Abstract
The Hippo pathway regulates tissue growth, and inactivation of any of four key components (HPO, WTS, SAV, and MATS) results in tissue overgrowth. Cell junctions may also modulate the pathway's activity.
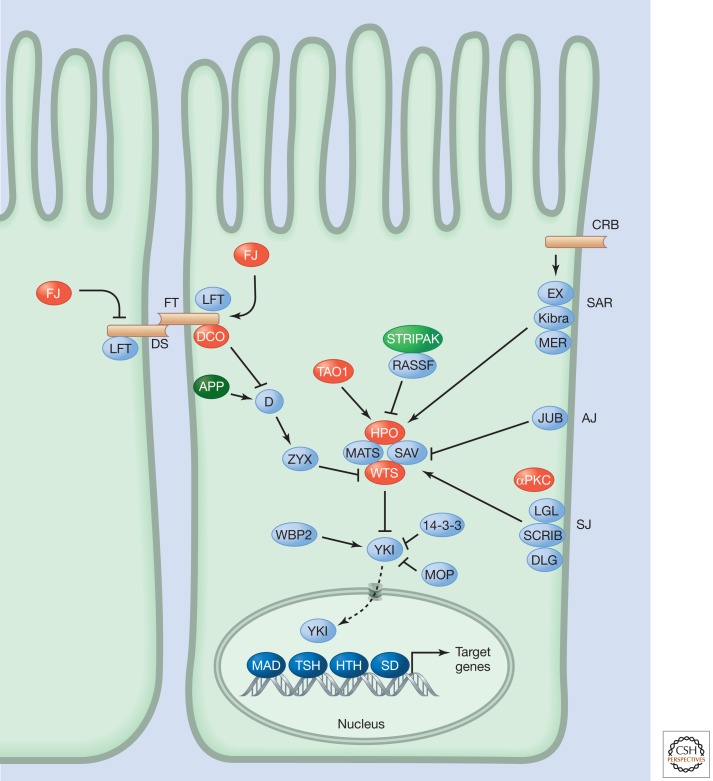
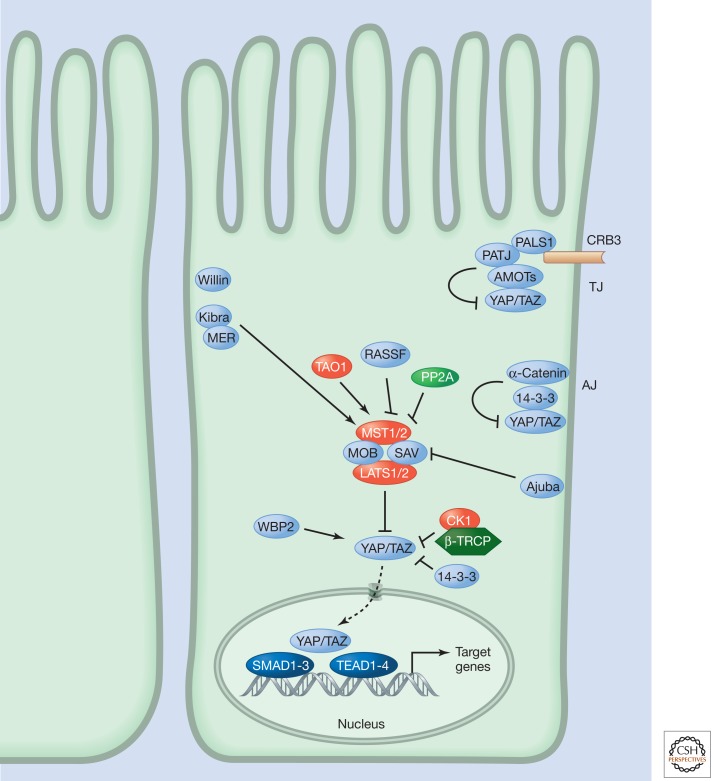
The Hippo pathway (Fig. 1), also known as the Salvador-Warts-Hippo pathway, regulates tissue growth in a wide variety of organisms (Harvey and Tapon 2007; Grusche et al. 2010; Oh and Irvine 2010; Pan 2010; Halder and Johnson 2011; Zhao et al. 2011). Many components of the pathway were identified as a result of mutations in the fruit fly Drosophila melanogaster that resulted in tissue overgrowth (Table 1). The pathway is conserved in vertebrates, including mammals (Fig. 2), and changing the activity of the pathway can result in dramatic changes in the size of certain organs, most notably the liver (Pan 2010; Halder and Johnson 2011). In addition to its role in regulating tissue growth, the pathway has been implicated in the control of other biological processes, such as cell-fate determination, mitosis, and pluripotency. Deregulation of Hippo pathway activity has been reported in many human cancers. The human homolog of D. melanogaster Merlin (MER), also known as Neurofibromatosis Type 2 (NF2) is a bona fide tumor suppressor, while altered activity of several Hippo pathway components has been implicated in human tumorigenesis (Harvey and Tapon 2007).
Figure 1.
The Drosophila Hippo pathway.
Table 1.
Components of the Drosophila melanogaster Salvador-Warts-Hippo pathway and their human homologues
| Drosophila | Human |
|---|---|
| Upstream | |
| Fat (FT) | FAT1—FAT4 |
| Dachsous (DS) | DCHS1 and DCHS2 |
| Discs overgrown (DCO) | CK1ε and CK1δ |
| Lowfat (LFT) | LIX1 and LIX1L |
| Four-jointed (FJ) | FJX1 |
| Dachs (D) | ? |
| Approximated (APP) | ZDHHC9, ZDHHC14 and ZDHHC18 |
| Zyxin (ZYX) | Zyxin, LPP and TRIP6 |
| Merlin (MER) | Merlin (also known as NF2) |
| Expanded (EX) | Willin/FRMD6 and FRMD1 |
| Kibra | Kibra |
| Crumbs (CRB) | CRB1—CRB3 |
| Lethal giant larvae (LGL) | LGL1 and LGl2 |
| Discs large (DLG) | DLG1—DLG4 |
| Scribble (SCRIB) | SCRIB |
| aPKC | aPKCι and aPKCζ |
| STRIPAK (PP2A) | PP2A (STRIPAK) |
| RASSF | RASSF1-RASSF6 |
| Myopic (MOP) | HD-PTP |
| JUB | Ajuba, LIMD1, WTIP |
| TAO1 | TAO1—TAO3 |
| Core | |
| Hippo (HPO) | MST1 and MST2 |
| Salvador (SAV) | SAV1 |
| Mats (MTS) | MOBKL1A and MOBKL1AB |
| Warts (WTS) | LATS1 and LATS2 |
| Downstream | |
| Yorkie (YKI) | YAP and TAZ |
| WBP2 | WBP2 |
| Scalloped (SD) | TEAD1—TEAD4 |
| MAD | SMADs |
| TSH | TSHZ1—TSHZ3 |
| HTH | MEIS1—MEIS3 |
Figure 2.
The mammalian Hippo pathway.
At the core of the pathway is a module composed of two kinases—Hippo (HPO) (Harvey et al. 2003; Jia et al. 2003; Pantalacci et al. 2003; Udan et al. 2003; Wu et al. 2003) and Warts (WTS; also known as LATS) (Justice et al. 1995; Xu et al. 1995)—and two other proteins—Salvador (SAV) (Kango-Singh et al. 2002; Tapon et al. 2002) and Mob as Tumor Suppressor (MATS) (Lai et al. 2005). HPO functions upstream of WTS and can directly phosphorylate it. Mutations that inactivate any of these four proteins result in tissue overgrowth. The first indication that some of these proteins might function in a pathway was the observation that sav and wts mutants display similar phenotypic abnormalities and that the two proteins can interact with each other (Tapon et al. 2002). More recently it has been shown that activity of this module can be regulated by RASSF, a scaffold protein that promotes tissue growth by recruiting the serine-threonine phosphatase complex STRIPAK to inhibit HPO autophosphorylation, and hence HPO activity (Ribeiro et al. 2010).
The main output of the module involves the transcriptional coactivator Yorkie (YKI) (Huang et al. 2005). Phosphorylation of YKI by WTS induces binding of 14-3-3 proteins to YKI that limit YKI activity by preventing nuclear accumulation. Phosphatases that counter the activity of WTS have not been discovered but the Myopic (MOP) tyrosine phosphatase regulates YKI activity, repressing it (Gilbert et al. 2011). YKI promotes tissue growth by increasing expression of positive regulators of cell growth and inhibitors of apoptosis. YKI, itself does not bind DNA but functions together with several transcription factors, including Scalloped (SD; the homolog of TEAD transcription factors in vertebrates), Homothorax (HTH), Teashirt (TSH), and Mothers against DPP (MAD). Transcriptional regulatory proteins such as WBP2 also control Hippo-pathway-dependent tissue growth (Zhang et al. 2011). WBP2 and other as-yet-unidentified proteins have been predicted to interact with YKI via its WW domains, which are important for YKI’s transcription activation function (Oh and Irvine 2010).
The HPO and WTS kinases appear to receive multiple inputs. The first upstream regulators to be discovered were the Band 4.1 proteins Expanded (EX) and MER (Hamaratoglu et al. 2006). These function together with the WW-domain-containing protein Kibra to activate the core kinase cassette by an unknown mechanism. EX is also thought to repress YKI by physical interaction and sequestration. The Fat/Dachsous branch of the pathway consists of the atypical cadherins Fat (FT) and Dachsous (DS) as well as the downstream effector proteins Discs overgrown (DCO, a serine-threonine kinase also known as casein kinase 1ε), Dachs (D, an atypical myosin), Approximated (APP, a palmitoyltransferase), Lowfat (LFT), and Zyxin (ZYX) (Grusche et al. 2010; Rauskolb et al. 2011). The Fat/Dachsous branch impinges on pathway activity by modulating the abundance of WTS and also modulates the Kibra-EX-MER branch by regulating EX levels. The sterile 20-like kinase, TAO1, phosphorylates and activates HPO (MST1/2 in mammals) although it is unclear whether TAO1 activity is regulated (Boggiano et al. 2011; Poon et al. 2011).
Increasing evidence underlines the importance of cell junctions for regulation of Hippo pathway activity. In D. melanogaster epithelial cells, many Hippo pathway proteins reside, at least partially, at the sub-apical region (SAR), adherens junction (AJ) or septate junction (SJ). Examples of such junctional proteins include the AJ protein Jub and the apical-basal polarity proteins Discs large (DLG), Lethal giant larvae (LGL), Scribble (SCRIB), Crumbs (CRB), and atypical protein kinase C (aPKC). In mammalian epithelial cells, several other junctional proteins regulate Hippo pathway activity (see Table 2), including angiomotin and α-catenin (Schlegelmilch et al. 2011; Zhao et al. 2011). The Hippo pathway may therefore help couple tissue growth to mechanical stresses or cell–cell contact, which might be important for organ size regulation.
Table 2.
Components of the human Salvador-Warts-Hippo pathway and their Drosophila melanogaster homologs
| Human | Drosophila |
|---|---|
| Upstream | |
| α-Catenin | α-Catenin |
| PATJ | Discs lost |
| PALS1 | Stardust |
| AMOTs | ? |
| β-TRCP | Slimb |
Footnotes
Editors: Lewis Cantley, Tony Hunter, Richard Sever, and Jeremy W. Thorner
Additional Perspectives on Signal Transduction available at www.cshperspectives.org
REFERENCES
- Boggiano JC, Vanderzalm PJ, Fehon RG 2011. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell 21: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MM, Tipping M, Veraksa A, Moberg KH 2011. A screen for conditional growth suppressor genes identifies the Drosophila homolog of HD-PTP as a regulator of the oncoprotein Yorkie. Dev Cell 20: 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusche FA, Richardson HE, Harvey KF 2010. Upstream regulation of the hippo size control pathway. Curr Biol 20: R574–582. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL 2011. Hippo signaling: Growth control and beyond. Development 138: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 8: 27–36. [DOI] [PubMed] [Google Scholar]
- Harvey K, Tapon N 2007. The Salvador-Warts-Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434. [DOI] [PubMed] [Google Scholar]
- Jia J, Zhang W, Wang B, Trinko R, Jiang J 2003. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev 17: 2514–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev 9: 534–546. [DOI] [PubMed] [Google Scholar]
- Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G 2002. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129: 5719–5730. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y 2005. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120: 675–685. [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD 2010. Yorkie: The final destination of Hippo signaling. Trends Cell Biol 20: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D 2010. The hippo signaling pathway in development and cancer. Dev Cell 19: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P 2003. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol 5: 921–927. [DOI] [PubMed] [Google Scholar]
- Poon CL, Lin JI, Zhang X, Harvey KF 2011. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell 21: 896–906. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Pan G, Reddy BV, Oh H, Irvine KD 2011. Zyxin links fat signaling to the hippo pathway. PLoS Biol 9: e1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro PS, Josue F, Wepf A, Wehr MC, Rinner O, Kelly G, Tapon N, Gstaiger M 2010. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol Cell 39: 521–534. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. 2011. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 144: 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber DA, Hariharan IK 2002. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110: 467–478. [DOI] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G 2003. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol 5: 914–920. [DOI] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W 1995. Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development 121: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Zhang X, Milton CC, Poon CL, Hong W, Harvey KF 2011. Wbp2 cooperates with Yorkie to drive tissue growth downstream of the Salvador-Warts-Hippo pathway. Cell Death Differ 18: 1346–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL 2011. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 13: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]