Abstract
Background:
Analyzing the structures and functions of different proteins of Wuchereria bancrofti is very important because till date no effective drug or vaccine has been discovered to treat lymphatic filariasis (LF). ATPase is one of the most important proteins of Wuchereria bancrofti. Adenosine triphosphate (ATP) converts into adenosine diphosphate (ADP) and a free phosphate ion by the action of these ATPase enzymes. Energy releases from these dephosphorylation reactions drive the other chemical reactions in the cell.
Materials and Methods:
In this study we worked on the protein ATPase of Wuchereria bancrofti which has been annotated from National Center for Biotechnology Information (NCBI). Various computational tools and databases have been used to determine the various characteristics of that enzyme such as physiochemical properties, secondary structure, three-dimensional (3D) structure, conserved domain, epitope, and their molecular evolutionary relationship.
Result:
Subcellular localization of ATPase was identified and we have found that 55.5% are localized in the cytoplasm. Secondary and 3D structure of this protein was also predicted. Both structure and function analysis of ATPase of Wuchereria bancrofti showed unique nonhomologous epitope sites and nonhomologous antigenicity sites. Moreover, it resulted in 15 ligand drug-binding sites in its tertiary structure.
Conclusion:
Structure prediction of these proteins and detection of binding sites and antigenicity sites from this study would indicate a potential target aiding docking studies for therapeutic designing against filariasis.
Keywords: ATPase, docking studies, epitope, Filariasis, Wuchereria bancrofti
INTRODUCTION
Wuchereria bancrofti is one of the most common human parasitic filarial nematode that is found mainly in tropical regions.[1] Wuchereria bancrofti is endemic in more than 78 nations and affects 128 million persons worldwide.[2,3] It is mainly found in Central Africa, South and Central America, Nile delta, and the tropical regions of Asia including Southern China and the Pacific. There are three parasites which cause filariasis and it is one of them.[4] It spreads by a mosquito vector and generally there are six genera and 70 species of mosquitoes responsible for spreading. Humans are the only recognized ultimate host of Wuchereria bancrofti. Although monkeys have been infected artificially, they are not hosted in the wild. It is responsible for about 90% of lymphatic filariasis (LF).[5] Filaria also causes chronic acute bacterial dermatolymphangioadenitis (ADLA) attacks which can cause renal disease, chyluria, nephritic syndrome, hematuria, proteinuria, and glomerulonephritis. Patients with LF can also have cystitis with urethral block, rheumatic problems, tropical vaginal hydroceles, fibrosing mediastinitis, and bladder pseudotumors. Typically ADLA occurs when a mature worm dies and the lymph vessels surrounding it are vexed due to the host's immune response.[6] Several therapeutic drugs are available, some of them are diethylcarbamazine (DEC), ivermectin, or albendazole. No effective drug or vaccine has been discovered to treat LF till date.[7] A mixture of albendazole and ivermectin or DEC and albendazoles can be used for eliminating microfilaria, but they only interrupt the mature females reproductive capability.[8,9] An indication of the efficiency of this combination treatment is still inadequate[10,11,12,13] and quantitative efficiency estimates are not yet available. Hence, the new antifilarial drug combinations are estimated to increase compliance, coverage, and decrease costs by familiarizing simple effective drug delivery and distribution methods. For this purpose to design new drugs or vaccines against an organism, its proteins can be used by detecting its domains and predicting ligand binding sites.[14] Among all these proteins, ATPase is one of the most significant proteins. It is a class of enzymes that are crucial for the formation of adenosine diphosphate (ADP) and a free phosphateion from decomposition of adenosine triphosphate (ATP). These dephosphorylation reaction releases energy, which the enzyme hitches to motivate other chemical reactions in the cell. It is also known as transmembrane ATPase, which has different types such as F-ATPases, A-ATPases, V-ATPases, P-ATPases, and E-ATPases. Among them V-ATPases function in the acidification of intracellular sections in eukaryotic cells.[15,16] Moreover, ATPases can be used as drugs or vaccine targets. ATPases are involved in various cellular functions and numerous human diseases. Therefore, gorgeous therapeutic drug targets and plentiful ATPase inhibitors are already on the marketplace. However, maximum of these drugs per form their activity without binding to the nucleotide-binding site. To design competitive ATP inhibitors are a substitute approach to inhibit ATPase's. This method has been successfully used to design protein-kinase inhibitors and it depends on the arrangement of the nucleotide-binding site.[17] In this study, we directed a bioinformatics investigation of structure and function of ATPase of Wuchereria bancrofti to predict the potential drug target sites and vaccine target site.
MATERIALS AND METHODS
Sequence retrieval
Amino acid sequences of ATPase protein of W. bancrofti was retrieved from National Center for Biotechnological Information (NCBI; http://www.ncbi.nlm.nih.gov/) and the accession number of this protein is EJW84114.1(gi | 402590183). Various computational tools and databases were used to analyze the different properties, like physicochemical, subcellular localization, secondary protein and three-dimensional (3D) structure, epitopes, and antigenicity sites.
Physiological–biochemical characterization
The Expasy Protparam server was used (http://us.expasy.org/tools/protparam) for the physicochemical characterization and to know the molecular weight, theoretical isoelectric point (pI), total number of negative and positive residues, aliphatic index, extinction coefficient instability index, and grand average hydropathicity (GRAVY) of this ATPase protein.[18]
Subcellular localization and signal peptide
Subcellular localization of the protein was identified by Psort (http://psort.nibb.ac.jp/form2.html). SignalP program (http://www.cbs.dtu.dk/services/SignalP/) was used to predict the signal peptide.[19]
Secondary structure prediction
PSIpred (http://bioinf.cs.ucl.ac.uk/psipred/) was done to analyze the secondary structure of this protein.[20] Secondary structural properties of the protein includes alpha helix, 310 helix, Pi helix, beta bridge, extended strand, beta turns, bend region, random coil, ambiguous states, etc.
Identification and comparison of conserved domain
Conserved Domain Database -Basic Local Alignment Search Tool (CDD-BLAST) (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi/) was done, to find out the conserved domain and to reveal any domain similarity with the HsATPase.[21]
Molecular evolution
Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) was used for searching evolutionary relationship among different organisms with the same protein ATPase.[22]
3D structure prediction
Phyre2, a web-based software (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id = index), was used to predict the 3D structure of this protein. Predicted structure was then refined by Modrefiner (http://zhanglab.ccmb.med.umich.edu/ModRefiner/). Finally the protein was visualized by Jmolserver and evaluated with PROCHEK server by Ramachandran plot analysis.[23,24,25]
Active site prediction
3DLigandSite (http://www.sbg.bio.ic.ac.uk/~3dligandsite/) is an automated software that was used to predict the ligand binding sites. Analysis of 3D ligand binding sites is a vital task for the modeled protein to do further work on its docking studies and it gives us an idea to make a grid before docking.[26,27]
B-cell epitope and antigenicity
The position of the linear B-cell epitopes was done by BepiPred (http://www.cbs.dtu.dk/services/BepiPred/) and Immune Epitope Database (IEDB) Analysis Resource (http://tools.immuneepitope.org/tools/bcell/iedb_input) was used to predict the antigenic sites.[28,29,30]
RESULT
Physical and chemical characteristics
The amino acid sequence of ATPase W. bancrofti (NCBI reference sequence: Gi | 402590183) contained 563 amino acid with a molecular weight of 64,160.1Da and the predicted pI is 9.37. The estimated half-lives of the protein in mammals, yeast, and E. coli were 30, >20, and >10 h, respectively, and its instability coefficient was 44.88, which was the characteristic of an unstable protein. The grand average of hydrophobicity (GRAVY of ATPase was − 0.644 and extinction coefficient 46,535. Therefore, the ATPase was an unstable and alkaline enzyme.
Molecular evolution and conserved domain
The molecular evolutionary analysis showed that the ATPase of W. bancrofti was more primitive in comparison to other nematodes and also from the host (Anopheles, Culex) and human [Figure 1]. It was largely destined from Homo sapiens. On the other hand, conserved domain analysis showed that it contains AAA superfamily, a DUF3523 multidomain, and a nonspecific superfamily PPV_E1_C [Figure 2].
Figure 1.

Phylogenetic analysis of adenosine triphosphate (ATP)ase of different species
Figure 2.
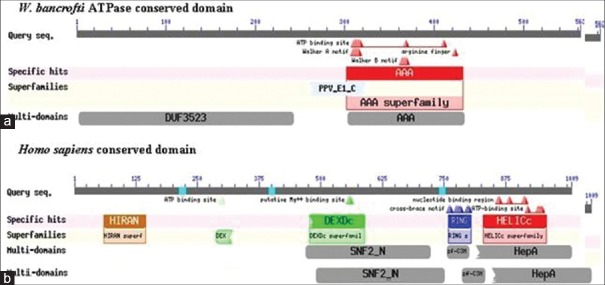
Comparison between (a) WbATPase and (b) HsATPaseconserved domain
Subcellular localization
ATPase of W. bancrofti was predicted to be localized 47.8% in the cytoplasm, 21.7% in mitochondria, 17.4% in the nucleus, and 4.3% each in the vacuoles, vesicles of secretory system, and in the endoplasmic reticulum. The prediction was done by k-nearest neighbors (KNN) prediction. Moreover the cytoplasmic location of this ATPase was 55.5% reliable according to Reinhardt's method for cytoplasmic/nuclear discrimination. Moreover, there was not any signal peptide.
Antigenicity sites and linear B-cell epitopes
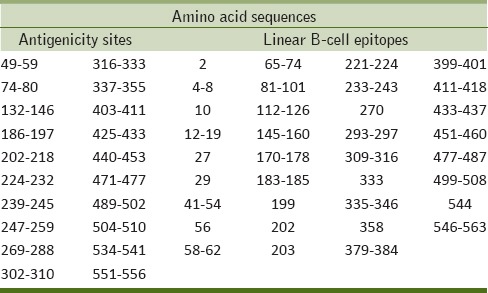
Bioinformatics tools predicated several antigenicity sites and linear B-cell epitopes. ATPase possesses 22 antigenicity sites, among them 16 sites were not homologous with the amino acid sequence of HsATPase, such as 49–59, 74–80, 132–146, 186–197, 202–218, 224–232, 239–245, 247–259, 361–368, 390–395, 425–433, 440–453, 471–477, 489–502, 504–510, and 551–556.
On the other hand 35 linear B-cell epitopes were found. Among them nine were homologous with HsATPase amino acid sequence, otherwise nonhomologous sequences are 12–19, 41–54, 65–74, 81–101, 170–178, 183–185, 221–224, 293–297, 379–384, 399–401, 451–460, 477–487, 499–508, 546–563, 2, 10, 56, 270, 333, and 544 [Table 1].
Table 1.
Linear B-cell epitopes and antigenicity sites of Wuchereria bancrofti
Structure of ATPase
The secondary structure analysis reveals that WbATPase consists of 67.5% α-helix, extend strand 7.28, and random coil 22.56. In case of solvent accessibility; 49.20% was buried, 15.10% intermediate accessibility, and 35.70% showed exposed accessibility [Figure 3]. Seventeen protein binding site were found. The tertiary structure of WbATPase was predicted by PHYRE2 which showed that the AAA superfamily and DUF3523 multidomain with PPV_E1_C nonspecific domain formed a large indentation [Figure 4]. Predicted model was then refined by Modrefiner and validated with PROCHEK server by Ramachandran plot analysis. PROCHEK analysis showed that 88.8% residues are positioned in the most favored region, 9.0% in additional allowed region, and 0.8% residues in the generously allowed region of the Ramachandran plot.
Figure 3.
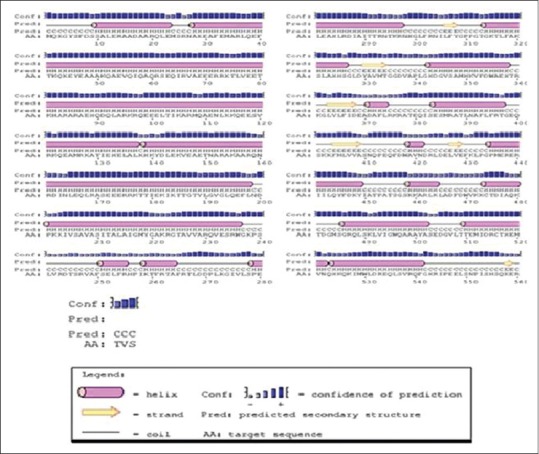
Secondary structure of ATPase
Figure 4.
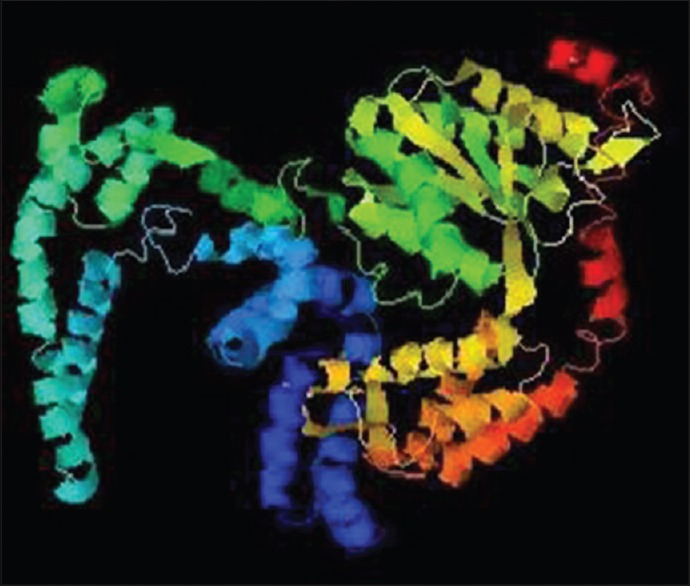
Refined three-dimensional (3D) structure of WbATPase
Ligand Binding Sites
3D ligand showed the 15 active sites of WbATPase with metallic to which drugs can bind. [Figure 5].
Figure 5.
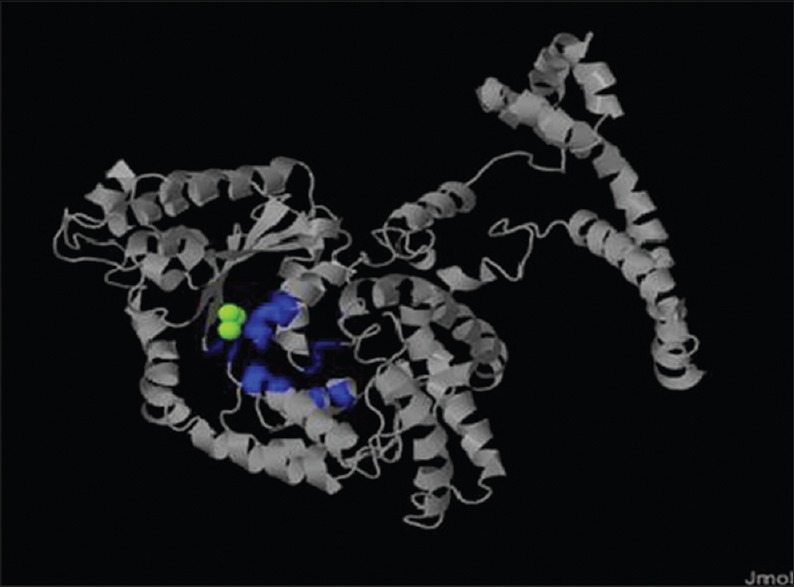
Three-dimensional (3D) ligand binding site. The blue color indicate the ligand binding site, the green color indicate the metallic heterogenes, and the ash color indicate the non-ligand binding site
DISCUSSION
The physiological–biochemical characterization of W. bancrofti ATPase showed that the pI of the protein is 9.87, which indicates that it is an alkaline enzyme. On the other hand the average hydrophobicity of this protein is −0.6, which means that it is an unstable protein in nature.[31]
The molecular phylogenetic analysis reveals that the ATPase of W. bancrofti is evolutionarily more ancient than the other nematodes as well as other organisms including the hosts (Ades, Culex, and Homo sapiens). But it is highly distant from the HsATPase that permits us to design new drugs or vaccines. The W. bancrofti ATPase possess conserved domains (AAA superfamily, a DUF3523 multidomain, and a nonspecific superfamily PPV_E1_C), which are not homologous with HsATPase conserved domains [Figure 2], that can be a potential target site of therapeutic agents.[32] The subcellular localization prediction shows that the ATPase is located in cellular cytoplasm and have segment of amino acids in NORs. This subcellular localization of ATPase may be one of the immune evasion techniques of W. bancrofti. The secondary structure annotation showed that 67.5% α-helical with 49.20% solvent accessibility and 17 protein binding sites which can also be used for molecular docking.[33] W. bancrofti ATPase is in cytoplasm of the cell with segments in the membrane and the drugs must inhibit and even kill the W. bancrofti, but will not be harmful for the normal cells of human. For this reason, drug targets must be some amino acid sequences that do not show the homology with HsATPase. The antigenicity sites—49-59, 74-80, 132-146, 186-197, 202-218, 224-232, 239-245, 247-259, 361-368, 390-395, 425-433, 440-453, 471-477, 489-502, 504-510, 551-556. And the B-cell Epitope sites 12-19, 41-54, 65-74, 81-101, 170-178, 183-185, 221-224, 293-297, 379-384, 399-401, 451-460, 477-487, 499-508, 546-563, 2, 10, 56, 270, 333, 544 without any homology with Human ATPase, can be used as ideal drugs or vaccines targets (Pasnik et al., 2005 and Dakappagari et al., 2000). In tertiary structure of W. bancrofti ATPase 3D ligand identified the 10 unique ligand binding site. It is highly notable that the available drugs of filarial DEC, ivermectin, and albendazole[34] are not against the ATPase or its related pathway in W. bancrofti. For this reason the ATPase of W. bancrofti can be a unique target site of drugs.[35,36,37] So we strongly believe our research/bioinformatics analysis will be helpful to design new vaccines or drugs against the W. bancrofti ATPase.
CONCLUSION
We have taken the ATPase sequence of W. bancroftifrom the NCBI database and used various bioinformatics tools to determine the various characteristics of that enzyme such as physiochemical properties, secondary structure, 3D structure, conserved domain, epitope, and their molecular evolution. The 3D structure shows superfamily and multidomain, which shows 15 ligand binding sites. The evolutionary relationships of epitopes are highly distant from the HsATPase that permits to design new drugs or vaccines. There are many epitopes and antigenicity sites present, from which the nonhomologous epitopes and antigenicity sites can be used as potential drug or vaccine target against W. bancrofti ATPase.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Melrose WD. Lymphatic filariasis- New insights into an old disease. Int J Parasitol. 2002;32:947–60. doi: 10.1016/s0020-7519(02)00062-0. [DOI] [PubMed] [Google Scholar]
- 2.Manguin S, Bangs MJ, Pothikasikorn J, Chareonviriyaphap T. Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infect Genet Evol. 2010;10:159–77. doi: 10.1016/j.meegid.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: Health impact after 8 years. PLoS Negl Trop Dis. 2008;2:317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreyer G, Noroes J, Figueredo-Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: A clinical perspective. Parasitol Today. 2004;16:544–8. doi: 10.1016/s0169-4758(00)01778-6. [DOI] [PubMed] [Google Scholar]
- 5.King CL, Freedman DP. “Filariasis”. In: Strickland GT, editor. Philadelphia: Hunter's tropical medicine and emerging infectious diseases; 2000. pp. 740–53. [Google Scholar]
- 6.Pfarr KM, Debrah AY, Specht S, Hoerauf A. Filariasis and lymphoedema. Parasite Immunol. 2009;31:664–72. doi: 10.1111/j.1365-3024.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomase VS, Chitlange NR, Sherkhane AS, Changbhale SS, Kale KV. Prediction of Wuchereria bancrofti troponin antigenic peptides: Application in synthetic vaccine design to counter lymphatic filariasis. J Vaccines Vaccin. 2007;4:69–73. [Google Scholar]
- 8.Hoti SL, Dhamodharan R, Subramaniyan K, Das PK. An allele specific PCR assay for screening for drug resistance among Wuchereria bancrofti populations in India. Indian J Med Res. 2009;130:193–9. [PubMed] [Google Scholar]
- 9.Bockarie MJ, Taylor MJ, Gyapong JO. Current practices in the management of lymphatic filariasis. Expert Rev Anti-Infect Ther. 2009;7:595–605. doi: 10.1586/eri.09.36. [DOI] [PubMed] [Google Scholar]
- 10.Ismail MM, Jayakody RL, Weil GJ, Fernando D, De Silva MS, De Silva GA, et al. Long-term efficacy of single-dose combinations of albendazole, ivermectin and diethylcarbamazine for the treatment of bancroftian filariasis. Trans R Soc Trop Med Hyg. 2001;95:332–7. doi: 10.1016/s0035-9203(01)90257-3. [DOI] [PubMed] [Google Scholar]
- 11.Dunyo SK, Simonsen PE. Ivermectin and albendazole alone and in combination for the treatment of lymphatic filariasis in Ghana: Follow-up after re-treatment with the combination. Trans R Soc Trop Med Hyg. 2002;96:189–92. doi: 10.1016/s0035-9203(02)90300-7. [DOI] [PubMed] [Google Scholar]
- 12.Makunde WH, Kamugisha LM, Massaga JJ, Makunde RW, Savael ZX, Akida J, et al. Treatment of co-infection with bancroftian filariasis and onchocerciasis: A safety and efficacy study of albendazole with ivermectin compared to treatment of single infection with bancrofti an filariasis. Filaria J. 2003;2:15. doi: 10.1186/1475-2883-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shenoy RK, John A, Babu BS, Suma TK, Kumaraswami V. Two-year follow-up of the microfilaraemia of asymptomatic brugian filariasis, after treatment with two, annual, single doses of ivermectin, diethylcarbamazine and albendazole, in various combinations. Ann Trop Med Parasitol. 2000;94:607–14. doi: 10.1080/00034983.2000.11813583. [DOI] [PubMed] [Google Scholar]
- 14.Wang YY, Nacher JC, Zhao XM. Predicting drug targets based on protein domains. Show Affiliations Mol Bio Syst. 2012;8:1528–34. doi: 10.1039/c2mb05450g. [DOI] [PubMed] [Google Scholar]
- 15.Cross RL, Müller V. The evolution of A-, F-, and V-type ATP synthases and ATPases: Reversals in function and changes in the H+/ATP coupling ratio. FEBS Lett. 2004;576:1–4. doi: 10.1016/j.febslet.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 16.Rappas M, Niwa H, Zhang X. Mechanisms of ATPase multidisciplinary approach. Curr Protein Pept Sci. 2004;5:89–105. doi: 10.2174/1389203043486874. [DOI] [PubMed] [Google Scholar]
- 17.Chene P. ATPases as drug targets: Learning from their structure. Nat Rev Drug Discov. 2002;1:665–73. doi: 10.1038/nrd894. [DOI] [PubMed] [Google Scholar]
- 18.Mohan R, Venugopal S. Computational structural and functional analysis of hypothetical proteins of Staphylococcus aureus. Bioinformation. 2012;8:722–8. doi: 10.6026/97320630008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardy JL, Laird MR, Chen F, Rey F, Walsh CJ, Ester M, et al. PSORTb v. 2.0:Expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2005;21:617–23. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- 20.Sabanadzovic S, Valverde RA, Brown JK, Martin RR, Tzanetakis IE. Southern tomato virus: The link between the families Totiviridae and Partitiviridae. Virus Res. 2009;140:130–7. doi: 10.1016/j.virusres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallel human genome analysis: Microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci U S A. 1996;93:10614–9. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swati S, Gandham VP, Kumar R, Mukhopadhyay CS, Brah CZ, Ansal M, et al. In silico analysis of evolutionary divergence of TLR9 transcript in Indian major carp Catlacatla. Herald J Biochem Bioinform. 2012;1:8–13. [Google Scholar]
- 23.Behjati M, Torktaz I, Mohammadpour M, Ahmadian M, Easton AJ. Comparative modeling of CCRL1, a key protein in masked immune diseases and virtual screening for finding inhibitor of this protein. Bioinformation. 2012;8:336–40. doi: 10.6026/97320630008336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herráez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Edu. 2006;34:255–61. doi: 10.1002/bmb.2006.494034042644. [DOI] [PubMed] [Google Scholar]
- 25.Hasan MA, Alauddin SM, Amin MA, Nur SM, Mannan A. In silico molecular characterization of cysteine protease yopt from yersinia pestis by homology modeling and binding site identification. Drug Target Insights. 2014;8:1–9. doi: 10.4137/DTI.S13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sibi P, Paul M. In silico docking analysis of constituents of Zingiber Officinale as antidepressant. J Pharmacogn Phytother. 2013;5:101–5. [Google Scholar]
- 27.Jambon M, Andrieu O, Combet C, Deléage G, Delfaud F, Geourjon C. The SuMo server: 3D search for protein functional sites. Bioinformatics. 2005;21:3929–30. doi: 10.1093/bioinformatics/bti645. [DOI] [PubMed] [Google Scholar]
- 28.Fan Z, Li K, Zhang L, Chen F, Wu Q, Li N, et al. Bioinformatics analysis of the structure and function of NADPHcytochrome p450 reductase of Plasmodium vivax. Biomed Rep. 2013;1:425–7. doi: 10.3892/br.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sealey KL, Kirk RS, Walker AJ, Rollinson D, Lawton SP. Adaptive radiation within the vaccine target tetraspanin-23 across nine Schistosoma species from Africa. Int J Parasitol. 2013;43:95–103. doi: 10.1016/j.ijpara.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Saffari B, Mohabatkar H, Sasan S. T and B-cell epitopes prediction of Iranian saffron (crocus sativus) profilin by bioinformatics tools. Protein Peptide Lett. 2008;15:280–5. doi: 10.2174/092986608783744270. [DOI] [PubMed] [Google Scholar]
- 31.Shi Q, Zhou Y, Sun Y. Influence of pH and ionic strength on the steric mass-action model parameters around the isoelectric point of protein. Biotechnol Prog. 2005;21:516–23. doi: 10.1021/bp049735o. [DOI] [PubMed] [Google Scholar]
- 32.Koch O. More than a rigid framework: Molecular design using secondary structure element information. J Cheminform. 2013;5:45. [Google Scholar]
- 33.Pasnik DJ, Evans JJ, Panangala VS, Klesius PH, Shelby RA, Shoemaker CA. Antigenicity of Streptococcus agalactiae extracellular products and vaccine efficacy. J Fish Dis. 2005;28:205–12. doi: 10.1111/j.1365-2761.2005.00619.x. [DOI] [PubMed] [Google Scholar]
- 34.Dakappagari NN, Douglas DB, Triozzi PL, Stevens VC, Kaumaya PT. Prevention of mammary tumors with a chimeric HER-2 B-cell epitope peptide vaccine. Cancer Res. 2000;60:3782–5. [PubMed] [Google Scholar]
- 35.Laurie R, Jackson AT, Richard M. Methods for the prediction of protein-ligand binding sites for structure-based drug design and virtual ligand screening. Curr Protein Pept Sci. 2006;7:395–406. doi: 10.2174/138920306778559386. [DOI] [PubMed] [Google Scholar]
- 36.Hasan MA, Hossain M, Alam MJ. A computational assay to design an epitope-based Peptide vaccine against Saint Louis encephalitis virus. Bioinform Biol Insights. 2013;7:347–55. doi: 10.4137/BBI.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomar NT, Singh V, Marla SS, Chandra R, Kumar R. Molecular docking studies with rabies virus glycoprotein to design viral therapeutics. Indian J Pharm Sci. 2010;72:486–90. doi: 10.4103/0250-474X.73905. [DOI] [PMC free article] [PubMed] [Google Scholar]


