Abstract
Breast cancer incidence is increasing worldwide, and breast cancer-related mortality is highest in women of African ancestry, who are more likely to have basal-like or triple-negative breast cancer (TNBC) than are women of European ancestry. Identification of cultural, epidemiological, and genetic risk factors that predispose women of African ancestry to TNBC is an active area of research. Despite the aggressive behaviour of TNBC, achievement of a pathological complete response with chemotherapy is associated with good long-term survival outcomes, and sensitivity to chemotherapy does not seem to differ according to ethnic origin. Discovery of the molecular signalling molecules that define TNBC heterogeneity has led to the development of targeted agents such as inhibitors of poly (ADP-ribose) polymerase-1 and mTOR and immunomodulatory drugs that are in the early stages of clinical testing. First, we summarise the existing published work on the differences reported on the epidemiology, biology, and response to systemic treatment of TNBC between women of African ancestry and white women, and identify some gaps in knowledge. Second, we review the opportunities for development of new therapeutic agents in view of the potential high clinical relevance for patients with TNBC irrespective of race or ethnic origin.
Introduction
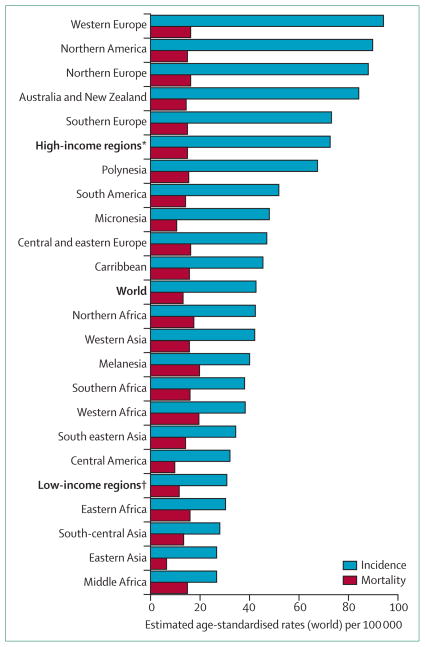
Breast cancer is the most common cancer in women worldwide, with around 1 676 633 new cases diagnosed in 2012 (figure 1).1 Breast cancer has become the most prevalent cancer in women in sub-Saharan Africa, and in most of the 22 countries of the Americas, including the Caribbean.2,3
Figure 1. Global breast cancer incidence and mortality in women in 2012.
*Includes all regions of Europe, Northern America, Australia, New Zealand, and Japan. †Includes all regions of Africa, Asia (excluding Japan), Latin America, the Caribbean, Melanesia, Federated States of Micronesia, and Polynesia. Region definitions from IARC WHO. Data from the International Agency for Research on Cancer, GLOBOCAN 2012.1
The annual incidence of breast cancer varies in women of African ancestry and accurate reporting of the data is affected by the inadequacy of cancer registries. The age-adjusted incidence ranges from 30 cases per 100 000 women in eastern Africa1 to 78·1 cases per 100 000 in a Caribbean population4 and 120·5 cases per 100 000 in African-American women.5 Since the publication of GLOBOCAN 2008 cancer statistics, breast cancer incidence has increased by more than 20% worldwide.1 In Africa, breast cancer incidence increased annually in Hare, Zimbabwe, by around 4·9% and in Kampala, Uganda, by about 4·5% between the early 1990s and mid-2000s.6,7 In addition to population growth and ageing, increases in breast cancer incidence in Africa, particularly in wealthy women, might be attributable to women having fewer children and giving birth for the first time later in their lives than in previous decades8 partly as a result of an increase in use of oral contraception, and to increases in prevalence of lifestyle risk factors, such as obesity and low levels of physical activity.9,10
Breast cancer is the main cause of cancer-related mortality in women worldwide; 324 000 deaths were reported in low-income countries in 2012,1 with 68 100 occurring in Africa.3 Breast cancer-related mortality in African-American women (30·8 deaths per 100 000)11 is quite similar by comparison with the age-standardised cancer-related mortality of women living in the non-Latin Caribbean who are predominantly of African ancestry (25·0 deaths per 100 000), despite decreased health-care capacity.2
Racial disparities in breast cancer-related mortality have also been noted in high-income countries with mixed racial populations. In the USA, African-American women have a 41% higher breast cancer-related death rate than do white women.11 In the UK, 5-year distant breast cancer relapse-free survival is 62·8% for young black women, compared with 77% for young white women with equal access to health care (p=0·0053).12 Racial disparities in breast cancer survival between and within countries are linked to the availability of early detection, access to diagnosis and treatment, cultural differences in lifestyle behaviours, socioeconomic factors, and differences in the biological characteristics of breast cancer.13
Subtypes of breast cancer
Breast cancer has traditionally been subtyped into oestrogen receptor (ER)-positive (60–70% of breast cancer cases) and ER-negative breast cancer (30–40% of cases).14 ER-positive breast cancer typically expresses the progesterone receptor (PR), another molecular marker of breast cancer. ER-positive or PR-positive breast cancer typically responds to ER-targeted therapy, such as selective ER modulators (eg, tamoxifen) or aromatase inhibitors, which lower the serum concentrations of oestrogen. ER-negative breast cancer is aggressive and is most common in women of African ancestry and women who carry BRCA1 mutations.15,16
15–20% of breast cancers amplify or overexpress the oncogene HER2/neu oncogene.14 When discovered, HER2-positive breast cancer—which can be either ER-positive or ER-negative—was associated with a poor prognosis.14 However, the advent of HER2-targeted treatments (eg, trastuzumab) have greatly improved the outcomes of women with HER2-positive breast cancer.14
These three molecular markers distinguish breast cancer into subgroups of ER-positive, PR-positive, or HER2- positive breast cancer. Breast cancer that does not express any of the three markers is commonly termed triple-negative breast cancer (TNBC). The recommended definition of ER-negative and PR-negative breast cancer are based on tests that distinguish immunoreactive cells; ER-negative and PR-negative breast cancers are those that express less than 1% immunoreactive cells, based on studies that have shown assocations of tumour response and clinical outcomes to endocrine therapy.17 However, many clinical trials and epidemiological studies often use a threshold of more than 10% immunoreactive cells to define receptor negativity, which was the previously defined threshold assumed to correspond with the cutoff point of cytosol protein 10 fmol per mg for the ligand-binding assay, the first test used to define receptor presence or absence based on the odds of response to endocrine treatment. TNBC accounts for about 10–15% of all breast cancers but it is much more common in women of African ancestry than any other ethnic origns, particularly in women who develop breast cancer before the age of 50 years.15
More recently, gene-expression profiling has also been used to subtype breast cancer, especially TNBC. Initial investigations done by Perou and colleagues18 and Sorlie and colleagues19 showed that breast cancer could be separated into five groups using RNA expression profiling: luminal A (ER-positive), luminal B (ER-positive), HER2 (HER2-positive), basal-like (mostly ER-negative), and normal-like breast cancer. Subsequently, Prat and colleagues20 showed that basal-like breast cancer included another subtype, termed claudin-low breast cancer. Basal-like and claudin-low breast cancer are both predominantly (but not exclusively) ER-negative and triple-negative.
TNBC subgroups, incidence, and prevelence
TNBC and basal-like breast cancer are more common in women of African ancestry than women of other ethnic origins.15,21 TNBC is often aggressive, and is associated with a higher mortality than the other subtypes of breast cancer (p<0·01).19 TNBC is characterised by high cell proliferation, poor cellular differentiation, many recurrent copy number imbalances, and, in most cases, mutations in the TP53 tumour suppressor gene.18,22,23
TNBC has been further subdivided by use of meta-analysis of gene-expression profiling data. Lehmann and colleagues24 analysed RNA expression profiling data from 14 breast cancer gene expression datasets that used breast tumours from women in the USA, Europe, and China as the training set to develop gene signatures for TNBC subgroups. They then validated these gene signatures by use of RNA profiling data from seven additional datasets of tumours from the USA and Europe. No breakdown of race or ethnic origin was provided in this analysis; however, due to the different ethnic population distributions of the countries, women of African ancestry were likely to be included in the US datasets but not in those from Europe or China. Their analysis shows that TNBC can be subdivided into six subgroups: basal-like 1, basal-like 2, immunomodulatory, mesenchymal-like, mesenchymal stem-like, and luminal androgen-receptor (AR) expressing. Lehmann and colleagues24 also showed that the breast cancer subtypes have varying sensitivity to therapeutic agents. Basal-like 1 and basal-like 2 cells were most sensitive to cisplatin, luminal AR cells to bicalutamide (an anti-androgen) and alvespimycin (an Hsp90 inhibitor), and the mesenchymal subgroups to the SRC inhibitor dasatinib and the phosphoinositol-3 kinase (PI3K)/mTOR inhibitor NVP-BEZ235. These results suggest that different forms of TNBC need different therapies, but no evidence suggests that subtypes of TNBC differ between racial groups of women.
Population-based incidence for TNBC in women of African ancestry have mainly been reported from the USA’s Surveillance, Epidemiology, and End Results (SEER) California Cancer Registry, which has collected data on the status of ER and PR subtypes since 1990 and on HER2/neu status since 1999.25 By use of the SEER California database, Clarke and colleagues25 showed that incidence of TNBC is higher in African-American women than other racial or ethnic origin groups at all ages (p<0·05).25 African-American women were twice as likely to be diagnosed with ER-positive, PR-positive, and HER2-negative breast cancer than TNBC; however, the ratio was substantially lower than reported for white (ratio 6·9:1) or Asian women (ratio 6·1:1). An analysis of the entire US SEER data of women diagnosed with breast cancer in 2010, added evidence to support this result, showing that African-American (odds ratio [OR] 1·4, 95% CI 1·2–1·6) and Hispanic women (1·3, 1·2–1·5) were more likely to be diagnosed with TNBC than were white women.26 Since mammography screening is more likely to detect ER-positive breast cancer,27 differences in screening patterns by race might account for the under-representation of ER-positive breast cancer in African- American women than in women of other ethnic origins. Data for population-based rates of mammography screening by race and ethnic origin are conflicting. Data from the 2010 National Health Interview Survey of self-reported mammography screening showed that black women were as likely as white women to report having had a mammogram in the past 2 years (73·2% and 72·8%, respectively), and 71·9% of all women aged 65–74 years reported mammography screening.28 Silber and colleagues29 used information from Medicare and the SEER database to show that in women diagnosed with breast cancer after the age of 66 years, black women were significantly less likely than white women (23·5% vs 35·7%, p<0·001) to have received any breast cancer screening in the 6–18 months before diagnosis. However, in women younger than 40 years in the USA, who are not eligible for population-based mammography screening, African-American women had an incidence of TNBC that was two times higher (4·1 per 100 000) than white (2·2 per 100 000) or Hispanic women (2·1 per 100 000 women). This result suggests that differential mammography screening rates and oversampling of ER-positive breast cancer might not fully explain racial and ethnic differences in the incidence of TNBC.30
Although the frequency of TNBC varies across regional populations of women of African ancestry, it is consistently higher than that reported in other racial or ethnic origin groups. In the population-based North Carolina Breast Cancer cohort of 878 African-American women with breast cancer, premenopausal African- American women had higher rates of basal breast cancer (39%) than did white women of a similar age (16%) or postmenopausal African-American women (14%).21 Similarly, Bowen and colleagues29 showed in a retrospective cohort study of patients with breast cancer in the UK that 22% of black women had TNBC compared with 15% of white women.31
Huo and colleagues32 investigated the distribution of molecular subtypes of invasive tumours in women (mean age 44·8 years) in different geographical regions in Nigeria and Senegal (507 women) and reported that TNBC, including basal-like TNBC, was the predominant type of cancer (27%).32 Of consecutive cases of breast cancer reported in a Bamako University hospital in Mali, the mean age of patients was 46 years (range 25–85 years) and 46% of tumours were triple-negative.33 In a large case study34 of 1216 women with breast cancer in Soweto, South Africa, 90% were black, and 20% of breast cancers were triple-negative, which is consistent with the frequency reported in African-American women. The frequency of TNBC was highest in women aged 50–59 years (29·2%), and was higher in women of African ancestry than in women of other ethnic origins (OR 2·2, 95% CI 1·1–3·8).34 Smaller, hospital-based studies have reported a higher prevalence of TNBC in a sample of Ghanaian women (79%) compared with African- American (32%) and white American women (10%).35
Overall, epidemiological data support the conclusion that although TNBC is not restricted to a specific age or ethnic group, this cancer is of a higher frequency and is a contributor to the survival disadvantage of women of African ancestry with breast cancer. Factors that might account for variations in the incidence and prevalence of TNBC in women of African ancestry include differences in methods of case ascertainment, population age structure, genetic and lifestyle risk factor distribution, and access to mammography screening.34,35 Overestimation of ER and PR negativity is of particular concern because poor or unreliable laboratory standards for tissue handling, type of fixation used, and the initiation and duration of fixation can affect the optimum performance of immunohistochemical testing and thus the results.17
Epidemiological risk factors
Few epidemiological studies have included large numbers of women of African ancestry to examine reproductive risk factors associated with TNBC or ER-negative breast cancer by ethnic origin. Most evidence is from studies done in the USA in which at least 10% of patients with breast cancer were of African ancestry, and suggests a positive association between higher parity or having more than one child and risk of development of TNBC, and a negative association between duration of breastfeeding and risk of development of TNBC (table).
Table.
Epidemiological risk factors for triple-negative breast cancer in women of African ancestry
| Number of cases of African ancestry (% of total cases) | Study design | Factors associated with triple-negative breast cancer | |
|---|---|---|---|
| Bauer et al (2007)15 | 636 (10%) | Cohort | Aged younger than 40 years (OR 1·53, 95% CI 1·37–1·70); low socioeconomic status (1·12, 1·01–1·24) |
| Millikan et al (2008)36 | 787 (43%) | Case-control | Parity (ptrend=0·04); short duration of breastfeeding (ptrend=0·03); large waist-to-hip ratio (ptrend=0·002) |
| Stead et al (2009)37 | 178 (43%) | Case-control | Overweight or obese (both non-significant) |
| Trivers et al (2009)38 | 116 (24%) | Case-control | Obesity (OR 1·89, 95% CI 1·22–2·92) |
| Ma et al (2010)39 | 849 (44%) | Case-control | Short duration of breastfeeding (ptrend=0·03); aged 45–65 years with oral contraceptive use before age 18 years (OR 2·87, 95% CI 1·44–5·74) |
| Shinde et al (2010)40 | 254 (10%) | Case-case | Parity (OR 1·12, 95% CI 1·06–1·20); short duration of breastfeeding (0·93, 0·90–0·97) |
| Ambrosone et al (2014)41 | 786 (100%) | Case-control | Parity (ptrend=0·03); shorter duration of breastfeeding (non-significant) |
OR=odds ratio. All studies were done in the USA.
The population-based North Carolina Breast Cancer study,36 which assessed 878 African-American women and 187 white women with breast cancer, showed that having both multiple children and not breastfeeding those children were risk factors for basal-like TNBC across ethnic groups. A population-based case study41 of black women from the USA and Caribbean showed that, although having more children was associated with an increased risk of TNBC (ptrend=0·03), the association with breastfeeding was not significant. Another large population-based, case-control study39 of 873 African- American and 1072 white women with breast cancer living in California reported a significant trend between longer durations of breastfeeding and reduced risk of development of TNBC (ptrend=0·02), and oral contraceptive use was associated with a 2·9 times increased risk of women aged 45–64 years developing TNBC.39 Development of TNBC in the absence or short duration of breastfeeding might be caused by progenitor cells in breast tissue not undergoing terminal differentiation and apoptosis that usually occurs with prolonged lactation, resulting in a persistent pool of progenitor cells that are at risk for carcinogenesis.40
Data for the relation between obesity and TNBC are conflicting.37,38 A meta-analysis42 of case-case and case-control studies, some of which included women of African ancestry, showed that obesity was associated with an increased risk of development of TNBC for all women (OR 1·20, 95% CI 1·03–1·40). An increased hip-to-waist ratio has also been associated with an increased risk of development of TNBC and ER/PR-negative breast cancer36,43 in African-American women. Additionally, a suggested role for diet in the risk of development of TNBC is derived from epidemiological studies that showed a slightly significant inverse association between high total vegetable intake (relative risk 0·82, 95% CI 0·74–0·90),44 or a diet high in fruits and vegetables (0·85, 0·76–0·95),45 and risk of development of ER-negative breast cancer.
Few epidemiological studies have investigated risk factors for breast cancer in African women, because routine assessment of ER, PR, and Her-2/neu on breast tumours is not done and thus few data are available about specific risk factors for subtypes. Similar causal associations are assumed to exist for breast cancer development in African-American women to women of African ancestry living elsewhere in the world.46 In view of the differences in the distribution of reproductive risk factors between African women and women of African ancestry in high-income countries, unknown risk factors could be associated with risk of developing TNBC in Africa. Galukande and colleagues47 reported a small case study of 113 women with breast cancer diagnosed in Uganda, with information available about ER and PR status. The prevalence of ER/PR-negative tumours was much the same between women younger than 50 years and women older than 50 years. No significant association between parity, breastfeeding, or body-mass index and ER/PR-positive versus ER/PR-negative disease was recorded; however, the sample size of this study was potentially too small to provide adequate power to detect associations.
The role that socioeconomic status has in the cause of TNBC is of interest because low socioeconomic status is associated with many of the shared characteristics of breast tumours that occur in women of African ancestry, including high grade, high clinical stage, and ER-negative status.15,21 Data from the California Cancer Registry showed that irrespective of race or ethnic origin, women living in areas of low socioeconomic status were more likely than women living in areas of high socioeconomic status to be diagnosed with TNBC than any other type of breast cancer.15 Socioeconomic status is intrinsically linked with race and lifestyle behaviours, such as physical activity, obesity, diet, reproductive experiences such as having more children, and screening behaviours, which vary in prevalence across different populations of women.48 Therefore, the challenge faced in assessment of the causes of TNBC in women of African ancestry is to distinguish the contributions of these factors, while also accounting for their different distributions across populations of women.
Genetic susceptibility
In view of the higher incidence of TNBC in women of African ancestry than of European ancestry, African ancestry might be associated with inherited genetic variants that predispose carriers to TNBC. By use of genome-wide admixture mapping in 1484 African- American women with invasive breast cancer, assessed for around 2400 ancestry informative markers (AIMs) that distinguish European and African ancestral origins, Fejerman and colleagues49 reported no association between African or European ancestry, for subtype-specific breast cancer risk at any specific loci. However, global ancestry results showed that women with a higher percentage of European ancestry, estimated from AIMs, had an increased risk of having ER-positive or PR-positive breast cancer rather than ER-positive or PR-negative breast cancer (OR 2·84, 95% CI 1·13–7·14 for a 0–100% change in European ancestry), and were more likely to be diagnosed at an earlier stage of disease.49 In another study of African-American women, Reding and colleagues50 estimated the percentage of African ancestry with 128 AIMs. They reported no variation within ethnic groups for risks of ER or PR subtypes, stage, or grade by percentage ancestry (≥95% African ancestry versus <80% African ancestry). Because of the large amounts of genetic variation in self-identified African-Americans, random samples of African-Americans recruited from different regions will probably have very different allele frequencies that might lead to inconsistent results in studies assessing genetic associations with TNBC.51
Women with a BRCA1 mutation have more than a 50% chance of development of TNBC, and unique and founder BRCA1 mutations have been identified in patients of African ancestry with breast cancer.52 The estimated prevalence of BRCA1 mutations in African-American women not selected for family history is low, at about 1·4%.53,54 In a cohort of 434 unselected Nigerian patients with breast cancer, 31 women (7·0%) had a BRCA1 mutation,55 which might suggest an increased contribution of deleterious mutations in BRCA1 causing TNBC in Nigerian women. A study56 of 214 Bahamian women with breast cancer who were not selected for age or family history reported that 52 (24%) carried BRCA1 mutation (of which, most mutations were African founder mutations), and is the highest prevalence reported so far in any population. Knowledge of the prevalence of this dominantly inherited allele, and its potential contribution to the higher incidence of TNBC in women of African ancestry, is restricted by the low rates of genetic testing in all women of this ethinic origin.
Low-penetrance genetic variants might contribute to genetic predisposition to TNBC. In a genome-wide association study57 of breast cancer in women of African ancestry (3153 cases and 11 330 controls), two novel risk loci for breast cancer were identified (rs432260 at chromosome 14q31, OR 1·18, p=4·3 × 10–6; and rs10510333 at chromosome 3p26, 1·15, p=1·5 × 10–5). However, in this study, the statistical power was too low to assess a potential association between single-nucleotide polymorphisms (SNPs) and ER-negative breast cancer risk, and so the associations did not meet the standard level of genome-wide significance.57 A meta-analysis58 of genome-wide association studies of ER-negative breast cancer using data from the breast cancer genome-wide association study in women of African ancestry and a European ancestry genome-wide association study identified a novel TERT-rs10069690 SNP in 5p15 for ER/PR-negative and TNBC risk. The risk allele frequency of rs10069690 was greater in women of African than European ancestry. These results were replicated in the Black Women’s Health Study (which examined only African-American women; 1199 patients and 1948 controls), and showed that the rs10069690 SNP in 5p13·33 was significantly associated with ER/PR-negative breast cancer (OR 1·29, 95% CI 1·04–1·59) and TNBC (1·42, 1·02–1·99). In this independent sample of African-American women, the mean percentage of African ancestry estimated from AIMs was also significantly higher in women with TNBC (83·0%) than in those with ER/PR-positive breast cancer (79·9%; p=0·008).59 Although the functional significance of these findings are unknown, these results support a genetic cause of TNBC that might partly contribute to racial or ethnic origin differences in the incidence of the disease.
Response to systemic treatments
Chemotherapy is the standard treatment for TNBC. Despite the aggressive course of TNBC disease, it can be very sensitive to cytotoxic drugs: the proportion of patients achieving a pathological complete response after treatment with modern adjuvant or neoadjuvant chemotherapy range from 30–45%.60,61 Preliminary reports suggest that the proportion of patients achieving a pathological complete response might be increased by adding platinum salts to standard anthracyline and taxane-based chemotherapy. In a large meta-analysis62 including 12 neoadjuvant studies, 389 (33·6%, 95% CI 30·9–36·4) of 1157 patients with TNBC achieved a pathological complete response.
Irrespective of breast cancer subtype, patients who achieve a pathological complete response after neo-adjuvant chemotherapy have improved long-term outcomes compared with patients with residual disease identified at the time of surgery.63,64 Despite the poor prognosis associated with TNBC, patients who achieve a pathological complete response have excellent long-term outcomes, with overall survival of about 94%. Furthermore, if a pathological complete response is achieved, patients with TNBC and other subtypes of breast cancer have similar overall survival (p=0·24). However, patients with TNBC and residual disease have worse outcomes than do patients with residual disease and other subtypes of breast cancer (3-year overall survival 68% vs 88%, p<0·0001; figure 2).63
Figure 2. Overall survival after breast cancer surgery.
Lines show survival as a function of response to chemotherapy (pathological complete response [pCR] vs residual disease [RD]) and triple-negative status (triple-negative breast cancer [TNBC] vs non-TNBC). Reproduced with permission from Liedtke and colleagues.63
TNBC represents a heterogeneous group of tumours. Masuda and colleagues61 assessed differential chemotherapeutic sensitivity according to Lehmann’s TNBC subtype classification. Of 130 patients, there was a strong correlation between TNBC subtype and proportion of patients achieving a pathological complete response. The proportion of all 130 assessable patients with TNBC who achieved a pathological complete response was 28%; basal-like 1 subtype had the highest proportion of achievement of a pathological complete response (52%), followed by mesenchymal-like (31%), immunomodulatory (30%), and mesenchymal stem subtypes (23%). 10% of patients with the luminal AR subtype achieved a pathological complete response, and no patients with basal-like 2 subtype achieved a pathological complete response. Despite the low proportion of patients with luminal AR TNBC achieving a pathological complete response, this subtype was associated with the best overall survival.61
Few data exist for the chemotherapeutic sensitivity of TNBC in women of African ancestry. In a retrospective study that aimed to exclusively assess patients with TNBC, Dawood and colleagues64 reported that 17% of African-American women achieved a pathological complete response, compared with a proportion of 25% for women of other ethnicities (p=0·09). After controlling for patient and tumour characteristics, ethnicity was not associated with relapse-free survival (hazard ratio [HR] 1·08, 95% CI 0·69–1·68), or overall survival (1·08, 0·69–1·68) in patients who were white or of another race compared with African-American patients.64
Similarly, we assessed the association between race or ethnic origin, and pathological complete response in 2074 patients treated with anthracycline-based and taxane-based chemotherapy.65 Of 490 patients with TNBC, 19·4% of patients attained a pathological complete response, and we noted no significant differences according to race or ethnic origin. The proportions of patients achieving a pathological complete response in patients who identified their race were 16·8% in African- American patients, 20·8% in Hispanic patients, 20·2% in white patients, and 16% in patients of other racial or ethnic backgrounds (p=0·843). Although these results show the excellent outcome for patients with TNBC and chemosensitive tumours, and similar proportions of patients achieving pathological complete response in African-American patients compared with patients of other racial or ethnic backgrounds, much remains to be learned from patients who have treatment-resistant tumours. In a retrospective study66 to assess predictors of tumour progression during neoadjuvant systemic chemotherapy, pretreatment clinical T3 status (OR 6·31, [95% CI 1·81–21·97]), ER negativity (ER-positive status with 0·24 [0·13–0·44]), and African ancestry (2·07 [1·12–3·84]) were the most important predictors of tumour progression.
Treatment options for patients with TNBC
No data suggest that patients with TNBC of African ancestry should be treated differently from patients of European or Asian ancestry; therefore, present therapy guidelines apply to all patients irrespective of race or ethnic origin. Data from clinical trials stratifying outcomes according to race or ethnic origin are sparse, and the number of participants of African ancestry is very small, usually ranging between 5% and 10% of participants, or up to 15% in a few trials. The challenge for future clinical trials in TNBC is to improve the racial and ethnic diversity of participant enrolment to enhance the applicability of the results to other populations. Development of evidence-based and locally appropriate strategies to treat TNBC in women of African ancestry living in Africa will need a commitment to capacity building in cancer research and continuous efforts towards attaining this important goal.67 For patients with early-stage or locally advanced TNBC, treatment with anthracycline and taxane-based regimens is preferred because it is associated with high rates of pathological complete response, which is a good surrogate marker for improved survival.63,64 In patients with metastatic disease, sequential single agents are frequently used, although combination chemotherapy is also part of the standard of care. No specific strategy is associated with improved survival; however, combination chemotherapy is associated with higher proportions of patients achieving an overall response, longer time to progression, and increased toxicity than are sequential single agents.
Ixabepilone and eribulin are some of the newest agents used in the treatment of metastatic TNBC. In the CA163- 046 trial 752 patients were randomly assigned to receive ixabepilone in combination with capecitabine, or capecitabine alone; subgroup analysis of the 187 patients with TNBC significantly favoured the combination group.68 In a similar study with 1221 patients with metastatic breast cancer, Sparano and colleagues69 reported that in the 256 patients with TNBC, the combination treatment was significantly associated with improved durations of progression-free survival (HR 0·64, 95% CI 0·48–0·98). A subgroup analysis of 40 African-American patients did not show any benefit from the combination treatment against capecitabine alone, but the conclusions were restricted by the small number of patients in this analysis.69
Tumours of BRCA mutation carriers have a deficiency in the repair of DNA double stranded breaks by homologous recombination and this phenotype has been termed BRCAness (or BRCA-like).70 30–50% of patients with TNBC are estimated to have BRCAness with loss of BRCA1 function. Identification of these BRCA-like tumours might have important therapeutic implications because of their frequent sensitivity to alkylating and platinum drugs. In patients with BRCA mutations, the reported proportions of patients achieving pathological complete responses with single-agent cisplatin were 83–100%.71,72 In a meta-analysis,73 investigators assessed the effect of addition of platinum salts to standard neoadjuvant chemotherapy, which doubled the increase in proportions of pathological complete response (19·6% vs 48·4%, p<0·0001) in patients with TNBC.73 The proportion of patients with BRCA mutations participating in these trials is unclear. The benefits of the addition of carboplatin or cisplatin could be driven by the very high proportions of pathological complete response in patients with BRCA mutations.
In the metastatic setting, the activity of single-agent platinum salts against TNBC is low, but the combination of platinum salts with other agents, such as gemcitabine, is synergistic. In a small phase 2 study74 to assess the combination of gemcitabine and carboplatin to treat metastatic breast cancer, the median time to progression was 5·3 months (95% CI 2·4–6·7 months) and the proportion of patients achieving an overall response was 31%.74 In patients with TNBC, the combination was associated with a similar proportion of patients achieving an overall response (27% for patients who had not previously been treated with taxane and 32% for those who were pretreated with taxane).75 These results are consistent with the proportion of patients achieving a clinical benefit of 35%, and the median duration of progression-free survival of 3·6 months recorded in the control group of a phase 2 study in which investigators assessed the use of gemcitabine and carboplatin with or without iniparib in patients with TNBC.76
New targeted therapies for TNBC
Different subtypes of breast cancer are now being treated with different molecularly targeted therapies (figure 3). The gene profiling and DNA mutation data of breast cancer subgroups have identified many molecular signalling molecules that could be targeted for the treatment of breast cancer, including those in women of African ancestry. A major aim of present research is to discover effective therapies for TNBC.
Figure 3. Oncogenic pathways associated with breast cancer.
Selected genes and pathways differentially associated with specific breast cancer subtypes. Green arrows show activating signals. Red lines show inhibitory effects.
Akt=akt proto-oncogene. AMPK=AMP-activated protein kinase. AR=androgen receptor. ARE=androgen response element. CDK=cyclin-dependent kinase. DSB=double-strand break. ER=oestrogen receptor. ERE=oestrogen response element. E2=oestrogen. IGFR=insulin-like growth factor receptor. IR=insulin receptor. MAPK=mitogen-activated protein kinase. MAPKK/MEK=mitogen-activated protein kinase kinase. P13K=phosphatidylinositol 3-kinase. PARP=poly ADP-ribose polymerase. pS6=ribosomal protein S6. pS6K=pS6 kinase. PTEN=phosphate and tensin homologue. Raf=raf-1 proto-oncogene. SRC=SRC proto-oncogene. TSC1=tuberous sclerosis 1. TSC2=tuberous sclerosis 2.
Signalling inhibitors have activity in preclinical or early clinical tests. Poly (ADP-ribose) polymerase-1 (PARP) plays a key part in the repair of DNA single-strand breaks through the base excision repair pathway. It binds directly to the site of DNA damage where the polymerase recruits additional DNA repair enzymes.77 PARP inhibitors cause specific DNA lesions that need homologous repair and are therefore interesting agents to target in cells that are deficient in repairing double-stranded DNA breaks. Data support the postulation that BRCA1-mutated tumours are sensitive to PARP inhibitors via synthetic lethality and drugs, including olaparib, have proven to have significant single-agent activity in patients with BRCA1 and BRCA2 mutations.78 Because of the important similarities between sporadic TNBC and BRCA-mutated breast cancers, the efficacy of these agents has been assessed in the treatment of TNBC. A randomised phase 2 study for patients with metastatic TNBC of iniparib in combination with gemcitabine and carboplatin seemed more effective than chemotherapy alone (progression-free-survival is 5·9 months [95% CI 4·5–7·2] for combination therapy vs 3·6 months [2·6–5·2] for chemotherapy alone; p=0·01 and overall survival 12·3 months [9·8–21·5] vs 7·7 months [6·5–13·3]; p=0·01);79 however, these results could not be reproduced in a phase 3 trial of identical design. PARP inhibitors are likely to be active in BRCA-mutant cancers, but its efficacy against sporadic TNBC has not been shown.
Additional promising molecular targets include the androgen receptor (for luminal androgen-receptor TNBC), src (for mesenchymal TNBC), and insulin-like growth factor receptor, PI3K, protein kinase B (AKT), and mTOR (all molecules that transduce growth signals from growth factor receptors).24 PI3K is especially promising because it is frequently mutated in human breast cancers.23 Another promising target is the cyclin-dependent kinases.80 These molecules phosphorylate crucial cell cycle regulators (cyclins) that regulate cancer cell growth. Specific small-molecule inhibitors of all these targets are being tested in early clinical trials for the treatment of breast cancer.
Immunomodulatory drugs are another important class of drugs that target the T-cell surface molecules CTLA4 (ipilimumab) or PD-1 (nivolumab). These drugs have shown great potential for treatment of melanoma and other cancers and are being considered for testing in breast cancer.81 The TP53 tumour suppressor gene is another important target. This critical cell-cycle regulatory gene is the most commonly mutated gene in ER-negative breast cancers (around 80% of cases of TNBC show TP53 gene mutations).23 The P53 protein has been very difficult to target because of its dynamic and complex protein– protein interactions and substantial number of DNA promotor binding.82 No P53-specific therapies are clinically available. However, development of P53-specific therapies is a major effort in pharmaceutical companies and research laboratories.
Conclusion
Breast cancer is an increasing health-care problem throughout the world with large populations of women of African ancestry, whose disease is diagnosed at an advanced stage and is associated with high mortality. Women of African ancestry have a disproportionately high frequency of the aggressive TNBC subtype (20–79%) and present with disease at a younger age than do women of European ancestry. In African-American women, premenopausal status, increased parity, and shorter duration of breastfeeding are positively associated with an increased risk of TNBC, but whether these represent shared risk factors across other populations of African women is unknown. Genetic markers of African ancestry that predispose women to TNBC independent of known environmental risk factors have not been identified, but this is an area of active investigation. TNBC or basal-like breast cancer is a heterogeneous disease with variable responsiveness to chemotherapy regardless of race or ethnic origin. High-priority clinical trials that combine novel targeted agents with standard chemotherapeutic regimens are being undertaken. An urgent need exists for collaborative multinational research that focuses on the causes, prevention, and treatment of TNBC in women of African ancestry.
Search strategy and selection criteria.
We searched PubMed for articles published in English between Jan 1, 2000, to July 1, 2014. Relevant articles were identified with the MeSH search terms “triple negative”, “basal”, “breast neoplasms”, “African continental ancestry group”, “African American”, “tumour markers/biology”, “epidemiology”, “genetics”, and “drug therapy”. We obtained additional statistics on breast cancer incidence and mortality from population-based registries available on the websites of the International Agency for Research on Cancer, Pan American Health Organization, and Surveillance Epidemiology, and End Results Program.
Acknowledgments
We thank Erica Goodoff for providing editorial assistance. AMB is supported by a US National Institute of Health (NIH) grant (1R01CA172511-01). PB is supported by NIH grants (5P30 CA016672-38; N01-CN-2012-00034, and N01-CN-35159). These funding sources had no role in the planning and writing of the review.
Footnotes
Contributors
All authors equally contributed to the literature research, content, and writing of the Review. All authors approved the final manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Ferlay JSI, Ervik M, Dikshit R, et al. IARC. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide: IARC Lyon. France: International Agency for Research on Cancer; 2013. [accessed Feb 1, 2014]. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. [Google Scholar]
- 2.PAHO. [accessed Feb 1, 2014];Cancer in the Americas: country profiles 2013. http://www.uicc.org/sites/main/files/private/Cancer-Country-Profiles-2013-ENG.pdf.
- 3.Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev. 2014;23:953–66. doi: 10.1158/1055-9965.EPI-14-0281. [DOI] [PubMed] [Google Scholar]
- 4.Hennis AJ, Hambleton IR, Wu SY, Leske MC, Nemesure B Barbados National Cancer Study G. Breast cancer incidence and mortality in a Caribbean population: comparisons with African- Americans. Int J Cancer. 2009;124:429–33. doi: 10.1002/ijc.23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader NNA, Krapcho M, Garshell J, et al. National Cancer Institute. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 6.Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer. 2013;133:721–29. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 7.Wabinga H, Parkin DM, Nambooze S, Amero J. Cancer survival in Kampala, Uganda, 1993–1997. IARC Sci Pub. 2011;162:243–47. [PubMed] [Google Scholar]
- 8.Creanga AA, Gillespie D, Karklins S, Tusui AO. Low use of contraception among poor women in Africa: an equity issue. Bull World Health Organ. 2011;89:258–66. doi: 10.2471/BLT.10.083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358:213–16. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 10.Sylla BS, Wild CP. A million africans a year dying from cancer by 2030: what can cancer research and control offer to the continent? Int J Cancer. 2012;130:245–50. doi: 10.1002/ijc.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 12.Copson E, Maishman T, Gerty S, et al. Ethnicity and outcome of young breast cancer patients in the United Kingdom: the POSH study. Br J Cancer. 2014;110:230–41. doi: 10.1038/bjc.2013.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342–49. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 14.Schiff R, Osborne CK, Fuqua SAWF. Clinical aspects of estrogen and progesterone receptors. In: Harris JR, Lippman ME, Morrow M, Osborne K, editors. Diseases of the Breast. 4. Philadelphia, USA: Lippincott Williams and Wilkins; 2010. pp. 408–42. [Google Scholar]
- 15.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–28. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 16.Stratton MR Breast Cancer Linkage Consortium. Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases. Lancet. 1997;349:1505–10. [PubMed] [Google Scholar]
- 17.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Thompson PA, Brewster AM, Kim-Anh D, et al. Selective genomic copy number imbalances and probability of recurrence in early-stage breast cancer. PLoS One. 2011;6:e23543. doi: 10.1371/journal.pone.0023543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke CA, Keegan TH, Yang J, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104:1094–101. doi: 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju055. pii: dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson SJ, Duffy SW, Blows FM, et al. Molecular characteristics of screen-detected vs symptomatic breast cancers and their impact on survival. Br J Cancer. 2009;101:1338–44. doi: 10.1038/sj.bjc.6605317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Cancer screening— United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–45. [PubMed] [Google Scholar]
- 29.Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–97. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 30.Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women: Implications for breast cancer screening recommendations. Cancer. 2011;117:2747–53. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowen RL, Duffy SW, Ryan DA, Hart IR, Jones JL. Early onset of breast cancer in a group of British black women. Br J Cancer. 2008;98:277–81. doi: 10.1038/sj.bjc.6604174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huo D, Ikpatt F, Khramtsov A, et al. Population differences in breast cancer: survey in indigenous African women reveals overrepresentation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515–21. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ly M, Antoine M, Dembele AK, et al. High incidence of triple-negative tumors in sub-Saharan Africa: a prospective study of breast cancer characteristics and risk factors in Malian women seen in a Bamako university hospital. Oncology. 2012;83:257–63. doi: 10.1159/000341541. [DOI] [PubMed] [Google Scholar]
- 34.McCormack VA, Joffe M, van den Berg E, et al. Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res. 2013;15:R84. doi: 10.1186/bcr3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stark A, Kleer CG, Martin I, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer. 2010;116:4926–32. doi: 10.1002/cncr.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stead LA, Lash TL, Sobieraj JE, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20:1071–82. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma H, Wang Y, Sullivan-Haley J, et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the Women’s contraception and reproductive experiences study. Breast feeding and contraception use. Cancer Res. 2010;70:575–87. doi: 10.1158/0008-5472.CAN-09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinde SS, Forman MR, Kuerer HM, et al. Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer. 2010;116:4933–43. doi: 10.1002/cncr.25443. [DOI] [PubMed] [Google Scholar]
- 41.Ambrosone CB, Zirpoli G, Ruszczyk M, et al. Parity and breastfeeding among African-American women: differential effects on breast cancer risk by estrogen receptor status in the Women’s Circle of Health Study. Cancer Causes Control. 2014;25:259–65. doi: 10.1007/s10552-013-0323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:307–14. doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 43.Bandera EV, Chandran U, Zirpoli G, et al. Body fatness and breast cancer risk in women of African ancestry. BMC Cancer. 2013;13:475. doi: 10.1186/1471-2407-13-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung S, Spiegelman D, Baglietto L, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013;105:219–36. doi: 10.1093/jnci/djs635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Link LB, Canchola AJ, Bernstein L, et al. Dietary patterns and breast cancer risk in the California Teachers Study cohort. Am J Clin Nutr. 2013;98:1524–32. doi: 10.3945/ajcn.113.061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Msolly A, Gharbi O, Ben Ahmed S. Impact of menstrual and reproductive factors on breast cancer risk in Tunisia: a case-control study. Med Oncol. 2013;30:480. doi: 10.1007/s12032-013-0480-4. [DOI] [PubMed] [Google Scholar]
- 47.Galukande M, Wabinga H, Mirembe F, Karamagi C, Asea A. Difference in risk factors for breast cancer by ER status in an indigenous African population. ISRN Oncol. 2013;2013:463594. doi: 10.1155/2013/463594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn BK, Agurs-Collins T, Browne D, Lubet R, Johnson KA. Health disparities in breast cancer: biology meets socioeconomic status. Breast Cancer Res Treat. 2010;121:281–92. doi: 10.1007/s10549-010-0827-x. [DOI] [PubMed] [Google Scholar]
- 49.Fejerman L, Haiman CA, Reich D, et al. An admixture scan in 1,484 African American women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:3110–17. doi: 10.1158/1055-9965.EPI-09-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reding KW, Carlson CS, Kahsai O, et al. Examination of ancestral informative markers and self-reported race with tumor characteristics of breast cancer among black and white women. Breast Cancer Res Treat. 2012;134:801–09. doi: 10.1007/s10549-012-2099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levran O, Awolesi O, Shen PH, Adelson M, Kreek MJ. Estimating ancestral proportions in a multi-ethnic US sample: implications for studies of admixed populations. Hum Genomics. 2012;6:2. doi: 10.1186/1479-7364-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Fackenthal JD, Zheng Y, et al. Recurrent BRCA1 and BRCA2 mutations in breast cancer patients of African ancestry. Breast Cancer Res Treat. 2012;134:889–94. doi: 10.1007/s10549-012-2136-z. [DOI] [PubMed] [Google Scholar]
- 53.John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298:2869–76. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 54.Malone KE, Daling JR, Doody DR, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 55.Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131:1114–23. doi: 10.1002/ijc.27326. [DOI] [PubMed] [Google Scholar]
- 56.Akbari MR, Donenberg T, Lunn J, et al. The spectrum of BRCA1 and BRCA2 mutations in breast cancer patients in the Bahamas. Clin Genet. 2014;85:64–67. doi: 10.1111/cge.12132. [DOI] [PubMed] [Google Scholar]
- 57.Chen F, Chen GK, Stram DO, et al. A genome-wide association study of breast cancer in women of African ancestry. Hum Genet. 2013;132:39–48. doi: 10.1007/s00439-012-1214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haiman CA, Chen GK, Vachon CM, et al. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. 2011;43:1210–14. doi: 10.1038/ng.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palmer JR, Ruiz-Narvaez EA, Rotimi CN, et al. Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev. 2013;22:127–34. doi: 10.1158/1055-9965.EPI-12-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 61.Masuda H, Baggerly KA, Wang Y, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–40. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 63.Liedtke C, Mazouni C, Hess KR, et al. Response to neo-adjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 64.Dawood S, Broglio K, Kau SW, et al. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27:220–26. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chavez-Macgregor M, Litton J, Chen H, et al. Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy: evaluating the effect of race/ethnicity. Cancer. 2010;116:4168–77. doi: 10.1002/cncr.25296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caudle AS, Gonzalez-Angulo AM, Hunt KK, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:1821–28. doi: 10.1200/JCO.2009.25.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adewole I, Martin DN, Williams MJ, et al. Building capacity for sustainable research programmes for cancer in Africa. Nat Rev Clin Oncol. 2014;11:251–59. doi: 10.1038/nrclinonc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–17. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 69.Sparano JA, Vrdoljak E, Rixe O, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–63. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–19. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 71.Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–79. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 72.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neo-adjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–53. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petrelli F, Coinu A, Borgonovo K, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144:223–32. doi: 10.1007/s10549-014-2876-z. [DOI] [PubMed] [Google Scholar]
- 74.Laessig D, Stemmler HJ, Vehling-Kaiser U, et al. Gemcitabine and carboplatin in intensively pretreated patients with metastatic breast cancer. Oncology. 2007;73:407–14. doi: 10.1159/000136796. [DOI] [PubMed] [Google Scholar]
- 75.Loesch D, Asmar L, McIntyre K, et al. Phase II trial of gemcitabine/carboplatin (plus trastuzumab in HER2-positive disease) in patients with metastatic breast cancer. Clin Breast Cancer. 2008;8:178–86. doi: 10.3816/CBC.2008.n.019. [DOI] [PubMed] [Google Scholar]
- 76.O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 77.Carey LA. Directed therapy of subtypes of triple-negative breast cancer. Oncologist. 2010;15 (suppl 5):49–56. doi: 10.1634/theoncologist.2010-S5-49. [DOI] [PubMed] [Google Scholar]
- 78.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 79.O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. New Eng J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 80.Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476–87. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Criscitiello C, Curigliano G. Immunotherapeutics for breast cancer. Curr Opin Oncol. 2013;25:602–08. doi: 10.1097/CCO.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 82.Wei CL, Wu Q, Vega VB, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–19. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]