 , the first term within the straight brackets reflects the probability that daughters inherit a
, the first term within the straight brackets reflects the probability that daughters inherit a 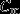 mitotype from a mating between a mother bearing the
mitotype from a mating between a mother bearing the 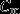 mitotype (with frequency
mitotype (with frequency 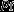 ) and a
) and a 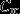 father (frequency
father (frequency 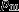 ). In this case, leakage does not affect transmission, as both parents carry the
). In this case, leakage does not affect transmission, as both parents carry the 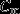 mitotype. Daughters also inherit the
mitotype. Daughters also inherit the 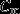 allele when a
allele when a 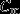 mother mates with a
mother mates with a 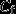 father and leakage does not occur with probability
father and leakage does not occur with probability  (second term), or when a
(second term), or when a 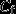 mother mates with a
mother mates with a 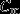 father and leakage occurs with probability
father and leakage occurs with probability  (third term). In all cases, daughters that inherit the
(third term). In all cases, daughters that inherit the 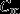 allele have a fitness of
allele have a fitness of  . Similar considerations govern the inheritance of
. Similar considerations govern the inheritance of 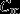 by sons, except that sons who inherit the
by sons, except that sons who inherit the 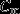 allele have a fitness of unity. Expressions for female and male mean fitness are given by
allele have a fitness of unity. Expressions for female and male mean fitness are given by
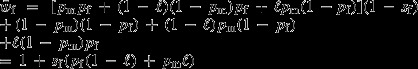 |
(2a) |
| (2b) |
yielding the corresponding difference equations  and
and 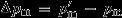 . Solving for
. Solving for 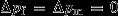 yields three equilibria: one in which
yields three equilibria: one in which 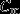 is extinct (
is extinct (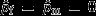 ), one where
), one where 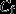 is extinct (
is extinct (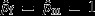 ) and an equilibrium where
) and an equilibrium where 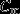 and
and 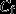 coexist:
coexist:
| (3a) |
| (3b) |
By evaluating the eigenvalues of the Jacobian matrix of the system in eqns (1a and 1b), we then assess how paternal leakage affects the stability of these equilibria (see below).
Result 1: mitochondrial polymorphism is only maintained when selection on males is strong
Consider a population that is fixed for 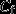 , the female-benefit, male-detriment mitochondrial allele (i.e.
, the female-benefit, male-detriment mitochondrial allele (i.e. 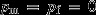 ). A rare mutant mitochondrion
). A rare mutant mitochondrion 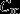 that benefits males but is detrimental to females invades when
that benefits males but is detrimental to females invades when
| (7) |
which reduces to the classical condition 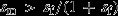 (Kidwell et al., 1977) for autosomal alleles (
(Kidwell et al., 1977) for autosomal alleles ( ). Unsurprisingly, when leakage is absent
). Unsurprisingly, when leakage is absent  , we find that
, we find that 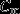 is unable to invade unless
is unable to invade unless  , in which case no male survives and the population goes extinct. However,
, in which case no male survives and the population goes extinct. However, 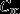 is able to invade for modest levels of leakage
is able to invade for modest levels of leakage 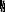 when the fitness effect of
when the fitness effect of 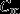 in females is modest relative to the deleterious fitness effects of the ’resident’ mitochondrion
in females is modest relative to the deleterious fitness effects of the ’resident’ mitochondrion 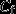 on males. Upon invasion,
on males. Upon invasion, 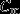 and
and 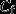 are both stably maintained in an equilibrium given by eqns (3a and 3b) when
are both stably maintained in an equilibrium given by eqns (3a and 3b) when
| (8) |
which is identical to the conditions for polymorphism identified by Kidwell et al. (1977) for autosomal loci  in the absence of dominance. Both conditions (7) and (8) are graphically summarized in Fig. 1 for realistic levels of leakage observed in natural populations. We conclude that, for modest levels of leakage
in the absence of dominance. Both conditions (7) and (8) are graphically summarized in Fig. 1 for realistic levels of leakage observed in natural populations. We conclude that, for modest levels of leakage  , mitochondrially linked male-benefit mutations will be unable to invade for a large region of the parameter space. This formalizes a previous verbal prediction by Wade & Brandvain (2009) (pp. 1087–1088) that low levels of leakage are unlikely to affect mitochondrial sexually antagonistic variation when selection is relatively weak. However, when selection on males is strong, we find that invasion of
, mitochondrially linked male-benefit mutations will be unable to invade for a large region of the parameter space. This formalizes a previous verbal prediction by Wade & Brandvain (2009) (pp. 1087–1088) that low levels of leakage are unlikely to affect mitochondrial sexually antagonistic variation when selection is relatively weak. However, when selection on males is strong, we find that invasion of 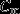 typically results in the stable maintenance of both
typically results in the stable maintenance of both 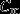 and
and 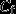 mitotypes (i.e. polymorphism). Lastly, for a much narrower range of the parameter space where leakage is considerable
mitotypes (i.e. polymorphism). Lastly, for a much narrower range of the parameter space where leakage is considerable  and selection on females is very weak relative to selection on males
and selection on females is very weak relative to selection on males 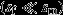 , we find that cytotypes that are deleterious for females are able to fix in the population.
, we find that cytotypes that are deleterious for females are able to fix in the population.
Figure 1.
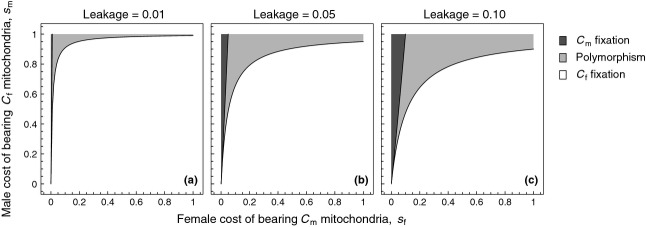
Regions depicting the effect of leakage on the maintenance of  mitochondria when individuals contain a single, haploid mitochondrion. Regions depict fixation of female-benefit
mitochondria when individuals contain a single, haploid mitochondrion. Regions depict fixation of female-benefit  mitochondria (white), polymorphism of
mitochondria (white), polymorphism of  and male-benefit
and male-benefit 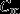 mitochondria (light grey), and fixation of
mitochondria (light grey), and fixation of 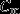 mitochondria (dark grey).
mitochondria (dark grey).
Model 2: multiple mitochondrial alleles per individual
Until now, we assumed that each individual contains only one mitochondrial allele. However, cells usually contain multiple mitochondria and multiple mtDNA molecules within each mitochondrion (Birky, 2001). Taking account of multiple alleles is essential given that paternal leakage is likely to result in heteroplasmy, in which different mitochondrial alleles coexist in a single individual (McCauley, 2013). We therefore investigate a model that allows for the presence of a finite number of M mitochondrial alleles within an individual (see also Hadjivasiliou et al., 2012). The dynamics of the number of cytoplasmic alleles per individual is then subject to a range of additional forces, including sampling due to drift (Ohta, 1980; Chapman et al., 1982), bottlenecks (Bergstrom & Pritchard, 1998), vegetative segregation (Birky, 2001), as well as selection arising from the epistatic interactions between different alleles within an individual.
Fitness: To model the effect of epistatic interactions between 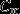 and
and 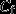 mitochondria on sexually antagonistic variation, let m be the number of
mitochondria on sexually antagonistic variation, let m be the number of 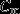 mitochondria present in an individual (and M−m the number of
mitochondria present in an individual (and M−m the number of 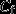 mitochondria). Following previous multilocus models that deal with sexual antagonism (e.g. Connallon & Clark, 2011; Hadjivasiliou et al., 2012), we assume that female and male fitnesses are then dependent on the proportion of male-benefit versus female-benefit alleles (see Table 1):
mitochondria). Following previous multilocus models that deal with sexual antagonism (e.g. Connallon & Clark, 2011; Hadjivasiliou et al., 2012), we assume that female and male fitnesses are then dependent on the proportion of male-benefit versus female-benefit alleles (see Table 1):
| (6a) |
| (6b) |
where k reflects the type of epistatic relationship between multiple mitochondrial alleles present in a single individual, in a similar fashion as dominance interactions have been modelled between alleles at a single locus (see also Fry, 2010; Connallon & Clark, 2011). When k = 1, mitochondria interact additively (see Fig. 2a). By contrast, when k > 1, 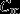 mitochondria are recessive to
mitochondria are recessive to 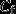 (solid lines in Fig. 2b) and when k < 1,
(solid lines in Fig. 2b) and when k < 1, 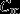 mitochondria are dominant relative to
mitochondria are dominant relative to 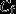 (solid lines in Fig. 2c). Importantly, the dominance of
(solid lines in Fig. 2c). Importantly, the dominance of 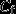 relative to
relative to 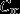 is the same across males and females (i.e. a ’constant dominance’ scenario; Connallon & Clark, 2011). Another way of thinking about these assumptions is that when
is the same across males and females (i.e. a ’constant dominance’ scenario; Connallon & Clark, 2011). Another way of thinking about these assumptions is that when 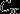 is dominant, there is strong fitness loss in females and strong fitness gain in males when
is dominant, there is strong fitness loss in females and strong fitness gain in males when 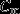 is at low frequency within an individual (solid lines in Fig. 2c). When
is at low frequency within an individual (solid lines in Fig. 2c). When 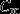 is recessive, the fitness loss in females and fitness gain in males is weak when
is recessive, the fitness loss in females and fitness gain in males is weak when 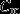 mitochondria are at low frequency (solid lines in Fig. 2). The fitness gain in one sex is exactly mirrored by the fitness loss in the other sex.
mitochondria are at low frequency (solid lines in Fig. 2). The fitness gain in one sex is exactly mirrored by the fitness loss in the other sex.
Figure 2.
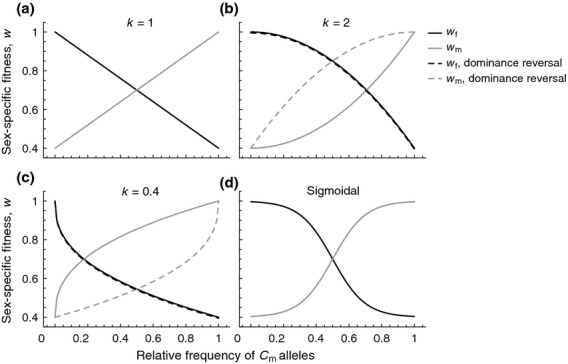
Male and female fitnesses when individuals contain more than a single cytoplasmic element. For all panels, solid lines indicate a scenario of ’constant dominance’ where the fitness gain in one sex is exactly mirrored by the fitness loss in the other sex. Dashed lines indicate a scenario of ’reverse dominance’, where the fitness gain in one sex is different from the fitness loss in the other sex. (a) k = 1, sex-specific fitness changes linearly with the proportion of  mitochondria in both males and females. (b, c) sex-specific fitness changes either in an accelerating or decelerating fashion with an increasing proportion of
mitochondria in both males and females. (b, c) sex-specific fitness changes either in an accelerating or decelerating fashion with an increasing proportion of  mitochondria. See Table 1 for formal descriptions of the different fitness functions. (d) sex-specific fitness changes in a sigmoidal fashion. Parameters for all panels:
mitochondria. See Table 1 for formal descriptions of the different fitness functions. (d) sex-specific fitness changes in a sigmoidal fashion. Parameters for all panels:  . Panel (d) k = 0.1.
. Panel (d) k = 0.1.
Alternatively we consider a fitness function for ’reversed dominance’ (e.g. Curtsinger et al., 1994; Connallon & Clark, 2011), where the dominance of 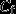 relative to
relative to 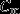 varies according to the sex of the carrier. Male fitness is now given by:
varies according to the sex of the carrier. Male fitness is now given by: 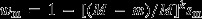 instead of eqn (6b), whereas female fitness is the same as in eqn (6a) (see Table 1). For example, when k > 1, a modest fitness loss in females could be mirrored by a strong fitness gain in males when
instead of eqn (6b), whereas female fitness is the same as in eqn (6a) (see Table 1). For example, when k > 1, a modest fitness loss in females could be mirrored by a strong fitness gain in males when 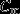 mitochondria are at low frequencies (dashed lines in Fig. 2b). Similarly, a strong fitness loss in females could be mirrored by weak fitness gain in males when the
mitochondria are at low frequencies (dashed lines in Fig. 2b). Similarly, a strong fitness loss in females could be mirrored by weak fitness gain in males when the 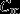 allele is at low frequency when k < 1 (dashed lines in Fig. 2c).
allele is at low frequency when k < 1 (dashed lines in Fig. 2c).
Lastly, we also assess a more complex fitness function, where 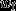 and
and 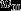 are given by a sigmoidal function (see Fig. 2d). Such a sigmoidal function reflects not only sexual antagonism, but also scenarios where the presence of polymorphism in
are given by a sigmoidal function (see Fig. 2d). Such a sigmoidal function reflects not only sexual antagonism, but also scenarios where the presence of polymorphism in 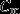 and
and 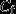 itself substantially reduces viability, conforming to recent findings that heteroplasmy (which reflects within-individual polymorphism) negatively affects fitness in certain cases (Lane, 2012; Sharpley et al., 2012; Wallace & Chalkia, 2013).
itself substantially reduces viability, conforming to recent findings that heteroplasmy (which reflects within-individual polymorphism) negatively affects fitness in certain cases (Lane, 2012; Sharpley et al., 2012; Wallace & Chalkia, 2013).
Life cycle: To model change in allele frequencies in the presence of multiple cytoplasmic elements, we assume a life cycle similar to that used by Hadjivasiliou et al. (2012) to model the evolution of uniparental inheritance of mitochondria (which we refer to for an in-depth discussion). The life cycle consists of five phases (see SI and Fig. 3): (i) at birth, an individual contains M cytoplasmic elements, (ii) individual mitochondria mutate independently of each other with probability μ, (iii) individuals then undergo sex-specific selection according to the equations in Table 1, (iv) surviving individuals enter a sexual phase in which they undergo meiosis and (v) syngamy to produce the next generation. Before meiosis, individuals undergo a bottleneck in which B mitochondria are sampled from the M original mitochondria (Birky, 1995, 2001), after which the population size of mitochondria is brought back up to M by randomly sampling with replacement from the B mitochondria. Although it is currently debated whether within-host purifying selection or bottlenecks are the underlying cause of rapid mtDNA segregation in animals (Stewart et al., 2008b; Wai et al., 2008; Samuels et al., 2010; Carling et al., 2011), the goal of the bottleneck stage is to assess the effect of within-host homogenization of cytoplasmic elements. After the bottleneck stage, each cell that undergoes meiosis produces 2M mitochondria, which are then reduced through two cell divisions to M/2 mitochondria present in each of the four gametes. Gametes then randomly fuse with each other, where the female gamete contributes 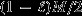 mitochondria (where
mitochondria (where  is the proportion of leakage), and the male gamete contributes
is the proportion of leakage), and the male gamete contributes 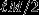 mitochondria. The resulting M/2 mitochondria are then doubled through sampling with replacement to arrive back at M mitochondria. The life cycle can be mathematically described in an exact manner, but the complexity of the model prohibits an analytical solution. We therefore use numerical simulations to find equilibrium frequencies of cytotypes
mitochondria. The resulting M/2 mitochondria are then doubled through sampling with replacement to arrive back at M mitochondria. The life cycle can be mathematically described in an exact manner, but the complexity of the model prohibits an analytical solution. We therefore use numerical simulations to find equilibrium frequencies of cytotypes 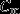 and
and 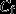 . For the sake of computational speed, we consider a conservative value of M = 200 copies of cytoplasmic genomes per cell, which is typically the lower bound of the number of mitochondrial copies in animals (e.g. Solignac et al., 1984; Cao et al., 2007). Moreover, a small value of M in combination with the aforementioned bottleneck is indicative of the fact that mitochondria typically have small effective population sizes (Ballard & Whitlock, 2004; Neiman & Taylor, 2009).
. For the sake of computational speed, we consider a conservative value of M = 200 copies of cytoplasmic genomes per cell, which is typically the lower bound of the number of mitochondrial copies in animals (e.g. Solignac et al., 1984; Cao et al., 2007). Moreover, a small value of M in combination with the aforementioned bottleneck is indicative of the fact that mitochondria typically have small effective population sizes (Ballard & Whitlock, 2004; Neiman & Taylor, 2009).
Figure 3.
Schematic depiction of the life cycle when individuals contain multiple mitochondria.
Result 2: cytoplasmic epistasis favours polymorphism and heteroplasmy
For individuals with multiple cytoplasmic elements (M = 200), polymorphism does not differ from the haploid model (where M = 1, see Fig. 1) when fitness effects are additive (see Fig. S1). Similar to the haploid model, higher levels of paternal leakage (large  ) together with strong selection on males (
) together with strong selection on males ( large) facilitate the presence of cytoplasmic polymorphism. By contrast, when fitness effects are nonlinear and felt similarly by males and females (solid lines in Fig. 2b and c), the degree of polymorphism differs. When
large) facilitate the presence of cytoplasmic polymorphism. By contrast, when fitness effects are nonlinear and felt similarly by males and females (solid lines in Fig. 2b and c), the degree of polymorphism differs. When  mitochondria are dominant in both sexes, they are much less likely to be maintained (Fig. 4a–c). However, when
mitochondria are dominant in both sexes, they are much less likely to be maintained (Fig. 4a–c). However, when  mitochondria are recessive in both sexes, differences with the haploid model are small (Fig. 4d–f). Likewise for sigmoidal nonadditive interactions (Fig. 2d), we find that the parameter space conducive to polymorphism is only slightly enhanced relative to the haploid model (see Fig. S2).
mitochondria are recessive in both sexes, differences with the haploid model are small (Fig. 4d–f). Likewise for sigmoidal nonadditive interactions (Fig. 2d), we find that the parameter space conducive to polymorphism is only slightly enhanced relative to the haploid model (see Fig. S2).
Figure 4.
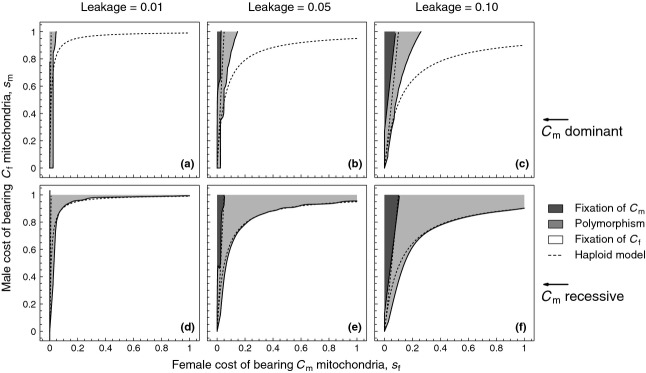
Cytoplasmic polymorphism for different levels of leakage  . Fitness effects are assumed to be nonlinear and felt similarly by males and females (see solid lines in Fig. 2a,b). Panels (a–c) fitness effects of a rare
. Fitness effects are assumed to be nonlinear and felt similarly by males and females (see solid lines in Fig. 2a,b). Panels (a–c) fitness effects of a rare 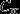 allele are dominant in both sexes (k = 0.25). Panels (d–f) fitness effects of a rare
allele are dominant in both sexes (k = 0.25). Panels (d–f) fitness effects of a rare  allele are recessive in both sexes (K=2). Parameters: M = 200,
allele are recessive in both sexes (K=2). Parameters: M = 200, 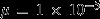 . Polymorphism is defined as
. Polymorphism is defined as 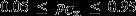 .
. 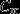 is considered fixed when
is considered fixed when 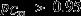 .
.
The conditions leading to sexually antagonistic polymorphism are strikingly different from the haploid model in the case of reverse dominance (Fig. 5). When the male-beneficial allele 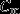 is recessive in males and hence dominant in females, we find that the region of polymorphism is markedly smaller relative to the haploid model (Fig. 5a–c). When
is recessive in males and hence dominant in females, we find that the region of polymorphism is markedly smaller relative to the haploid model (Fig. 5a–c). When 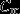 successfully invades, it nearly always achieves fixation, as the fitness benefit to males increases more than linearly the more common it gets (dashed line for
successfully invades, it nearly always achieves fixation, as the fitness benefit to males increases more than linearly the more common it gets (dashed line for 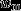 and solid line for
and solid line for 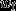 in Fig. 2c). By contrast, when the male-beneficial allele
in Fig. 2c). By contrast, when the male-beneficial allele 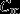 is dominant in males and recessive in females, we find that
is dominant in males and recessive in females, we find that 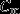 can invade for considerably lower values of
can invade for considerably lower values of 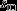 relative to the haploid case ( Fig. 5d–f). The
relative to the haploid case ( Fig. 5d–f). The 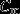 allele invades as its fitness when rare is largely masked in females, whereas it provides a large fitness benefit in males. However, it will typically not achieve fixation, as the fitness cost for females (which transmit the majority of mitochondria) rises markedly with increasing frequency of
allele invades as its fitness when rare is largely masked in females, whereas it provides a large fitness benefit in males. However, it will typically not achieve fixation, as the fitness cost for females (which transmit the majority of mitochondria) rises markedly with increasing frequency of 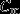 (dashed line for
(dashed line for 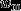 and solid line for
and solid line for 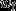 in Fig. 2b). Consequently, polymorphism is expected to occur in the presence of leakage for male-benefit alleles that are dominant in males, but recessive in females.
in Fig. 2b). Consequently, polymorphism is expected to occur in the presence of leakage for male-benefit alleles that are dominant in males, but recessive in females.
Figure 5.
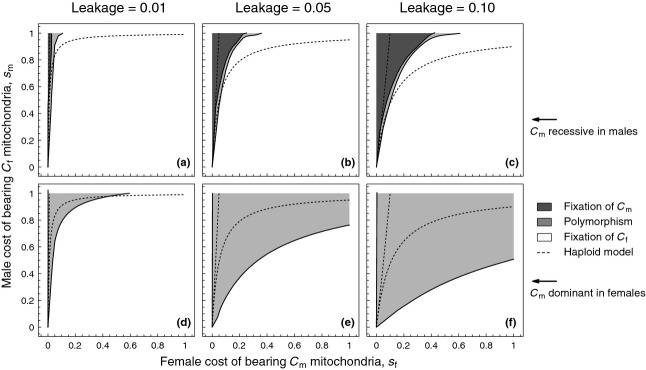
Cytoplasmic polymorphism for different levels of leakage  . Epistatic interactions among cytoplasmic elements reflect a scenario of ’reverse dominance’ (dashed lines in Fig. 2b, c). Panels (a–c) rare
. Epistatic interactions among cytoplasmic elements reflect a scenario of ’reverse dominance’ (dashed lines in Fig. 2b, c). Panels (a–c) rare 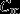 mitochondria are recessive in males and dominant in females (k = 0.25). Panels (d–f) rare
mitochondria are recessive in males and dominant in females (k = 0.25). Panels (d–f) rare 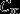 mitochondria are dominant in males and recessive in females (k = 2). Parameters: M = 200,
mitochondria are dominant in males and recessive in females (k = 2). Parameters: M = 200, 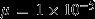 . Polymorphism is defined as
. Polymorphism is defined as 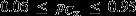 .
. 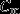 is considered fixed when
is considered fixed when 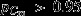 .
.
The degree of polymorphism is also reflected by heteroplasmy, the variation of mitochondria within an individual. For a null scenario without any leakage and additive fitness effects (solid line in Fig. 6a), heteroplasmy is only caused by recurrent mutation and increases in the frequency of 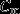 alleles are due to drift. Consequently, the majority of individuals only bear
alleles are due to drift. Consequently, the majority of individuals only bear 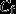 mitochondria, whereas heteroplasmic individuals are very rare and a small fraction of individuals are fixed for
mitochondria, whereas heteroplasmic individuals are very rare and a small fraction of individuals are fixed for 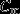 . When leakage is nonzero and fitness is additive, we find a slight increase in heteroplasmy (dashed line in Fig. 6a). Most individuals are still homogeneous for
. When leakage is nonzero and fitness is additive, we find a slight increase in heteroplasmy (dashed line in Fig. 6a). Most individuals are still homogeneous for 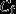 mitochondria, however, as selection on males is not strong enough to favour much polymorphism when fitness is additive. As a result, the overall frequency of
mitochondria, however, as selection on males is not strong enough to favour much polymorphism when fitness is additive. As a result, the overall frequency of 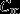 mitochondria does not exceed our threshold of P = 0.05 that demarcates the zone of polymorphism (Fig. S2).
mitochondria does not exceed our threshold of P = 0.05 that demarcates the zone of polymorphism (Fig. S2).
Figure 6.
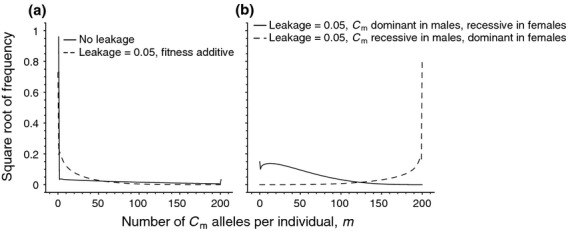
Example distributions of the frequency (square-root transformed) of individuals bearing m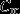 -alleles. Panel (a) when leakage is absent or fitness effects are additive, heteroplasmy is nearly negligible and most individuals contain only
-alleles. Panel (a) when leakage is absent or fitness effects are additive, heteroplasmy is nearly negligible and most individuals contain only 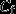 mitochondria. Panel (b) when leakage is present and fitness effects reflect a scenario of ’reverse dominance’ (dashed lines in Fig. 2b, c), levels of heteroplasmy can be substantial, particularly when
mitochondria. Panel (b) when leakage is present and fitness effects reflect a scenario of ’reverse dominance’ (dashed lines in Fig. 2b, c), levels of heteroplasmy can be substantial, particularly when 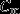 is dominant in males, but recessive in females (solid line). By contrast, when
is dominant in males, but recessive in females (solid line). By contrast, when 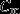 is recessive in males, yet dominant in females, levels of polymorphism are substantially lower. For the restrictive range of parameters for which
is recessive in males, yet dominant in females, levels of polymorphism are substantially lower. For the restrictive range of parameters for which 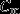 invades (when selection on males is very strong), it will achieve fixation (dashed line). Parameters throughout:
invades (when selection on males is very strong), it will achieve fixation (dashed line). Parameters throughout: 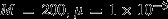 ; panel (a)
; panel (a)  ; dashed line:
; dashed line: 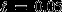 . Panel (b)
. Panel (b)  (solid line).
(solid line).  (dashed line).
(dashed line).
The scope for higher frequencies of 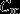 alleles is enhanced with reverse dominance ( Fig. 6b). The selection pressure when the
alleles is enhanced with reverse dominance ( Fig. 6b). The selection pressure when the 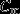 allele is dominant in males and hence recessive in females is now strong enough to lead to high levels of heteroplasmy (solid line in Fig. 6b). This is not true in the opposite case when the
allele is dominant in males and hence recessive in females is now strong enough to lead to high levels of heteroplasmy (solid line in Fig. 6b). This is not true in the opposite case when the 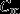 allele is recessive in males and dominant in females, as selection in favour of
allele is recessive in males and dominant in females, as selection in favour of 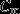 tends to lead to a situation where most individuals are fixed for
tends to lead to a situation where most individuals are fixed for 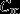 mitochondria (dashed line in Fig. 6b).
mitochondria (dashed line in Fig. 6b).
Finally, we consider how mechanisms that increase within-individual homogeneity of mitochondria (e.g. bottlenecks) impact on the parameter space in which polymorphism is found under paternal leakage. Considering an illustrative bottleneck size of B = 10, we find that the domain of polymorphism is hardly affected when fitness effects are additive (Fig. S3) or  is dominant (Fig. S4). However, under reverse dominance in which the area favouring polymorphism is greater (Fig. 5d–f), we find that results are sensitive to within-individual homogenization of mitochondria due to bottlenecks (Fig. 7), reducing the area favouring polymorphism.
is dominant (Fig. S4). However, under reverse dominance in which the area favouring polymorphism is greater (Fig. 5d–f), we find that results are sensitive to within-individual homogenization of mitochondria due to bottlenecks (Fig. 7), reducing the area favouring polymorphism.
Figure 7.
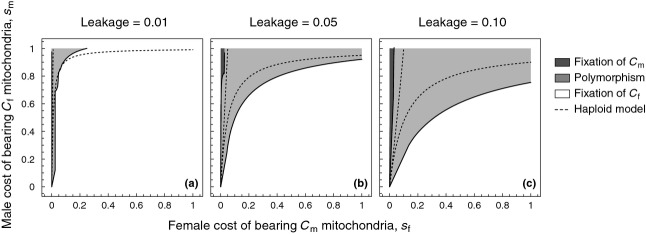
The occurrence of cytoplasmic polymorphism for different levels of leakage  , when multiple cytoplasmic elements are present per individual and a bottleneck B = 10. Epistatic interactions among cytoplasmic elements reflect a scenario of ’reverse dominance’ in which
, when multiple cytoplasmic elements are present per individual and a bottleneck B = 10. Epistatic interactions among cytoplasmic elements reflect a scenario of ’reverse dominance’ in which  mitochondria in males are dominant over
mitochondria in males are dominant over  mitochondria (k = 2), similar to Fig. 5d–f. Parameters: M = 200,
mitochondria (k = 2), similar to Fig. 5d–f. Parameters: M = 200, 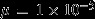 .
.
Discussion
The matrilineal transmission of cytoplasmic elements – such as mitochondria or endosymbiotic bacteria like Wolbachia – is widely understood to lead to mother's curse (Frank & Hurst, 1996; Gemmell et al., 2004), where selection cannot stop the build-up of cytoplasmic mutations that are detrimental to males. Given the importance of mitochondria for male metabolism, we would expect mother's curse to be severe. Indeed, a number of examples exist that are in line with mother's curse (Camus et al., 2012; Yee et al., 2013; Beekman et al., 2014), but other studies have failed to find sex-specific fitness asymmetries of cytoplasmic elements (Friberg & Dowling, 2008; Schaefer et al., 2008; Mossman et al., 2010, 2012; Schon et al., 2012). A number of resolutions have been suggested to explain the limited occurrence of mother's curse, such as the presence of inbreeding and sperm limitation (Wade & Brandvain, 2009; Zhang et al., 2012) or recruitment of compensatory autosomal mutations to counter detrimental male fitness effects (Beekman et al., 2014). Here, we assess whether low levels of paternal mtDNA leakage – which have been recorded in an increasing number of species (McCauley, 2013) – could potentially provide another explanation for the limited generality of mother's curse.
The current analysis shows that for some of the fitness functions considered, haploid models of cytoplasmic inheritance (Frank & Hurst, 1996; Unckless & Herren, 2009; Wade & Brandvain, 2009) provide a robust description of cytoplasmic variation. This is particularly the case when fitness is linear or symmetric across the sexes (e.g. solid lines in Fig. 2a–d). In these cases, leakage only favours cytoplasmic mutations that benefit males when fitness effects are strong relative to fitness effects on females. This suggests that there is limited scope for paternal leakage as a mechanism to ameliorate mother's curse, given that selection coefficients measured in nature are generally weak (Kingsolver et al., 2001). However, when the shape of the fitness function differs between the sexes (dashed lines in Fig. 2b–d), we find that the haploid model fails. It is unable to capture the fine-grained costs and benefits of carrying a small number of 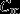 mitochondria (favoured in males but deleterious in females), or to predict the resulting levels of heteroplasmy, that in principle should be easily measured. This echoes previous work on the evolution of the sexes (Hadjivasiliou et al., 2012, 2013), which showed that taking account of multiple mitochondria provides a more subtle picture of the evolution of mitochondrial uniparental inheritance in comparison with previous studies that ignored the evolution of within-individual frequency of mutant mitochondria (Hurst & Hamilton, 1992; Randerson & Hurst, 1999).
mitochondria (favoured in males but deleterious in females), or to predict the resulting levels of heteroplasmy, that in principle should be easily measured. This echoes previous work on the evolution of the sexes (Hadjivasiliou et al., 2012, 2013), which showed that taking account of multiple mitochondria provides a more subtle picture of the evolution of mitochondrial uniparental inheritance in comparison with previous studies that ignored the evolution of within-individual frequency of mutant mitochondria (Hurst & Hamilton, 1992; Randerson & Hurst, 1999).
Using this more sophisticated model to track multiple mitochondria per individual, leakage coupled to nonadditive fitness interactions leads to some plausible conditions that favour the spread of male-benefit mutations. In particular, when a rare mutant is dominant in males and recessive in females, a broader range of selective conditions lead to polymorphism (see dashed line in Fig. 2b). This effect is most prominent when the levels of leakage are relatively large ( ), but polymorphism can still occur at lower levels of leakage (
), but polymorphism can still occur at lower levels of leakage (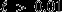 ). Current measured values of mtDNA leakage in animal and plant species are typically small, in the range between
). Current measured values of mtDNA leakage in animal and plant species are typically small, in the range between  to 0.06 (Wagner et al., 1991; Svab & Maliga, 2007; Wolff et al., 2008; McCauley, 2013; Nunes et al., 2013). However, we are still far from a conclusive picture about the prevalence of leakage, as paternal inheritance of mtDNA has only been assessed for a limited number of species under a limited number of environmental conditions (reviewed in McCauley, 2013; Greiner et al., 2015). Chloroplast inheritance patterns appear to be more variable, with biparental inheritance of cpDNA found in 3 of 10 species measured so far (see Table 1 in Greiner et al., 2015). Consequently, more studies of leakage patterns in both mitochondria and chloroplasts in natural populations are likely to shed more light on the possibility of leakage leading to polymorphism.
to 0.06 (Wagner et al., 1991; Svab & Maliga, 2007; Wolff et al., 2008; McCauley, 2013; Nunes et al., 2013). However, we are still far from a conclusive picture about the prevalence of leakage, as paternal inheritance of mtDNA has only been assessed for a limited number of species under a limited number of environmental conditions (reviewed in McCauley, 2013; Greiner et al., 2015). Chloroplast inheritance patterns appear to be more variable, with biparental inheritance of cpDNA found in 3 of 10 species measured so far (see Table 1 in Greiner et al., 2015). Consequently, more studies of leakage patterns in both mitochondria and chloroplasts in natural populations are likely to shed more light on the possibility of leakage leading to polymorphism.
The finding that polymorphism is particularly likely when male-benefit alleles impose a small fitness detriment on females, yet lead to large fitness benefits in males, begs the question whether this is likely in nature. One mechanism that could lead to such an asymmetric fitness landscape is the differential segregation of male- and female-benefit mitochondria to sex-specific tissues. For example, male-benefit mitochondria could segregate preferentially to male-specific reproductive tissues (e.g. sexual ornaments, parts of male genitalia not involved in gamete production, male muscular tissue, male parts of plants). Consequently, the majority of detrimental fitness effects of these male-benefit mitochondria would be masked in females (as they lack these tissues), while positively contributing to fitness in males, for example by allowing for a rate of mitochondrial biogenesis and energy metabolism closer to the male optimum for such tissues.
A mechanism based on preferential segregation of different mitochondrial types to different somatic tissues is supported by a number of recent studies using highly sensitive genetic techniques. These have shown that mitochondria in heteroplasmic individuals do indeed segregate in a tissue-specific fashion (Battersby et al., 2003; Magnacca & Brown, 2010; Burgstaller et al., 2014) and the frequency of mitochondrial mutants depends on tissue type (Dunbar et al., 1995; Chinnery et al., 1999; Samuels et al., 2013). For example, in heteroplasmic individuals from four species of Hawaiian bees, mitochondrial haplotypes present in the abdomen differ from those haplotypes in legs and thoracic flight muscle (Magnacca & Brown, 2010). Similar findings have been reported in bats (Brunet & Rossinni, 2004). Consequently, differential tissue segregation may be a plausible means through which the aforementioned fitness difference between the sexes can be achieved. Doubly uniparental inheritance in bivalve molluscs such as Mytilus, in which male-benefit mitochondria are specifically targeted to male gonads and female-benefit mitochondria to male somatic tissues, demonstrates that the segregation of mitochondria to male-specific tissues can and does occur (Breton et al., 2007; Zouros, 2013).
Differential tissue segregation need not be specific for male tissues if the fitness effects of male-benefit mitochondria are recessive in female tissues but dominant in male tissues with high energetic demand, including reproductive tissues. Given that males typically have higher metabolic demands (Mittwoch, 2004), one would predict that male-benefit mitochondria ought to be capable of sustaining a higher maximal metabolic rate; but that should not be especially detrimental to females. One possible female detriment is a greater leak of reactive oxygen species (ROS) from male-benefit mitochondria in female tissues (e.g. Ballard et al., 2007). If so, higher ROS leak could be diminished by either mild respiratory uncoupling (i.e. loss of coupling between respiration and ATP production; Lane, 2011b) or the production of relatively few male-benefit mitochondria, making them recessive in females. We would therefore expect to find higher rates of mitochondrial polymorphism or even fixation of male-benefit mitochondria in species with strong selection for male stamina.
We also showed that the severe homogenizing effect of bottlenecks in mitochondrial number could be important in those cases that favoured polymorphism. Bottlenecks are considered to be widespread in animals (Bergstrom & Pritchard, 1998; Stewart et al., 2008b; Lee et al., 2012), although the actual mechanism (i.e. the size of the bottleneck) that underlies this homogenization is still poorly understood (Carling et al., 2011). In plants, such homogenizing effects appear to be much rarer (Kmiec et al., 2006; Galtier, 2011, but see Morgan & Maliga, 1987). Consequently, the scope for cytoplasmically linked sexually antagonistic variation in the presence of leakage is likely to be substantially enhanced in plants relative to animals. However, as the literature on the size and cause of mitochondrial homogenizing effects in animals is scattered, mired in debate and focuses on a few species only (Carling et al., 2011; Wallace, 2013), it remains to be seen whether bottlenecks are the norm in animals.
Although considerable attention has been devoted to the study of interactions between cytoplasm and nucleus (e.g. Rand et al., 2004; Dowling et al., 2007a, 2008), little is known about epistatic interactions among the cytoplasmic elements themselves. In animals, studies of heteroplasmy have suggested that the mere presence of different types of mitochondria in a single individual can have negative fitness consequences (Wallace, 1994; Zeviani & Antozzi, 1997; Lane, 2011a; Lane, 2012; Sharpley et al., 2012). Again, plants appear to be the most amenable model organisms to test the fitness consequences of heteroplasmy, as substantial levels of heteroplasmy in both mtDNA (e.g. Städler & Delph, 2002) and chloroplast DNA (Frey et al., 2005) are found across a range of study systems (reviewed in McCauley, 2013). From our point of view, it would be particularly interesting to relate pollen and ovule production in plants to varying levels of heteroplasmy in cytoplasmic elements. Such experiments not only would allow one to assess whether different relative compositions of cytoplasmic elements have sex-specific effects, but also would provide measures of the epistatic fitness function (e.g. see Fig. 2). More generally, experiments like these are needed to assess whether selection on mitochondria is a general force rather than an exception (Ballard & Melvin, 2010).
Our study opens up several avenues for future work. Foremost, the current study considers leakage to be a fixed parameter, whereas it is likely to be a heritable character amenable to selection and evolutionary change. Modelling the evolution of leakage may shed light on the question of whether leakage is a sporadic aberration that mainly occurs in outbred or hybrid crosses (e.g. Fontaine et al., 2007; Hoarau et al., 2009; Hoolahan et al., 2011), or whether it is adaptive in its own right. Burt & Trivers (2006) suggested that paternal leakage is selectively favoured by the nucleus in the presence of cytoplasmic male sterility (CMS), as this favours symbionts that do not cause CMS (see also Wade & McCauley, 2005; McCauley, 2013). Even for male-detriment alleles with a less deleterious phenotype than CMS (e.g. Dowling et al., 2007b; Innocenti et al., 2011), we predict that the nucleus may favour a certain level of leakage, as accepting cytoplasmic elements from successful males ensures a lower degree of male-detriment effects in the cytoplasm. Nonetheless, leakage may also reintroduce costs that are associated with competition for replication between different cytoplasmic elements, for which sake uniparental inheritance may have evolved in the first place (Hurst & Hamilton, 1992). Consequently, whether leakage will indeed evolve needs to be addressed by formal studies. Models like these may also shed light on the striking variation in cytoplasmic inheritance patterns found in plants (Mogensen, 1996; McCauley, 2013), where maternal inheritance predominates, but multiple cases of paternal cytoplasmic inheritance (Neale et al., 1989; Havey, 1997) or biparental inheritance are known (Mogensen, 1996; Havey, 1997). All in all, the evolution of cytoplasmic inheritance and its consequences are still far from understood.
Acknowledgments
This study was funded by an EPSRC-funded 2020 Science fellowship (EP/I017909/1). We thank Andy Gardner and two anonymous reviewers for comments on the manuscript. The authors acknowledge the use of the UCL Legion High Performance Computing Facility (Legion@UCL), and associated support services, in the completion of this work. NL acknowledges support from the Provost's Venture Research Fellowship and the Leverhulme Trust, and AP from the EPSRC (EP/F500351/1, EP/I017909/1, EP/K038656/1) and NERC (NE/G00563X/1).
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Cytoplasmic polymorphism with multiple mitochondria per individual and the shape of male and female fitness functions is given by a linear function (additive).
Figure S2 Cytoplasmic polymorphism when multiple mitochondria per individual are present and the shape of male and female fitness functions is given by a sigmoidal function (see Fig. 2d).
Figure S3 Cytoplasmic polymorphism when the shape of male and female fitness functions is additive and the size of the bottleneck B = 10.
Figure S4 Cytoplasmic polymorphism when the shape of male and female fitness functions is given by a scenario of constant dominance (solid lines in Fig. 2b and c) and the size of the bottleneck B = 10.
Data S1 Model with multiple mitochondria.
References
- Ballard JWO, Melvin RG. Linking the mitochondrial genotype to the organismal phenotype. Mol. Ecol. 2010;19:1523–1539. doi: 10.1111/j.1365-294X.2010.04594.x. [DOI] [PubMed] [Google Scholar]
- Ballard JWO, Whitlock MC. The incomplete natural history of mitochondria. Mol. Ecol. 2004;13:729–744. doi: 10.1046/j.1365-294x.2003.02063.x. [DOI] [PubMed] [Google Scholar]
- Ballard JWO, Melvin RG, Miller JT, Katewa SD. Sex differences in survival and mitochondrial bioenergetics during aging in Drosophila. Aging Cell. 2007;6:699–708. doi: 10.1111/j.1474-9726.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- Battersby BJ, Loredo-Osti J, Shoubridge EA. Nuclear genetic control of mitochondrial DNA segregation. Nat. Genet. 2003;33:183–186. doi: 10.1038/ng1073. [DOI] [PubMed] [Google Scholar]
- Beekman M, Dowling DK, Aanen DK. The costs of being male: are there sex-specific effects of uniparental mitochondrial inheritance? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130440. doi: 10.1098/rstb.2013.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom CT, Pritchard J. Germline bottlenecks and the evolutionary maintenance of mitochondrial genomes. Genetics. 1998;149:2135–2146. doi: 10.1093/genetics/149.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky CW. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc. Natl. Acad. Sci. USA. 1995;92:11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky CW. The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annu. Rev. Genet. 2001;35:125–148. doi: 10.1146/annurev.genet.35.102401.090231. [DOI] [PubMed] [Google Scholar]
- Breton S, Beaupré HD, Stewart DT, Hoeh WR, Blier PU. The unusual system of doubly uniparental inheritance of mtDNA: isn't one enough? Trends Genet. 2007;23:465–474. doi: 10.1016/j.tig.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Brunet, Rossinni AK. Testing the free radical theory of aging in bats. Ann. NY. Acad. Sci. 2004;1019:506–508. doi: 10.1196/annals.1297.093. [DOI] [PubMed] [Google Scholar]
- Burgstaller J, Johnston I, Jones N, Albrechtová J, Kolbe T, Vogl C, et al. mtDNA segregation in heteroplasmic tissues is common in vivo and modulated by haplotype differences and developmental stage. Cell Rep. 2014;7:2031–2041. doi: 10.1016/j.celrep.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt A, Trivers R. Genes in Conflict: The Biology of Selfish Genetic Elements. Cambridge, MA: Belknap Press; 2006. [Google Scholar]
- Camus MF, Clancy DJ, Dowling DK. Mitochondria, maternal inheritance, and male aging. Curr. Biol. 2012;22:1717–1721. doi: 10.1016/j.cub.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Cao L, Shitara H, Horii T, Nagao Y, Imai H, Abe K, et al. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat. Genet. 2007;39:386–390. doi: 10.1038/ng1970. [DOI] [PubMed] [Google Scholar]
- Carling PJ, Cree LM, Chinnery PF. The implications of mitochondrial DNA copy number regulation during embryogenesis. Mitochondrion. 2011;11:686–692. doi: 10.1016/j.mito.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Chapman RW, Stephens JC, Lansman RA, Avise JC. Models of mitochondrial DNA transmission genetics and evolution in higher eucaryotes. Genet. Res. 1982;40:41–57. doi: 10.1017/s0016672300018899. [DOI] [PubMed] [Google Scholar]
- Chinnery PF, Zwijnenburg PJ, Walker M, Howell N, Taylor RW, Lightowlers RN, et al. Nonrandom tissue distribution of mutant mtDNA. Am. J. Med. Genet. 1999;85:498–501. [PubMed] [Google Scholar]
- Connallon T, Clark AG. The resolution of sexual antagonism by gene duplication. Genetics. 2011;187:919–937. doi: 10.1534/genetics.110.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby K, Smith DR. Does the mode of plastid inheritance influence plastid genome architecture? PLoS One. 2012;7:e46260. doi: 10.1371/journal.pone.0046260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JW, Service PM, Prout T. Antagonistic pleiotropy, reversal of dominance, and genetic polymorphism. Am. Nat. 1994;144:210–228. [Google Scholar]
- Dowling DK, Friberg U, Hailer F, Arnqvist G. Intergenomic epistasis for fitness: within population interactions between cytoplasmic and nuclear genes in Drosophila melanogaster. Genetics. 2007a;175:235–244. doi: 10.1534/genetics.105.052050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling DK, Larkeson, Nowostawski A, Arnqvist G. Effects of cytoplasmic genes on sperm viability and sperm morphology in a seed beetle, implications for sperm competition theory? J. Evol. Biol. 2007b;20:358–368. doi: 10.1111/j.1420-9101.2006.01189.x. [DOI] [PubMed] [Google Scholar]
- Dowling DK, Friberg U, Lindell J. Evolutionary implications of non-neutral mitochondrial genetic variation. Trends Ecol. Evol. 2008;23:546–554. doi: 10.1016/j.tree.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Dunbar DR, Moonie PA, Jacobs HT, Holt IJ. Different cellular backgrounds confer a marked advantage to either mutant or wild-type mitochondrial genomes. Proc. Natl. Acad. Sci. USA. 1995;92:6562–6566. doi: 10.1073/pnas.92.14.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine KM, Cooley JR, Simon, C C. Evidence for paternal leakage in hybrid periodical cicadas (Hemiptera: Magicicada spp) PLoS One. 2007;2:e892. doi: 10.1371/journal.pone.0000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA, Hurst LD. Mitochondria and male disease. Nature. 1996;383:224–224. doi: 10.1038/383224a0. [DOI] [PubMed] [Google Scholar]
- Frey JE, Frey B, Forcioli D. Quantitative assessment of heteroplasmy levels in Senecio vulgaris chloroplast DNA. Genetica. 2005;123:255–261. doi: 10.1007/s10709-004-3711-y. [DOI] [PubMed] [Google Scholar]
- Friberg U, Dowling DK. No evidence of mitochondrial genetic variation for sperm competition within a population of Drosophila melanogaster. J. Evol. Biol. 2008;21:1798–1807. doi: 10.1111/j.1420-9101.2008.01581.x. [DOI] [PubMed] [Google Scholar]
- Fry JD. The genomic location of sexually antagonistic variation: some cautionary comments. Evolution. 2010;64:1510–1516. doi: 10.1111/j.1558-5646.2009.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier N. The intriguing evolutionary dynamics of plant mitochondrial DNA. BMC Biol. 2011;9:61. doi: 10.1186/1741-7007-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell NJ, Metcalf VJ, Allendorf FW. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004;19:238–244. doi: 10.1016/j.tree.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Greiner S, Sobanski J, Bock R. Why are most organelle genomes transmitted maternally. BioEssays. 2015;37:80–94. doi: 10.1002/bies.201400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U, Wharton D, Josefsson A, Wilson AC. Paternal inheritance of mitochondrial DNA in mice. Nature. 1991;352:255–257. doi: 10.1038/352255a0. [DOI] [PubMed] [Google Scholar]
- Hadjivasiliou Z, Pomiankowski A, Seymour RM, Lane N. Selection for mitonuclear coadaptation could favour the evolution of two sexes. Proc. R. Soc. Lond. B Biol. Sci. 2012;279:1865–1872. doi: 10.1098/rspb.2011.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjivasiliou Z, Lane N, Seymour RM, Pomiankowski A. Dynamics of mitochondrial inheritance in the evolution of binary mating types and two sexes. Proc. R. Soc. Lond. B Biol. Sci. 2013;280:20131920. doi: 10.1098/rspb.2013.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havey MJ. Predominant paternal transmission of the mitochondrial genome in cucumber. J. Hered. 1997;88:232–235. [Google Scholar]
- Hoarau G, Coyer JA, Olsen JL. Paternal leakage of mitochondrial DNA in a FucusPhaeophyceae) hybrid zone. J. Phycol. 2009;45:621–624. doi: 10.1111/j.1529-8817.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- Hoolahan AH, Blok VC, Gibson T, Dowton M. Paternal leakage of mitochondrial DNA in experimental crosses of populations of the potato cyst nematode Globodera pallida. Genetica. 2011;139:1509–1519. doi: 10.1007/s10709-012-9650-0. [DOI] [PubMed] [Google Scholar]
- Hurst LD, Hamilton WD. Cytoplasmic fusion and the nature of sexes. Proc. R. Soc. Lond. B Biol. Sci. 1992;247:189–194. [Google Scholar]
- Innocenti P, Morrow EH, Dowling DK. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science. 2011;332:845–848. doi: 10.1126/science.1201157. [DOI] [PubMed] [Google Scholar]
- Kidwell JF, Clegg MT, Stewart FM, Prout T. Regions of stable equilibria for models of differential selection in the two sexes under random mating. Genetics. 1977;85:171–183. doi: 10.1093/genetics/85.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, et al. The strength of phenotypic selection in natural populations. Am. Nat. 2001;157:245–261. doi: 10.1086/319193. [DOI] [PubMed] [Google Scholar]
- Kmiec B, Woloszynska M, Janska H. Heteroplasmy as a common state of mitochondrial genetic information in plants and animals. Curr. Genet. 2006;50:149–159. doi: 10.1007/s00294-006-0082-1. [DOI] [PubMed] [Google Scholar]
- Lane N. Energetics and genetics across the prokaryote-eukaryote divide. Biol. Direct. 2011a;6:35. doi: 10.1186/1745-6150-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N. The evolution of oxidative stress. In: Pantapoulos K, Schipper H, editors. Free Radical Biomedicine. Volume 1. Hauppauge: Nova Science Publishers; 2011b. pp. 1–17. In: Principles of. [Google Scholar]
- Lane N. The problem with mixing mitochondria. Cell. 2012;151:246–248. doi: 10.1016/j.cell.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- Lee HS, Ma H, Cervera, Juanes R, Tachibana M, Sparman M, Woodward J, et al. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Rep. 2012;1:506–515. doi: 10.1016/j.celrep.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnacca KN, Brown MJF. Tissue segregation of mitochondrial haplotypes in heteroplasmic Hawaiian bees: implications for DNA barcoding. Mol. Ecol. Resour. 2010;10:60–68. doi: 10.1111/j.1755-0998.2009.02724.x. [DOI] [PubMed] [Google Scholar]
- Martin W. Early evolution without a tree of life. Biol. Direct. 2011;6:36. doi: 10.1186/1745-6150-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley DE. Paternal leakage, heteroplasmy, and the evolution of plant mitochondrial genomes. New Phytol. 2013;200:966–977. doi: 10.1111/nph.12431. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. The elusive action of sex-determining genes: mitochondria to the rescue? J. Theor. Biol. 2004;228:359–365. doi: 10.1016/j.jtbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Mogensen HL. The hows and whys of cytoplasmic inheritance in seed plants. Am. J. Bot. 1996;83:383–404. [Google Scholar]
- Morgan A, Maliga P. Rapid chloroplast segregation and recombination of mitochondrial DNA in Brassica cybrids. Mol. Gen. Genet. 1987;209:240–246. doi: 10.1007/BF00329649. [DOI] [PubMed] [Google Scholar]
- Mossman JA, Slate J, Birkhead TR. Mitochondrial haplotype does not affect sperm velocity in the zebra finch Taeniopygia guttata. J. Evol. Biol. 2010;23:422–432. doi: 10.1111/j.1420-9101.2009.01913.x. [DOI] [PubMed] [Google Scholar]
- Mossman JA, Slate J, Birkhead TR, Moore HD, Pacey AA. Mitochondrial haplotype does not influence sperm motility in a UK population of men. Hum. Reprod. 2012;27:641–651. doi: 10.1093/humrep/der438. [DOI] [PubMed] [Google Scholar]
- Mullon C, Pomiankowski A, Reuter M. The effects of selection and genetic drift on the genomic distribution of sexually antagonistic alleles. Evolution. 2012;66:3743–3753. doi: 10.1111/j.1558-5646.2012.01728.x. [DOI] [PubMed] [Google Scholar]
- Nakada K, Sato A, Yoshida K, Morita T, Tanaka H, Inoue SI, et al. Mitochondria-related male infertility. Proc. Natl. Acad. Sci. USA. 2006;103:15148–15153. doi: 10.1073/pnas.0604641103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale DB, Marshall KA, Sederoff RR. Chloroplast and mitochondrial DNA are paternally inherited in Sequoia sempervirens D. Don Endl. Proc. Natl. Acad. Sci. USA. 1989;86:9347–9349. doi: 10.1073/pnas.86.23.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman M, Taylor DR. The causes of mutation accumulation in mitochondrial genomes. Proc. R. Soc. Lond. B Biol. Sci. 2009;276:1201–1209. doi: 10.1098/rspb.2008.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MDS, Dolezal M, Schlötterer C. Extensive paternal mtDNA leakage in natural populations of Drosophila melanogaster. Mol. Ecol. 2013;22:2106–2117. doi: 10.1111/mec.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. Two-locus problems in transmission genetics of mitochondria and chloroplasts. Genetics. 1980;96:543–555. doi: 10.1093/genetics/96.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CB, Larsson NG. Mitochondrial DNA mutations in disease and aging. J. Cell Biol. 2011;193:809–818. doi: 10.1083/jcb.201010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand DM, Haney RA, Fry AJ. Cytonuclear coevolution: the genomics of cooperation. Trends Ecol. Evol. 2004;19:645–653. doi: 10.1016/j.tree.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Randerson JP, Hurst LD. Small sperm, uniparental inheritance and selfish cytoplasmic elements: a comparison of two models. J. Evol. Biol. 1999;12:1110–1124. [Google Scholar]
- Rice WR. Sex chromosomes and the evolution of sexual dimorphism. Evolution. 1984;38:735–742. doi: 10.1111/j.1558-5646.1984.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Samuels DC, Wonnapinij P, Cree LM, Chinnery PF. Reassessing evidence for a postnatal mitochondrial genetic bottleneck. Nat. Genet. 2010;42:471–472. doi: 10.1038/ng0610-471. [DOI] [PubMed] [Google Scholar]
- Samuels DC, Li C, Li B, Song Z, Torstenson E, Boyd, Clay H, et al. Recurrent tissue specific mtDNA mutations are common in humans. PLoS Genet. 2013;9:e1003929. doi: 10.1371/journal.pgen.1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, Taylor RW, et al. Prevalence of mitochondrial DNA disease in adults. Ann. Neurol. 2008;63:35–39. doi: 10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat. Rev. Genet. 2012;13:878–890. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Vissing J. Paternal inheritance of mitochondrial DNA. N. Engl. J. Med. 2002;347:576–580. doi: 10.1056/NEJMoa020350. [DOI] [PubMed] [Google Scholar]
- Sharpley MS, Marciniak C, Eckel-mahan K, Mcmanus M, Crimi M, Waymire K, et al. Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell. 2012;151:333–343. doi: 10.1016/j.cell.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solignac M, Génermont J, Monnerot M, Mounolou JC. Genetics of mitochondria in Drosophila: mtDNA inheritance in heteroplasmic strains of D. mauritiana. Mol. Gen. Genet. 1984;197:183–188. [Google Scholar]
- Städler T, Delph LF. Ancient mitochondrial haplotypes and evidence for intragenic recombination in a gynodioecious plant. Proc. Natl. Acad. Sci. USA. 2002;99:11730–11735. doi: 10.1073/pnas.182267799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JB, Freyer C, Elson JL, Larsson NG. Purifying selection of mtDNA and its implications for understanding evolution and mitochondrial disease. Nat. Rev. Genet. 2008a;9:657–662. doi: 10.1038/nrg2396. [DOI] [PubMed] [Google Scholar]
- Stewart JB, Freyer C, Elson JL, Wredenberg A, Cansu Z, Trifunovic A, et al. Strong purifying selection in transmission of mammalian mitochondrial DNA. PLoS Biol. 2008b;6:e10. doi: 10.1371/journal.pbio.0060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z, Maliga P. Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its impact on transgene containment. Proc. Natl. Acad. Sci. USA. 2007;104:7003–7008. doi: 10.1073/pnas.0700063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata N, Maruyama T. A mathematical model of extranuclear genes and the genetic variability maintained in a finite population. Genet. Res. 1981;37:291–302. [Google Scholar]
- Takahata N, Slatkin M. Evolutionary dynamics of extranuclear genes. Genet. Res. 1983;42:257–265. [Google Scholar]
- Unckless RL, Herren JK. Population genetics of sexually antagonistic mitochondrial mutants under inbreeding. J. Theor. Biol. 2009;260:132–136. doi: 10.1016/j.jtbi.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Wade MJ, Brandvain Y. Reversing mother's curse: selection on male mitochondrial fitness effects. Evolution. 2009;63:1084–1089. doi: 10.1111/j.1558-5646.2009.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade MJ, McCauley DE. Paternal leakage sustains the cytoplasmic polymorphism underlying gynodioecy but remains invasible by nuclear restorers. Am. Nat. 2005;166:592–602. doi: 10.1086/491660. [DOI] [PubMed] [Google Scholar]
- Wagner D, Dong J, Carlson M, Yanchuk A. Paternal leakage of mitochondrial DNA in Pinus. Theor. Appl. Genet. 1991;82:510–514. doi: 10.1007/BF00588607. [DOI] [PubMed] [Google Scholar]
- Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet. 2008;40:1484–1488. doi: 10.1038/ng.258. [DOI] [PubMed] [Google Scholar]
- Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol. Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA sequence variation in human evolution and disease. Proc. Natl. Acad. Sci. USA. 1994;91:8739–8746. doi: 10.1073/pnas.91.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial bioenergetic etiology of disease. J. Clin. Invest. 2013;123:1405–1412. doi: 10.1172/JCI61398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 2013;5:a021220. doi: 10.1101/cshperspect.a021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D, Singh G, Lott M, Hodge J, Schurr T, Lezza A, et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Wolff JN, Gandre S, Kalinin A, Gemmell NJ. Delimiting the frequency of paternal leakage of mitochondrial DNA in chinook salmon. Genetics. 2008;179:1029–1032. doi: 10.1534/genetics.107.085274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JN, Nafisinia M, Sutovsky P, Ballard JWO. Paternal transmission of mitochondrial DNA as an integral part of mitochondrial inheritance in metapopulations of Drosophila simulans. Heredity. 2013;110:57–62. doi: 10.1038/hdy.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee WK, Sutton KL, Dowling DK. In vivo male fertility is affected by naturally occurring mitochondrial haplotypes. Curr. Biol. 2013;23:R55–R56. doi: 10.1016/j.cub.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Zeviani M, Antozzi C. Mitochondrial disorders. Mol. Hum. Reprod. 1997;3:133–148. doi: 10.1093/molehr/3.2.133. [DOI] [PubMed] [Google Scholar]
- Zhang H, Guillaume F, Engelstädter J. The dynamics of mitochondrial mutations causing male infertility in spatially structured populations. Evolution. 2012;66:3179–3188. doi: 10.1111/j.1558-5646.2012.01675.x. [DOI] [PubMed] [Google Scholar]
- Zouros E. Biparental inheritance through uniparental transmission: the doubly uniparental inheritance (DUI) of mitochondrial DNA. Evol. Biol. 2013;40:1–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Cytoplasmic polymorphism with multiple mitochondria per individual and the shape of male and female fitness functions is given by a linear function (additive).
Figure S2 Cytoplasmic polymorphism when multiple mitochondria per individual are present and the shape of male and female fitness functions is given by a sigmoidal function (see Fig. 2d).
Figure S3 Cytoplasmic polymorphism when the shape of male and female fitness functions is additive and the size of the bottleneck B = 10.
Figure S4 Cytoplasmic polymorphism when the shape of male and female fitness functions is given by a scenario of constant dominance (solid lines in Fig. 2b and c) and the size of the bottleneck B = 10.
Data S1 Model with multiple mitochondria.


