Abstract
Scope:
The aim of the presented study was to investigate the mycotoxin exposure of Ivorian population related to the consumption patterns of maize, peanuts, millet, and cassava product (attiéké).
Materials and Methods:
Maize flour samples (n = 51) were purchased from all Abidjan local markets, in the south of Ivory Coast, and urine (n = 99) was collected during the same reference period (July–September 2011) from volunteers living in Abidjan and Daloa cities. Reversed-phase liquid chromatography coupled with electrospray ionization triple quadrupole mass spectrometry (LC-ESI-MS/MS) was used to analyze aflatoxins (AFB1, AFB2, AFG1, and AFG2), ochratoxin A (OTA), fumonisins (FB1, FB2), deoxynivalenol (DON), zearalenone (ZEA), and T-2 and HT-2 toxins in maize flour samples, and their relevant biomarkers (AFM1, DON, DON + de-epoxydeoxynivalenol (DOM-1), FB1, α-zearalenol (ZOL), β-ZOL, and OTA) in urine samples.
Results:
Critical maize contamination was observed by AFs occurrence (total AFs 4.5 – 330.0 μg/kg) while OTA was found in 13% of samples analyzed. AFM1 was detected in 40% of urines samples (0.06 – 14.11 ng/ml), OTA in 37% (0.01 – 0.42 ng/ml), FB1 in 27% (0.07 to 15.31 ng/ml) and, DON was found in 21% of samples at levels up to 10.0 ng/ml. The correlation coefficients (R2) obtained by plotting the percentage of biomarker occurrence (positive samples) versus the frequency of food consumption revealed maize, peanuts, millet and attiéké were strongly linked to AFB1 and OTA exposure with values of R2 ranged from 0.462 to 0.956.
Conclusion:
The present study provided data on mycotoxin risk in Ivory Coast, revealing a frequent co-exposure to the major mycotoxins such as AFs, OTA, and fumonisins, which appeared to be related to the frequency of peanuts, maize, millet and attiéké consumption.
Keywords: Human exposure, Ivory Coast, mycotoxins, multi-biomarker, maize-foods
INTRODUCTION
Mycotoxins are secondary metabolites produced by different fungal species that can contaminate agricultural commodities in field, during harvest and/or in storage, and can reach human beings through contaminated food.
In Ivory Coast (Côte d’Ivoire), studies on mycotoxin occurrence in agricultural products concern essentially cocoa and coffee, since these products are largely exported to Europe, America, or Japan; countries having strict regulations for mycotoxins in foodstuffs. In particular, available data revealed natural contamination of coffee and cocoa byaflatoxins (AFs) and ochratoxin A (OTA).[1,2,3] Few studies have been performed on food largely consumed by local population such as maize, millet, peanuts, and rice. Surveys carried out in Ivory Coast in the period 1998–2004 revealedhigh levels of mycotoxins such as AFs and OTA in maize.[4,5] Certainly, peanuts and millet had been found to be contaminated by mycotoxins. But, concentrations of OTA, zearalenone (ZEA), and fumonisin B1 (FB1) were low and may not cause problems per se. In contrast, maize exhibited the highest OTA contamination levels, which were in the range 3–1,738 μg/kg, whereas other co-occurring mycotoxins such as AFs, FB1, and ZEA were detected in ranges of 1.5–20, 20–50, and 0.3–1.5 μg/kg, respectively.[4] Worldwide, maize is the crop most frequently exposed to the risk of simultaneous contamination by all major mycotoxins. Thus, in particular for maize intended for direct human consumption, the European Commission has established maximum permitted levels for aflatoxins (AFB1, 2 μg/kg; total AFs, 4 μg/kg), OTA (3 μg/kg), ZEA (100 μg/kg), deoxynivalenol (DON, 750 μg/kg), total FBs (1,000 μg/kg, FB1 + FB2), andrecommended levels for the sum of T-2 and HT-2 (100 μg/kg).[6,7,8] Maize susceptibility to contamination by toxigenic molds of the genera Aspergillus, Penicillium, and Fusarium has been documented in west African countries such as Ivory Coast and Nigeria, where high humidity and temperature conditions are favorable to fungal growth and mycotoxin production.[9,10,11] Therefore, there is the need to investigate on mycotox in occurrence in maize and its derived foods, as well as their impact on the health of populations living in these regions. Among these, Ivory Coast has been focused in the present work.
Mycotoxin exposure through consumption of contaminated foods may be directly evaluated by the analysis of their specific biomarkers in biological fluids, for example urine or blood. The measurement of urinary AFM1 has been used to assess human exposure to AFB1 in China, South America, and Africa;[12,13,14] whereas, urinary DON and DON + de-epoxydeoxynivalenol (DOM-1) have been extensively used to assess human exposure to DON in Europe.[15,16,17,18] For OTA exposure, the OTA level in plasma has often been used.[19] Urinary FB1 has been used as a biomarker of fumonisin intake in several studies conducted in Mexico, China, and South Africa.[20,21,22] The occurrence of ZEA biomarkers (ZEA, α-zearalenol (ZOL), β-ZOL, and ZEA-glucuronide) in human urine has been recently reported;[23,24,25] whereas to the best of our knowledge, no data on the presence of urinary HT-2 (potential biomarker of T-2 and HT-2 toxins) is available for humans. Concerning Ivorian population, available data on mycotoxin biomarkers are limited to one study on blood biomarker of OTA exposure.[26] Aim of the present study was to provide data enabling a more accurate evaluation of the mycotoxin risk of exposure in Ivory Coast.
MATERIAL AND METHODS
Chemicals and reagents
Acetonitrile, methanol (all HPLC grade), and glacial acetic acid were purchased from Mallinckrodt Baker (Milan, Italy). Ultrapure water was produced by a Milli-Q system (Millipore, Bedford, MA, USA). Ammonium acetate (for mass spectrometry (MS)) from Sigma-Aldrich (Milan, Italy). Standard mycotoxin solutions from Biopure Referenzensubstanzen GmbH (Tulln, Austria). β-Glucuronidase/sulfatase type H-2 from Helix pomatia (specific activity 130,200 units/ml and β-glucuronidase 709 units/ml sulfatase) from Sigma-Aldrich (Milan, Italy). What man GF/A glass microfiber filter papers were obtained from Whatman International (Maidstone, UK). Myco6in1TM immunoaffinity columns were obtained from VICAM (Watertown, MA, USA). Oasis HLB® cartridges (60 mg, 3 ml) used for biomarker analysis were purchased from Waters (Milford, MA, USA) and regenerated cellulose filters (0.45 μm) were purchased from Sartorius Stedim Biotech, (Goettingen, Germany). Phosphate-buffered solution at pH 7.4 (PBS) was prepared by dissolving commercial phosphate-buffered saline tablets (Sigma-Aldrich) in distilled water.
Samples collection
The presence of mycotoxin biomarkers has been investigated in urine samples collected from volunteers living in Abidjan and Daloa cities. The possible correlation between the presence of urinary biomarkers and the frequency of consumption of selected foods (maize, peanuts, millet, and cassava product-attiéké) has been studied. Furthermore, the occurrence of major mycotoxins (AFs, OTA, deoxynivalenol, T-2 and HT-2 toxins, fumonisins, and ZEA) has been investigated in maize flour samples, collected from all Abidjan local markets in the same reference period.
Maize samples
Maize sampling was carried out in the markets of 10 communes of Abidjan. Maize consumed by Abidjan population comes from sellers of maize flour, that is, maize finely ground in all markets of Abidjan. This maize is directly consumed by population after boiling for 30–under an air stream at 55° C, 60 min.
Abidjan area was divided into five areas according to their proximity:
Seri 1 (S1): Yopougon, the biggest commune of Abidjan (10 samples)
Seri 2 (S2): Cocody and Abobo (nine samples)
Seri 3 (S3): Port Bouet (14 samples)
Seri 4 (S4): Adja me Attécoubé and Plateau (10 samples)
Seri 5 (S5): Treichville, Marcory, and Koumassi (eight samples).
All the samples (100–150 g) were collected and kept frozen at −20°C until liquid chromatography (LC)-MS/MS analysis.
Participants and urine samples
One hundred volunteers (50 male and 50 female), with an age range of 2–70 years, were recruited from Daloa and Abidjan cities. All participants were apparently healthy and were not subjected to any specific medical treatments. The project was approved by the Ivorian National Ethical Committee and consent was obtained from all participants. A questionnaire was administered to each participant to obtain information on age, sex, place of residence, and frequency of consumption (times per week) of various locally and readily available foods, with emphasis on potential sources of mycotoxin intake; namely maize, peanuts, millet, and attiéké. First-voided urine was collected by each volunteer in the early Monday morning in September 2011 and kept frozen at −20°C until LC-MS/MS analysis.
Sample preparation (extraction and cleanup)
For mycotoxinin maize
Mycotoxin analysis in maize samples was performed according to Lattanzio et al.[27] Ground maize samples (5 g) were first extracted with 25 ml PBS, by shaking for 60 min on an orbital shaker. After centrifugation (3,000 g, 10 min), 17.5 ml of PBS extract (extract A) were collected and filtered through a glass microfiber filter. Then 17.5 ml of methanol was added to the remaining and the sample was extracted again by shaking for 60 min. After centrifugation (3,000 g, 10 min), 10 ml of methanol/PBS extract was diluted with 90 ml PBS to reduce the organic fraction and filtered through a glass microfiber filter (extract B). For extract clean up, 50 ml of extract B were passed through the Myco6in1TM immunoaffinity column from VICAM (Watertown, MA, USA), then the column was washed with 20 ml PBS. After passing 5 ml of extract A, the column was washed with 10 ml distilled water. Toxins were eluted from the column as follows. 1.5 mL of methanol was applied on the column, and passed through by gravity. The eluate was collected in a 10 mL glass tube vial. A second portion of 1.5 mL of methanol was applied and eluted by gravity after waiting for 5 min. Then 2 mL water were passed through and collected in the same tube. The eluate was dried under an air stream at 50°C and reconstituted with 400 μl methanol/water 40:60, containing 1 mM ammonium acetate and 0.1% acetic acid. Volumes of 20 μl were analyzed by LC-MS/MS.
For mycotoxin in urine
Mycotoxin analysis in urine samples was performed according to Solfrizzo et al.[28] Enzymatic deconjugation of mycotoxins in urine samples was performed by adding 300 μl β-glucuronidase/sulfatase solution to 6 ml urine which was then incubated under static conditions at 37°C overnight. After incubation, urine samples were purified by applying the following procedure. An Oasis HLB® column from Waters (Milford, MA, USA) was conditioned by passing 2 ml methanol and 2 ml water and then attached under a Myco6in1TM immunoaffinitycolumn. The two stacked columns were positioned on a vacuum manifold. Urine samples were centrifuged at 3,000 g for 5 min at 4°C to remove particulate matter. The urine (6 ml) was subsequently diluted with 6 ml ultrapure water, mixed, and then slowly passed through the two stacked purifying columns. After sample elution, the two columns were treated separately as follows. The immunoaffinity column was washed with 4 ml water, then gently vacuum-dried for 15 s. The toxins were eluted with 3 ml methanol and 2 ml water in a vial as described above for mycotoxins in maize. The Oasis HLB® column was washed with 1 ml methanol–water (20:80), then gently vacuum-dried for 15 s. The toxins were eluted from the column by gravity with 1 ml methanol–water (40:60) and collected in the vial containing the eluates from the immunoaffinity column. The column was gently vacuum-dried to completely elute the remaining liquid. The combined final eluates were dried under an air stream at 55°C, reconstituted in 200 μl methanol–water (20:80) containing 0.5% acetic acid, and filtered through a regenerated cellulose filter. Volumes of 20 μL (equivalent to 0.6 ml urine sample) were analyzed by LC-MS/MS.
Matrix-matched calibration
For mycotoxin in maize
A mixed mycotoxin standard solution was prepared by drying down appropriate amounts of stock solutions and dissolving them with methanol to obtain the following final concentrations: DON, FB1, and FB2 10 μg/ml; T-2, HT-2, and ZEA 2.5 μg/ml; and OTA, AFG2, AFB2, AFG1, and AFB1 0.25 μg/ml.
Calibrant solutions for matrix-matched calibration curves were prepared in blank maize extract solutions cleaned up through the Myco6in1TM column. Appropriate amounts of the mixed standard solution were added to the eluates which were then dried and reconstituted with the LC mobile phase. Standard- and matrix-matched calibration curves (five points) were acquired in the following ranges: DON, FB1, and FB2 100–2,000 μg/kg; T-2, HT-2, and ZEA 25–500 μg/kg; OTA, AFG2, AFB2, AFG1, and AFB1 2.5–50 μg/kg.
For mycotoxin biomarkers in urine
A mixed standard solution with a final concentration of 300 ng/ml DON, α-ZOL, and β-ZOL; 150 ng/ml DOM-1; 30 ng/ml AFM1; 60 ng/ml FB1; and 3 ng/ml OTA was prepared in acetonitrile by diluting and mixing appropriate amounts of commercially available standard solutions of each toxin. For matrix-matched calibration curves, five aliquots of the mixed standard solution were added to the combined eluates from the Myco6in1TM and Oasis HLB® columns of blank urine which were then dried and reconstituted with 200 μl initial LC mobile phase. The toxin concentration ranges were, therefore: 30–450 ng/ml for DON, α-ZOL, and β-ZOL; 15–225 ng/ml for DOM-1; 3–45 ng/ml for AFM1; 6–90 ng/ml for FB1; and 0.3–4.5 ng/ml for OTA. Considering that 20 μl calibrant solution or purified sample extract (equivalent to 0.6 ml urine) were injected for LC-MS/MS analysis, the equivalent toxin concentrations in urine ranged between: 1 and 15 ng/ml for DON, α-ZOL, and β-ZOL; 0.5 and 7.5 ng/ml for DOM-1; 0.1 and 1.5 ng/ml for AFM1; 0.2 and 3 ng/ml for FB1; and 0.01 and 0.15 ng/ml for OTA.
LC-MS/MS equipment and parameters
LC-MS/MS analyses were performed on a QTrap MS/MS system (AB Sciex, Framingham, Massachusetts, USA), equipped with an electrospray ionization (ESI) interface and a 1100 series micro-LC system comprising a binary pump and a microauto sampler from Agilent Technologies (Waldbronn, Germany).
LC conditions
The analytical column was a Gemini C18 column (150 mm × 2 mm, 5 μm particles; Phenomenex, Torrance, CA, USA), preceded by a Gemini C18 guard column (4 mm × 2 mm, 5 μm particles). The column oven was set at 40°C. The flow rate of the mobile phase was 200 μl/min and the injection volume was 20 μl. The column effluent was directly transferred into the ESI interface, without splitting.
For mycotoxins in maize
Eluent A was water and eluent B was methanol, both containing 0.5% acetic acid and 1 mM ammonium acetate. A gradient elution was performed by changing the mobile phase composition as follows. After 3 min at 20% eluent B, the proportion was set at 40%, then linearly increased to 63% in 35 min, and kept constant for 11 min. The column was re-equilibrated with 20% eluent B for 10 min.
For biomarkers in urines
Eluent A was water and eluent B was methanol, both containing 0.5% acetic acid. A gradient elution was performed as follows: From 20 to 40% methanol in 3 min, then to 48% methanol in 12 min, to 80% methanol in 15 min, and then maintained at 80% methanol for 8 min (total run 38 min). The column was then brought to 20% methanol in 1 min and left to equilibrate for 7 min before the next run.
MS conditions
The ESI interface was used by switching from positive to negative mode depending on the mycotoxin, with the following settings: Temperature (TEM) 350°C; curtain gas (CUR) pressure, nitrogen, 30 psi; nebulizer gas pressure (GS1), air, 10 psi; heater gas pressure (GS2), air, 30 psi; ionspray voltage +4,500 V or −4,500 V. The mass spectrometer operated in selected reaction monitoring (SRM) mode, by monitoring three transitions (one quantifier and two qualifiers) for each mycotoxin, with a dwell time of 100 ms. Quantification of the mycotoxins was performed by measuring peak areas in the SRM chromatogram, and comparing these with the relevant matrix-matched calibration curve. All relevant details can be found in Lattanzio et al.,[30] and Solfrizzo et al.[31]
Statistical analysis
Median, mean, standard deviation (SD), and detectable range were calculated for concentrations of all biomarkers measured. For comparisons, t-tests or Wilcoxon tests were used as appropriate to examine differences between biomarker data. Regression analysis was used to compare foods (maize, peanuts, millet, and attiéké) frequency of consumption with urinary mycotoxin biomarkers. A P value ≤0.05 (two-tailed) was considered significant.
RESULTS
The present study was focused on high maize consumers; therefore, the occurrence of mycotoxin contamination in maize samples collected from representative local markets was assessed in a preliminary phase. However, among inclusion criteria the frequency of consumption of peanuts, attiéké, and millet was also included since these foods could represent an additional source of mycotoxin intake.
Occurrence of mycotoxins in maize flour from Abidjan communes markets
Values of mycotoxin concentrations in maize flour (51 samples) are summarized in Tables 1 and 2.
Table 1.
Summary of mycotoxin levels (μg/kg) in maize flour samples (n=51) collected in markets of 10 communes of Abidjan
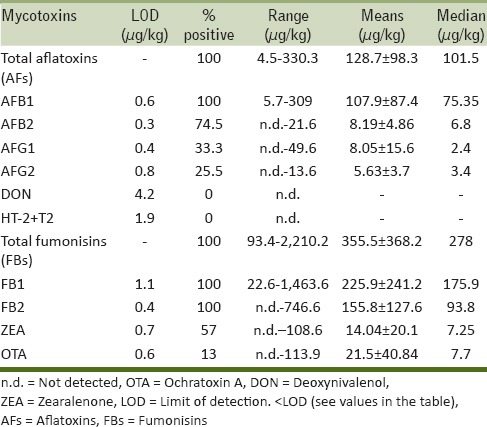
Table 2.
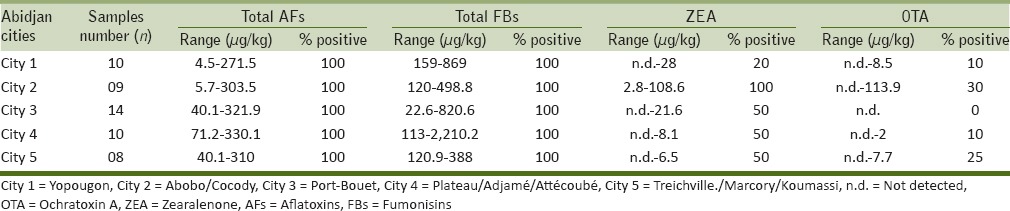
Mycotoxin occurrence and contamination ranges in maize flour samples from each selected Abidjan city
All analyzed maize samples were contaminated by AFs. AFB1 was the most frequently occurring AF (100% of positive samples) with levels up to 324 μg/kg, followed by AFB2 (94% of positive samples) with levels up to 21.6 μg/kg. AFG1 and AFG2 showed lower incidence but levels of AFG1 were up to 49 μg/kg. Total AFs values ranged from 4.5 to 330 μg/kg with mean of 128.7 ± 98.3 μg/kg and median of 101.5 μg/kg.
All maize samples were also contaminated by fumonisins. FB1 concentrations ranged from 32.3 to 1463.6 μg/kg, and FB2 concentrations from 18.5 to 746.6 μg/kg. Total fumonisins levels ranged from 45.3 to 2210.2 μg/kg with mean of 355.5 ± 368.2 μg/kg and median of 278 μg/kg. It is worth noting that only two samples showed total fumonisins levels above 1000 μg/kg (i.e., the EC maximum permitted level in maize for human consumption).
Trichothecenes (DON, T-2, and HT-2 toxins) were not detected in any of the analyzed maize samples, whereas a widespread contamination of ZEA (57% positive samples) was found with levels ranging from 2.3 to 108.6 μg/kg. Only one maize sample was contaminated at level above 100 μg/kg (the EC maximum permitted level in maize for human consumption). The other samples showed low ZEA levels ranging from 2.3 to 28 μg/kg. Mean and median values were 14.0 ± 20.1 and 7.2 μg/kg, respectively. The highest frequency (100% positive samples) and contamination levels of ZEA were detected at Abobo/Cocodycities.
Only 12% of maize samples resulted to be contaminated by OTA with concentrations ranging from 2.0 to 113.9 μg/kg. OTA was not detected at all in maize samples from Port Bouet city.
Mycotoxin biomarkers in urine from high cereal consumers living in Ivory Coast
An overview of results of biomarker analysis is presented in Table 3. Analysis of urinary biomarkersin samples from Abidjan and Daloa revealed that aflatoxin biomarker (AFM1) was present in 40 out of the 99 urine samples (40%), fumonisins biomarker (FB1) in 27 out of the 99 samples (27%), and ochratoxin A biomarker (OTA), in 35 out of the 99 urine samples (35%). The biomarker of deoxynivalenol, DON, was detected in 21 out of the 99 urine samples (21%), whereas DOM-1 was not detected. The rank order in the frequency of detection was AFM1 ( 40%) > OTA (35%) > FB1 ( 27.3%) > DON (21.2%). Figure 1 shows the chromatogram (total ion chromatogram, sum of Multiple reaction monitoring MRM transitions) of a urine sample naturally contaminated by DON, 8.0 ng/ml; AFM1, 14.1 ng/ml; FB1, 12.1 ng/ml; and OTA, 0.1 ng/ml.
Table 3.
Overall occurrence and contamination range of urinary mycotoxin biomarkers in the urine sample set (n=99) collected from volunteers from Daloa and Abidjan

Figure 1.
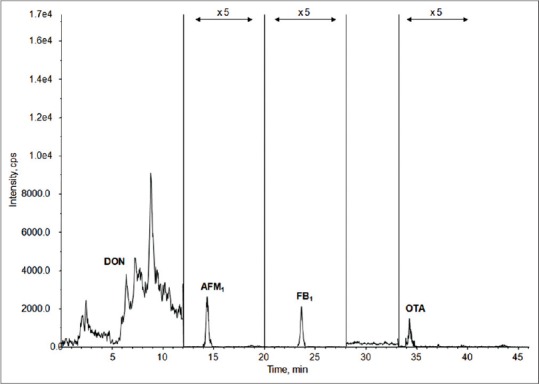
Total ion chromatogram (TIC of MRM) of a urine sample naturally contaminated by DON, 8.0 ng/ml; AFM1, 14.1 ng/ml; FB1, 12.1 ng/ml; and OTA, 0.1 ng/ml. OTA = Ochratoxin A, DON = deoxynivalenol, AF = aflatoxin, FB = fumonisin
Table 4 compares the occurrence of detected biomarkers (percentage of positive samples) and relevant median values in Daloa and Abidjan. AFM1 was detected with similar frequency in Daloa and Abidjan, but with higher urinary levels (median 1.13 ng/ml) in Abidjan. Both FB1 and OTA were detected with higher frequency in Daloa region; urinary levels of FB1 were higher in Daloa (median 0.79 ng/ml); whereas, OTA levels were similar in the two regions (median 0.09 and 0.11 ng/ml in Daloa and Abidjan, respectively).
Table 4.
Occurrence of mycotoxin biomarkers (percentage of positive samples) and median values according to sample origin (Daloa or Abidjan), age of voluntaires, and their sex

Finally, DON was detected with higher frequency and levels in Abidjan with 25.5% of positive samples (median 2.35 ng/ml) versus 16.3% positive samples (median 0.14 ng/ml) in Daloa. The highest urinary levels of DON and FB1, as well as the highest frequency of detection of AFM1 were found in urine samples collected from young volunteers (age ≤ 16 years) that also showed 90.9% of occurrence of at least one mycotoxin biomarker [Table 4].
Correlation between food consumption and occurrence of mycotoxin biomarkers
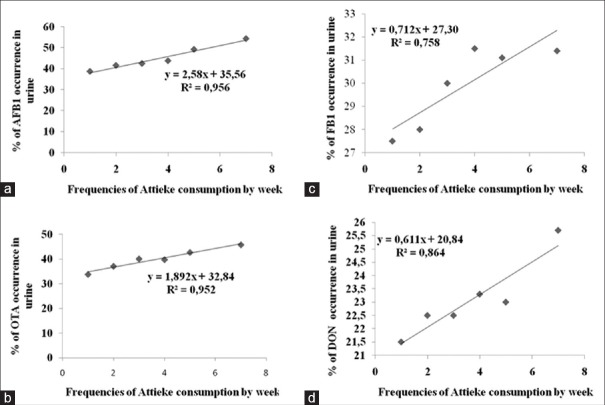
Tables 5 and 6 give an overview of the percentage of mycotoxin biomarker occurrence and relevant median values in relation to the frequency of consumption of selected foods (maize, peanuts, millet, and attiéké). The correlation coefficients (R2) obtained by plotting the percentage of biomarker occurrence (positive samples) versus the frequency of food consumption gave some information on the possible correlation between the consumption of the selected foods and mycotoxin exposure. These results are summarized in Table 7; whereas, Figure 2 shows an example of good correlation between biomarker occurrence and attiéké consumption. All volunteers consumed attiéké at least once a week. A good correlation between biomarker occurrence and attiéké consumption was observed for AFs, fumonisins, OTA, and DON biomarkers; indicating this food as a potential source of exposure to all major mycotoxins. Concerning the other food commodities considered in this study, some general consideration could be drawn. A good correlation between OTA biomarker and food consumption was observed for all other selected food commodities (maize, peanuts, and millet), indicating all these foods as potential source of OTA intake in Ivory Coast. The good correlation between FB1 and maize was definitely in agreement with the widespread fumonisins contamination observed in maize, as well as the absence of correlation between FB1 and peanuts or millet consumption was also expected, since these food commodities are not likely to be contaminated by fumonisins. Good correlation was observed between DON biomarker and frequency of millet consumption. Interestingly, DON biomarker occurrence did not correlate with maize consumption. This was in agreement with the absence of DON contamination found in maize samples [Table 1], and supported the assumption that the source of DON intake could be another food. Even though data on maize contamination presented herein are limited to Abidjan region only, based on correlation data it could be assumed that maize from Daloa markets could also be free of DON contamination.
Table 5.
Correlation between frequencies of maize or peanuts consumption and occurrence of mycotoxins urinary biomarkers (% of positive samples)
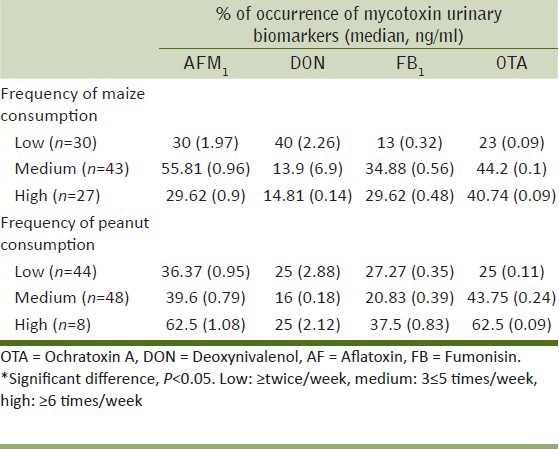
Table 6.
Correlation between frequency of millet or attiéké consumption and occurrence of mycotoxins urinary biomarkers
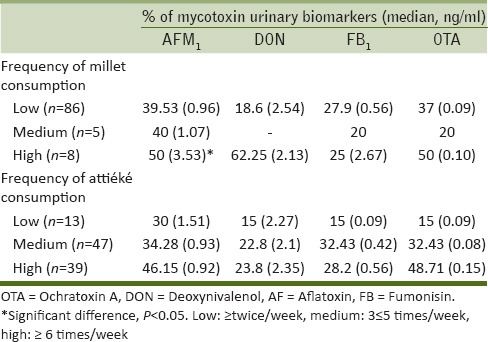
Table 7.
Summary of coefficients of correlation (R2) obtained by plotting the frequency of consumption of four selected foods versus the percentage of positive urine samples
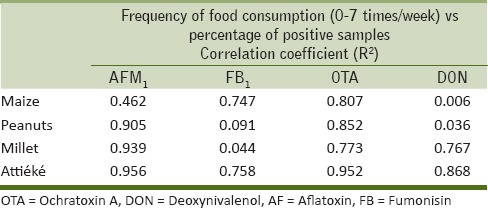
Figure 2.
Correlation of cassava product (attiéké) consumption and occurrence (percentage of positive samples) of urinary mycotoxin biomarkers for (a) AFM1, (b) OTA, (c) FB1, and (d) DON
DISCUSSION
Aim of the present study was to provide information on mycotoxin risk by Ivorian population through the assessment of levels of urinary biomarkers in high maize consumers. Maize is a widely consumed food in Ivory Coast in particular at Abidjan, the biggest town having a population of over 5 million. Therefore, a preliminary investigation on the co-occurrence of major mycotoxins was carried out in a set of maize samples collected from all markets of Abidjan, to have a representative set of the real mycotoxin contamination.
Maize samples intended for human consumption were collected from August to October 2011. Results from the study revealed the predominance of AFs with extremely high levels (4.8–334 μg/kg) when compared to those reported in previous study,[4] limited to 10 maize samples from Abidjan, and showing AFB1 levels in the range 1.5–20 μg/kg. Further recent studies focused on West African regions reported relatively low levels of AF in foods. Low AF levels have been reported in Nigerian foods, and in particular in maize the levels were in the range 0.11–0.20 μg/kg.[29] Slightly higher values of AF contamination in maize (up to 15 μg/kg) have been reported in Cameroon.[30,31] In agreement with our findings, AF levels up to 636 μg/kg were detected in maize samples from Burkina Faso.[32]
Fumonisins were detected in all investigated maize samples from Abidjan with levels (45.3–2,210.2 μg/kg total fumonisins) very similar to those recently reported in other west African regions having similar environmental and ecological conditions. Total fumonisin levels of about 2,000 μg/kg have been reported in maize samples from Burkina Faso and Cameroon.[30,32] Even though generally lower than the maximum permitted level set in EC (1,000 μg/kg total fumonisins), this widespread contamination (100% positive samples) may represent a relevant risk of chronic exposure by Ivorian population, as already observed for nearby regions.[33] A recent survey carried out in south western Nigeria revealed that 73% of maize samples analyzed were contaminated by FB1 with concentrations ranging from 10 to 779 μg/kg, and that Fusarium verticillioides was frequently isolated from maize seeds.[10]
ZEA appeared as the third major mycotoxin in maize from Ivory Coast, but with levels ranging from 2.3 to 28 μg/kg, lower than those previously reported in maize samples from Cameroon (up to 309 μg/kg) or Nigeria (up to 799 μg/kg).[10,31,31] On the other hand, low ZEA levels were reported also in maize from Burkina Faso (up to 16 μg/kg).[32] The low levels of ZEA in maize flour from Ivory Coast in comparison with maize seeds from Nigeria could be explained by the process of flour preparation which consists to wash maize seeds by water before grinding. This step could reduce ZEA contamination like other mycotoxins.[33] Despite the presence of ZEA in maize, neither type-A nor -B trichothecenes were detected in the samples analyzed in this study. To date further data on trichothecene occurrence in Ivory Coast is not available for comparison purposes.
Concerning OTA, incidence and contamination levels, up to 40 μg/kg, were lower than those found for AFs, but in some cases quite higher than the EC maximum permitted level (3 μg/kg). The low incidence of OTA contamination in maize in West Africa has also been documented by other studies.[4,30,31,32] As a general conclusion, the present study revealed a natural and wide spread co-occurrence of major mycotoxins in maize flour intended to human consumption in Ivory Coast.
On the basis of maize contamination, the presence of multiple mycotoxin biomarkers in human urines collected within this study was expected. The incidence of urinary AFM1 was 40% with levels up to 14.1 ng/ml in positive samples that could be considered quite high if compared with those observed in similar studies performed in Cameroon,[24] Ghana,[34] Egypt,[35] or Guinea.[35] Therefore, based on AFM1 levels, Ivory Coast could be recognized as a high risk region for AFs exposure like other previously investigated sub-Saharan countries.[24] Studies conducted in Benin in 2002 and 2003 have shown a high prevalence of AF exposure in the population.[36,37] This observation of high exposure to AF was attributed to high consumption of contaminated staple foods such as maize, cassava, and groundnut. Results of recent studies, focused on other West African regions, showed that fresh cassava is frequently contaminated by Aspergillus flavus, but it is unlikely to be contaminated by AFs.[38,39] Anti-AF properties of cassava have been evidenced in these studies. However, it has been also shown that processing may alter its ability to block toxigenesis leading to secondary contamination. This conclusion is in agreement with positive correlation found in this work between attiéké (cassava processed product) consumption and presence of AF urinary biomarker.
Levels of FB1 ranged from 0.05 to 15.3 ng/ml. These values were quite similar to those reported in studies carried out in Cameroon (FB1 urinary levels up to 14.8 ng/ml),[24] and higher than those reported in South Africa[20] where urinary FB1 levels ranged from 0.144 to 0.350 ng/ml. Therefore, with respect to fumonisins too, Ivory Coast like other West African countries appeared to be at higher risk of exposure if compared to other investigated regions.
DON biomarker was detected with lower frequency (about 20% samples) at concentrations ranging from 0.8 to 10.0 ng/ml. Given the absence of DON contamination in the analyzed maize samples collected in Abidjan, the presence of urinary DON was supposed to be related to others foods which need to be better identified to draft possible intervention strategies. The good correlation observed between the presence of DON biomarker and the frequency of millet and attiéké consumption suggested that these foods could be a source of DON intake.
The incidence of OTA biomarker in urine samples appeared to be related to the assumption of all four selected food commodities [Table 7]. Besides data on maize contamination reported in this study [Table 1], the natural occurrence of OTA in millet, maize, and peanuts in Ivory Coast has also been reported in previous studies.[4,5] A similar incidence (35% positive samples) has been reported for OTA in blood samples from apparently healthy Ivorian people.[26] Although a direct comparison between urinary and blood biomarker cannot be made, these data support the results obtained in this work relevant to the risk of OTA exposure in Ivory Coast. Regarding the correlation between mycotoxin exposure and age, our study showed the highest frequency of OTA and AFs biomarkers in volunteers having age <16 years. Such high risk of exposure in young population may be explained by high frequency of cereals and attiéké (cassava product) consumption in contrast to older voluntaries. Similarly, women seemed to be more exposed to mycotoxins. Indeed, in Ivory Coast, young population and women have a similar diet based on cereals and attiéké which have been found to increase mycotoxin exposure.
CONCLUSIONS
Data on human exposure to multiple mycotoxins in West Africa are limited, but the few available studies indicated that these populations are chronically exposed to major mycotoxins, and particularly to AFs, fumonisins, and OTA. The present study provided new data on mycotoxin risk in Ivory Coast, revealing for the first time a frequent coexposure to different mycotoxins in this country. A validated multi-biomarker approach was applied[28,40] as a valuable tool for direct assessment of mycotoxin exposure. A possible correlation between mycotoxin exposure and food consumption patterns has been evaluated showing highly consumed foods, such as maize, millet, peanuts, and attiékéé as potential source of mycotoxin intake.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Manda P, Dano SD, Kouadio JH, Diakité A, Sangaré-Tigori B, Ezoulin MJ, et al. Impact of industrial treatments on ochratoxin A content in artificially contaminated cocoa beans. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2009;26:1081–8. doi: 10.1080/02652030902894397. [DOI] [PubMed] [Google Scholar]
- 2.Dano DS, Manda P, Kouadio JH, Dembélé AA, Diakité A. Incidence of roasting on ochratoxin A residue in coffee. Eur J Sci Res. 2009;26:393–401. [Google Scholar]
- 3.Dembele A, Coulibaly A, Traoré SK, Mamadou K, Silue N, Abba Touré A. Assessment of cocoa beans from Ivory Coast contamination level by ochratoxin A intended to exportation. Tropicultura. 2009;27:26–30. [Google Scholar]
- 4.Sangare-Tigori B, Moukha S, Kouadio HJ, Betbeder AM, Dano DS, Creppy EE. Co-occurrence of aflatoxin B1, fumonisin B1, ochratoxin A and zearalenone in cereals and peanuts from Cote d’Ivoire. Food Addit Contam. 2006;23:1000–7. doi: 10.1080/02652030500415686. [DOI] [PubMed] [Google Scholar]
- 5.Sangare-Tigori B, Dem AA, Kouadio HJ, Betbeder AM, Dano DS, Moukha S, et al. Preliminary survey of ochratoxin A in millet, maize, rice and peanuts in Cote d’Ivoire from 1998 to 2002. Hum Exp Toxicol. 2006;25:211–6. doi: 10.1191/0960327106ht605oa. [DOI] [PubMed] [Google Scholar]
- 6.Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official J Eur Union. 2006 L364/5. [Google Scholar]
- 7.Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Official J Eur Union. 2007 255/14. [Google Scholar]
- 8.Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Official J Eur Union. 2013 L91/12. [Google Scholar]
- 9.Jimoh KO, Kolapo AL. Mycoflora and aflatoxin production in market samples of some selected Nigerian foodstuffs. Res J Microbiol. 2008;3:169–74. [Google Scholar]
- 10.Oluwafemi F, Nnanna II. Microbial contamination of seven major weaning foods in Nigeria. J Health Popul Nutr. 2011;29:415–9. doi: 10.3329/jhpn.v29i4.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adejumo TO, Hettwer U, Karlovsky P. Survey of maize from south-western Nigeria for zearalenone, alpha- and beta-zearalenols, fumonisin B1 and enniatins produced by Fusarium species. Food Addit Contam. 2007;24:993–1000. doi: 10.1080/02652030701317285. [DOI] [PubMed] [Google Scholar]
- 12.Zhu JQ, Zhang LS, Hu X, Xiao Y, Chen JS, Xu YC, et al. Correlation of dietary aflatoxins B1 levels with excretion of aflatoxins M1 in human urine. Cancer Res. 1987;47:1848–52. [PubMed] [Google Scholar]
- 13.Romero AC, Ferreira TR, Dias CT, Calori-Domingues MA, Gloria EM. Occurrence of AFM1 in urine samples of a Brazilian population and association with food consumption. Food Control. 2009;21:554–8. [Google Scholar]
- 14.Jolly P, Jiang Y, Ellis W, Awuah R, Nnedu O, Phillips T, et al. Determinants of aflatoxin levels in Ghanaians: Sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. Int J Hyg Environ Health. 2006;209:345–58. doi: 10.1016/j.ijheh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Meky FA, Turner PC, Ashcroft AE, Miller JD, Qiao YL, Roth MJ, et al. Development of a urinary biomarker of human exposure to deoxynivalenol. Food Chem Toxicol. 2003;41:265–73. doi: 10.1016/s0278-6915(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 16.Turner PC, Hopton RP, White KL, Fisher J, Cade JE, Wild CP. Assessment of deoxynivalenol metabolite profiles in UK adults. Food Chem Toxicol. 2011;49:132–5. doi: 10.1016/j.fct.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner PC, Hopton RP, Lecluse Y, White KL, Fisher J, Lebailly P. Determinants of urinary deoxynivalenol and de-epoxy deoxynivalenol in male farmers from Normandy, France. J Agric Food Chem. 2010;58:5206–12. doi: 10.1021/jf100892v. [DOI] [PubMed] [Google Scholar]
- 18.Turner PC, White KL, Burley VJ, Hopton RP, Rajendram A, Fisher J, et al. A comparison of deoxynivalenol intake and urinary deoxynivalenol in UK adults. Biomarkers. 2010;15:553–62. doi: 10.3109/1354750X.2010.495787. [DOI] [PubMed] [Google Scholar]
- 19.Scott PM. Biomarkers of human exposure to ochratoxin A. Food Addit Contam. 2005;22:99–107. doi: 10.1080/02652030500410315. [DOI] [PubMed] [Google Scholar]
- 20.van Der Westhuizen L, Shephard GS, Burger HM, Rheeder JP, Gelderblom WC, et al. Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiol Biomarkers Prev. 2011;20:483–9. doi: 10.1158/1055-9965.EPI-10-1002. [DOI] [PubMed] [Google Scholar]
- 21.Gong YY, Torres-Sanchez L, Lopez-Carrillo L, Peng JH, Sutcliffe AE, White KL, et al. Association between tortilla consumption and human urinary fumonisin B1 levels in a mexican population. Cancer Epidemiol Biomarkers Prev. 2008;17:688–94. doi: 10.1158/1055-9965.EPI-07-2534. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Cai Q, Tang L, Wang S, Hu X, Su J, et al. Evaluation of fumonisin biomarkers in a cross-sectional study with two high-risk populations in China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27:1161–9. doi: 10.1080/19440049.2010.481638. [DOI] [PubMed] [Google Scholar]
- 23.Bandera EV, Chandran U, Buckley B, Lin Y, Isukapalli S, Marshall I, et al. Urinary mycoestrogens, body size and breast development in New Jersey girls. Sci Total Environ. 2011;409:5221–7. doi: 10.1016/j.scitotenv.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abia AW, Warth B, Sulyok M, Krska R, Tchana A, Njobeh PB, et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem Toxicol. 2013;62:927–34. doi: 10.1016/j.fct.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Solfrizzo M, Gambacorta L, Visconti A. Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxins. 2014;6:523–38. doi: 10.3390/toxins6020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangare-Tigori B, Moukha S, Kouadio JH, Dano DS, Betbeder AM, Achour A, et al. Ochratoxin A in human blood in Abidjan, Côte d’Ivoire. Toxicon. 2006;47:894–900. doi: 10.1016/j.toxicon.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Lattanzio VM, Solfrizzo M, Powers S, Visconti A. Simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in maize by liquid chromatography/tandem mass spectrometry after multitoxin immunoaffinity cleanup. Rapid Commun Mass Spectrom. 2007;21:3253–61. doi: 10.1002/rcm.3210. [DOI] [PubMed] [Google Scholar]
- 28.Solfrizzo M, Gambacorta L, Lattanzio VM, Powers S, Visconti A. Simultaneous LC-MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem. 2011;401:2831–41. doi: 10.1007/s00216-011-5354-z. [DOI] [PubMed] [Google Scholar]
- 29.Adejumo O, Atanda O, Raiola A, Somorin Y, Bandyopadhyay R, Ritieni A. Correlation between aflatoxin M1 content of breast milk, dietary exposure to aflatoxin B1 and socioeconomic status of lactating mothers in Ogun State, Nigeria. Food Chem Toxicol. 2013;56:171–7. doi: 10.1016/j.fct.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Abia WA, Wart B, Sulyok M, Ksrka R, Tchana AN, Njobeh PB, et al. Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC-MS/MS) Food Control. 2013;31:438–53. [Google Scholar]
- 31.Njobeh PB, Dutton MF, Kock SH, Chuturgoon AA, Stoev SD, Mosonik JS. Simultaneous occurrence of mycotoxins in human food commodities from Cameroon. Mycotoxin Res. 2010;26:47–57. doi: 10.1007/s12550-009-0039-6. [DOI] [PubMed] [Google Scholar]
- 32.Warth B, Parich A, Atehnkeng J, Bandyopadhyay R, Schumacher R, Sulyok M, et al. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J Agric Food Chem. 2012;60:9352–63. doi: 10.1021/jf302003n. [DOI] [PubMed] [Google Scholar]
- 33.Fandohan P, Zoumenou D, Hounhouigan DJ, Marasas WF, Wingfield MJ, Hell K. Fate of aflatoxins and fumonisins during the processing of maize into food products in Benin. Int J Food Microbiol. 2005;98:249–59. doi: 10.1016/j.ijfoodmicro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Obuseh FA, Jolly PE, Jiang Y, Shuaib FM, Waterbor J, Ellis WO, et al. Aflatoxin B1 albumin adducts in plasma and aflatoxin M1 in urine are associated with plasma concentrations of vitamins A and E. Int J Vitam Nutr Res. 2010;80:355–68. doi: 10.1024/0300-9831/a000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polychronaki N, Christopher P, Wild C, Hannu M, Hassan A, Mosaad A, et al. Urinary biomarkers of aflatoxin exposure in young children from Egypt and Guinea. Food Chem Toxicol. 2008;46:519–26. doi: 10.1016/j.fct.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, et al. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ. 2002;325:20–1. doi: 10.1136/bmj.325.7354.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong YY, Egal S, Hounsa A, Turner PC, Hall AJ, Cardwell KF, et al. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa; the critical role of weaning. Int J Epidemiol. 2003;32:556–62. doi: 10.1093/ije/dyg109. [DOI] [PubMed] [Google Scholar]
- 38.Gnonlonfin GJ, Adjovi CS, Katerere DR, Shephard GS, Sanni A, Brimer L. Mycoflora and absence of aflatoxin contamination of commercialized cassava chips in Benin, West Africa. Food Control. 2012;23:333–7. [Google Scholar]
- 39.Adjovi YC, Bailly S, Gnonlonfin BJ, Tadrist S, Querin A, Sanni A, et al. Analysis of the contrast between natural occurrence of toxigenic Aspergilli of the Flavi section and aflatoxin B1 in cassava. Food Microbiol. 2014;38:151–9. doi: 10.1016/j.fm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Shephard GS, Burger HM, Gambacorta L, Gong YY, Krska R, Rheeder JP, et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem Toxicol. 2013;62:217–25. doi: 10.1016/j.fct.2013.08.040. [DOI] [PubMed] [Google Scholar]