Abstract
Introduction:
Present study was designed to evaluate the protective effects of ethanolic extract of Dioscorea alata L. (DA) on hematological and biochemical changes in aniline-induced spleen toxicity in rats.
Materials and Methods:
Wistar rats of either sex (200–250g) were used in the study and each group contains six rats. Splenic toxicity was induced in rats by administration of aniline hydrochloride (AH; 100 ppm) in drinking water for a period of 30 days. Treatment groups received DA (50 and 100 mg/kg/day, po) along with AH. At the end of treatment period, various serum and tissue parameters were evaluated.
Result:
Rats administered with AH (100 ppm) in drinking water for 30 days showed a significant alteration in general parameters (organ weight, body weight, water intake, feed consumption, and fecal matter content), hematological parameters (red blood cell (RBC), white blood cell (WBC), and hemoglobin content), and biochemical parameters (total iron content, lipid peroxidation, reduced glutathione (GSH), and nitric oxide (NO) content) of spleen. Treatment with DA (50 and 100 mg/kg/day, po) for 30 days along with AH showed significant recovery in aniline-induced splenic toxicity.
Conclusion:
The present result showed that involvement of oxidative and nitrosative stress in aniline-induced splenic toxicity and DA protects the rats from the toxicity, which might be due to its antioxidant property and the presence of different phytochemicals.
Keywords: Aniline, antioxidants, dioscorea alata, spleen toxicity
INTRODUCTION
Aniline is a toxic aromatic amine, widely used in chemical industry, particularly in the manufacture of dyes, resins, varnishes, perfumes, pigments, herbicides, fungicides, explosives, isocyanates, hydroquinone, and rubber chemicals.[1] Chronic exposure to aniline leads to the development of splenomegaly, increased erythropoietic activity, hyper pigmentation, hyperplasia, and fibrosis.[2,3,4] The clinical symptoms of aniline exposure such as cyanosis, weakness, dizziness, headache, stupor, loss of coordination, and coma occur rapidly (within 1–3 h) after ingestion or skin contact.[3] Earlier studies have shown that aniline exposure leads to the formation of oxidative and nitrosative stress which is due to iron overload and induction of lipid peroxidation. The excess production of free radicals could attack proteins and nucleic acid leading to structural and functional changes in the spleen.[5]
Natural products having antioxidant property are gaining importance in disease prevention where oxidative stress has been involved. D. alata L. (DA); popularly known as white, water, or winged yam; is a tuberous root vegetable which belongs to the genus of Dioscorea. DAis mainly cultivated for its large white edible flesh roots which have high carbohydrate content. The yams contain steroid saponins along with sapogenins, such as diasogenin. It is reported to possess anticarcinogenic,[6] antimicrobial,[7] antihypertensive,[8] antioxidant,[9] and reno- and hepatoprotective activities.[10] The tubers of some Dioscorea species have been used in traditional medicine for diarrhea, diabetes, skin problems, and rheumatism. They are also used as tonic for the spleen, stomach, lung, and kidney disorders.[11] Based on its potent antioxidant activity and traditional uses, the present study was designed to assess the effect of (DA) on aniline exposure-induced spleen toxicity in rats by evaluating different biochemical parameters.
MATERIALS AND METHODS
Drugs and chemicals
Standardized extracts of (DA) was obtained as gift sample from Green Chem Pvt Ltd, Bangalore, India, along with certificate of analysis. Aniline hydrochloride (AH); 2,2’-dipyridy l, 5,5’- dithiobis-(2-nitrobenzoic acid); and N-(1-Napthyl) ethylenediamine dihydrochloride were purchased from HiMedia Lab. Pvt Ltd, Mumbai. All the other chemicals used in the study were of analytical grade and procured from standard supplier.
Animals
Wistar rats of either sex (200–250g) were used in the study and each group contains six rats (three male and three female). The animals were procured from LACSMI Biofarms, AUNDH (Pune). Rats were placed separately in polypropylene cages (four per cage) with paddy husk as bedding. The animals were maintained under standard laboratory conditions at temperature 23 ± 1°C, relative humidity 45–55, and 12 h light and 12 h dark cycles throughout the experiments. The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) of SSDJ College of Pharmacy, Neminagar, Chandwad (approval no. SSDJ/IAEC/2012/028).
Experimental protocol
Animals were divided into different groups (n = 6). Group I served as normal control and received 1% carboxymethyl cellulose (CMC), orally as vehicle. Group II rats received AH (100 ppm) in drinking water for 30 days. Group III and IV rats received AH (100 ppm) via drinking water and DA in two different doses (50 and 100 mg/kg/po) for 30 days. Group Vrats received DA (100 mg/kg/po) for 30 days.
Biochemical evaluation
General parameters like body weight, liver and spleen weight, water intake, feed intake, and fecal matter content (using metabolic cages) were studied in between and at the end of study.
At the end of treatment period, blood was withdrawn from retroorbital plexus using glass capillary and serum was separated. Blood sample was used for the estimation of hemoglobin (Sahli's hemometer method), and red blood cell (RBC) and white blood cell (WBC) using hemocytometer;[12] and serum sample was used for the estimation of iron content[13] and protein content using Span Diagnostic kit.
Assessment of tissue parameters
Tissues homogenization
The animals were euthanized using humane procedure, spleen and liver quickly transferred to ice-cold Tris-hydrochloric buffered saline (pH 7.4). It was blotted free of blood and tissue fluids, weighed on a Single Pan Electronic Balance (Precisa 205 ASCS). The organs were cross-chopped with surgical scalpel into fine slices, suspended in chilled0.25M sucrose solution, and quickly blotted on a filter paper. The tissues were then minced and homogenized in chilled Tris-hydrochloride buffer (10 mM, pH 7.4) to a concentration of 10% w/v. Prolonged homogenization under hypotonic condition was designed to disrupt, as far as possible, the structure of the cells so as to release soluble proteins. The homogenate was centrifuged at 10,000 rpm at 0°C for 15 min using Remi C-24 high speed cooling centrifuge. The clear supernatant was used for the determination of lipid peroxidation and reduced glutathione (GSH) level.
Assessment of lipid peroxidation
Two milliliter of the tissue homogenate (supernatant) was added to 2.0 ml of freshly prepared 10% w/v trichloroacetic acid (TCA) and the mixture was allowed to stand in an ice bath for 15 min. After 15 min, the precipitate was separated by centrifugation (2,000 rpm for 10 min) and 2.0 ml of clear supernatant solution was mixed with 2.0 ml of freshly prepared thiobarbituric acid (TBA). The resulting solution was heated in a boiling water bath for 10 min. It was then immediately cooled in an ice bath for 5 min. The color developed was measured at 532nm against reagent blank. Different concentrations (0–23 nM) of standard malondialdehyde (MDA; prepared from 1,1,3,3-tetraethoxypropane, obtained from Sigma Chemicals, St Louis, MO, USA) were taken and processed as above for standard graph. The values were expressed as nM of MDA/mg tissue.[14]
Assessment of GSH content
Equal volumes of tissue homogenate (supernatant) and 20% TCA were mixed. The precipitated fraction was centrifuged and to 0.25 ml of supernatant, 2ml of 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) reagent was added. The final volume was made up to 3ml with phosphate buffer. The color developed was read at 412 nm against reagent blank. A standard graph was plotted using standard Glutathione obtained from Sigma Chemicals, St Louis, MO, USA. Protein content was estimated using Lowry C and Folin's phenol reagent in the spleen. The amount of GSH was expressed as microgram of GSH/mg protein.[15]
Assessment of nitric oxide (NO) contents
To 1 ml of tissue homogenate, add 1 ml of Griess reagent and incubate it for 15 min at 37°C. Read the absorbance at 540 nm against a Griess reagent blank. Sodium nitrite solution was used as the standard. The amount of nitrite present in the samples was estimated from the standard curves obtained.[16]
Statistical analysis
All the values are presented as mean ± standard error of the mean (SEM). Statistical significance between more than two groups was tested using one-way analysis of variance (ANOVA) followed by the Tukey's multiple comparison test as appropriate using computer-based fitting program (Prism 5). Differences were considered to be statistical significant when P < 0.05.
RESULTS
Effect of DA on body weight, liver and spleen weight, water intake, feed intake, and fecal matter content
As shown in Table 1, at the end of 30th day body weight, water intake and feed consumption was monitored and it was found to be moderately changed in AH-treated group as compared to control group. Whereas fecal matter content was found to be unchanged in AH-treated group. Chronic (30 Days) treatment with DA (100 mg/kg, po) showed significant alteration in body weight, water intake, and feed consumption feed as compared to AH-treated rats.
Table 1.
Effect of Discorea alata L. on body weight, water intake, feed intake, and fecal matter content in aniline-treated rats after 30 days

Spleen and liver weight was monitored at the end of study [Table 2], that is, on 31st day. A significant increased (P < 0.001) in the weight of liver and spleen was observed in AH-treated animals as compared to control rats. Chronic treatment with DA (50 and 100 mg/kg) for 30 days significantly (P < 0.001) restored the weight of spleen and liver as compared to aniline-treated groups. DA (100 mg/kg) showed significant changes in spleen weight as compared to lower dose, that is, 50 mg/kg [Table 2].
Table 2.
Effect of Discorea alata L. on spleen and liver weight in aniline-treated rats

Effect of DA on RBC, WBC, and hemoglobin level
The RBCs count was significantly (P < 0.01) decreased and WBC count was significantly (P < 0.01) increased in aniline-treated rats as compared to control animals. The chronic (30 days) treatment with DA (100 mg/kg) showed a significant (P < 0.01) increase in RBC count and significant (P < 0.01) decrease in WBC count compared to aniline-treated rats. DA (50 mg/kg) was found to be ineffective in maintaining the level of RBC and WBC. DA alone-treated rats did not show any significant alteration in RBC and WBC count [Table 3].
Table 3.
Effect of Discorea alata L. on RBC and WBC count in aniline-treated rats

The level of hemoglobin was found to be significantly (P < 0.01) decreased in aniline-treated rats as compared to control group. Chronic treatment of DA (50 and 100 mg/kg) for 30 days significantly (P < 0.05 and P < 0.01, respectively) increased the level of hemoglobin as compared to aniline-treated rats [Figure 1].
Figure 1.
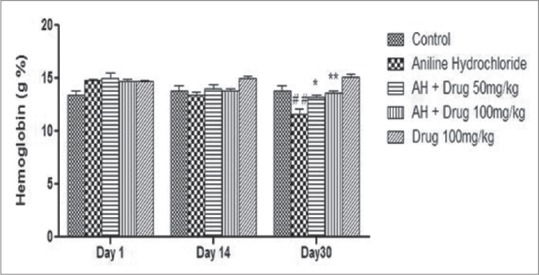
Effect of Discorea alata L. on hemoglobin level in aniline-treated rats. Values are expressed as mean ± standard error of the mean (SEM). Level of significance is considered as #P < 0.05, ##P < 0.01, ###P < 0.001 compared to control group. *P < 0.05, **P < 0.01, ***P < 0.001 compared to AH-treated group. AH = Aniline hydrochloride
Effect of DA on serum total protein and total iron content
Total protein contents and serum iron content were monitored and shown in Figures 2 and 3. A significant (P < 0.001) decreased in the level of serum protein and a significant (P < 0.001) increased in total iron content was observed in aniline-treated group as compared to control group. Treatment with DA (50 and 100 mg/kg) for 30 days showed significant (P < 0.001) increased in total protein contents and significant (P < 0.001) decreased in total iron content as compared to aniline-treated rats.
Figure 2.
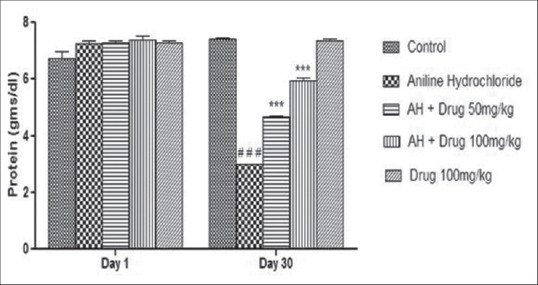
Effect of Discorea alata L. on protein content in aniline-treated rats. Values are expressed as mean ± SEM. Level of significance is considered as #P < 0.05, ##P < 0.01, ###P < 0.001 compared to control. *P < 0.05, **P < 0.01, ***P < 0.001 compared to AH-treated group
Figure 3.
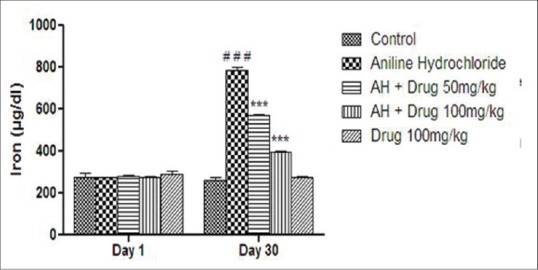
Effect of Discorea alata L. on iron content in aniline-treated rats. Values are expressed as mean ± SEM. Level of significance is considered as #P < 0.05, ##P < 0.01, ###P < 0.001 compared to control. *P < 0.05, **P < 0.01, ***P < 0.001 compared to AH-treated group
Effect of DA on tissue lipid peroxide (LPO), GSH, and serum NO levels
The level of endogenous antioxidants like LPO and GSH were measured in spleen and liver tissue homogenate; whereas, NO level was also monitored [Table 4]. LPO levels was found to be significantly (P < 0.001) increased in spleen and liver tissue in aniline-treated rats as compared to control group; whereas, GSH level was significantly decreased in both the tissue. Chronic treatment with DA (50 and 100 mg/kg) showed significant (P < 0.001) decrease in LPO levels and significant (P < 0.001) increased in GSH level as compared to aniline-treated group. DA (100 mg/kg) was found to be more effective in maintaining the antioxidant status in aniline-treated rats [Table 4]. NO levels was significantly (P < 0.001) increased in aniline-treated group as compared to control. Treatment with DA showed significant (P < 0.001) decrease in the level of NO as compared to aniline-treated rats [Table 4].
Table 4.
Effect of Discorea alata L. on lipid peroxidation, reduced glutathione, and nitric oxide levels in aniline-treated rats

DISCUSSION
Aniline and substituted aniline exposure leads to the development of selective splenic toxicity in rats. Studies have shown that exposure to aniline produces substantial increases in total iron content and oxidative stress in rats.[2,3,17] AH in rats causes an association between erythrocyte damage and the severity of the splenotoxicity. In the present study, splenic toxicity was induced by chronic administration of AH (100 ppm) via drinking water. Toxicity of spleen was confirmed by evaluating the hemoglobin level for alternate days for 30 days. At 30th day, hemoglobin level was significantly decreased indicating the development of spleen toxicity. Significant decreased in body weight, feed consumption, water intake, and fecal matter in aniline-treated rats might be due to toxicity of aniline which decreased the food consumption and can be directly correlated to decreased body weight. One of the important features of this study was increase in the weights of spleen (splenomegaly) and liver in AH-treated rats. DA is reported to play a major role in the treatment of various conditions such as hypertension, diabetes, hepatotoxicity, and inflammation. DA was reported to exhibit antioxidant activity which can modify serum lipid level.[18] In the present study, DA treatments reverse the changes in body weight, feed consumption, and water intake in AH-treated animals. The alteration of general parameters suggested the positive effect of DA in AH toxicity.
Changes in the level of hemoglobin, RBC, and WBC were observed. These changes are due to excessive deposition of phenylhydroxylamine (PHA)-modified erythrocytes.[19] The changes in WBC count might be due to excessive generation of oxidative and nitrosative stress. The changes observed in the blood parameters were similar to earlier studies on aniline and its derivatives.[5] Treatment with DA showed significant alteration of hemoglobin level and RBC and WBC content, which might be due to the strong antioxidant/free radical scavenging activity of DA.[18]
In the present study, AH-administered rats displayed significant increase in iron load and decrease in protein contents. Iron plays a significant role as a mediator of AH-induced splenotoxicity.[2,3] Aniline treatment causes remarkable accumulation of iron which may catalyze excessive formation of reactive oxygen species, which react with and damage proteins, nucleic acids, and lipids; leading to cellular dysfunction.[19] The aniline exposure leads to iron overload and induction of lipid peroxidation (oxidative stress) in the spleen. Lipid peroxidation and protein oxidation are at least two important early biochemical events in AH-induced splenic toxicity.
In the present study, markers of oxidative stress such as lipid peroxidation, glutathione, and NO were evaluated. AH-induced group showed a significant increase in LPO and NO (it forms a part of reactive nitrogen species); whereas, a significant decrease in GSH level in spleen and liver was observed. Oxidative stress plays vital role in splenic toxicity induced by aniline. Aniline induces lipid peroxidation and protein oxidation suggesting the role of oxidative stress in the splenic toxicity.[19] It suggested that involvement of oxidative stress in the splenic toxicity induced by aniline. Aniline treatment resulted in significant formation in LPO and GSH, suggested that lipid peroxidation produces structural modification of native proteins, which can alter their functional properties and, thus, contribute to splenic toxicity induced by aniline. Iron deposition in the spleen may result in the formation of reactive oxygen species, which can react with and damage cellular components.[2] The numerous biological activities of DA have been attributed to their rich phytochemicals including phenolics, saponins, polysaccharides, and mucilage. DA treatment showed the attenuation of splenic toxicity induced by aniline which might be due to its inhibitory potential of reactive oxygen species as well as potent free radical scavenging activity.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Khan MF, Boor PJ, GU Y, Alcock NW, Ansari GA. Oxidative stress in the splenotoxicity of aniline. Fundam Appl Toxicol. 1997;35:22–30. doi: 10.1006/faat.1996.2259. [DOI] [PubMed] [Google Scholar]
- 2.Firoze Khan M, Wu X, Wang J. Up-regulation of transforming factor- β1 in the spleen of aniline-treated rats. Toxicol Appl Pharmacol. 2003;187:22–8. doi: 10.1016/s0041-008x(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 3.Khan MF, Kannan S, Wang J. Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol Appl Pharmacol. 2006;210:86–93. doi: 10.1016/j.taap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Pauluhn J. Subacute inhalation toxicity of aniline in rats: Analysis of time-dependence and concentration-dependence of hematotoxic and splenic effects. Toxicol Sci. 2004;81:198–215. doi: 10.1093/toxsci/kfh187. [DOI] [PubMed] [Google Scholar]
- 5.Bus JS, Popp JA. Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally related compounds. Food Chem Toxicol. 1987;25:619–26. doi: 10.1016/0278-6915(87)90024-x. [DOI] [PubMed] [Google Scholar]
- 6.Miyoshi N, Nagasawa T, Mabuchi R, Yasui Y, Wakabayashi K, Tanaka T, et al. Chemoprevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by freezed-dried yam sanyaku and its constituents diosgenin. Cancer Prev Res (Phila) 2011;4:924–34. doi: 10.1158/1940-6207.CAPR-10-0279. [DOI] [PubMed] [Google Scholar]
- 7.Atindehou KK, Kone M, Terreaux C, Traore D, Hostettmann K, Dosso M. Evaluation of the antimicrobial potential of medicinal plants from the Ivory Coast. Phytother Res. 2002;16:497–502. doi: 10.1002/ptr.970. [DOI] [PubMed] [Google Scholar]
- 8.Liu YH, Lin YS, Liu DZ, Han CH, Chen CT, Fan M, et al. Effect of different types of yam (Dioscorea alata) products on the blood pressure of spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2009;73:1371–6. doi: 10.1271/bbb.90022. [DOI] [PubMed] [Google Scholar]
- 9.Farombi EO, Britton G, Emerole GO. Evaluation of the antioxidant activity and partial characterization of extracts from browned yam flour diet. Food Res Int. 2000;33:493–9. [Google Scholar]
- 10.Lee SC, Tsai CC, Chen JC, Lin CC, Hu ML, Lu S. The evaluation of reno- and hepatoprotective effects of huai-shan-yao (Rhizome Dioscoreae) Am J Chin Med. 2002;30:609–16. doi: 10.1142/S0192415X02000624. [DOI] [PubMed] [Google Scholar]
- 11.Dykman KD, Tone C, Ford C, Dykman RA. The effect of nutritional supplement on the symptoms of fibromyalgia and chronic fatigue syndrome. Integr Physiol Behav Sci. 1998;33:61–71. doi: 10.1007/BF02688676. [DOI] [PubMed] [Google Scholar]
- 12.Godkar PB, Godkar DP. 2nd ed. Mumbai, India: Published by Balani Publishing House; 2008. Determination of Hemoglobin. Text Book of Medical Laboratory Technology; pp. 726–31. [Google Scholar]
- 13.Ramsay WN. The determination of total iron-binding capacity of serum. Clin Chim Acta. 1957;2:221–6. doi: 10.1016/0009-8981(57)90106-7. [DOI] [PubMed] [Google Scholar]
- 14.Slater TF, Sawyer BC. The stimulatory effect of carbon tetrachloride and other halogenoalkanes or peroxidative reaction in the rat liver functions in vitro. General features of the systems used. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 16.Guevara I, Iwanejko J, Dembinska-kiec A, Pankiewicz J, Wanat A, Anna P, et al. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta. 1998;274:177–88. doi: 10.1016/s0009-8981(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 17.Khan MF, Wu X, Ansari GA. Contribution of nitrosobenzene to splenic toxicity of aniline. J Toxicol Environ Health A. 2000;60:263–73. [PubMed] [Google Scholar]
- 18.Lubag A, Laurena C, Mendoza E. Antioxidant of purple and white greater yam (Dioscorea alata L.) varieties from the Philippines. Philippine J Sci. 2008;137:61–7. [Google Scholar]
- 19.Khan MF, Green SM, Ansari GA, Boor PJ. Phenylhydroxylamine: Role in aniline-associated splenic oxidative stress and induction of subendocardial necrosis. Toxicol Sci. 1998;42:64–71. doi: 10.1006/toxs.1997.2420. [DOI] [PubMed] [Google Scholar]


