Abstract
Peptide YY (PYY) and neuropeptide Y (NPY) have been proposed to participate in the control of energy homeostasis. Since these activities show circadian variations, we explored the circadian pattern of locomotor, exploratory and ingestive behaviour in male and/or female mice with disrupted genes for PYY (PYY−/−), NPY (NPY−/−) as well as PYY plus NPY (PYY+NPY−/−). The effect of bacterial lipopolysaccharide (LPS, 0.1 mg/kg intraperitoneally) on these behaviours was also examined. The animals were housed singly in cages fitted with sensors for water and food intake and two infrared frames for recording ambulation and rearing under a 12 h light/dark cycle for 4 days. Locomotor and exploratory behaviour was decreased in female NPY−/− as well as male and female PYY+NPY−/− mice during the photo- and scotophase, and in male PYY−/− mice during the scotophase. Significant decreases in water and food intake were seen in female NPY−/− as well as male and female PYY+NPY−/− mice during the photophase. The effect of LPS to attenuate ingestive behaviour during the light and/or dark phase was most pronounced in PYY−/− and NPY−/− mice. These findings attest to a circadian cycle- and gender-related role of NPY and PYY in the control of behaviours that balance energy intake and energy expenditure. Both peptides stimulate feeding and drinking to balance the energy demand that they generate by enforcing the circadian pattern of locomotion and exploration. In addition, they counteract the anorectic and antidipsogenic effects of immune challenge.
Keywords: Neuropeptide Y, peptide YY, circadian pattern, locomotion, exploration, feeding, drinking, energy intake, energy expenditure, energy homeostasis, bacterial lipopolysaccharide
INTRODUCTION
Peptide YY (PYY) and neuropeptide Y (NPY) belong to a family of peptides that also includes pancreatic polypeptide. These peptides share a number of structural and functional similarities, and their biological actions are mediated by 5 types of receptors, termed Y1, Y2, Y4, Y5 and y6, which are coupled to Gi/o signalling pathways [1,2]. PYY is secreted postprandially from endocrine L cells of the gastrointestinal tract, these cells occurring most abundantly in the hindgut [3,4]. The major circulating form of this peptide, PYY(3-36), is thought to be a satiety signal and to reduce food intake in rodents and humans primarily via binding to autoinhibitory Y2 receptors in the arcuate nucleus of the hypothalamus [3-5], although corticolimbic areas are also involved [6].
In contrast, NPY is a messenger widely distributed in the peripheral and central nervous system. Its many functional implications include the control of sympathetic nervous activity and the central regulation of energy balance, seizure activity, cognition, mood, anxiety and stress sensitivity [7-12]. Haplotype-driven expression of NPY in humans predicts brain responses to emotional and stress challenges and inversely correlates with trait anxiety [13].
The functional implications of PYY and NPY have been explored by gene knockout approaches and, where available, pharmacological antagonism of Y receptors. Knockout of the PYY gene was found to increase the daily cumulative food intake, enhance re-feeding after an overnight fast and raise body weight (BW) and size [14,15]. The phenotype of NPY knockout (NPY−/−) mice is characterized by subtle changes in feeding behaviour, energy homeostasis and emotional-affective behaviour [10,16,17]. In addition, there is evidence for a gender-dependent increase in anxiety-related behaviour, especially in male NPY−/− mice, and a decrease in locomotion [17,18].
Locomotion, exploration, water and food intake are activities that are subject to circadian regulation. Although PYY and NPY have been implicated in the circadian system [19-22], the effect of PYY and NPY gene knockouts on the circadian cycle of locomotion, exploration and ingestive behaviour has not yet been examined in a systematic manner. Therefore, the first aim of this study was to characterize the circadian pattern of locomotion, exploration, drinking and feeding in PYY knockout (PYY−/−), NPY knockout (NPY−/−) and PYY plus NPY knockout (PYY+NPY−/−) mice, relative to wild-type (WT) mice. This goal was pursued with the newly developed LabMaster system (TSE Systems, Bad Homburg, Germany), and it was the second aim of this study to evaluate the utility and potential of this system in the circadian phenotyping of genetically modified mice.
The third aim was to explore the effect of immune challenge by intraperitoneal injection of a low dose of bacterial lipopolysaccharide (LPS) on the circadian cycle of PYY−/−, NPY−/− and PYY+NPY−/− mice. Systemic LPS is known to induce proinflammatory cytokines which elicit an illness response involving fever, anorexia and hypolocomotion, among other symptoms [23,24]. There is evidence that NPY-expressing neurons in the arcuate nucleus and paraventricular nucleus of the hypothalamus participate in the illness response to proinflammatory cytokines [25-27]. It was therefore tested whether knockout of the PYY and/or NPY genes modifies the ability of LPS to reduce locomotor, exploratory and ingestive behaviour in a circadian cycle-dependent fashion.
METHODS
Experimental animals
The study was conducted with adult mice of four different genotypes: WT mice, PYY−/− mice and PYY+NPY−/− mice of either gender as well as female NPY−/− mice, all on a mixed C57BL/6:129/SvJ (1:1) background. Germline PYY−/− mice were generated and their genotype determined by polymerase chain reaction as reported [15]. The generation of germline NPY−/− mice has also been described [18], and the two knockout strategies were combined to generate germline double knockout (PYY+NPY−/−) mice. Male and female WT, PYY−/− and PYY+NPY−/− mice were bred at the Institute of Experimental and Clinical Pharmacology of the Medical University of Graz. NPY−/− mice were obtained from the Institute of Pharmacology of the Medical University of Innsbruck and allowed to acclimatize in the animal house of the Medical University of Graz for a minimum of 4 weeks. For practical reasons, only female NPY−/− mice were provided for this study.
Before the experiments, the animals were housed in groups of 2 – 5 per cage, whereas in the experiments they were housed singly in the test cages. In either case, the animals were maintained under conditions of controlled temperature (set point 24 °C), controlled relative air humidity (set point 50 %) and a 12-h light/dark cycle (lights on at 07:00 h, lights off at 19:00 h). All experiments were approved by an ethical committee at the Federal Ministry of Science and Research of the Republic of Austria and conducted according to the Directive of the European Communities Council of 24 November 1986 (86/609/EEC). The experiments were designed in such a way that the number of animals used and their suffering was minimized.
LabMaster system
The circadian pattern of locomotion, exploration, drinking and feeding was assessed with the LabMaster system (TSE Systems, Bad Homburg, Germany), which allowed continuous recording of these parameters [28] for up to 10 days while the animals remained undisturbed by any investigator. The system consisted of six recording units, each unit comprising a test cage (type III, 42 cm × 26.5 cm × 15 cm, length × width × height), two external infrared frames and a cage lid fitted with two weight transducers. These devices were connected to a personal computer which was used to collect and analyze the data with the LabMaster software. The hardware sampling rate at the infrared frames was 100 Hz, while that at the drinking and feeding sensors was 1 Hz. In contrast, the minimal sampling interval of the LabMaster software was 1 min, which means that the recordings taken by the hardware over 1 min (6,000 and 60, respectively) were summed up at 1 min intervals. In other terms, 720 values of each test parameter were collected over a 12 h interval.
The two weight transducers were employed to quantify ingestive behaviour. To this end, a feeding bin filled with standard rodent chow (altromin 1324 FORTI; Altromin, Lage, Germany) and a drinking bottle filled with tap water were each attached to a transducer on the cage lid, and the animals were allowed to drink and feed ad libitum. The drinking flasks were equipped with a special nipple that prevented the spontaneous leaking of water from the bottle. Water and food intake over time was measured in ml and g, respectively. For data analysis, the amount of water and food ingested over select time intervals was normalized to the BW of the animals (ml/g BW, g/g BW).
For recording locomotion and exploration, the two external infra-red frames were positioned in a horizontal manner above one another at a distance of 4.3 cm, with the lower frame being fixed 2.0 cm above the bedding floor. The bottom frame was used to record horizontal locomotion (ambulatory movements) of the mice, while the top frame served to record vertical movements (rearing, exploration). The measures of activity (locomotion, exploration) were derived from the light beam interruptions (counts) of the corresponding infra-red frames. An ambulatory movement was defined as temporally subsequent interruption of any two different light beams in one axis, and the total locomotor activity was calculated by summing up the counts in both the x- and y-axes over select time intervals.
Experimental protocols
In order to enable the mice to adapt to the test room conditions, the group-housed animals were transferred to the test room at least one week before the experiments in the LabMaster system were started on day 0. Two to three days before day 0, the group-housed animals were also habituated to the drinking bottles used in the LabMaster system. On day 0, the mice were weighed and then placed singly in the test cages and maintained there for 4 days. The light intensity in the centre of the test cages during the photophase was 230 – 340 lux.
The transfer of the animals to the test cages took place in the morning or early afternoon of day 0. The remaining photophase and the following scotophase of day 0 were allowed for habituation of the mice to the novel environment and, for this reason, were not included in the statistical analysis of the results, although the data of scotophase 0 are shown to illustrate the circadian time course of the test parameters.
Given the multitude of values (720) collected for each test parameter during a 12 h interval, the results were subjected to the following data reduction procedure. First, the data for each test parameter and animal collected during the photophase of days 1 and 2 as well as the data collected during the scotophase of days 1 and 2 were summed up. The respective sum values for the photophase and scotophase on days 1 and 2 were then averaged for each animal. Finally, the mean values of each test parameter during the photophase and scotophase were used for statistical analysis of differences between the genotypes.
In the morning of day 3 (9.00 – 9.30 h), female animals of each genotype received an intraperitoneal injection of LPS (0.1 mg/kg, 10 ml/kg). For comparison, a group of WT mice received an intraperitoneal injection of vehicle (sterile saline, 10 ml/kg). Following the intraperitoneal treatment, the test parameters were recorded for another 24 h. The effect of LPS and its vehicle on the test parameters measured during the photophase and scotophase of day 3 was expressed as a percentage of the respective parameters measured in the photophase and scotophase of day 2.
Substances and solutions
LPS extracted from E. coli 0127:B8 (Sigma, Vienna, Austria) was dissolved in pyrogen-free sterile saline (0.9 % NaCl; Fresenius Kabi Austria, Graz, Austria) at a concentration of 0.01 mg/ml.
Statistics
Statistical analysis was performed on SPSS 14.0 (SPSS Inc., Chicago, IL, USA). Explorative data analysis revealed a violation of normality assumptions for some of the test parameters. This can be explained by the small number of animals in each group. Nevertheless, parametric tests were performed because the F statistics are relatively robust against this kind of violation [29]. Statistical differences among genotypes were determined with one-way ANOVA for the respective test parameters. To check homogeneity the Levene test was used. Post-hoc analysis of group differences was performed with Tukey’s test in case of variance homogeneity and with the Games-Howell test in case of variance inhomogeneity. Gender and treatment differences in a particular genotype were evaluated with the two-sample t-test. The paired t-test was used to evaluate any treatment effect of LPS or its vehicle in a particular genotype. Probability values ≤ 0.05 were considered as statistically significant, while probability values ≤ 0.1 were rated as indicative of a statistical trend. All data are presented as means ± SEM, n referring to the number of mice in each group.
RESULTS
General observations and body weight
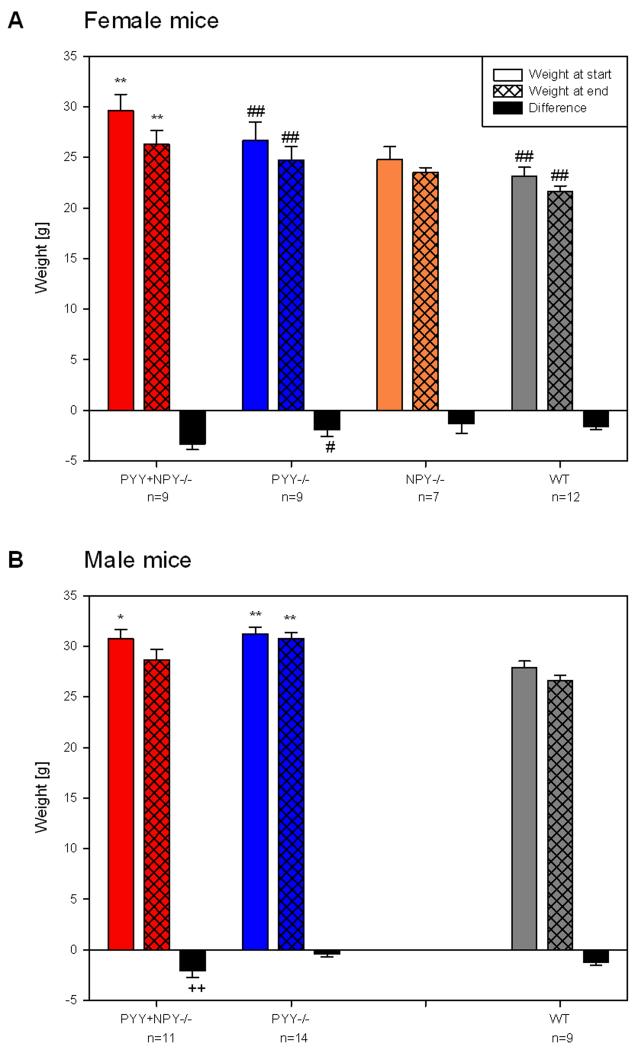
As reported previously for animals deficient in PYY or NPY [15,18], PYY−/−, NPY−/− and PYY+NPY−/− mice did not show any gross abnormalities or obvious signs of sensory deficits and appeared healthy. The experiments were carried out with adult mice as defined by Crawley [30], i.e., mice aged 6.0 ± 0.3 months (mean ± SEM, n = 82). Their average BW was 27.1 ± 0.5 g (mean ± SEM, n = 82), but there was a significant difference in the BW between the four genotypes under study, both before and after the trials (Figure 1). At the beginning of the test session, male PYY−/− and female PYY+NPY−/− mice weighed significantly more than the respective WT mice, while the BW of male PYY+NPY−/− animals showed only a trend (P < 0.1) to be higher than that of male WT mice. In addition, there was a gender difference in BW, since male PYY−/− and WT mice were significantly heavier than the respective females. During the test session all animals lost weight, and this change was nominally more pronounced in female than in male animals (Figure 1). The fall of BW in female PYY−/− mice differed from that in male PYY−/− mice at P < 0.1, and the weight loss in male PYY+NPY−/− mice was statistically more marked than in male PYY−/− mice.
Figure 1.
Body weight at the beginning (day 0) and end (day 4) of the experimental trial and weight loss during the experimental trial in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The values represent means ± SEM, n as indicated below the abscissa. * P ≤ 0.1, ** P ≤ 0.05 vs. WT mice of the same gender, ++ P ≤ 0.05 vs. PYY−/− mice of the same gender (one-way ANOVA); # P ≤ 0.1, ## P ≤ 0.05 vs. male mice of the same genotype (two-sample t-test).
Locomotion and exploration
Circadian pattern
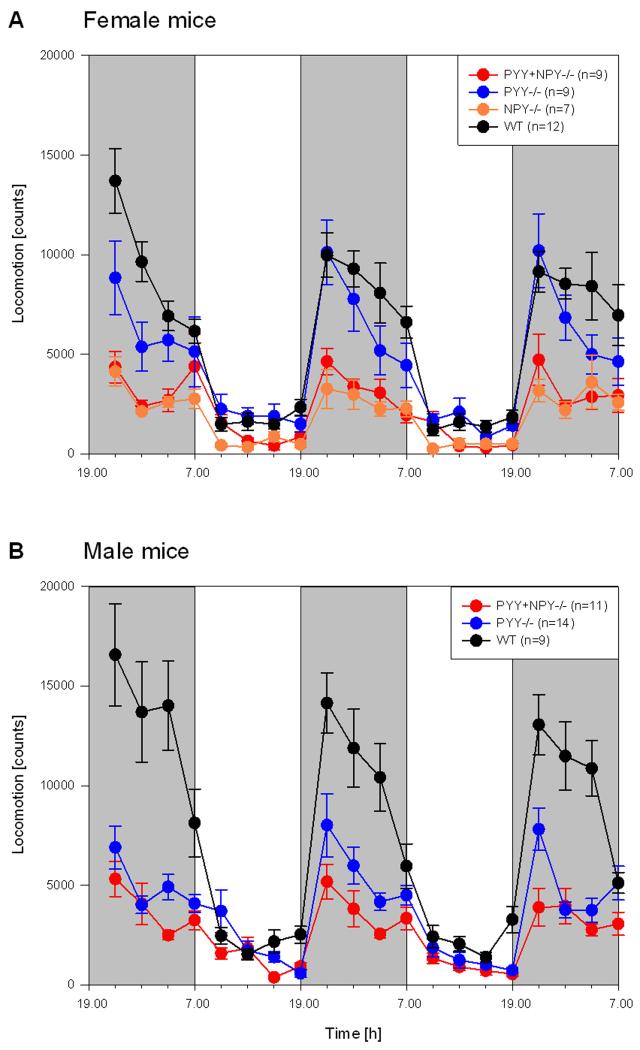
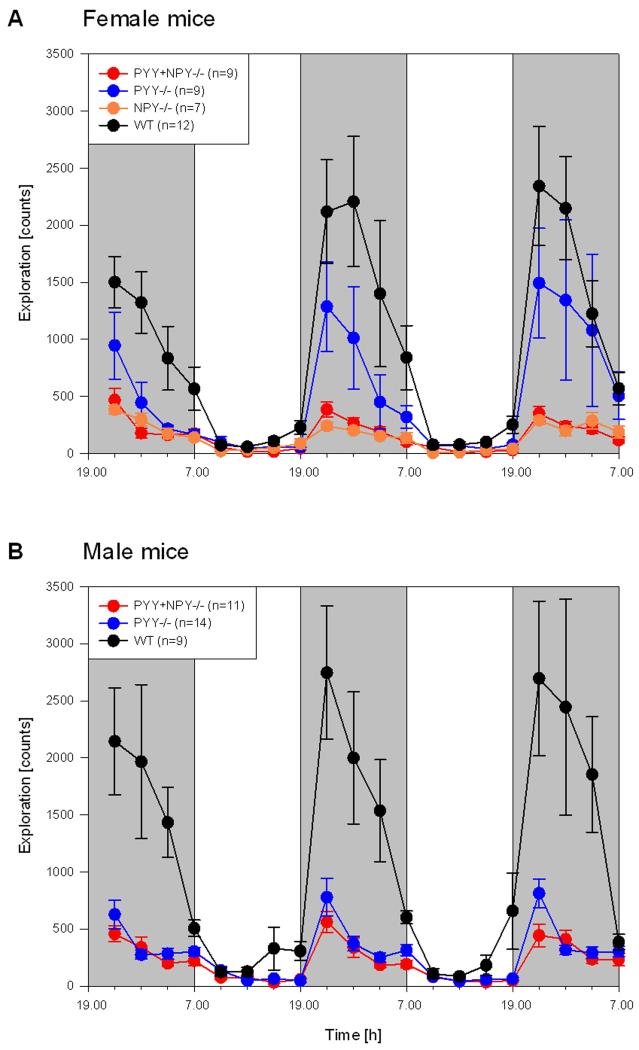
The locomotor (ambulatory) and exploratory (rearing) behaviour as recorded during the scotophase of days 0, 1 and 2 and the photophase of days 1 and 2 showed a characteristic circadian time course (Figures 2 and 3). As was expected for nocturnal animals, the activity of the mice was considerably higher during the scotophase than during the photophase. While this circadian pattern of activity was seen in all genotypes of either gender, the magnitude of nocturnal activity differed considerably with genotype and gender (Figures 2 and 3). It is also worth noting that both female and male WT mice moved more and explored less during the scotophase of day 0, compared with the respective activities in the dark phase of days 1 and 2. This variation, interpreted as a response to the novel environment of the test cages, was also seen with regard to the exploratory behaviour of PYY−/− mice but was largely absent in PYY+NPY−/− and NPY−/− mice (Figures 2 and 3).
Figure 2.
Time course of circadian locomotor (ambulatory) activity in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The graphs show the counts of light beam crossings summed up at intervals of 3 h for three consecutive dark phases (shaded areas, experimental days 0, 1 and 2) and two intervening light phases (white areas, experimental days 1 and 2). The values represent means ± SEM, n as indicated in brackets.
Figure 3.
Time course of circadian exploratory (rearing) activity in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The graphs show the counts of light beam crossings summed up at intervals of 3 h for three consecutive dark phases (shaded areas, experimental days 0, 1 and 2) and two intervening light phases (white areas, experimental days 1 and 2). The values represent means ± SEM, n as indicated in brackets.
Time course of locomotion
The locomotion of female WT and PYY−/− mice during the scotophase was markedly higher than that of female PYY+NPY−/− and NPY−/− mice (Figure 2A). Locomotion peaked during the first quarter of the scotophase and subsequently declined, this decrease in activity being more pronounced in PYY−/− than in WT mice (Figure 2A). Locomotor activity during the photophase was low in all four genotypes, although even in this phase female WT and PYY−/− mice appeared to move more than female PYY+NPY−/− and NPY−/− mice (Figure 2A).
The circadian variation of locomotor activity in male mice followed the pattern seen in female mice (Figure 2A,B). However, male PYY−/− and PYY+NPY−/− mice moved considerably less during the scotophase than male WT mice (Figure 2B). When compared with female WT mice, locomotion in male WT mice was higher during the first three quarters of the scotophase but fell markedly in the last quarter of the dark phase (Figure 2A,B). In contrast, male PYY−/− mice moved clearly less during the first half of the scotophase than female PYY−/− mice (Figure 2A,B). The locomotor activity of the male mice during the photophase was low and did not show pronounced genotype-related differences (Figure 2B).
Time course of exploration
Like locomotion, exploration (rearing) showed a typical circadian cycle, with increased activity during the scotophase and few rearings during the photophase (Figure 3A,B). The magnitude of activity, however, varied widely with genotype and gender. Among the females, WT mice exhibited the highest level of nocturnal exploration, with a peak during the first half of the dark phase and a progressive decline during the second half of the scotophase (Figure 3A). Female PYY−/− mice reared considerably less than female WT mice but markedly exceeded PYY+NPY−/− and NPY−/− mice which explored at a comparatively low level even during the dark phase.
Like female WT mice, male WT mice showed the highest rearing activity during the scotophase (Figure 3B). While female PYY−/− mice reared at an intermediate level but were appreciably more active than female PYY+NPY−/− mice during the dark phase (Figure 3A), male PYY−/− mice exhibited much less nocturnal exploration than female PYY−/− mice and in this parameter did not differ from PYY+NPY−/− mice (Figure 3B). The exploratory behaviour during the photophase was very low and did not exhibit any conspicuous genotype- and gender-related differences, except that both female and male WT mice increased exploration towards the end of the photophase (Figure 3A,B).
Quantitative differences
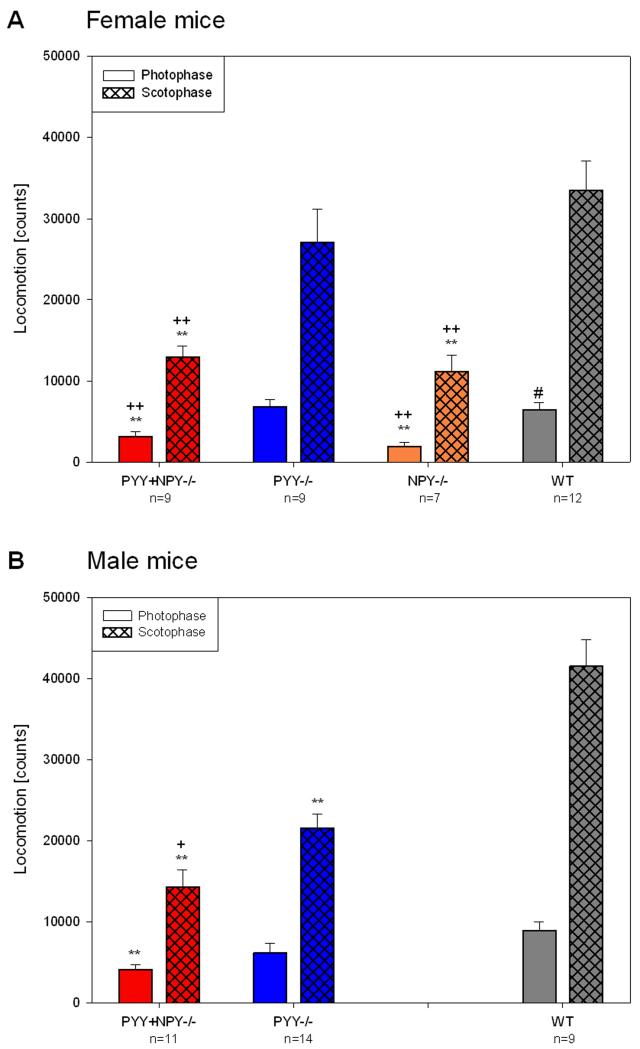
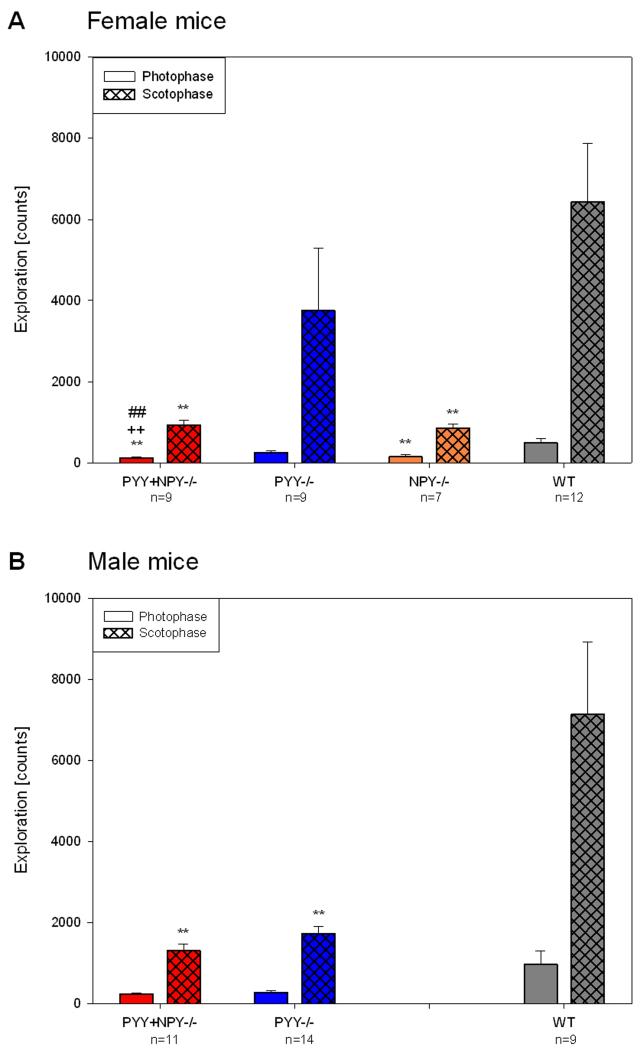
Quantitative estimates of locomotor and exploratory activity during the photo- and scotophase in female and male WT, PYY−/−, PYY+NPY−/− and female NPY−/− mice were obtained by summing up the counts of light beam crossings for the whole photophase and scotophase, respectively, and averaging the counts of experimental days 1 and 2.
As regards locomotion, female PYY+NPY−/− and NPY−/− mice moved significantly less than female WT mice during both the scoto- and photophase, whereas female PYY−/− mice did not differ in these parameters (Figure 4A). As a consequence, both nocturnal and diurnal locomotion of female PYY+NPY−/− and NPY−/− mice was significantly attenuated when compared with that of female PYY−/− mice. In contrast, male PYY−/− mice moved significantly less than male WT mice during the dark phase but did not significantly differ from male WT mice during the photophase (Figure 4B). Male PYY+NPY−/− mice resembled their female counterparts as they moved less than male WT mice throughout the circadian cycle. During the scotophase, the locomotor activity of male PYY+NPY−/− mice was also smaller (P < 0.1) than that of male PYY−/− mice (Figure 4B). The only gender difference in locomotion concerned female WT mice which during the photophase moved less than male WT mice (Figure 4A,B).
Figure 4.
Quantitative estimates of locomotor (ambulatory) activity during the photo- and scotophase in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The bars represent the counts of light beam crossings summed up and averaged for the photophase and scotophase, respectively, of experimental days 1 and 2. The values represent means ± SEM, n as indicated below the abscissa. ** P ≤ 0.05 vs. WT mice of the same gender, + P ≤ 0.1, ++ P ≤ 0.05 vs. PYY−/− mice of the same gender (one-way ANOVA); # P ≤ 0.1 vs. male mice of the same genotype (two-sample t-test).
The genotype and gender-related differences in locomotion were mirrored by similar differences in exploratory behaviour (Figure 5A,B). Thus, female PYY+NPY−/− and NPY−/− mice reared significantly less than female WT mice throughout the day, whereas female PYY−/− mice did not differ in this respect (Figure 5A). In addition, female PYY+NPY−/−, but not NPY−/−, mice explored less than female PYY−/− mice during the photophase (Figure 5A). The nocturnal exploration of male PYY−/− and PYY+NPY−/− was significantly decreased when compared with that of male WT mice, while during the photophase no significant differences emerged (Figure 5B). The only gender difference in exploration concerned PYY+NPY−/− mice in which diurnal rearing was more frequent in male than in female animals (Figure 5A,B).
Figure 5.
Quantitative estimates of exploratory (rearing) activity during the photo- and scotophase in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The bars represent the counts of light beam crossings summed up and averaged for the photophase and scotophase, respectively, of experimental days 1 and 2. The values represent means ± SEM, n as indicated below the abscissa. ** P ≤ 0.05 vs. WT mice of the same gender, ++ P ≤ 0.05 vs. PYY−/− mice of the same gender (one-way ANOVA); ## P ≤ 0.05 vs. male mice of the same genotype (two-sample t-test).
Ingestive behaviour
Time course
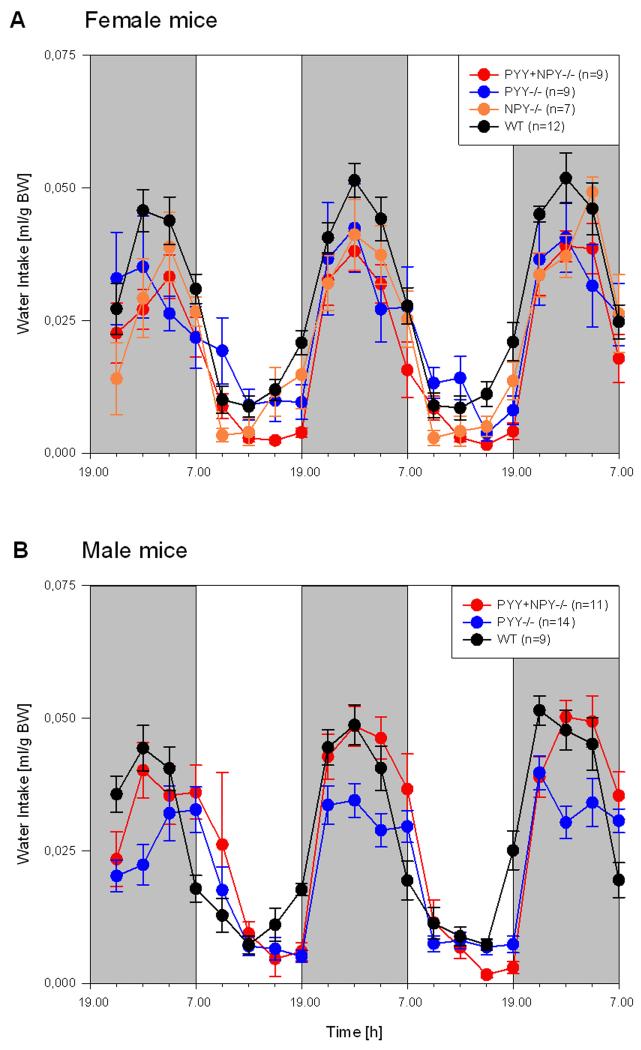
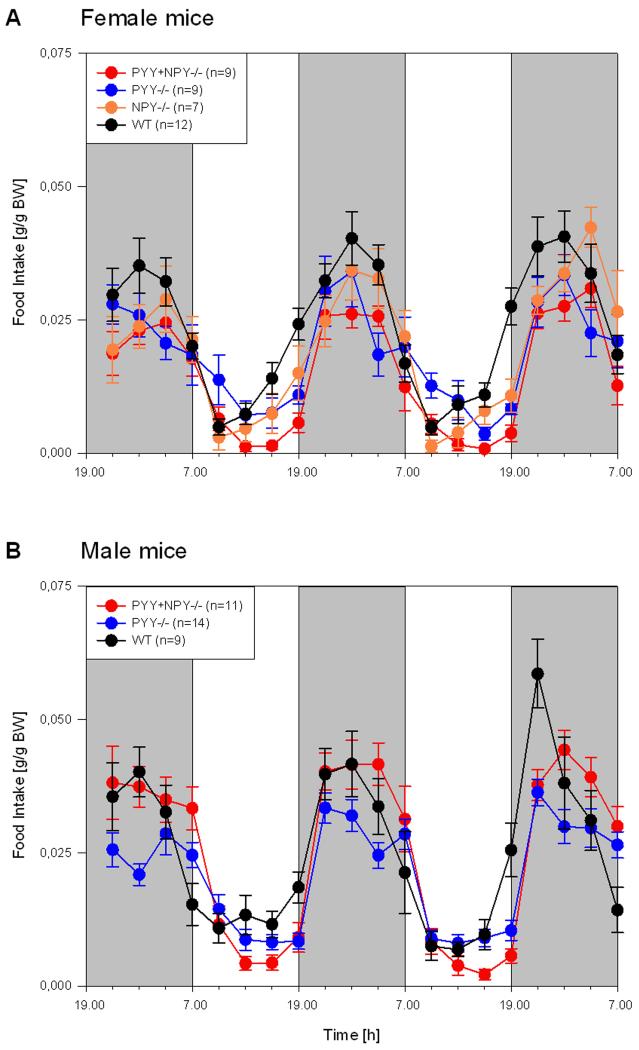
Like locomotion and exploration, drinking and feeding followed a characteristic circadian pattern, peak activity occurring during the scotophase (Figures 6 and 7). The circadian time course of water and food intake in the different genotypes under study showed considerable overlap, and there was no conspicuous difference between male and female animals (Figures 6 and 7). It is, however, worth noting that ingestive behaviour peaked roughly in the middle of the dark phase. Of further note is the finding that during the second half of the photophase both WT and NPY−/− mice progressively increased their water and food consumption (Figures 6 and 7). Another noteworthy observation concerned nocturnal ingestion in male PYY−/− mice: while peak feeding and drinking appeared to be attenuated relative to that of male WT mice, male PYY−/− mice drank and ate at a fairly constant level throughout the dark phase (Figures 6 and 7).
Figure 6.
Time course of circadian water intake in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The graphs show the water consumption summed up at intervals of 3 h for three consecutive dark phases (shaded areas, experimental days 0, 1 and 2) and two intervening light phases (white areas, experimental days 1 and 2). Water consumption is expressed as ml/g BW. The values represent means ± SEM, n as indicated in brackets.
Figure 7.
Time course of circadian food intake in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The graphs show the food consumption summed up at intervals of 3 h for three consecutive dark phases (shaded areas, experimental days 0, 1 and 2) and two intervening light phases (white areas, experimental days 1 and 2). Food consumption is expressed as g/g BW. The values represent means ± SEM, n as indicated in brackets.
Quantitative differences
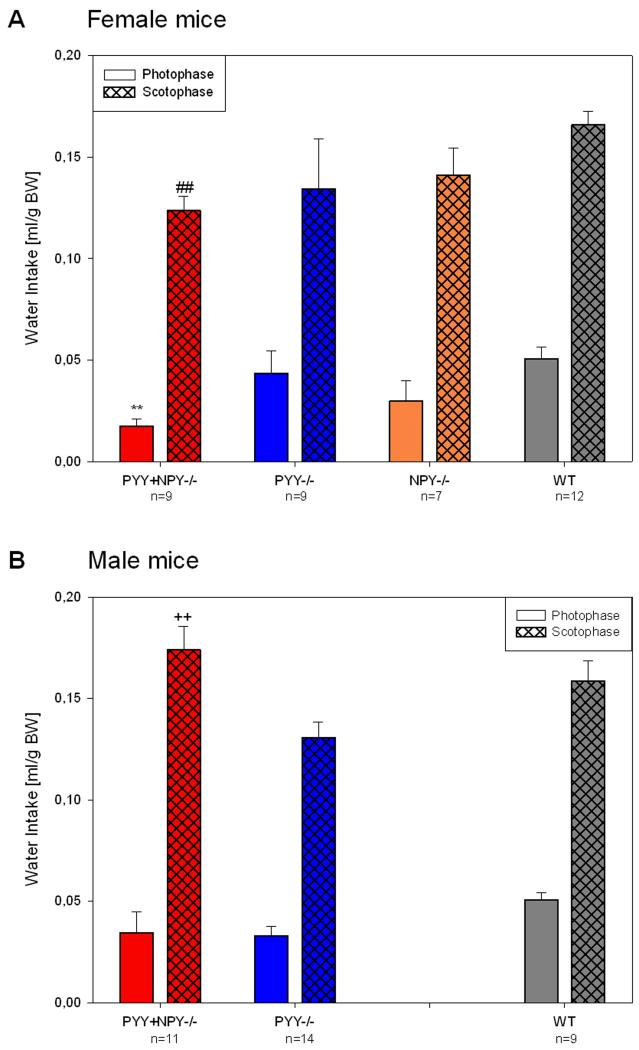
Quantitative estimates of ingestion during the photo- and scotophase in female and male WT, PYY−/−, PYY+NPY−/− and female NPY−/− mice were obtained by summing up drinking and feeding during the whole photophase and scotophase, respectively, and averaging the counts of experimental days 1 and 2. The consumption rates were expressed relative to the BW determined at the beginning of the experiments.
Water intake was little influenced by deletion of the PYY and NPY genes (Figure 8A,B). The only differences that emerged from quantitative analysis concerned female PYY+NPY−/− mice, which during the photophase drank less than female WT mice (Figure 8A), and male PYY+NPY−/− mice which during the dark phase drank more than male PYY−/− mice (Figure 8B). In addition, nocturnal drinking of male PYY+NPY−/− mice was higher than that of their female counterparts. No other significant differences between the genotypes under study were found, both in respect to scoto- and photophase as well as gender (Figure 8A,B).
Figure 8.
Quantitative estimates of water intake during the photo- and scotophase in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The bars represent the water consumption summed up and averaged for the photophase and scotophase, respectively, of experimental days 1 and 2. Water consumption is expressed as ml/g BW. The values represent means ± SEM, n as indicated below the abscissa. ** P ≤ 0.05 vs. WT mice of the same gender, ++ P ≤ 0.05 vs. PYY−/− mice of the same gender (one-way ANOVA); ## P ≤ 0.05 vs. male mice of the same genotype (two-sample t-test).
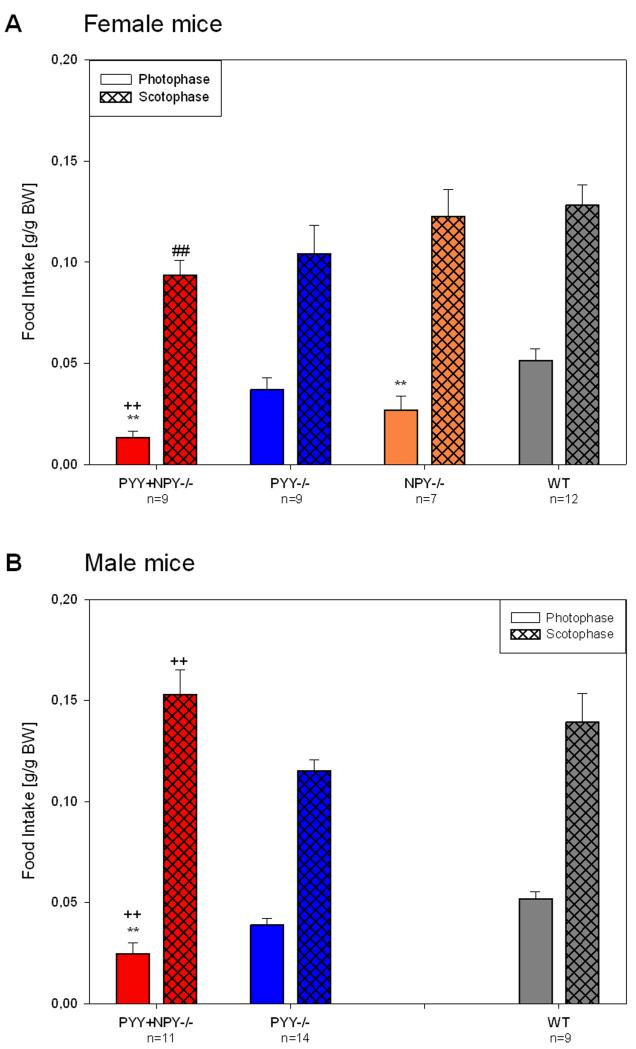
While nocturnal food intake of female PYY−/−, PYY+NPY−/− and NPY−/− mice did not differ from that of WT mice, food consumption during the light phase was reduced in PYY+NPY−/− and NPY−/− mice, but not in PYY−/− mice (Figure 9A). In addition, female PYY+NPY−/− mice ate significantly less than female PYY−/− mice during the photophase. As was true for female PYY−/− mice, the circadian food intake in male PYY−/− mice did not differ from that of male WT mice (Figure 9B). Male PYY+NPY−/− mice likewise did not differ from male WT mice in their nocturnal food consumption but ate significantly more than male PYY−/− mice during the scotophase. Furthermore, the nocturnal food intake of male PYY+NPY−/− mice was significantly higher than that of their female counterparts (Figure 9A,B). During the photophase, male PYY+NPY−/− mice ate significantly less than male WT and male PYY−/− mice (Figure 9B).
Figure 9.
Quantitative estimates of food intake during the photo- and scotophase in female (A) and male (B) WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. The bars represent the food consumption summed up and averaged for the photophase and scotophase, respectively, of experimental days 1 and 2. Food consumption is expressed as g/g BW. The values represent means ± SEM, n as indicated below the abscissa. ** P ≤ 0.05 vs. WT mice of the same gender, ++ P ≤ 0.05 vs. PYY−/− mice of the same gender (one-way ANOVA); ## P ≤ 0.05 vs. male mice of the same genotype (two-sample t-test).
In addition to the diurnal and nocturnal food consumption, the cumulative daily intake of food was also calculated. In this way it was found that male PYY−/− and female PYY+NPY−/− mice ate less than WT mice of the same gender (Table 1). The only gender difference in daily food intake was observed in PYY+NPY−/− mice, given that males consumed more food than females (Table 1).
Table 1.
Cumulative daily food intake
| Genotype | Gender | n | Food intake (g/g BW) | t-test | ANOVA |
|---|---|---|---|---|---|
| WT | female | 12 | 0.18 ± 0.01 | n.s. | - |
| male | 9 | 0.19 ± 0.01 | - | ||
| PYY−/− | female | 9 | 0.14 ± 0.02 | n.s. | n.s. |
| male | 14 | 0.15 ± 0.01 | P ≤ 0.1 | ||
| PYY+NPY−/− | female | 9 | 0.11 ± 0.01 | P ≤ 0.05 | P ≤ 0.05 |
| male | 11 | 0.18 ± 0.01 | n.s. | ||
| NPY−/− | female | 7 | 0.15 ± 0.01 | - | n.s. |
The cumulative daily food intake was calculated by summing up the food intake during the photo- and scotophase and averaging the daily food consumption during experimental days 1 and 2. The values represent means ± SEM, n as indicated. Gender differences within the genotypes were analyzed with the t-test, whereas differences between WT and knockout animals of the same gender were evaluated with one-way ANOVA; n.s., not significant.
Effect of intraperitoneal LPS
The effect of intraperitoneally injected LPS (0.1 mg/kg) on locomotion, exploration, feeding and drinking during the following photo- and scotophase was evaluated on female mice of each genotype, and any change in these parameters was expressed as a percentage of those measured on the preceding day. The effect of intraperitoneal vehicle (sterile saline, 10 ml/kg) was tested on female WT mice to check for any effect of the experimental manipulation and injection procedure.
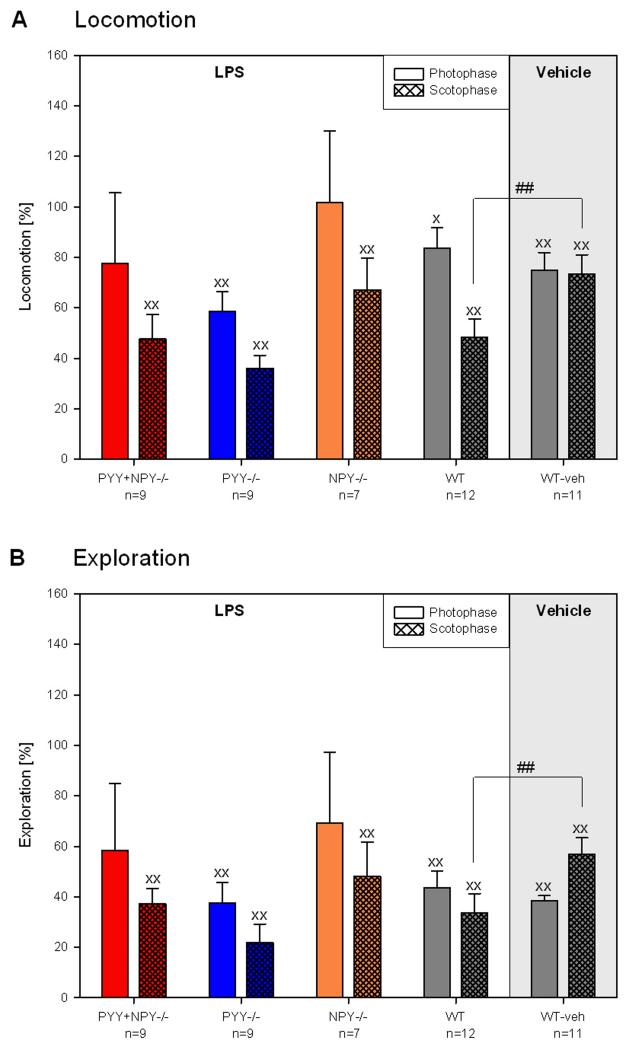
As shown in panels A and B of Figure 10, injection of vehicle to WT mice significantly reduced locomotion and exploration both during the photo- and scotophase. Administration of LPS to WT mice also attenuated the ambulatory and rearing behaviour during the light and dark phase, the effect of LPS during the scotophase being significantly more pronounced than that of vehicle. It is also evident from Figure 10A,B that LPS decreased nocturnal locomotor and exploratory activity in PYY−/−, PYY+NPY−/− and NPY−/− mice to a significant extent, whereas during the light phase these test parameters were diminished in PYY−/− mice only. One-way ANOVA did not reveal any genotype-related differences in locomotion and exploration during the photo- and scotophase following LPS administration.
Figure 10.
Effect of intraperitoneal LPS (0.1 mg/kg) and vehicle (saline; 10 ml/kg) on locomotion (A) and exploration (B) of female WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. LPS or vehicle was injected in the morning of day 3 (9.00 – 9.30 h), and locomotion and exploration recorded during the following photo- and scotophase. The effect of LPS and its vehicle during the photophase and scotophase of day 3 was expressed as a percentage of the respective parameters measured in the photophase and scotophase of day 2, respectively. The values represent means ± SEM, n as indicated below the abscissa. x P ≤ 0.1, xx P ≤ 0.05 vs. day 2 (paired t-test); ## P ≤ 0.05 vs. vehicle-treated WT mice (two-sample t-test).
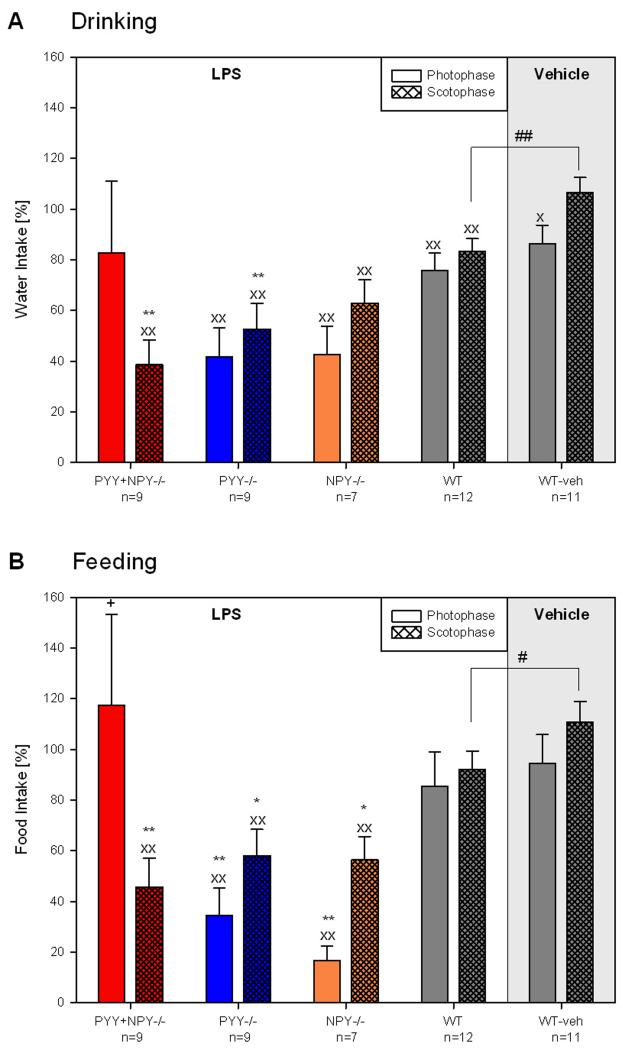
With regard to ingestive behaviour, the only effect of vehicle in WT mice was a reduction of water intake during the photophase that followed the injection (Figure 11A,B). In contrast, LPS administration to WT mice decreased the water intake during the photo- and scotophase to a significant extent, with nocturnal drinking in LPS-treated WT mice being significantly less than that in vehicle-treated WT mice (Figure 11A). Although LPS did not reduce food intake in WT mice, nocturnal feeding in LPS-treated WT mice was less than in vehicle-treated WT mice (Figure 11B).
Figure 11.
Effect of intraperitoneal LPS (0.1 mg/kg) and vehicle (saline; 10 ml/kg) on the water (A) and food (B) intake of female WT, PYY−/−, PYY+NPY−/− and NPY−/− mice. LPS or vehicle was injected in the morning of day 3 (9.00 – 9.30 h), and drinking and feeding recorded during the following photo- and scotophase. The effect of LPS and its vehicle during the photophase and scotophase of day 3 was expressed as a percentage of the respective parameters measured in the photophase and scotophase of day 2, respectively. The values represent means ± SEM, n as indicated below the abscissa. x P ≤ 0.1, xx P ≤ 0.05 vs. day 2 (paired t-test); # P ≤ 0.1, ## P ≤ 0.05 vs. vehicle-treated WT mice (two-sample t-test); * P ≤ 0.1, ** P ≤ 0.05 vs. WT mice, + P ≤ 0.1 vs. NPY−/− mice (one-way ANOVA).
LPS administration to PYY−/− and NPY−/− mice attenuated water and food intake during the light and dark phase, whereas PYY+NPY−/− mice drank and ate less only during the scotophase (Figure 11A,B). There were also genotype-related differences in the effect of LPS on ingestive behaviour. Thus, LPS diminished nocturnal drinking in PYY−/− and PYY+NPY−/− mice to a larger degree than in WT mice (Figure 11A). The LPS-induced attenuation of diurnal and nocturnal feeding in PYY−/− and NPY−/− mice was more pronounced than in WT mice. In PYY+NPY−/− mice only nocturnal food consumption was reduced by LPS to a significantly larger degree than in WT mice, whereas diurnal feeding in the double knockout mice remained unchanged by LPS (Figure 11B). It is worth noting, however, that diurnal food intake of female PYY+NPY−/− mice was higher than that of female NPY−/− mice.
DISCUSSION
General considerations
This study set out to explore the circadian pattern of locomotion, exploration, drinking and feeding in PYY−/−, NPY−/− and PYY+NPY−/− mice, relative to WT animals. Additional aims were to test the utility of the LabMaster system in this task and to explore whether the system would be able to detect changes in the parameters under study following intraperitoneal injection of a low dose of LPS. The major findings of the study indicate that knockout of PYY and/or NPY alters locomotor, exploratory and ingestive behaviour in a genoytype-, gender- and circadian cycle-related manner. These results have a direct bearing on the proposed implications of the PYY and NPY systems in energy homeostasis. By recording feeding and drinking as well as locomotion and exploration it is possible to obtain information on the balance between energy intake and expenditure, parameters that are affected by appetite and have an impact on BW.
Since NPY is also involved in the control of anxiety and mood, most information on the implications of the NPY system in locomotion and exploration has been obtained from tests of emotional-affective behaviour, many of which rely on the recording of locomotor and exploratory activity during a limited period of observation [31,32]. However, these test paradigms represent stressors that are likely to bias the parameters under study as, for instance, the anorectic effect of intraperitoneally injected PYY(3-36) is inhibited by stress [33]. To circumvent these limitations, we chose to record the ingestive and motor behaviour continuously for several days, keeping the animals undisturbed in the same cage so that they could become familiar with their new environment.
The ability to continuously record ingestive and motor activity and the opportunity to analyze the circadian pattern of these activities are among the major advantages of the LabMaster system. On the other hand, the animals need be kept singly in the test cages, which entails social deprivation. There is evidence for a gender-related difference in the reaction to single housing which appears to be a stressor for female, but not male, mice [34]. This issue need be kept in mind when the current results are compared with data in the literature and when gender-related differences in our observations are discussed.
There are several possibilities to explore the implications of the PYY and NPY system in ingestive and motor behaviour. The pharmacological approach is limited because these peptides have affinities for more than one of the five Y receptor subtypes and the availability of selective receptor antagonists is confined to the Y1, Y2 and Y5 subtypes [2,3]. In the present study, a genetic approach based on germline PYY−/−, NPY−/− and PYY+NPY−/− mice was chosen. Although the data of the study attest to distinct roles of PYY and NPY in energy intake and expenditure activities, it must not be neglected that developmental compensations may mask the full implication of PYY and NPY in the functions under study.
Circadian locomotion and exploration
Locomotor and exploratory behaviour was significantly decreased in NPY−/− and PYY+NPY−/− mice during the photo- and scotophase, and in male PYY−/− mice during the scotophase. The phenotypic variations became obvious not only as overall activity changes during the light and dark phases but also as distinct differences in the time course of the respective parameters. This observation confirms the tenet that circadian phase is important to consider in the phenotyping of animals with regard to behaviours that undergo a circadian cycle [35].
The results obtained in NPY−/− animals are consistent with observations in the open field in which locomotor and exploratory activities are attenuated in male and female NPY-deficient mice [17,18]. It follows that NPY provides a stimulant impact on the maintenance of the circadian pattern of locomotor and exploratory activity and, as reported previously [19-22], can modify the setting of the circadian clock. In order to achieve this, NPY is likely to act via multiple Y receptors with opposing actions [2,9,19], an instance that may explain why intracerebroventricularly administered NPY does not facilitate but rather depresses open field locomotion and homecage activity in rats [31]. Our finding of reduced locomotion and exploration during the scotophase in male, but not female, PYY−/− mice extends the report by Boey et al. [15] who failed to observe changes in locomotor activity in the open field and home cage (48 h) in PYY-deficient mice of either gender. While the difference between their and our results may be related to the different experimental settings and analysis procedures, both sets of results attest to a gender-dependent involvement of PYY in the regulation of nocturnal locomotion and exploration. It is worth noting in this respect that the implication of PYY in certain metabolic processes is likewise gender-dependent [15].
Despite this discrepancy, our data support the inference that PYY is less relevant than NPY to the control of circadian ambulation and rearing. This conclusion is affirmed by a comparison of the circadian locomotion and exploration patterns over the genotypes studied here, the patterns being considerably more similar between NPY−/− mice and PYY+NPY−/− mice than between of PYY−/− mice and PYY+NPY−/− mice. Although we did not study male NPY−/− mice, a similar inference is likely to apply to male NPY-deficient animals because Karl et al. [18] reported a gender-independent hypoactive phenotype of NPY−/− mice.
Ingestive behaviour and body weight
Significant decreases in water and food intake were found in NPY−/− and PYY+NPY−/− mice during the light but not dark phase. In contrast, a tendency towards increased BW was associated with knockout of the PYY and/or NPY genes, female PYY+NPY−/− and male PYY−/− mice being significantly heavier than the respective WT mice. Although these observations have a bearing on the proposed role of NPY and PYY in the regulation of appetite and energy homeostasis [3,4,36,37], our results regarding water and food intake as well as BW in PYY−/− and NPY−/− mice are not directly congruent with the ability of centrally administered NPY to stimulate and peripherally administered PYY to inhibit food intake [3,4,36,37]. The decrease in spontaneous diurnal feeding in female NPY−/− mice, however, is consistent with a decrease in fasting-induced feeding in male NPY−/− mice [17]. Since in NPY−/− mice the decrease in diurnal ingestion is combined with an attenuation of circadian locomotion and exploration, it would appear that endogenous NPY stimulates energy intake and expenditure in a balanced manner. As a result, the BW remains unaltered as found in the present and a previous study [16].
Although PYY−/− mice did not significantly differ from WT mice in their circadian ingestion and cumulative daily intake of food was nominally decreased, male PYY−/− mice weighed more than the respective WT mice. Together with the finding that male PYY−/− mice moved less during the scotophase, our observations indicate that endogenous PYY shifts energy balance towards storage rather than expenditure. The present findings are, at least in part, at variance with those of two other studies, the results of which also disagree with each other. One of the reports holds that male PYY−/− mice are hyperphagic and that both male and female PYY−/− mice (aged 5 – 26 weeks) are overweight [14], whereas the other report found that the cumulative daily intake of food and water is decreased in female PYY−/− mice aged 11 weeks, but neither in female PYY−/− mice aged 24 weeks nor in male PYY−/− mice of any age [15]. In addition, female PYY−/− mice aged 4 – 28 weeks were found to be overweight whereas male PYY−/− mice were not [15]. Although these discrepancies are at present not understood but likely to reflect multiple differences between the experimental settings, two general conclusions can be drawn. Firstly, the implications of PYY in the control of ingestion and energy homeostasis are subject to gender differences and circadian variations. Secondly, the role of PYY in energy homeostasis involves not only alterations in energy intake and expenditure but also changes in processes such as insulin release, glucose homeostasis and metabolic rate [15].
The decrease in diurnal ingestion and cumulative daily food intake, the decrease in circadian locomotion and exploration and the enhancement of BW encountered in PYY+NPY−/− mice further attests to the functional implications of both NPY and PYY in metabolic and energy homeostasis. Overall, NPY may play a greater role than PYY in controlling these functions because the phenotypic overlap between NPY−/− and PYY+NPY−/− mice appeared to be greater than that between PYY−/− and PYY+NPY−/− mice. The gender-related role of these peptides is emphasized by the findings that the enhancement of BW and decrease in cumulative daily food intake is particularly pronounced in female PYY+NPY−/− mice.
During the test session all animals lost weight, yet two aspects of this observation deserve consideration. Firstly, the weight loss was nominally more pronounced in female than in male mice and, secondly, largest in female PYY+NPY−/− mice. This gender difference is likely to reflect that single housing is more stressful to female than to male mice [34] and causes weight loss most likely by enhanced metabolic demand [38]. This may in particular apply to female PYY+NPY−/− mice which loose weight concomitantly with a reduction in ingestion, locomotion and exploration. It may be worth exploring how PYY+NPY−/− mice fare in their stress coping capacity.
Effect of intraperitoneal LPS
Knockout of the PYY and/or NPY genes altered the ability of intraperitoneal LPS to modify ingestion, locomotion and exploration to a differential extent. Anorexia and a decrease in locomotion are two components of the sickness response that is induced by systemic administration of LPS via induction of proinflammatory cytokines [23,24]. Interleukin-1, for instance, has been found to alter the concentration of NPY in distinct brain nuclei [25], and there is evidence that NPY-expressing neurons in the arcuate and paraventricular nuclei of the hypothalamus participate in the anorectic component of the illness response to proinflammatory cytokines [25-27]. Further support for this inference comes from the finding that knockout of Y2 receptors alters several aspects of the acute sickness response to LPS [39].
The present study involving LPS was complicated by the ability of intraperitoneally injected vehicle to reduce locomotion by about 20 % and exploration by some 40 – 60 % in WT mice. This observation attests to the sensitivity of the LabMaster system to pick up stress-induced behavioural disturbances such as those caused by handling and injecting the animals, yet represents a problem that is likely to obscure the effects of LPS. When injected at the low dose of 0.1 mg/kg [39], the endotoxin indeed failed, relative to LPS-treated WT mice, to significantly alter ambulation and rearing in PYY−/−, NPY−/− and PYY+NPY−/− mice. LPS, however, caused a significant attenuation of nocturnal ambulation and rearing in WT mice, and a similar albeit not significant trend was observed in PYY−/−, NPY−/− and PYY+NPY−/− mice. Apart from the adverse impact of handling and injection, the effect of LPS to reduce mobility could also have been masked by the fact that NPY−/− and PYY+NPY−/− mice per se display a significant reduction of circadian locomotion and exploration.
In contrast to its influence on mobility, the effect of LPS to decrease ingestion was more easily demonstrated with the LabMaster system because intraperitoneally injected vehicle influenced feeding and drinking in WT mice only to a minor degree. Specifically, LPS reduced nocturnal drinking in PYY−/− and PYY+NPY−/− mice to a larger extent than in WT animals, whereas the effect of LPS to attenuate feeding was most pronounced in PYY−/− and NPY−/− mice during the photophase and in PYY+NPY−/− animals during the scotophase. It seems that PYY can antagonize the antidipsogenic effect of LPS and that both PYY and NPY can counteract the anorectic response to LPS in a circadian cycle-dependent manner. This interpretation is consistent with the ability of intracerebroventricularly administered NPY to reverse anorexia caused by interleukin-1 [26]. The observation that the diurnal intake of water and food remained unaltered by LPS in PYY+NPY−/− mice awaits further analysis.
CONCLUSIONS
The current data reveal that knockout of the PYY and/or NPY genes alters the circadian pattern of locomotor, exploratory, drinking and feeding behaviour in a differential and gender-related manner. These phenotypic traits can be sensitively and continuously recorded by the LabMaster system which in conjunction with alterations in BW provides information on the balance between energy intake and locomotion-associated energy expenditure. By analyzing the data of this study three major conclusions can be drawn. One, endogenous NPY provides a stimulant impact on the maintenance of the circadian pattern of locomotor and exploratory activity, a contribution that is shared by endogenous PYY to some degree. Second, both peptides stimulate feeding and drinking to balance the energy demand that they generate by enforcing mobility. While NPY stimulates energy intake and locomotion-associated energy expenditure in a balanced manner, the role of PYY in energy homeostasis involves not only alterations in energy intake and locomotion-associated expenditure but also changes in metabolic processes. Third, both PYY and NPY are able to counteract the anorexigenic and antidipsogenic effects of immune challenge.
ACKNOWLEDGEMENTS
This study was supported by the Zukunftsfonds Steiermark (grant 262) and the Austrian Scientific Research Funds (FWF grant L25-B05). The help of Professor Günther Sperk, Institute of Pharmacology at the Medical University of Innsbruck, in providing NPY−/− mice is gratefully acknowledged. The authors thank Dr. Birgit Ebner and Dr. Christian Gülly for genotyping the animals and Professor Andreas Tiran for his support to conduct the study at the Centre for Medical Research of the Medical University of Graz.
REFERENCES
- [1].Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T., XVI International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–50. [PubMed] [Google Scholar]
- [2].Redrobe JP, Carvajal C, Kask A, Dumont Y, Quirion R. Neuropeptide Y and its receptor subtypes in the central nervous system: emphasis on their role in animal models of psychiatric disorders. In: Michel MC, editor. Handbook of Experimental Pharmacology: Neuropeptide Y and Related Peptides: Bd 162. Springer; Berlin: 2004. pp. 101–36. [Google Scholar]
- [3].McGowan BMC, Bloom SR. Peptide YY and appetite control. Curr Opin Pharmacol. 2004;4:583–8. doi: 10.1016/j.coph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- [4].Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul Pept. 2008;145:12–6. doi: 10.1016/j.regpep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- [5].Abbott CR, Small CJ, Sajedi A, Smith KL, Parkinson JRC, Broadhead LL, Ghatei MA, Bloom SR. The importance of acclimatisation and habituation to experimental conditions when investigating the anorectic effects of gastrointestinal hormones in the rat. Int J Obes (Lond) 2006;30:288–92. doi: 10.1038/sj.ijo.0803137. [DOI] [PubMed] [Google Scholar]
- [6].Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SCR. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–9. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- [7].Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- [8].Kask A, Harro J, von Hörsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–83. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- [9].Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–24. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- [10].Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [11].Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front Neuroendocrinol. 2006;27:308–39. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [12].Karl T, Herzog H. Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides. 2007;28:326–33. doi: 10.1016/j.peptides.2006.07.027. [DOI] [PubMed] [Google Scholar]
- [13].Zhou Z, Zhu G, Hariri AR, Enoch M, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu X, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen P, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta J, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–33. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [15].Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, Couzens M, Slack K, Dallmann R, Sainsbury A, Herzog H. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia. 2006;49:1360–70. doi: 10.1007/s00125-006-0237-0. [DOI] [PubMed] [Google Scholar]
- [16].Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–21. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- [17].Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- [18].Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. European Journal of Neuroscience. 2008;28:173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- [19].Gribkoff VK, Pieschl RL, Wisialowski TA, van den Pol AN, Yocca FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J Neurosci. 1998;18:3014–22. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yannielli PC, Harrington ME. Neuropeptide Y in the mammalian circadian system: effects on light-induced circadian responses. Peptides. 2001;22:547–56. doi: 10.1016/s0196-9781(01)00356-4. [DOI] [PubMed] [Google Scholar]
- [21].Harrington M, Molyneux P, Soscia S, Prabakar C, McKinley-Brewer J, Lall G. Behavioral and neurochemical sources of variability of circadian period and phase: studies of circadian rhythms of npy−/− mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1306–14. doi: 10.1152/ajpregu.00383.2006. [DOI] [PubMed] [Google Scholar]
- [22].Kim HJ, Harrington ME. Neuropeptide Y-deficient mice show altered circadian response to simulated natural photoperiod. Brain Res. 2008;1246:96–100. doi: 10.1016/j.brainres.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- [24].Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- [25].McCarthy HD, Dryden S, Williams G. Interleukin-1 beta-induced anorexia and pyrexia in rat: relationship to hypothalamic neuropeptide Y. Am J Physiol. 1995;269:E852–7. doi: 10.1152/ajpendo.1995.269.5.E852. [DOI] [PubMed] [Google Scholar]
- [26].Sonti G, Ilyin SE, Plata-Salamán CR. Neuropeptide Y blocks and reverses interleukin-1 beta-induced anorexia in rats. Peptides. 1996;17:517–20. doi: 10.1016/0196-9781(96)00016-2. [DOI] [PubMed] [Google Scholar]
- [27].McMahon CD, Buxton DF, Elsasser TH, Gunter DR, Sanders LG, Steele BP, Sartin JL. Neuropeptide Y restores appetite and alters concentrations of GH after central administration to endotoxic sheep. J Endocrinol. 1999;161:333–9. doi: 10.1677/joe.0.1610333. [DOI] [PubMed] [Google Scholar]
- [28].Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schürmann A, Szanto I, Tschöp MH, Rohner-Jeanrenaud F. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–93. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kirk RE. Experimental Design: Procedures for Behavioral Sciences: Procedures for the Behavioral Sciences. Wadsworth Inc Fulfillment; 1995. p. 99. [Google Scholar]
- [30].Crawley JN. What’s Wrong With My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley & Sons; 2000. p. 25. [Google Scholar]
- [31].Heilig M, Murison R. Intracerebroventricular neuropeptide Y suppresses open field and home cage activity in the rat. Regul Pept. 1987;19:221–31. doi: 10.1016/0167-0115(87)90278-3. [DOI] [PubMed] [Google Scholar]
- [32].Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–9. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- [33].Halatchev IG, Ellacott KLJ, Fan W, Cone RD. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004;145:2585–90. doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- [34].Palanza P, Gioiosa L, Parmigiani S. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav. 2001;73:411–20. doi: 10.1016/s0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- [35].Beeler JA, Prendergast B, Zhuang X. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests. Physiol Behav. 2006;87:870–80. doi: 10.1016/j.physbeh.2006.01.037. [DOI] [PubMed] [Google Scholar]
- [36].Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33:329–38. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- [37].Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knockout models. Eur J Pharmacol. 2003;480:21–9. doi: 10.1016/j.ejphar.2003.08.089. [DOI] [PubMed] [Google Scholar]
- [38].van Leeuwen SD, Bonne OB, Avraham Y, Berry EM. Separation as a new animal model for self-induced weight loss. Physiol Behav. 1997;62:77–81. doi: 10.1016/s0031-9384(97)00144-3. [DOI] [PubMed] [Google Scholar]
- [39].Painsipp E, Herzog H, Holzer P. Implication of neuropeptide-Y Y2 receptors in the effects of immune stress on emotional, locomotor and social behavior of mice. Neuropharmacology. 2008;55:117–126. doi: 10.1016/j.neuropharm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]