Abstract
BACKGROUND
Critical illness is often accompanied by hypercortisolemia, which has been attributed to stress-induced activation of the hypothalamic–pituitary–adrenal axis. However, low corticotropin levels have also been reported in critically ill patients, which may be due to reduced cortisol metabolism.
METHODS
In a total of 158 patients in the intensive care unit and 64 matched controls, we tested five aspects of cortisol metabolism: daily levels of corticotropin and cortisol; plasma cortisol clearance, metabolism, and production during infusion of deuterium-labeled steroid hormones as tracers; plasma clearance of 100 mg of hydrocortisone; levels of urinary cortisol metabolites; and levels of messenger RNA and protein in liver and adipose tissue, to assess major cortisol-metabolizing enzymes.
RESULTS
Total and free circulating cortisol levels were consistently higher in the patients than in controls, whereas corticotropin levels were lower (P<0.001 for both comparisons). Cortisol production was 83% higher in the patients (P=0.02). There was a reduction of more than 50% in cortisol clearance during tracer infusion and after the administration of 100 mg of hydrocortisone in the patients (P≤0.03 for both comparisons). All these factors accounted for an increase by a factor of 3.5 in plasma cortisol levels in the patients, as compared with controls (P<0.001). Impaired cortisol clearance also correlated with a lower cortisol response to corticotropin stimulation. Reduced cortisol metabolism was associated with reduced inactivation of cortisol in the liver and kidney, as suggested by urinary steroid ratios, tracer kinetics, and assessment of liver-biopsy samples (P≤0.004 for all comparisons).
CONCLUSIONS
During critical illness, reduced cortisol breakdown, related to suppressed expression and activity of cortisol-metabolizing enzymes, contributed to hypercortisolemia and hence corticotropin suppression. The diagnostic and therapeutic implications for critically ill patients are unknown. (Funded by the Belgian Fund for Scientific Research and others; ClinicalTrials.gov numbers, NCT00512122 and NCT00115479; and Current Controlled Trials numbers, ISRCTN49433936, ISRCTN49306926, and ISRCTN08083905.)
Critical illness, an example of severe acute physical stress, is often accompanied by hypercortisolemia that is proportionate to the severity of illness.1,2 This observation has traditionally been attributed to stress-induced activation of the hypothalamic–pituitary–adrenal (HPA) axis and increased corticotropin-driven cortisol production.3 However, this stress response may not be sufficient for a good prognosis in patients with relative adrenal insufficiency.4-7 Moreover, Vermes et al.8 reported only transiently elevated levels of corticotropin during critical illness, whereas cortisol levels remained high, a paradoxical dissociation between cortisol and corticotropin levels that has also been observed in other stress conditions.9
In addition to alternative activators of cortisol production, such as proinflammatory cytokines,9,10 another explanation for hypercortisolemia in the presence of suppressed corticotropin could be reduced cortisol removal. The principal routes of cortisol clearance occur in the liver (through A-ring reductases [5β-reductase and 5α-reductase]) and kidney (through 11β-hydroxysteroid dehydrogenase type 2 [11β-HSD2], which converts cortisol to cortisone). This removal is offset by the regeneration of cortisol from cortisone through 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in liver and adipose tissue.11,12 The regulation of these enzymes is complex.12,13 In addition, in critically ill patients, elevated circulating levels of bile acids could be powerful suppressors of the expression and activity of cortisol-metabolizing enzymes.14-17 We hypothesized that cortisol metabolism is reduced during critical illness, contributing to sustained hypercortisolemia with enhanced negative-feedback inhibition of corticotropin.
METHODS
STUDY DESIGN
To test our hypothesis, we performed five clinical studies comparing 158 consecutively screened patients in the intensive care unit (ICU) with 64 demographically matched controls (Table 1, and Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).18,19 In these studies, we measured daily levels of corticotropin and cortisol; plasma cortisol clearance, metabolism, and production during infusion of deuterium-labeled steroid hormones as tracers; plasma clearance of 100 mg of hydrocortisone; urinary cortisol metabolites; and levels of messenger RNA (mRNA) and protein in liver and adipose tissue to assess major cortisol-metabolizing enzymes.
Table 1. Characteristics of the Patients and Controls at Baseline in the Five Studies.*.
| Characteristic | Plasma Corticotropin–Cortisol Time Course | D4-Cortisol Tracer | Plasma Clearance of Therapeutic Cortisol | Cortisol-Metabolizing Enzymes in Urine | Cortisol-Metabolizing Enzymes in Tissue | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (N = 47) | Controls (N = 12) | Patients (N = 11) | Controls (N = 9) | Patients (N = 20) | Controls (N = 8) | Patients (N = 36) | Controls (N = 15) | Patients (N = 44) | Controls (N = 20) | |
| Male sex — no. (%) | 27 (57) | 5 (42) | 7 (64) | 5 (56) | 8 (40) | 5 (62) | 25 (69) | 9 (60) | 28 (64) | 14 (70) |
| Age — yr | 63.7±18.0 | 60.4±4.7 | 68.5±8.3 | 62.6±4.1 | 64.4±13.2 | 60.3±4.3 | 66.4±10.8 | 61.1±8.3 | 71.2±12.0 | 70.4±11.6 |
| Body-mass index† | 26.2±4.2 | 24.4±3.4 | 28.5±4.9 | 25.2±4.1 | 26.3±5.8 | 24.1±1.8 | 26.7±5.4 | 25.2±5.3 | 24.8±3.6 | 25.0±2.6 |
| APACHE II score‡ | 28±10 | 29±11 | 34±7 | 31±6 | 29±9 | |||||
| Systemic inflammatory response syndrome — no. (%)§ | 20 (43) | 7 (64) | 20 (100) | 22 (61) | 35 (80) | |||||
| Sepsis — no. (%) | 16 (34) | 5 (45) | 15 (75) | 17 (47) | 26 (59) | |||||
| C-reactive protein — mg/liter | 107±86 | 199±131 | 143±106 | 109±81 | 185±91 | |||||
| Inotropes administered — no. (%) | 6 (13) | 3 (27) | 8 (40) | 2 (7) | 24 (55) | |||||
| Vasopressors administered — no. (%) | 17 (36) | 7 (64) | 20 (100) | 19 (53) | 30 (68) | |||||
| 24-hr urine output — liters | 1.8±1.0 | 1.6±0.5 | 0.9±0.7 | 1.7±0.7 | 1.0±1.3 | |||||
| Blood lactate — mmol/liter | 0.93±0.28 | 1.18±0.35 | 2.54±2.84 | 1.27±1.85 | 3.50±1.16 | |||||
| Treatment with opioids — no. (%) | 28 (60) | 10 (91) | 15 (75) | 16 (44) | 34 (77) | |||||
| Treatment with therapeutic anticoagulation — no. (%) | 5 (11) | 0 | 0 | 0 | 2 (6) | |||||
| Days in ICU — no. | 18±16 | 26±16 | 24±32 | 22±25 | 14±17 | |||||
| Day of sampling in ICU — no. | 1 to 7 | 7±5 | 9±15 | 10±17 | 14±17 | |||||
| Death in ICU — no. (%) | 6 (13) | 2 (18) | 7 (35) | 5 (14) | 44 (100) | |||||
Plus–minus values are means ±SD. ICU denotes intensive care unit.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Scores on the Acute Physiology and Chronic Health Evaluation II (APACHE II) range from 0 to 71, with higher scores indicating a greater severity of illness.18
The presence of the systemic inflammatory response syndrome was determined by the criteria in Bone et al.19
Excluded from the study were patients and controls who had predisposing risk factors for HPA-axis dysfunction, who were receiving contraindicated drugs, or who were undergoing extracorporeal membrane oxygenation or therapy with a circulatory-assist device (for details, see Methods Sections S1 and S2 and Table S2 in the Supplementary Appendix). All samples were stored at −80°C.
All study protocols were approved by the institutional review board at KU Leuven. The study protocol and statistical analysis plan are available at NEJM.org. No commercial entity provided support for this study. All participants or their representatives provided written informed consent.
CORTICOTROPIN–CORTISOL TIME COURSE
Morning blood samples were collected daily from 47 patients for 7 days after admission and from 12 controls (Table 1). Samples were collected in prechilled EDTA tubes, placed on ice, and centrifuged at 4°C. Total levels of cortisol (Immunotech) and transcortin (DiaSource) were quantified on radioimmunoassay and corticotropin levels on double-monoclonal immunoradiometric assays (Brahms Diagnostics).20,21
PLASMA CORTISOL CLEARANCE AND PRODUCTION
A total of 11 patients and 9 controls (Table 1) received intravenous deuterated cortisol (D4-cortisol, Cambridge Isotopes)22 as a 0.7-mg priming bolus, followed by continuous infusion of 0.35 mg per hour for 3 hours between 10 a.m. and 1 p.m. to achieve steady state in the circulation.11,23,24 After 100 minutes of D4-cortisol infusion, participants also received deuterated cortisone (D2-cortisone, Cambridge Isotopes)25 as a 0.08-mg priming bolus, followed by continuous infusion of 0.1053 mg per hour. Blood samples were obtained 5 minutes before D4-cortisol infusion and at intervals of 60, 120, 140, 160, 165, 170, 175, and 180 minutes after infusion. In preinfusion samples, plasma levels of corticotropin were measured (as described above), and levels of tumor necrosis factor α (TNF-α) and interleukin-6 were measured by means of enzyme-linked immunosorbent assay (Invitrogen). Plasma cortisol was quantified on liquid chromatography–tandem mass spectrometry. Tracer analyses and calculations of cortisol kinetics are described in Methods Section S3 in the Supplementary Appendix.11,25 Ten patients un derwent a short corticotropin-stimulation test (injection of 250 μg) within 24 hours after tracer infusion.
PLASMA CLEARANCE OF A THERAPEUTIC CORTISOL DOSE
A total of 20 patients and 8 controls (Table 1) received a 100-mg intravenous bolus of hydrocortisone (Solu-Cortef). Blood samples were taken every 10 minutes for 1 hour and then every hour for 4 hours. Cortisol was quantified on radioimmunoassay, as described above, and pharmacokinetic characteristics were calculated (as described in Methods Section S4 in the Supplementary Appendix).
ACTIVITY OF CORTISOL-METABOLIZING ENZYMES
We collected 24-hour urine samples from 36 patients and 15 controls, followed by a morning blood sample (Table 1). Patients requiring renal-replacement therapy were excluded. We used liquid chromatography–tandem mass spectrometry to estimate levels of urinary cortisol, cortisone, 5α-tetrahydrocortisol, 5β-tetrahydrocortisol, and tetrahydrocortisone, which were quantified on gas chromatography–mass spectrometry.26 Activities of A-ring reductases and 11β-HSD enzymes were estimated (for details, see Methods Section S5 in the Supplementary Appendix).26-30 Total bile acids were quantified by means of enzymatic cycling (Diazyme Laboratories).
TISSUE EXPRESSION OF CORTISOL-METABOLIZING ENZYMES
A morning blood sample was obtained on the last ICU day, and liver and adipose tissue samples were obtained immediately after death from 44 patients (see Methods Section S6 in the Supplementary Appendix). For comparison, tissue-biopsy and blood samples were collected from 20 controls undergoing elective abdominal surgery (Table 1). Tissue samples were snap-frozen in liquid nitrogen.
Total tissue mRNA was purified, complementary DNA was quantified in real time, and the results were analyzed (for details, see Methods Section S7 in the Supplementary Appendix). Western blotting was performed and the results were analyzed (for details, see Methods Section S8 in the Supplementary Appendix). Enzyme activity of 11β-HSD1 and 5β-reductase was determined in vitro in hepatic tissue, and activity of 11β-HSD1 was determined in adipose tissue.31
Total cortisol was quantified with the use of chemiluminescence (Immulite 2000, Diagnostic Products), and total bile acids were measured, as described above.
STATISTICAL ANALYSIS
All data are presented as means ±SD or medians with interquartile ranges. We used Wilcoxon rank-sum tests for data that did not have a normal distribution and unpaired Student’s t-tests for normally distributed data. Proportions were compared with the use of chi-square tests. Associations were analyzed with linear regression after transformation to approximate normal distribution when required. We used analysis of variance to calculate the significance of Pearson’s determination coefficients (r2). A two-sided P value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed with the use of JMP software, version 9.0.0 (SAS Institute).
RESULTS
PATIENTS VERSUS CONTROLS
Differences between patients and controls applied to survivors and nonsurvivors and were not influenced by illness severity, illness duration at the time of blood or tissue sampling, or status with respect to the use of opioids or anticoagulant agents (Tables S2 through S6 in the Supplementary Appendix).
PLASMA CORTICOTROPIN AND CORTISOL TIME COURSE
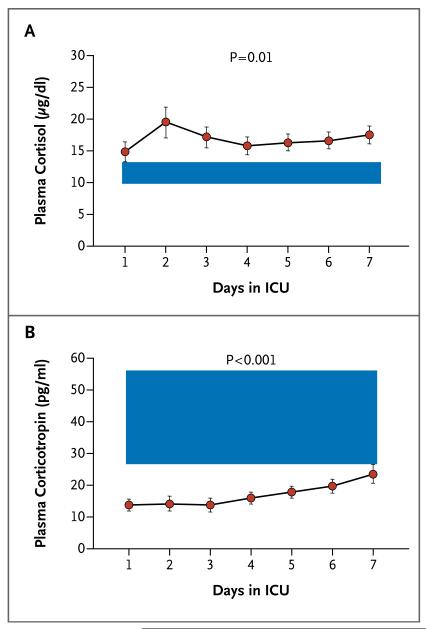
In the presence of elevated total cortisol levels (averaged over 7 days, P=0.01), patients had lower corticotropin levels than did controls (P<0.001) (Fig. 1). Levels of cortisol did not correlate with corticotropin levels. Mean levels of transcortin were lower in the patients than in the controls, with values for patients on day 1 of 31.6±10.4 mg per liter (P=0.001) and on day 7 of 47.4±11.6 mg per liter (P<0.001), as compared with a single measure of 67.8±8.7 mg per liter in the controls. As a consequence, calculated median free cortisol levels were higher in the patients than in the controls, with values for patients on day 1 of 1.0 μg per deciliter (interquartile range, 0.4 to 2.7 [28 nmol per liter; interquartile range, 11 to 74]) and on day 7 of 0.9 μg per deciliter (interquartile range, 0.6 to 1.6 [25 nmol per liter; interquartile range, 17 to 44]), as compared with a single measure of 0.4 μg per deciliter (interquartile range, 0.3 to 0.4 [11 nmol per liter; interquartile range, 8 to 11]) in the controls (P≤0.001 for both comparisons).
Figure 1. Dissociation between Corticotropin and Cortisol Levels among Patients in the Intensive Care Unit (ICU).
Shown are mean values for cortisol (Panel A) and corticotropin (Panel B) in 47 patients from day 1 to day 7 in the ICU. The shaded area represents the interquartile range of values in 12 healthy controls. The overall mean cortisol levels over the 7-day period were 16.8±7.8 μg per deciliter (464±215 nmol per liter) for patients and 11.9±2.3 μg per deciliter (328±63 nmol per liter) for controls (P=0.01). The overall mean corticotropin levels over the 7-day period were 16.9±9.5 pg per milliliter (4±2 pmol per liter) for patients and 49.6±37.9 pg per milliliter (11±8 pmol per liter) for controls (P<0.001). To convert values for cortisol to nanomoles per liter, multiply by 27.6. To convert values for corticotropin to picomoles per liter, multiply by 0.22. The I bars indicate standard errors.
CORTISOL KINETICS DURING INFUSION OF STABLE ISOTOPE TRACERS
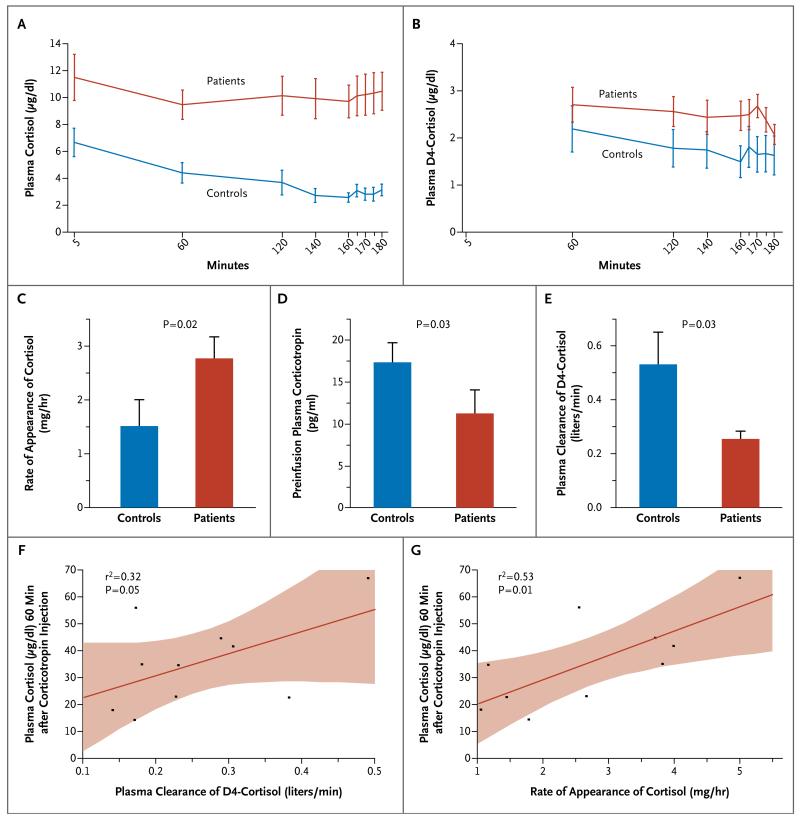
Steady-state levels and enrichments of D4-cortisol and D2-cortisone were achieved from 60 minutes onward. In the patients, as compared with the controls, endogenous cortisol levels were increased by a factor of 3.5 (P<0.001), and the rate of appearance of cortisol (hereafter referred to as cortisol production) was increased by 83% (P=0.02), but preinfusion corticotropin levels were reduced by 65% (P=0.03) (Fig. 2A, 2C, and 2D). There was no significant difference in cortisol production between patients who were treated with catecholamines and those who were not treated with catecholamines (2.7±1.3 mg per hour and 2.9±1.4 mg per hour, respectively; P=0.86).
Figure 2. Results of Infusion of D4-Cortisol Tracer.
Shown are mean plasma levels of endogenous cortisol (Panel A) and D4-cortisol tracer (Panel B), which reached steady state during the study period, in 11 patients and 9 controls. The I bars indicate standard errors. To convert values for D4-cortisol to nanomoles per liter, multiply by 27.3. Also shown are the mean rate of appearance of cortisol (Panel C), mean preinfusion plasma corticotropin levels (Panel D), and mean D4-cortisol clearance (Panel E). In Panels C, D, and E, the T bars indicate standard errors. Plasma clearance of D4-cortisol (Panel F) and the rate of appearance of cortisol (Panel G) were correlated with plasma cortisol responses, measured for 60 minutes after the injection of corticotropin (250 μg) in the patients. In Panels F and G, the red lines indicate the regression lines, and the shaded areas represent 95% confidence intervals.
In the patients, as compared with the controls, plasma levels of TNF-α were increased by 49% (P=0.001), and plasma interleukin-6 levels were increased by more than a factor of 200 (P<0.001). Cytokine levels correlated positively with cortisol production, with a coefficient of determination of 0.26 for TNF-α (P=0.02) and 0.30 for interleukin-6 (P=0.01); there also was a positive correlation after correction for corticotropin levels, with a coefficient of determination of 0.28 for TNF-α (P=0.03) and 0.30 for interleukin-6 (P=0.02). Consistent with these correlations was the finding that patients with the systemic inflammatory response syndrome19 had 90% higher cortisol production than those who did not have the syndrome (3.4±1.1 mg per hour vs. 1.8±1.1 mg per hour, P=0.04); the latter value did not differ significantly from that of controls (P=0.39).
In the patients, as compared with the controls, D4-cortisol levels during infusion were 57% higher (P=0.04) and plasma clearance of D4-cortisol was reduced by 53% (P=0.03) (Fig. 2B and 2E). Reduced cortisol clearance did not correlate with markers of organ perfusion (Table S7 in the Supplementary Appendix).
We tested whether decreased cortisol clearance was associated with evidence of adrenal insufficiency in relation to reduced corticotropin stimulation. A lower cortisol response to corticotropin stimulation in the patients correlated both with lower cortisol production and with lower D4-cortisol clearance (Fig. 2F and 2G). Patients with a cortisol response to corticotropin of less than 21 μg per deciliter (579 nmol per liter), a level that is considered indicative of absolute adrenal insufficiency,32 had substantially lower D4-cortisol plasma clearance (0.15±0.02 liters per minute) than did patients who had a normal response to corticotropin (0.28±0.11 liters per minute; P=0.01). Cortisol production in patients with adrenal insufficiency (1.4±0.5 mg per hour) was indistinguishable from that in controls, whereas it was elevated (3.0±1.3 mg per hour) in patients with a normal response to corticotropin (P=0.03). Circulating levels of cortisol before corticotropin stimulation were similar in these two groups of patients (9.5±1.5 μg per deciliter [262±41 nmol per liter] and 11.5±6.5 μg per deciliter [317±179 nmol per liter], respectively; P=0.51).
Tracer analysis also allowed dissection of the contribution of 11β-HSD enzymes to altered cortisol clearance.11,25 The patients had a lower net rate of appearance of cortisone than the controls (0.07±0.02 mg per hour for every microgram per deciliter vs. 0.14±0.07 mg per hour for every microgram per deciliter, P=0.01). However, there was no significant between-group difference in the level of regeneration of cortisol by 11β-HSD1, as measured by the rate of appearance of D3-cortisol (0.42±0.12 mg per hour and 0.49±0.12 mg per hour, respectively; P=0.23). These findings are consistent with impaired conversion of cortisol to cortisone by 11β-HSD2.
PLASMA CLEARANCE OF CORTISOL
We tested whether altered cortisol metabolism in critically ill patients occurs at supraphysiological concentrations after the administration of therapeutic hydrocortisone. The calculated plasma clearance after the administration of 100 mg of hydrocortisone in the patients (0.04±0.02 liters per minute) was 60% lower than that in the controls (0.10±0.02 liters per minute, P<0.001), with a distribution volume that was 37% higher (22±10 liters vs. 16±5 liters, P=0.03) (Fig. S1 in the Supplementary Appendix). Cortisol clearance was even more suppressed in nonsurvivors (0.02±0.01 liters per minute) than in survivors (0.05±0.03 liters per minute, P=0.03).
ACTIVITY OF CORTISOL-METABOLIZING ENZYMES
We addressed the contribution of 11β-HSD enzymes and A-ring reductases to impaired cortisol clearance in the patients. There were no correlations between urinary levels of cortisol metabolites and creatinine clearance or daily urine output, which was similar in patients and controls (1936±655 ml vs. 1713±734 ml in 24 hours, P=0.29).33,34 Analysis on liquid chromatography– tandem mass spectrometry suggested substantial changes in relative excretion of cortisol metabolites (Table S8 in the Supplementary Appendix). These findings were further quantified on gas chromatography–mass spectrometry, which showed that the daily urinary excretion of cortisol was increased by a factor of 3.2 in the patients, as compared with the controls, and urinary excretion of cortisone was 73% higher in the patients (P<0.001 for both comparisons). In contrast, levels of 5α-tetrahydrocortisol and 5β-tetrahydrocortisol were similar in patients and controls, whereas the level of tetrahydrocortisone was reduced by 69% in the patients (P<0.001) (Table S9 in the Supplementary Appendix).
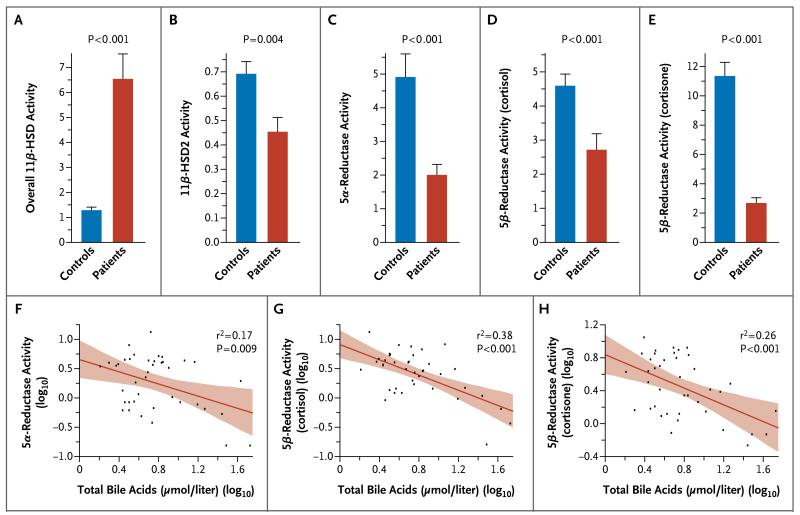
Decreased levels of 11β-HSD2 were confirmed in the patients, in whom the ratio of urinary cortisone to cortisol, which reflects the renal 11β-HSD2 level, and the ratio of 5α-tetra hy drocortisol and 5β-tetrahydrocortisol combined to tetrahydrocortisone, which reflects the conversion of cortisol to cortisone, was markedly altered in favor of cortisol (Fig. 3A and 3B). In addition, the ratios reflecting activities of A-ring reductases were markedly reduced in the patients, as compared with the controls (Fig. 3C, 3D, and 3E). Nonsurvivors had lower estimated median 5α-reductase activity than survivors (ratio of 5α-tetrahydrocortisol to cortisol, 0.6 [interquartile range, 0.2 to 0.8] vs. 1.4 [interquartile range, 0.8 to 4.2], P=0.01) but similar 5β-reductase activity (ratio of 5β-tetrahydrocortisol to cortisol, 1.2 [interquartile range, 0.2 to 2.5] and 1.9 [interquartile range, 1.3 to 3.3], respectively; P=0.14).
Figure 3. Activity of Cortisol-Metabolizing Enzymes, as Estimated from Ratios of Cortisol Metabolites in 24-Hour Urine Samples.
Enzyme activities were estimated in 36 patients and 15 controls on the basis of urinary metabolites quantified with the use of gas chromatography–mass spectrometry. The overall activity of 11β-hydroxysteroid dehydrogenases (11β-HSDs) was calculated as the ratio of a combination of 5α-tetrahydrocortisol and 5β-tetrahydrocortisol to tetrahydrocortisone (Panel A), which reflects the relative balance of the cortisone–cortisol interconversion. The activity of renal 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) was estimated by calculating the ratio of cortisone to cortisol (Panel B). The activity of 5α-reductase was estimated by calculating the ratio of 5α-tetrahydrocortisol to cortisol (Panel C). The activity of 5β-reductase was estimated by calculating the ratio of 5β-tetrahydrocortisol to cortisol (in Panel D) and the ratio of tetrahydrocortisone to cortisone (Panel E). In Panels A through E, the bars represent means, and the T bars standard errors. The lower panels show the correlations of the level of total bile acids (in log10 values) with the activity of 5α-reductase as estimated by calculating the ratio of 5α-tetrahydrocortisol to cortisol (Panel F), the activity of 5β-reductase activity as estimated by calculating the ratio of 5β-tetrahydrocortisol to cortisol (Panel G), and the activity of 5β-reductase as estimated by calculating the ratio of tetrahydrocortisone to cortisone (Panel H). The red lines in the three lower panels indicate the regression lines, and the shaded areas represent 95% confidence intervals.
Levels of total bile acids were increased by a factor of 2.4 in the patients, as compared with the controls (10.9±2.5 μmol per liter [4.3±1.0 μg per milliliter] vs. 4.6±1.8 μmol per liter [1.8±0.7 μg per milliliter]) (P=0.04) and correlated inversely with urinary ratios reflecting A-ring reductase activities (Fig. 3F, 3G, and 3H).
TISSUE EXPRESSION OF CORTISOL-METABOLIZING ENZYMES
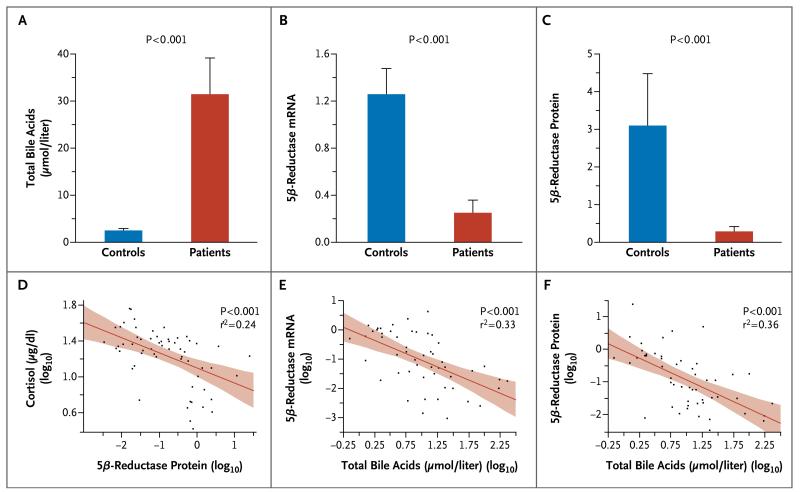
To test the inferences from urinary metabolite ratios and tracer kinetics, we studied cortisol-metabolizing enzymes in tissue-biopsy samples. Circulating levels of total cortisol in the patients were three times as high as those in the controls (24.3 μg per deciliter; interquartile range, 19.6 to 30.3 [670 nmol per liter; interquartile range, 541 to 836] vs. 7.4 μg per deciliter; interquartile range, 4.9 to 14.7 [204 nmol per liter; interquartile range, 135 to 406], P<0.001). Levels of total bile acids were substantially higher in the patients than in the controls (P<0.001) (Fig. 4A) and correlated positively with cortisol levels (r2=0.26, P<0.001).
Figure 4. Tissue Expression of Cortisol-Metabolizing Enzymes in Relation to Circulating Levels of Cortisol and Bile Acids.
Shown are the results, for 44 patients and 20 controls, of studies evaluating total bile acids (Panel A), 5β-reductase messenger RNA (mRNA) (Panel B), and 5β-reductase protein (Panel C). In Panels A, B, and C, the bars represent means, and the T bars standard errors. The lower panels show the correlations of the 5β-reductase protein level or total bile acid level (in log10 values) with the plasma level of cortisol (Panel D), 5β-reductase mRNA (Panel E), and 5β-reductase protein (Panel F). The red lines in the three lower panels indicate regression lines, and the shaded areas represent 95% confidence intervals. The mRNA data, which have been normalized for glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) expression, are expressed as the factor difference from the mean value for the controls. The protein data, which have been normalized for cytokeratin 18 protein expression, are also expressed as the factor difference from the mean value for the controls.
In liver specimens from the patients, the mRNA level and protein expression of 5β-reductase were reduced by 80 to 91%, as compared with controls (Fig. 4B and 4C), and enzyme activity was reduced by 58% (0.022 pmol per minute per milligram [interquartile range, 0.012 to 0.029]) vs. 0.053 pmol per minute per milligram [interquartile range, 0.033 to 0.067], P=0.01). The mRNA level correlated positively with protein expression of 5β-reductase (r 2=0.46, P<0.001), which in turn had a negative correlation with circulating cortisol levels (Fig. 4D). The level of total bile acids correlated inversely with the mRNA level and protein expression of 5β-reductase (Fig. 4E and 4F).
The liver mRNA level of 5α-reductase was reduced by 77% in patients, as compared with controls (ratio, 0.23 [interquartile range, 0.12 to 0.36] vs. 1.01 [interquartile range, 0.66 to 1.65], P<0.001). (The mRNA data, which have been normalized for glyceraldehyde-3-phosphate-dehydrogenase [GAPDH] expression, are expressed as the factor difference from the mean value for the controls.) The hepatic mRNA 5α-reductase level also correlated negatively (albeit more weakly than 5β-reductase) with circulating bile acids (r2=0.22, P<0.001) and cortisol (r2=0.13, P=0.005).
Levels of 11β-HSD1 mRNA in liver were reduced by 80% in patients, as compared with controls (ratio, 0.20 [interquartile range, 0.12 to 0.37] vs. 1.01 [interquartile range, 0.62 to 1.36], P<0.001), whereas 11β-HSD1 protein and enzyme activity were unaltered. These levels were unrelated to the elevated cortisol levels, despite an inverse correlation with bile acids (r2=0.26 for mRNA, P<0.001; r2=0.16 for protein, P=0.005).
Levels of 11β-HSD1 mRNA in adipose tissue were reduced by 73% in patients, as compared with controls (ratio, 0.27 [interquartile range, 0.16 to 0.46] vs. 1.00 [interquartile range, 0.60 to 2.09], P=0.003) and by 82% in subcutaneous adipose tissue (ratio, 0.18 [interquartile range, 0.07 to 0.30] vs. 1.00 [interquartile range, 0.56 to 2.17], P<0.001). These levels were unrelated to elevated cortisol levels, despite an inverse correlation with bile acids (r2=0.28 for subcutaneous adipose tissue, P=0.008). Levels of 11β-HSD1 protein and in vitro enzyme activity in adipose tissue were unaltered.
DISCUSSION
In our study, elevated cortisol levels in critically ill patients were only partially explained by an increase of 83% in cortisol production, as compared with controls. Since corticotropin levels were paradoxically low in the patients, a pituitary-independent mechanism was suggested. We showed that impaired cortisol clearance contributed to hypercortisolemia, as suggested by studies conducted in the 1950s before the advent of ICUs.35,36 Reduced cortisol clearance could be explained by suppressed levels of A-ring reductases and 11β-HSD2.
In other circumstances of reduced cortisol metabolism, such as congenital 11β-HSD2 deficiency,37 negative feedback on the HPA-axis results in compensatory down-regulation of cortisol secretion, with lower corticotropin levels and adrenocortical atrophy. Elevated levels and production of cortisol in patients being treated in the ICU must reflect an ongoing stimulus to cortisol secretion. In the presence of low corticotropin levels, increased sensitivity to corticotropin might play a role. However, this seems unlikely during critical illness, since cortisol responses to corticotropin stimulation are not increased. More likely candidates are neuropeptides, catecholamines, or cytokines,10 especially since cytokine levels were substantially elevated and were positively correlated with cortisol production. The role of cytokines is further corroborated by the finding that only patients with pronounced inflammation had a level of cortisol production that was higher than the level in controls, whereas cortisol clearance was suppressed regardless of the inflammatory status. It remains to be investigated whether adrenocortical atrophy is associated with a sustained reduction in the activation of corticotropin receptors on adrenocortical cells in patients with reduced cortisol clearance. However, such a mechanism would explain the high incidence of adrenal vascular instability in surgical patients with prolonged critical illness38 and is supported by our observation that the patients with the least response to corticotropin stimulation had the lowest cortisol production and the lowest cortisol clearance, despite a similar baseline cortisol level, as compared with the other patients.
Although in isolation each of the separate studies is suggestive, the corroboration of the findings with the use of multiple approaches is helpful for making conclusions. Urinary excretion of cortisol was elevated in the critically ill patients, but cortisol metabolite levels were normal or low, despite increased cortisol production; this pattern is quite different from that in Cushing’s syndrome.29,39 The ratios of urinary cortisol metabolites suggested reduced activity of the A-ring reductases in the critically ill patients and a net suppression of cortisol-to-cortisone conversion. This interpretation was corroborated by low mRNA and protein levels and low activity of the A-ring reductases in liver-biopsy samples. Unfortunately, kidney samples were unavailable to quantify 11β-HSD2 levels. However, the stable-isotope study showed impaired cortisone generation in the critically ill patients, indicating suppressed 11β-HSD2 activity. Moreover, 11β-HSD1 protein and enzyme activity in biopsy samples and in vivo D3-cortisol generation were unaltered, so it is unlikely that altered regeneration of cortisol from cortisone played a role in the patients.
Although hypoperfusion of cortisol-metabolizing organs could theoretically reduce cortisol breakdown, this factor does not explain our findings. In contrast, bile acids are known to be competitive inhibitors and transcriptional suppressors of cortisol-metabolizing enzymes.13-15 Observations in patients and animals with cholestasis support the inhibition of glucocorticoid metabolism by bile acids.14,15,40 The negative correlation between the expression and activity of the A-ring reductases and circulating bile acid levels suggests that elevated levels of bile acids may reduce cortisol metabolism in critically ill patients, a hypothesis that should be further investigated.
Our studies have some limitations. First, it would have been ideal to document all the changes in a single patient population; this was not feasible, in part for ethical reasons. However, the five groups of patients were matched, and the results of all studies corroborated our hypothesis of reduced cortisol breakdown. Second, biopsy samples were obtained on autopsy, which may have introduced bias. However, reduced cortisol clearance was clearly also present in the patients who survived.
These findings have clinical implications. The contribution of reduced cortisol breakdown to hypercortisolemia during critical illness changes our understanding of the stress response. Reduced inactivation of cortisol may be important not only to increase circulating levels but also to potentiate cortisol levels and activity within the vital tissues that express inactivating enzymes. More pragmatically, the data suggest that “stress doses” of hydrocortisone (200 mg per day), which are advocated to replace cortisol production in critically ill patients who are presumed to have adrenal failure, are at least three times too high.21 Finally, our data suggest that a low cortisol response to corticotropin stimulation does not necessarily reflect adrenal failure, since cortisol production in critically ill patients is not subnormal and the suppressed clearance maintains hypercortisolemia. Our results may therefore help to explain why studies investigating the effect of the daily administration of 200 mg of hydrocortisone in patients with sepsis (on the basis of a low cortisol response to corticotropin stimulation) have had conflicting results.5,7
In conclusion, in critically ill patients in the ICU, reduced cortisol breakdown appeared to contribute to abnormal blood cortisol levels. This finding has potential implications for the diagnosis of adrenal failure and its treatment in the ICU setting.
Supplementary Material
Acknowledgments
Supported by grants from the Belgian Fund for Scientific Research (to Dr. Van den Berghe) and the British Heart Foundation (to Dr. Walker); long-term structural research support from the Methusalem Program, funded by the Flemish government (to Dr. Van den Berghe through the Catholic University of Leuven); and a European Research Council Advanced Grant from the Ideas Program of the European Union 7th Framework Program (AdvG-2012-321670, to Dr. Van den Berghe).
We thank the patients, their relatives, and the healthy volunteers for participating; attending physicians, nurses, and research assistants (Alexandra Hendrickx, Sylvia Van Hulle, and Katrien Reyniers) for their help; Inge Derese, Annelies Aertgeerts, Jill Harrison, Carolynn Cairns, and Alison Rutter for technical assistance; Fabian Guiza Grandas for computer assistance; Dr. Roger Bouillon for critical review of an earlier draft of the manuscript; and the Wellcome Trust Clinical Research Facility Mass Spectrometry Core Laboratory in Edinburgh.
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Mesotten D, Vanhorebeek I, Van den Berghe G. The altered adrenal axis and treatment with glucocorticoids during critical illness. Nat Clin Pract Endocrinol Metab. 2008;4:496–505. doi: 10.1038/ncpendmet0921. [DOI] [PubMed] [Google Scholar]
- 2.Widmer IE, Puder JJ, König C, et al. Cortisol response in relation to the severity of stress and illness. J Clin Endocrinol Metab. 2005;90:4579–86. doi: 10.1210/jc.2005-0354. [DOI] [PubMed] [Google Scholar]
- 3.Vermes I, Beishuizen A. The hypothalamic-pituitary-adrenal response to critical illness. Best Pract Res Clin Endocrinol Metab. 2001;15:495–511. doi: 10.1053/beem.2001.0166. [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Sébille V, Troché G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–45. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- 5.Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–71. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]; JAMA. 2008;300:1652. Erratum. [Google Scholar]
- 6.Selye H. The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol Metab. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- 7.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 8.Vermes I, Beishuizen A, Hampsink RM, Haanen C. Dissociation of plasma adrenocorticotropin and cortisol levels in critically ill patients: possible role of endothelin and atrial natriuretic hormone. J Clin Endocrinol Metab. 1995;80:1238–42. doi: 10.1210/jcem.80.4.7714094. [DOI] [PubMed] [Google Scholar]
- 9.Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–80. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Bornstein SR, Chrousos GP. Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab. 1999;84:1729–36. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- 11.Stimson RH, Andersson J, Andrew R, et al. Cortisol release from adipose tissue by 11beta-hydroxysteroid dehydrogenase type 1 in humans. Diabetes. 2009;58:46–53. doi: 10.2337/db08-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomlinson JW, Walker EA, Bujalska IJ, et al. 11Beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–66. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 13.Langlois VS, Zhang D, Cooke GM, Trudeau VL. Evolution of steroid-5alpha-reductases and comparison of their function with 5beta-reductase. Gen Comp Endocrinol. 2010;166:489–97. doi: 10.1016/j.ygcen.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Ackermann D, Vogt B, Escher G, et al. Inhibition of 11beta-hydroxysteroid dehydrogenase by bile acids in rats with cirrhosis. Hepatology. 1999;30:623–9. doi: 10.1002/hep.510300303. [DOI] [PubMed] [Google Scholar]
- 15.McNeilly AD, Macfarlane DP, O’Flaherty E, et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J Hepatol. 2010;52:705–11. doi: 10.1016/j.jhep.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stauffer AT, Rochat MK, Dick B, Frey FJ, Odermatt A. Chenodeoxycholic acid and deoxycholic acid inhibit 11 beta-hydroxysteroid dehydrogenase type 2 and cause cortisol-induced transcriptional activation of the mineralocorticoid receptor. J Biol Chem. 2002;277:26286–92. doi: 10.1074/jbc.M201556200. [DOI] [PubMed] [Google Scholar]
- 17.Vanwijngaerden YM, Wauters J, Langouche L, et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology. 2011;54:1741–52. doi: 10.1002/hep.24582. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 19.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 20.Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26:197–202. doi: 10.1016/0022-4731(87)90071-9. [DOI] [PubMed] [Google Scholar]
- 21.Vanhorebeek I, Peeters RP, Vander Perre S, et al. Cortisol response to critical illness: effect of intensive insulin therapy. J Clin Endocrinol Metab. 2006;91:3803–13. doi: 10.1210/jc.2005-2089. [DOI] [PubMed] [Google Scholar]
- 22.Andrew R, Smith K, Jones GC, Walker BR. Distinguishing the activities of 11beta-hydroxysteroid dehydrogenases in vivo using isotopically labeled cortisol. J Clin Endocrinol Metab. 2002;87:277–85. doi: 10.1210/jcem.87.1.8157. [DOI] [PubMed] [Google Scholar]
- 23.Andrew R, Westerbacka J, Wahren J, Yki-Järvinen H, Walker BR. The contribution of visceral adipose tissue to splanchnic cortisol production in healthy humans. Diabetes. 2005;54:1364–70. doi: 10.2337/diabetes.54.5.1364. [DOI] [PubMed] [Google Scholar]
- 24.Basu R, Basu A, Grudzien M, et al. Liver is the site of splanchnic cortisol production in obese nondiabetic humans. Diabetes. 2009;58:39–45. doi: 10.2337/db08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]; Diabetes. 2009;58:1936. Errata. [Google Scholar]; 2010;59:1283. [Google Scholar]
- 25.Hughes KA, Manolopoulos KN, Iqbal J, et al. Recycling between cortisol and cortisone in human splanchnic, subcutaneous adipose, and skeletal muscle tissues in vivo. Diabetes. 2012;61:1357–64. doi: 10.2337/db11-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Best R, Walker BR. Additional value of measurement of urinary cortisone and unconjugated cortisol metabolites in assessing the activity of 11 beta-hydroxysteroid dehydrogenase in vivo. Clin Endocrinol (Oxf) 1997;47:231–6. doi: 10.1046/j.1365-2265.1997.2471061.x. [DOI] [PubMed] [Google Scholar]
- 27.Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab. 1998;83:1806–9. doi: 10.1210/jcem.83.5.4951. [DOI] [PubMed] [Google Scholar]
- 28.Ulick S, Tedde R, Wang JZ. Defective ring A reduction of cortisol as the major metabolic error in the syndrome of apparent mineralocorticoid excess. J Clin Endocrinol Metab. 1992;74:593–9. doi: 10.1210/jcem.74.3.1740492. [DOI] [PubMed] [Google Scholar]
- 29.Palermo M, Shackleton CH, Mantero F, Stewart PM. Urinary free cortisone and the assessment of 11 beta-hydroxysteroid dehydrogenase activity in man. Clin Endocrinol (Oxf) 1996;45:605–11. doi: 10.1046/j.1365-2265.1996.00853.x. [DOI] [PubMed] [Google Scholar]
- 30.Walker BR. How will we know if 11beta-hydroxysteroid dehydrogenases are important in common diseases. Clin Endocrinol (Oxf) 2000;52:401–2. doi: 10.1046/j.1365-2265.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 31.Drake AJ, Livingstone DE, Andrew R, Seckl JR, Morton NM, Walker BR. Reduced adipose glucocorticoid reactivation and increased hepatic glucocorticoid clearance as an early adaptation to high-fat feeding in Wistar rats. Endocrinology. 2005;146:913–9. doi: 10.1210/en.2004-1063. [DOI] [PubMed] [Google Scholar]
- 32.Trainer PJ, Besser M. The Bart’s endocrine protocols. Churchill Livingstone; New York: 1995. p. 52. [Google Scholar]
- 33.Fenske M. Urinary free cortisol and cortisone excretion in healthy individuals: influence of water loading. Steroids. 2006;71:1014–8. doi: 10.1016/j.steroids.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Mericq MV, Cutler GB., Jr High fluid intake increases urine free cortisol excretion in normal subjects. J Clin Endocrinol Metab. 1998;83:682–4. doi: 10.1210/jcem.83.2.4555. [DOI] [PubMed] [Google Scholar]
- 35.Melby JC, Spink WW. Comparative studies on adrenal cortical function and cortisol metabolism in healthy adults and in patients with shock due to infection. J Clin Invest. 1958;37:1791–8. doi: 10.1172/JCI103772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandberg AA, Eik-Nes K, Migeon CJ, Samuels LT. Metabolism of adrenal steroids in dying patients. J Clin Endocrinol Metab. 1956;16:1001–16. doi: 10.1210/jcem-16-8-1001. [DOI] [PubMed] [Google Scholar]
- 37.White PC, Mune T, Agarwal AK. 11 Beta-hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev. 1997;18:135–56. doi: 10.1210/edrv.18.1.0288. [DOI] [PubMed] [Google Scholar]
- 38.Barquist E, Kirton O. Adrenal insufficiency in the surgical intensive care unit patient. J Trauma. 1997;42:27–31. doi: 10.1097/00005373-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Stewart PM, Walker BR, Holder G, O’Halloran D, Shackleton CH. 11 Beta-hydroxysteroid dehydrogenase activity in Cushing’s syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80:3617–20. doi: 10.1210/jcem.80.12.8530609. [DOI] [PubMed] [Google Scholar]
- 40.Quattropani C, Vogt B, Odermatt A, Dick B, Frey BM, Frey FJ. Reduced activity of 11 beta-hydroxysteroid dehydrogenase in patients with cholestasis. J Clin Invest. 2001;108:1299–305. doi: 10.1172/JCI12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.