Abstract
Free radicals are one of the frequent products of normal cellular metabolism. Disparity of metabolism and excessive generation of free radicals predisposes to disorders like Parkinson's disease, Alzheimer's disease, and aging phenomenon. Curculigo orchioides Gaertn. is known for “adaptogen” and “aphrodisiac” activity and has been proved for antiasthmatic, estrogenic, antiosteoporotic activity along with protection from cisplatin-induced cell damage. C. orchioides was powdered and subjected to soxhlet extraction using methanol. Phytochemical studies and estimation of polyphenols and flavonoids was performed. Acute toxicity studies were performed by Organization for Economic Cooperation and Development OECD guidelines. Animals were treated with cyclophosphamide to induce neurotoxicity. Curculigo orchioides was powdered and subjected to soxhlet extraction using methanol. Catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation were estimated by reported methods. C. orchioides (400 mg/kg) significantly promoted restoration of catalase (P < 0.005), superoxide dismutase (P < 0.005), and glutathion (P < 0.05) levels. Similarly, a very significant decrease (P < 0.005) in the levels of malondialdehyde was observed. In all cases as mentioned previously, C. orchioides at dose 200 mg/kg promoted significant (P < 0.05) restoration of enzyme levels. C. orchioides (Kali Musli) is rich source of phytochemicals like flavonoids and polyphenols. Flavonoids and polyphenols are reputed to demonstrate neuroprotective effect. These phytochemicals in the present study might be responsible to demonstrate neuroprotective effect.
Keywords: Antioxidant enzymes, brain, Curculigo orchioides, neuroprotective
INTRODUCTION
Free radicals are one of the frequent products of normal cellular metabolism. Imbalance in antioxidants defense mechanism and overproduction of free radicals due to environmental stress is ultimately responsible for neurodegeneration. Imbalance of metabolism and excessive generation of free radicals predisposes to disorders like Parkinson's disease, Alzheimer's disease, and aging phenomenon.[1] Free-radicals-mediated lipid peroxidation is known to change structure cell membrane and its activity. Increase in production of free radicals stimulates lipid peroxidation which leads to increased levels of malondialdehyde.[2,3] Plant sources could be considered as novel source of phytochemicals out of which flavonoids and polyphenols are of great importance that may be helpful to cope up with such oxidative stress.
Curculigo orchioides Gaertn. (family Amaryllidaceae), one of the jeopardized Indian “rasayan” herb, is commonly known as “Kali Musli.” The plant is known for “adaptogen” and “aphrodisiac activity. The plant also finds use in Kampo and Chinese medicines.[4] Scientifically, plant has proved for antiasthmatic[5], estrogenic[6], antiosteoporotic[7] activity. It also protects from cisplatin-induced cell damage.[8]
The current work was undertaken to evaluate neuroprotective potential of C. orchioides against cyclophosphamide-induced neurotoxicity.
EXPERIMENTAL
Plant collection, identification, and extraction
Curculigo orchioides was purchased from Bhopal region and was authenticated at Pinnacle Biomedical Research Institute, Bhopal. Dried Curculigo orchioides was powdered and subjected to soxhlet extraction using methanol.
Phytochemical screening
Phytochemical testing was performed to identify presence of different phytoconstituents.[9]
Phytoanalytical studies
Determination of total phenolic compounds
Total soluble phenolic compounds in the methanol extract were determined with Folin-Ciocalteu reagent according to reposted method[10] using pyrocatechol as a standard phenolic compound. Briefly, 1 ml of HEE (1000 μg/ml) in a volumetric flask was diluted with distilled water (46 ml). One milliliter of Folin-Ciocalteu reagent was added and the content of the flask was mixed thoroughly. After 3 minutes, Na2CO3 (3 ml, 2% w/v) was added and then allowed to stand for 2 hours with intermittent shaking. The absorbance was measured at 760 nm in a spectrophotometer (Shimadzu-1700). The total concentration of phenolic compounds in the extract determined as microgram of pyrocatechol equivalent by using an equation that was obtained from standard pyrocatechol graph:
Absorbance = 0.0053 × total phenols (pyrocatechol equivalent [μg]) −0.0059
Assay for total flavonoids content
Total flavonoid content was determined using the method given elsewhere.[10] Briefly, aluminium trichloride (1 ml, 2% w/v) in methanol was mixed with the same volume of the HEE (1 ml, 2000 μg/ml). Absorption readings at 415 nm were taken after 10 minutes against a blank sample consisting of a methanol extract (1 ml, 2000 μg/ml) with methanol (1 ml) without AlCl3. The concentrations of flavonoid compounds were calculated according to the following equation that was obtained from the standard quercetin graph:
Absorbance = 0.0338 (quercetin [μg]) −0.0002; R2 = 0.9969
Acute oral toxicity studies
Acute oral toxicity study was carried out in mice as per OECD-423 guidelines. The four fixed dose levels were selected as 5, 50, 300, 2000 mg/kg body weight. The mice were continuously observed for their mortality and behavioral response for 24 hours and thereafter once in a day for 14 days.[11] Hence, a dose of 200 mg/kg and 400 mg/kg was used in this study.
Animal grouping and treatment
Animals were divided into four groups containing five animals each. Animals were dosed as
Group I : Normal control
Group II: Toxic control [Cyclophosphamide (50 mg/kg i.p.)]
Group III: Extract treated [Cyclophosphamide (50 mg/kg i.p.) +200 mg/kg extract]
Group IV: Extract treated [Cyclophosphamide (50 mg/kg i.p.) +400 mg/kg extract]
Cyclophosphamide[12] was administered once on the first day; extract was administered for 5 consecutive days.
Brain isolation and brain antioxidants study
After euthanasia, brains from animals were isolated and rinsed with ice-cold normal saline, followed by ice-cold 0.15 M tris HCl (pH 7.4). For estimation of antioxidant markers, viz., superoxide dismutase, catalase, glutathione, and malondialdehyde, a 10% w/v tissue homogenate in 0.15 M tris HCl and centrifuged at 15,000 rpm for 15 minutes at 4°C, supernatant was used for analysis.
Catalase[13], superoxide dismutase[14], glutathione peroxidase[15], and lipid peroxidation[16] were estimated by reported methods.
Statistical analysis
All results were analyzed by one-way analysis of variance (ANOVA), and post-hoc analysis was performed with Bonferroni's test. Value of P < 0.05 was considered to be statistically significant in all the cases.
RESULTS
Phytochemical screening and phytoanalytical studies
Phytochemical screening of extract demonstrated presence of flavonoids, polyphenolics, and alkaloids. The total amount of phenolic content present in extract was found to be 752.23 ± 5.78 mg pyrocatechol equivalent (PE)/100 g. By using the standard curve of quercetin (R2 = 0.9998), the total flavonoid content of extract was found to be 203.52 ± 4.56 mg quercetin equivalent (QE)/100 g.
Acute toxicity studies
C. orchioides extract did not showed any toxicity at a dose of 2000 mg/kg as evidenced by observations. No signs of abnormal behavior or mortality were observed during the study period. Thus, dose of 200 mg/kg and 400 mg/kg of extract were selected for further studies.
Brain antioxidants study
As evident by Figure 1, C. orchioides (400 mg/kg) significantly promoted the restoration of catalase (P < 0.005), superoxide dismutase (P < 0.005), and glutathion (P < 0.05) levels. Similarly, a very significant decrease (P < 0.005) in the levels of malondialdehyde was observed. In all cases as mentioned previously, C. orchioides at dose 200 mg/kg promoted significant (P < 0.05) restoration of enzyme levels.
Figure 1.
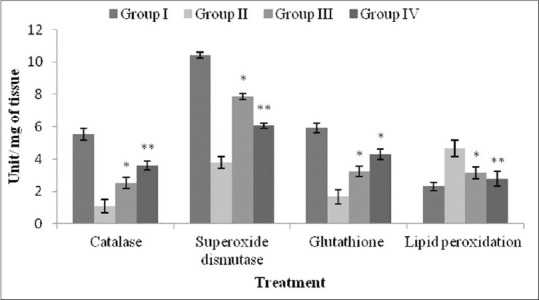
Protective effect of Curculigo orchioides extract on brain antioxidants in rats. Values are mean ± S.E.M. (n = 5), *P < 0.05 (significant); ** P < 0.01 (very significant) when compared to toxic control group
DISCUSSION
Etiopathogenesis of various nerve-racking situations lead to generation of several “psychotic disorders” where normal physiological functions of neurotransmitters are altered. Cyclophosphamide, an anticancer agent is reported to produce adverse effects on brain[17] which is possibly due to development of oxidative stress. Antioxidant is any molecule that is capable of alleviating effects of free radicals prior to attack to cell. Human body contains a variety of antioxidant systems and enzymes that continuously protect body from harm full effects of free radicals and oxidative stress. Supplementation with antioxidant-rich food could be useful to provide adequate protection.[18]
Superoxide dismutase, an antioxidant enzyme, catalyzes translation of superoxide free radical to hydrogen peroxide and water, and serves to be useful in inhibiting oxidative stress.[19] Catalase is an important enzyme accountable for disposition of H2O2. Diminution of activity of this enzyme is associated with increased activity of free radicals, which may lead to alter the activity of cell membranes.[20] In the present study, restoration in the levels of antioxidant enzymes by extract demonstrated protective effect.
Basically, there exists equilibrium between production of reactive oxygen species and antioxidant defense system which regulates homeostasis towards oxidative stress of cell. Due to low levels of antioxidant enzymes, hippocampal neurons are predominantly vulnerable to oxidative stress. Glutathione can be regarded as a chief “intracellular non-protein thiol compound” and act as scavenger of free radicals. Thus, glutathione could be regarded as a first line of antioxidant defense.[21] Glutathione depletion therefore may cause death of nerve cells after ischemia of forebrain.[22] Flavonoids from plants are widely recognized to prevent membrane damage and thus protect the integrity of cell.[23]
Curculigo orchioides (Kali Musli) is rich source of phytochemicals like flavonoids and polyphenols. Flavonoids[24,25] and polyphenols[26,27] are reputed to demonstrate neuroprotective effect. These phytochemicals in the present study might be responsible to demonstrate neuroprotective effect. However, more systematic studies along isolation and characterization of phytoconstituents responsible for activity seem to be necessary.
Footnotes
Source of Support: Self.
Conflict of Interest: None declared.
REFERENCES
- 1.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senthilkumar S, Yogeeta SK, Subashini R, Devaki T. Attenuation of cyclophosphamide induced toxicity by squalene in experimental rats. Chem Biol Interact. 2006;160:252–60. doi: 10.1016/j.cbi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Oboh G, Rocha JB. Polyphenols in red pepper (Capsicum annuum var. aviculare) and their protective effect on some pro-oxidants induced lipid peroxidation in brain and liver. Eur Food Res Technol. 2007;225:239–47. [Google Scholar]
- 4.Chauhan NS, Sharma V, Thakur M, Dixit VK. Curculigo orchioides: The black gold with numerous health benefits. Zhong Xi Yi Jie He Xue Bao. 2010;8:613–23. doi: 10.3736/jcim20100703. [DOI] [PubMed] [Google Scholar]
- 5.Pandit P, Singh A, Bafna AR, Kadam PV, Patil MJ. Evaluation of Antiasthmatic Activity of Curculigo orchioides Gaertn. Rhizomes. Indian J Pharm Sci. 2008;70:440–4. doi: 10.4103/0250-474X.44590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayanarayana K, Rodrigues RS, Chandrashekhar KS, Subrahmanyam EV. Evaluation of estrogenic activity of alcoholic extract of rhizomes of Curculigo orchioides. J Ethnopharmacol. 2007;114:241–5. doi: 10.1016/j.jep.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Jiao L, Cao DP, Qin LP, Han T, Zhang QY, Zhu Z, et al. Antiosteoporotic activity of phenolic compounds from Curculigo orchioides. Phytomedicine. 2009;16:874–81. doi: 10.1016/j.phymed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Kang TH, Hong BN, Jung SY, Lee JH, So HS, Park R, et al. Curculigo orchioides protects cisplatin-induced cell damage. Am J Chin Med. 2013;41:425–41. doi: 10.1142/S0192415X13500316. [DOI] [PubMed] [Google Scholar]
- 9.Harborne JB. New York: Chapman and Hall; 1983. Phytochemical method: A guide to modern techniques of plants analysis; pp. 11–23. [Google Scholar]
- 10.Mustafa RA, Abdul Hamid A, Mohamed S, Bakar FA. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J Food Sci. 2010;75:C28–35. doi: 10.1111/j.1750-3841.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- 11.Paris: OECD Guidelines; 2001. Organization for Economic Cooperation and development (OECD). Guideline 423 for testing chemicals; pp. 1–14. [Google Scholar]
- 12.Nitharwal RK, Patel H, Karchuli MS, Ugale RR. Chemoprotective potential of Coccinia indica against cyclophosphamide-induced toxicity. Indian J Pharmacol. 2013;45:502–7. doi: 10.4103/0253-7613.117783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gornal AC, Bardawill CJ, David MM. Determination of serum protein by means of biuret reaction. J Biol Chem. 1949;177:751–66. [PubMed] [Google Scholar]
- 14.Liu J, Simon LM, Phillips JR, Robin ED. Superoxide dismutase (SOD) activity in hypoxic mammalian systems. J Appl Physiol Respir Environ Exerc Physiol. 1977;42:107–10. doi: 10.1152/jappl.1977.42.1.107. [DOI] [PubMed] [Google Scholar]
- 15.Khynriam D, Prasad SB. Changes in endogenous tissue glutathione level in relation to murine ascites tumor growth and the anticancer activity of cisplatin. Braz J Med Biol Res. 2003;36:53–63. doi: 10.1590/s0100-879x2003000100008. [DOI] [PubMed] [Google Scholar]
- 16.Rekka EA, Kourounakis AP, Kourounakis PN. Investigation of the effect of chamazulene on lipid peroxidation and free radical processes. Res Commun Mol Pathol Pharmacol. 1996;92:361–4. [PubMed] [Google Scholar]
- 17.Oboh G, Akomolafe TL, Adetuyi AO. Inhibition of cyclophosphamide-induced oxidative stress in brain by dietary inclusion of red dye extracts from sorghum (Sorghum bicolor) stem. J Med Food. 2010;13:1075–80. doi: 10.1089/jmf.2009.0226. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao G, Fong TH, Tzu NH, Lin KH, Chou DS, Sheu JR. A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo. 2004;18:351–6. [PubMed] [Google Scholar]
- 19.Malstrom B, Andreasson L, Reinhammer B, editors. NewYork: Academic Press; 1975. The Enzymes.X11B; p. 533. [Google Scholar]
- 20.Cheng L, Kellogg EW, 3rd, Packer L. Photoinactivation of catalase. Photochem Photobiol. 1981;34:125–9. [PubMed] [Google Scholar]
- 21.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 22.Al-Majed AA, Sayed-Ahmed MM, Al-Omar FA, Al-Yahya AA, Aleisa AM, Al-Shabanah OA. Carnitine esters prevent oxidative stress damage and energy depletion following transient forebrain ischaemia in the rat hippocampus. Clin Exp Pharmacol Physiol. 2006;33:725–33. doi: 10.1111/j.1440-1681.2006.04425.x. [DOI] [PubMed] [Google Scholar]
- 23.Essa MM, Subramanian P. Protective role of Pongamia Pinnata leaf extract on tissue antioxidant status and lipid peroxidation in Ammonium chloride induced hyperammonemic rats. Toxicol Mech Methods. 2006;16:477–83. doi: 10.1080/15376510600885067. [DOI] [PubMed] [Google Scholar]
- 24.Abbasi E, Nassiri-Asl M, Shafeei M, Sheikhi M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem Biol Drug Des. 2012;80:274–8. doi: 10.1111/j.1747-0285.2012.01400.x. [DOI] [PubMed] [Google Scholar]
- 25.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008;3:115–26. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basli A, Soulet S, Chaher N, Mérillon JM, Chibane M, Monti JP, et al. Wine polyphenols: Potential agents in neuroprotection. Oxid Med Cell Longev 2012. 2012 doi: 10.1155/2012/805762. 805762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mo H, Chen Y, Huang L, Zhang H, Li J, Zhou W. Neuroprotective effect of tea polyphenols on oxyhemoglobin induced subarachnoid hemorrhage in mice. Oxid Med Cell Longev 2013. 2013 doi: 10.1155/2013/743938. 743938. [DOI] [PMC free article] [PubMed] [Google Scholar]


