Abstract
Background:
Oral therapy for pulmonary tuberculosis (TB) treatment suffers from the limitation of hepatic metabolism leading insufficient concentration of antitubercular (anti-TB) drugs in alveolar macrophage which harbors Mycobacterium tuberculosis (MTB). Targeted aerosol delivery of antituberculous drug to lung is efficient for treating local lung TB infection.
Objective:
The present study was aimed to evaluate rifapentine (RPT) loaded proliposomal dry powder for inhalation (RLDPI) for anti-TBactivity and cytotoxicity in vitro. In vivo toxicity study was also undertaken in Wistar rats to determine safe concentration of RLDPI for administration.
Materials and Methods:
Anti-TB activity of developed RLDPI was assessed using drug susceptibility testing (DST) on Mycobacteria growth indicator tube (MGIT) method. In vitro cytotoxicity was performed in A549 cell lines and IC50 values were used to compare the cytotoxicity of formulation with pure RPT. In vivo repeated dose toxicity study was undertaken using Wistar rats at three different doses for 28-days by intratracheal insufflations method.
Results:
The results of DST study revealed sensitivity of tubercle bacteria to RLDPI at concentration equivalent to 10 μg/mL of RPT. This study confirmed anti-TB potential of RPT in spray-dried RLDPI, though the spray drying method is reported to reduce activity of drugs. Cytotoxicity study in A549 cells demonstrated that RPT when encapsulated in liposomes as RLDPI was safe to cells as compared to pure RPT. In vivo toxicity study revealed that RPT in the form of RLDPI was safe at 1 and 5 mg/kg dose. However, mortality was seen at higher dose (10 mg/kg), possibly because of liver and kidney damage.
Conclusion:
Thus, these studies demonstrated safety of RLDPI for the treatment of pulmonary TB.
Keywords: A549 cell line, drug susceptibility testing on Mycobacteria growth indicator tube, pulmonary tuberculosis, rifapentine, toxicity
INTRODUCTION
Tuberculosis (TB) is a global epidemic infectious disease after HIV/AIDS, and is caused by deposition of Mycobacterium tuberculosis (MTB) in the lung(s).[1] More than 80% of the TB population suffers from pulmonary TB alone. Since oral administration of anti-tubercular (anti-TB) drug is associated with extensive first pass metabolism, very less amount reaches the lung, and thereby, alveolar macrophage (Mϕ) of target cells which harbor the MTB resulting in failure of the therapy. This requires administration of very high doses of the drug which may cause systemic toxicity.[2,3,4] Further, prolongedoral administration of systemic doses of single or combined antibiotics is associated with unwanted side effects resulting in poor patient compliance. Thus, to overcome drawbacks of oral therapy, the anti-TB drugs should be targeted to the Mϕ, which will eventually improve efficacy and reduce systemic side effects.[5,6] Muttil et al., have reported 20-fold higher concentrations of isoniazid and rifabutin in intra-macrophages when encapsulated within poly (lactic acid) and administered as aerosols than oral, intravenous, and intratracheal instillation of pure drug.[7] Liposomal formulations are readily accepted because of the biological safety and compatibility of liposomes and lung surfactant.[8] Lung surfactant comprises of phospholipid (78–90%), protein (5–10%), and neutral lipid (such as cholesterol; 4–10%). The most abundant component of lung surfactant is phosphatidyl choline (PC; 70–80% of total lipid) and 50–70% of the PC corresponds to dipalmitoyl PC (DPPC).[9]
Several strategies have been reported in the literature to target actives to the macrophage by alteration of lipid composition of the liposome. Liposomal properties such as size, charge, and inclusion of surface ligands (viz., proteins, peptides, antibodies, polysaccharides, glycolipids, glycoproteins, and lectins) can be modified to target the drug molecules to the macrophage.[10] Vyas et al., have reported higher dug concentration of negatively charged liposomes over plain drug and conventional liposomes within the cells of male albino rats.[11] However, efficient intracellular drug delivery can be achieved by electrostatic interaction between cationic liposomes and negatively charged cell surface proteoglycans.[12] Liposomal dry powder for inhalation (LDPI) has been proved to be advantageous in targeting particles to respiratory tract. Arikace (liposomal amikacin for inhalation) has been approved as an orphan drug and has received orphan drug designation in the Europe for the treatment of bronchopulmonary Pseudomonas aeruginosa infecton in cystic fibrosis (CF) patients in July 2006.[13] Also, US Food and Drug Administration (FDA) has approved ciprofloxacin for inhalation (Aradigm Corporation, US) for the management of bronchiectasis (BE) as an orphan drug. Currently, amikacin (Arikace; Insmed, NJ) and ciprofloxacin (Bayer) as inhalation liposomal particles are under the phase III and phase IIb clinical trial development stage, respectively.[14,15]
Rifapentine (RPT) is a piperazinyl hydrazone derivative of 3-formyl rifamycin with antimicrobial spectrum similar to rifampicin and is approved by USFDA for the treatment of pulmonary TB. It acts by specifically inhibiting bacterial DNA-dependent RNA polymerase activity in susceptible cells without affecting mammalian enzyme. The two limitations which restricts the use of RPT by oral route are, extensive first pass metabolism of the drug and variable bioavailability upon oral administration (high fat meal required for better absorption).[16,17] Hence, in our previous study, we have successfully applied the principles of quality by design (QbD) to develop RPT loaded proliposomal dry powder for inhalation (RLDPI) in single step for the treatment of TB by pulmonary administration. In the said study, we have demonstrated that administering RPT as proliposomal dry powder for inhalation achieved highest targeting potential (of 13.995) with improved pulmokinetic parameters.[18]
The present study was undertaken to evaluate the toxic potential of RLDPI at different doses in vitro in lung cellline (A549) and in vivo in Wistar rats by intratracheal insufflations. Also, anti-TB activity of the prepared formulation by spray drying method was confirmed using in vitro drug susceptibility testing (DST) in Mycobacteria growth indicator tube (MGIT) method.
MATERIALS AND METHODS
MTB strain H37 Rv (Lot # 27294) was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Bactec 1213 Mycobacteria culture vials (Becton Dickinson and Co., Sparks, Maryland) containing Middlebrook 7H12 broth were used for thenon-radiometric assay on MGIT 960 system. Fetal bovine serum (FBS), antibiotics (penicillin, streptomycin and neomycin) and Ham's F12K medium were procured from Gibco®, life technologies (Grand Island, NY, USA). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was purchased from Sigma (Sigma, St Louis, MO, USA). The A549 human NSCLC cell line was obtained from ATCC (Rockville, MD, USA), was grown in media supplemented with 10% FBS. Antimycotic antibiotics such as penicillin (5,000 U/mL), streptomycin (0.1 mg/mL), and neomycin (0.2 mg/mL) were added to all tissue culture media. The cells were maintained at 37°C in the presence of 5% of CO2 and a fully humidified atmosphere. All other chemicals used were of analytical grade.
RLDPI was prepared by spray drying method. 1:2:1 molar ratio of RPT: Hydrogenated soyaphosphatidylcholine (HSPC): Cholesterol was selected to prepare RLDPI. Stearyl amine was used as the charged lipid in the concentration of 30% of total lipid. The liposomal vesicle size, mass median diameter (MMD) and mass median aerodynamic diameter (MMAD) of prepared proliposomes were 578 ± 4.9 nm, 5.32 ± 0.32 μm, and 1.56 ± 0.16 μm, respectively as determined by Malvern MasterSizer (2000 HydroSM and Scirocco 2000, Malvern Instruments, Malvern UK) with 72.08 ± 1.9% of encapsulation efficiency. MMAD of the RLDPI was determined using 8-stage non-viable cascade impactor (Westech Scientific Instruments, Bedfordshire, UK). Sustained drug release profile with approximately 90% RPT release was achieved at the end of 24 h from RLDPI. As a control, proliposomal dry powder for inhalation (placebo-LDPI) was prepared excluding RPT.[18]
In vitro anti-TB activity of RLDPI
In vitro anti-TB activity of RLDPI was conducted as per previous report.[19]
Preparation of inocula
The standard protocol recommended by the manufacturer for first-line anti-TB drugs was followed. In brief, the bacterial suspension was prepared in 4 mL of Middlebrook 7H9 broth (Hi Media Laboratories Ltd, Mumbai) and the turbidity was adjusted to 0.5 McFarland standard. From this suspension, 1.0 mL was diluted with 4.0 mL of sterile saline (1:5 dilution). Half a milliliter of this (1:5) dilution was used to inoculate each of the drug containing MGITs. Subsequently, 100 μL of 1:5 dilution was pipetted into 10.0 mL of sterile saline to obtain a final dilution of 1:500; of which 500 μL was used to inoculate MGIT growth control (GC) tubes without drug. All the MGIT tubes were labeled appropriately and 0.8 mL of MGIT 960 AST Supplement was added. Further, 0.1 mL of drug concentrations to be tested for each drug (RLDPI aqueous suspensions equivalent to 5 and 10 μg/mL of RPT and pure RPT solution in ethylene glycol at 5 and 10 μg/mL) were aseptically added and finally 0.5 mL of the test inoculum was added to each 7 mL MGIT tube. The GC tube with supplement and without drug was inoculated with 0.5 mL of GC inoculum.
All inoculated MGIT 960 tubes were placed in the DST set carrier and entered into the MGIT 960 instrument as “unknown drugs” using the DST entry feature. The instrument flagged the DST set “complete” when the GC reached a growth unit (GU) value of 400. If the GU of the drug-containing tube was more than 100 when the GU of the GC was 400, the results were defined as resistant. If the GU values of the drug-containing tubes were equal to or less than 100, the results were considered susceptible.[20]
In vitro cytotoxicity study
The in vitro cytotoxicity of RLDPI was evaluated in the A549 cellline (adenocarcinomic human alveolar basal epithelial cells) by MTT assay as reported earlier.[21,22] For comparison the RPT solution was prepared by dissolving RPT in dimethylsulfoxide (DMSO) and subsequently diluted with cell culture medium with final concentration of DMSO less than 1%. A 100 μL of A549 cells were cultured in 96-well microtiter plates at a density of 1 × 106 cells/well and after overnight incubation the cells were treated with 100 μL of various concentrations of RPT solution, placebo LDPI, and RLDPI formulations diluted in F12K medium (20–100 μg/mL). After 24 h incubation of cells with samples at 37 ± 0.2°C in a 5% CO2-jacketed incubator, 100 μL supernatant was replaced by sterilized MTT solution (20 μL of 5 mg/mL in PBS) was added into each well which were further incubated for 4 h at 37°C. The supernatant was carefully removed and the formazan crystals were dissolved by adding 100 μL of DMSO and mixed thoroughly. The absorbance was recorded at 540 nm using the microplate reader (SpectraMax Plus 384, Molecular Devices, CA, USA). The proportion of viable cells in treated wells was compared to the untreated well (control). The graph of percentage of cell survival (% cell viability) as a function of drug concentration was plotted to determine the IC50 value (the drug concentration needed to prevent cell proliferation by 50%).
Subacute repeated dose pulmonary toxicity study
Toxicity studies were performed at the Poona College of Pharmacy, Pune, India. Animal handling was performed according to Good Laboratory Practice. Protocol for experiments on animal was prepared as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and was approved by the Institutional Animal Ethics Committee (IAEC) of Poona College of Pharmacy, Pune.
Animals
Wistar rats of either sex (150–200 g, 10–12 weeks) were obtained from National Toxicology Centre (NTC), Pune, India and were housed in polypropylene cages (five animals per cage) at standard conditions of temperature (24 ± 1°C) and relative humidity (55 ± 10%) for 12 h light/dark cycles throughout the experiment. Animals had access to commercially available standard pellet diet (Pranav Agro Industries, Sangli, Maharashtra, India) and filtered water, ad libitum. Animalswere acclimatized for 1 week prior to the initiation of treatment. During this acclimatization period, the health status of the rats was monitored daily.
Study protocol
Animals were randomly divided into four groups containing five rats per group. Group I served as the control which did not receive any treatment and Group II animals received placebo-LDPI. These group animals received amount of lipid phase present in higher dose of the RPT taken for the study. Group III, IV, and V rats received RLDPI equivalent to 1 (low), 5 (medium), and 10 (high) mg/kg of RPT by pulmonary administration as intratracheal insufflation for 28 days at a prefixed time daily under anesthesia (80 mg/kg of ketamine), respectively. Intratracheal insufflation on the rats was performed by earlier reports.[23]
Throughout the study period, animals were observed in their cages daily for mortality and signs of any toxic effects. Effect of treatment on general health of the animals, body weight, and behavior was also noted.
Body weight of animals and food intake
Before administration of RLDPI, the animals were weighed using a calibrated balance. The weight of the animals was recorded daily throughout the experimental period at a fixed time. For recording the food consumption, a weighed amount of standard rat food pellets was placed in the food tray of the cage. The unconsumed pellets were weighed and replaced with fresh pellets in each tray every day. The time of providing the feed was fixed throughout the study.
Analysis of bronchoalveolar (BAL) fluid
The biomarkers, lactate dehydrogenase (LDH) and polymorphonuclear neutrophils (PMN) were estimated in BAL to evaluate pulmonary cytotoxicity and inflammation as reported earlier with few modifications.[24,25,26] BAL fluid was collected aseptically by intratracheally injecting 10 mL PBS (pH 7.4) in parts which was prewarmed to 37°C and withdrawn by gentle aspiration after 2 min. This BAL fluid was centrifuged (Allegra™ 64R Centrifuge, BeckmanCoulter India Pvt. Ltd., Andheri (E) at 700 g for 15 min at 4°C, Mumbai, Maharashtra), supernatant was collected and stored at − 70°C till further analysis. LDH released in the BAL fluid was measured using commercial kits (Transasia Bio-Medicals Ltd, Bangalore) on auto analyzer (Erba EM-360 Chemistry Analyzer, Transasia Bio-Medicals Ltd, Bangalore). For the determination of neutrophil, Leishman stain (Coral Pvt. Ltd., Goa) was used. Neutrophils as purple colored nuclei with pink cytoplasm were counted in atleast five squares of hemocytometer.
Blood sampling and biochemical assay
At the end of the 28 day study period, the animals were fasted overnight. On day 29, each animal was anesthetized using anesthetic ether and blood samples were collected from the retro-orbital plexus of all the rats. The serum for biochemical assay was obtained by centrifugation of the whole blood at 3,000 rpm for 15 min. Biochemical parameters, viz. serum glutamic-pyruvic transaminases (SGPT, U/L), serum glutamic-oxalacetic transaminases (SGOT, U/L), creatinine (mg/dL), andurea (mg/dL) were assayed using a Merck Microlab 300 semi-automated clinical chemistry analyzer (Vital Scientific, Netherlands).
Histopathological observations
The animal groups treated with placebo-LDPI, RLDPI, and the control were sacrificed at the end of study and the lung was rapidly dissected out, washed twice in sterile PBS, and carefully weighed on an analytical balance. The lung was cut into small pieces and preserved in 10% formalin for 24 h. The specimen was successively dehydrated with alcohol of 70, 80, and 100% each for 1 h. Tissues were cleaned by treating with xylene each time for 1 h. Infiltration and impugnation was done by treating with paraffin wax twice, each time for 1 h. Paraffin ‘L’-shaped molds were prepared. Specimens were cut into sections of 3–5 μm in thickness stained by hematoxylin and eosin. The sections were mounted using disterene phthalate xylene. Sections from all processed tissues of control, placebo-LDPI, and RLDPI-treated groups were examined under light microscope (Nikon Coolpix camera mounted on a Nikon Eclipse 50i microscope, Japan).
Statistical analysis
The data are presented as the mean ± standard error (SE). Results were analyzed statistically using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. The minimum level of significance was setat P < 0.05.
RESULTS AND DISCUSSION
Direct delivery of anti-TB drug such as RPT by inhalation route in the form of RLDPI to the lung reduces systemic toxicity and achieves higher local drug concentration at the site of infection. Also, inhalation route bypasses first pass metabolism in the liver. Sustained and controlled release formulations such as liposomes have high patient compliance, can target drug to the Mϕs by inhalation of LDPI and reduced total drug dose exposure (due to reduction in total amount of drug and frequency) reduces systemic side effects.[3,5]
In vitro anti-TBactivity
RPT is a well-known anti-TB drug with its established activity against MTB bacteria. However, a drug was processed into RLDPI formulation by spray drying method. Processing of the drugs by spray drying method may reduce the activity or degrade the drug. Hence, in order to check the anti-TB activity of the drug after processing into formulation by spray drying method, the DST on MGIT was undertaken. MGIT determines whether or not TB bacteria are susceptible to anti-TB drugs. Resistant bacteria grow, whereas susceptible bacteria cannot grow in presence of the drugs. The MGIT, a liquid culture system is developed by Becton, Dickinson (BD, USA). It consists of a small tube with the medium containing oxygen and fluorescence substance at the bottom. Anti-TB drug and TB bacterium are added to these tubes followed by incubation period. During incubation period if bacteria grows and consumes oxygen, fluorescence material glows, indicating bacteria is resistant to the specific anti-TB drug; whereas if fluorescence is not observed, indicates susceptible bacteria which could not grow in presence of the anti-TB drug added in the tube.[27] The reported minimum inhibitory concentration (MIC) for RPT is 6 μg/mL.[28] Therefore, in vitro anti-TB activity was studied at the concentration of 1 and 5 μg/mL. The results of in vitro anti-TB activity showed that M. tuberculosis control strain (H37Rv) were sensitive to the pure RPT as well as RLDPI concentrations equivalent to 1 and 5μg/mL of RPT. Thus, from the study, it can be concluded that processing of liposomally encapsulated RPT by spray drying maintained its activity against MTB.
In vitro cytotoxicity study
In vitro cytotoxicity of RPTand RLDPI at different concentrations was evaluated on human lung adenocarcinoma (A549) cell line [Figure 1] at the end of 24 h. A549 cells were used to study the in vitro cytotoxicity because lung cells represent the primary biological target for the inhaled particles. From the graph of percentage cell viability as a function of RPT concentration, IC50 values for pure RPT and RLDPI were found to be 72.57 ± 3.63 and 105.28 ± 5.26 μg/mL, respectively. Also, to evaluate the cytotoxicity of the liposomal vesicular carrier, the cells were also challenged with equivalent amount of lipids (placebo-LDPI) carrying the dose of 75μg/mL of the RPT.1.44-fold increase in IC50 was obtained for RPT after encapsulation of it in the liposomal vesicular carrier. Almost 100% cell viability observed for placebo-LDPI demonstrated the nontoxicity of lipids used and the potential of the liposomes as the carrier for anti-TB drug for pulmonary application.
Figure 1.
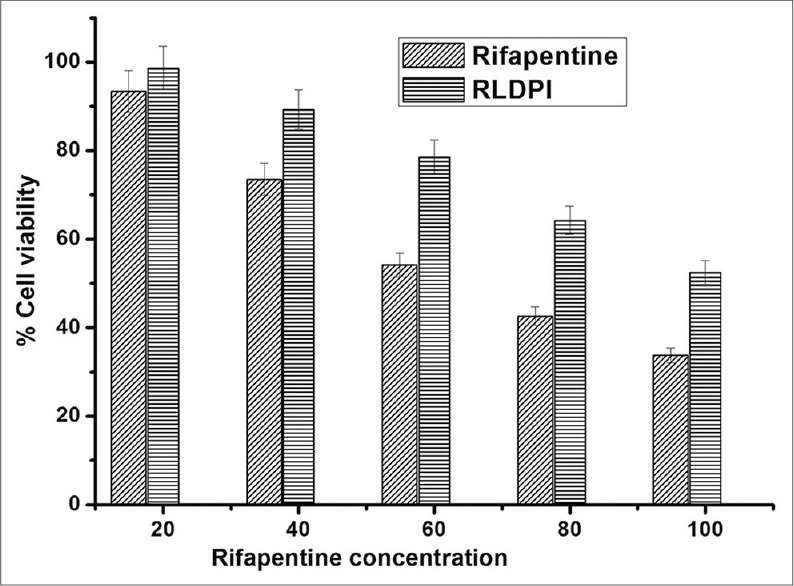
In vitro cytotoxicity profile of RPT in A549 cells after 24 h. Cells were treated with increasing concentrations of RPT and RLDPI. Cytotoxicity was determined using MTT assay as discussed under methods. IC50 value for RPT and RLDPI was determined from the plot of % cell viability as a function of RPT concentration (values represent the mean ± SD; n = 6). RPT = rifapentine, RLDPI = RPT loaded proliposomal dry powder for inhalation, MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, IC50 = drug concentration needed to prevent cell proliferation by 50%, SD = standard deviation
The reduction in cytotoxicity of RLDPI can be correlated with in vitro RPT release from the RLDPI. For the improved efficacy, liposomes can be tailor-made to control and sustain the release of the entrapped drug. The in vitro release study of RLDPI revealed approximately 90% release of RPT from RLDPIat the end of 24 h.[18] Thus, amount of free drug available from RLDPI was found to be 54μg/mL. Similar results have been reported by Rojanarat et al., for the safety of levofloxacin (LEV) in the form of LEV-proliposomes on respiratory associated cells.[22] The results of cytotoxicity with the RLDPI formulation could mainly be due to controlled release of drug and endocytosis of nanoparticles slowly over a prolonged period of time.[21] It was also noted that amount of the RPT released in the cell line medium at the end of 24 h was approximately eight times greater than the MIC (6 μg/mL) reported for the drug in the literature.[27]
Subacute repeated dose pulmonary toxicity study (28 day)
Animal observation, food intake and effect on body weight
During the study period detailed physical examinations for signs of morbidity were conducted every week. It was observed that the animals fed with placebo-LDPI, 1 and 5 mg/kg doses (Groups II, III, and IV) of RLDPI were healthy with respect to the behavior as well as food intake. There was statistically insignificant difference (P > 0.05) in increase in body weight between the Group I, II, III, and IV animals (P < 0.05) [Table 1]. This indicated that low and medium dose of RPT in the form of LDPI did not have any effect on food intake of the animals.
Table 1.
Analysis of bronchoalveolar fluid for total cell count, polymorphonuclear neutrophils and lactose dehydrogenase after repeated dose 28-day toxicity study (mean±SD; n=5)
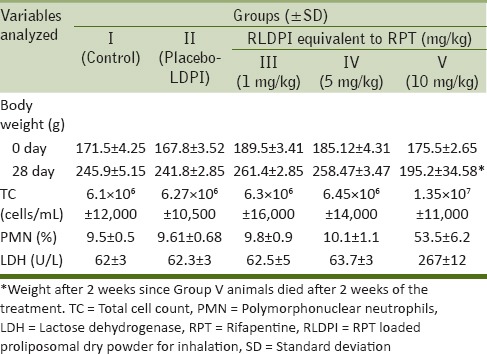
A 10 mg/kg dose (Group V) was found to affect the usual activities of all the rats in the group. The activities of Group V rats were slowed with major effect on food consumption and in turn on weight of animals. All the Group V animals showed drastic decrease in body weight (P < 0.01) when compared with control (Group I) and placebo-LDPI (Group II) group rats. This confirmed the reduced food consumption of rat as compared to the control group animals. It was noticed that out of six animals of Group V, two animals died on day 15 and remaining four rats died on day 16 after the dose.
BAL fluid analysis
Injury to endothelial cell basolateral membrane induces release of LDH into the alveolar interstitium [Table 1]. Also, acute inflammation influx of neutrophils increases permeability of the alveolar/capillary barrier resulting into cellular cytotoxicity. Therefore, BAL fluid LDH and PMN are used as biochemical indices of pulmonary damage.[29] A slight but nonsignificant (P > 0.05) increase in TC was detected in BAL fluid of Group II, III, and IV animals against Group I (control) and amongst Group II and III animals. Whereas, a significant increase in TC was noticed in animals treated with 10 mg/kg RPT (Group V, P < 0.001), when compared with control as well as Groups III and IV.
The presence of higher percentage of PMN in BAL fluid is used as a marker for the detection of pulmonary inflammation. Intratracheal insufflation of RLDPI equivalent to 1 and 5 mg/kg of RPT did not elicit pulmonary irritation as revealed from the PMN values [Table 1] for the respective groups compared with control group. On the other hand, Group V animals showed significant (P < 0.001 against Group I) airway inflammation as demonstrated from the five fold increase in PMN value within 15 days.
A 28-day repeated dose pulmonary intratracheal insufflation of RLDPI at low and medium doses produced no significant cytotoxic response as measured by LDH release [Table 1] in BAL fluid compared with control group animals. The animals belonging to Groups III and IV were healthy throughout study without no behavioral changes, whereas all animals of Group V died within 14–16 days of study period. A significant increase in BAL fluid LDH levels in Group V animals (P < 0.001) confirmed an inflammatory response at 10 mg/kg dose of RPT in the form of RLDPI and could be responsible for the death of animals.
The results of the study implies that pro-inflammatory potential in the lung was absent for low and medium dose of RLDPI, whereas, presence of significantly higher amount of RPT in high dose resulted in inflammatory response as revealed from the values of PMN and LDH in BAL fluid of the lung.
Biochemical analysis
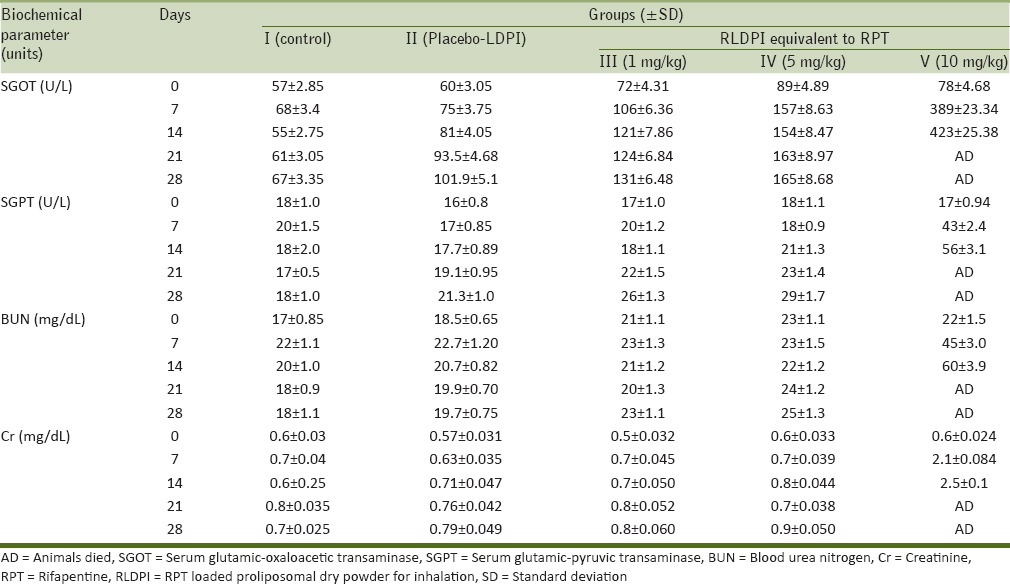
There was no significant difference in all the biochemical parameters analyzed (SGOT, SGPT, blood urea level (BUL), and serum creatinine) for Group II, III, and IV rats for all days as compared to control (Group I) suggesting normal functioning of the liver as well as kidney of these respective groups [Table 2]. SGOT values for these treated groups were slightly greater than control group for Groups II, III, and IV; but it was within the normal range. For Group V all the biochemical parameter values were significantly higher than the control group (P < 0.001) as well as Groups III and IV. These results suggested liver as well as kidney failure at 10 mg/kg dose of RPT equivalent RLDPI by pulmonary insufflation within 15 days (2 weeks) and this could be the reason for death of this group of animals.
Table 2.
Serum biochemical parameters a) SGPT, b) SGOT, c) BUL, and d) creatinine of control, placebo-LDPI and RLDPI treated (equivalent to 1, 5, and 10 mg/kg RPT) Wistar rats for 28 day intratracheal instillation toxicity study (mean±SD; n=5)
Histopathological examination
Lungs of the control and RPT-treated groups were observed histopathologically to examine changes occurred in lungs after exposure to RLDPI by pulmonary intratracheal insufflation every day for 28 days.
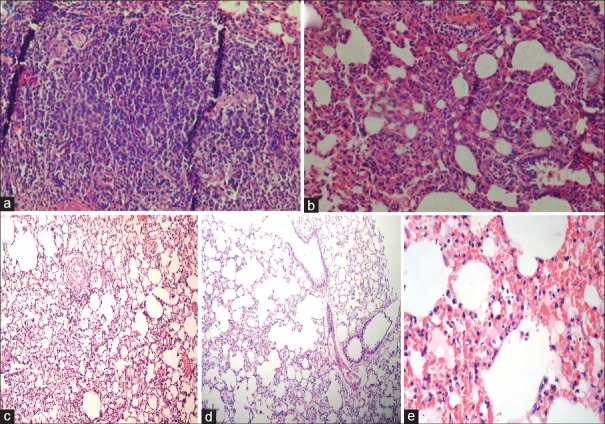
No abnormality was detected in lungs of Group I, II, III, and IV animal shistopathologically. However, some focal changes were noticed in Group III and IV lungs, but these focal changes did not alter functional capacity of the lung [Figure 2]. Group V rat's lung when microscopically examined, demonstrated minimal changes in mononuclear cells (MNC) infiltration in alveoli lining, emphysema in alveoli, and pneumonic changes with consolidation of lung parenchyma. Also, moderate to severe congestion, hemorrhages in parenchyma and hyperplasia of bronchiolar epithelium were revealed in the same histomicrographs which affects the function of lung.
Figure 2.
Light photomicrographs of lung tissues after 28 days administration of RLDPI by intratracheal instillation. The images depicted here are from the (a) control (Group I), (b) placebo-LDPI (Group II), and RLDPI-treated groups; (c) 1 mg/kg (Group III), (d) 5 mg/kg (Group IV), and (e) 10 mg/kg (Group V) equivalent to RPT
In vivo 28-day repeated dose toxicity study demonstrated no abnormality in lung of animals treated with placebo-LDPI and at the doses 1 and 5 mg/kg. However, a dose of RLDPI equivalent to 10 mg/kg of RPT revealed moderate to severe changes in the lung. The present study also confirmed the safety of liposome as a carrier for pulmonary administration. Thus, the study demonstrated inflammatory and cytotoxic effects of 10 mg/kg dose of RPT administrated as intratracheal insufflation.
CONCLUSION
In summary, RLDPI prepared for pulmonary inhalation by spray drying method retained its anti-TB potential even after processing at very high temperature by spray drying. RLDPI treated animals showed dose-dependent lung toxicity. Also, encapsulation of RPT in liposomes was found to increase the cytotoxic threshold (IC50) as compared to pure RPT in A549 cell lines. Thus, this study supports the potential of RLDPI for the treatment of pulmonary TB.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organisation. Tuberculosis fact sheet. [Last accessed on 2014 Apr 28]. Available from: http://www.whi.int/inffs/en/fact104.html .
- 2.Pandey R, Khuller GK. Antitubercular inhaled therapy: Opportunities, progress and challenges. J Antimicrob Chemotherapy. 2005;55:430–5. doi: 10.1093/jac/dki027. [DOI] [PubMed] [Google Scholar]
- 3.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 2005;172:1487–90. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma R, Saxena D, Dwivedi AK, Misra A. Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharm Res. 2001;18:1405–10. doi: 10.1023/a:1012296604685. [DOI] [PubMed] [Google Scholar]
- 5.Suarez S, O’Hara P, Kazantseva M, Newcomer CE, Hopfer R, McMurray DN, et al. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: Screening in an infectious disease model. Pharm Res. 2001;18:1315–9. doi: 10.1023/a:1013094112861. [DOI] [PubMed] [Google Scholar]
- 6.Sethuraman VV, Hickey AJ. Powder properties and their influence on dry powder inhaler delivery of an anti-tubercular drug. AAPS Pharm Sci Tech. 2002;3:E28. doi: 10.1208/pt030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muttil P, Kaur J, Kumar K, Yadav AB, Sharma R, Misra A. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur J Pharm Sci. 2007;32:140–50. doi: 10.1016/j.ejps.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, et al. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb) 2010;91:71–81. doi: 10.1016/j.tube.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Weibel ER. Berlin: Springer Verlag: Academic Press; 1963. Review: Morphometry of the human lung; p. 3. [Google Scholar]
- 10.Kelly C, Jefferies C, Cryan SA. Targeted liposomal drug delivery to monocytes and macrophages. J Drug Deliv 2011. 2011 doi: 10.1155/2011/727241. 727241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyas SP, Kannan ME, Jain S, Mishra V, Singh P. Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. Int J Pharm. 2004;269:37–49. doi: 10.1016/j.ijpharm.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Wiethoff CM, Smith JG, Koe GS, Middaugh CR. The potential role of proteoglycans in cationic lipid-mediated gene delivery. Studies of the interaction of cationic lipid-DNA complexes with model glycosaminoglycans. J Biol Chem. 2001;276:32806–13. doi: 10.1074/jbc.M007940200. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency, Orphan designation. EU/3/06/387 [Google Scholar]
- 14. [Last accessed on 2014 Feb 28]. Available from: http://clinicaltrials.gov/ct2/show/NCT01315678?term=ARIKACE and rank=5 .
- 15. [Last accessed on 2014 Feb 28]. Available from: http://www.aradigm.com/products_3100.html .
- 16.Jarvis B, Lamb HM. Rifapentine. Drugs. 1998;56:607–16. doi: 10.2165/00003495-199856040-00008. [DOI] [PubMed] [Google Scholar]
- 17.Drugs @ FDA. [Last accessed on 2014 Jan 02]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/1998/21024lbl.pdf .
- 18.Patil-Gadhe A, Pokharkar V. Single step spray drying method to develop proliposomes for inhalation: A systematic study based on quality by design approach. Pulm Pharmacol Ther. 2014;27:197–207. doi: 10.1016/j.pupt.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Lin SY, Desmond E, Bonato D, Gross W, Siddiqi S. Multicenter evaluation of Bactec MGIT 960 system for second-line drug susceptibility testing of Mycobacterium tuberculosis complex. J Clin Microbiol. 2009;47:3630–4. doi: 10.1128/JCM.00803-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rüsch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. Multicenter laboratory valuation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol. 2006;44:688–92. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patlolla RR, Chougule M, Patel AR, Jackson T, Tata PN, Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Control Release. 2010;144:233–41. doi: 10.1016/j.jconrel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojanarat W, Nakpheng T, Thawithong E, Yanyium N, Srichana T. Levofloxacin-proliposomes: Opportunities for use in lung tuberculosis. Pharmaceutics. 2012;4:385–412. doi: 10.3390/pharmaceutics4030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brain JD, Knudson DE, Sorokin SP, Davis MA. Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ Res. 1976;11:13–33. doi: 10.1016/0013-9351(76)90107-9. [DOI] [PubMed] [Google Scholar]
- 24.Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97:163–80. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- 25.Sanna V, Kirschvink N, Gustin P, Gavini E, Roland I, Delattre L, et al. Preparation and in vivo toxicity study of solid lipid microparticles as carrier for pulmonary administration. AAPS Pharm Sci Tech. 2004;5:e27. doi: 10.1208/pt050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warheit DB, Carakostas MC, Hartsky MA, Hansen JF. Development of a short-term inhalation bioassay to assess pulmonary toxicity of inhaled particles: Comparisons of pulmonary responses to carbonyl iron and silica. Toxicol Appl Pharmacol. 1991;107:350–68. doi: 10.1016/0041-008x(91)90215-z. [DOI] [PubMed] [Google Scholar]
- 27. [Last accessed on 2015 Feb 28]. Available form: http://tbonline.info/posts/2011/11/29/drug-susceptibility-testing-mgit-system/
- 28.Venkataraman P, Paramasivan CN, Prabhakar R. In vitro activity of rifampicin, rifapentine and rifabutin against south Indian isolates of mycobacterium tuberculosis. Indian J Tub. 1993;40:17–20. [Google Scholar]
- 29.Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–42. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]