Abstract
Mammalian cells depend on phospholipid (PL) and fatty acid (FA) transport to maintain membrane structure and organization, and to fuel and regulate cellular functions. In mammary glands of lactating animals, copious milk secretion, including large quantities of lipid in some species, requires adaptation and integration of PL and FA synthesis and transport processes to meet secretion demands. At present few details exist about how these processes are regulated within the mammary gland. However, recent advances in our understanding of the structural and molecular biology of membrane systems and cellular lipid trafficking provide insights into the mechanisms underlying the regulation and integration of PL and FA transport processes the lactating mammary gland. This review discusses the PL and FA transport processes required to maintain the structural integrity and organization of the mammary gland and support its secretory functions within the context of current molecular and cellular models of their regulation.
Keywords: Biosynthesis, Lactation, Lipids, Mammary gland, Membranes, Transport mechanisms
Introduction
Lipids, including phospholipids, sphingolipids, glycerol lipids, fatty acids, and cholesterol, are continuously transported within cells for renewal and biogenesis of membrane systems, for organelle function, for macromolecule trafficking, and for energy homeostasis [1, 2]. The processes mediating these transport functions are genetically and mechanistically diverse, and tailored by evolution to match a cell’s physiological roles [3, 4]. Within glandular systems, there are additional requirements for directionally organized lipid transport to maintain the cells apical-basal polarity and to achieve their secretory functions [5].
In the mammary gland, copious milk secretion requires efficient phospholipid transport for synthesis, packaging, and apical movement of milk cargo. In addition, the marked expansion of the endoplasmic reticulum (ER) and Golgi associated with secretory differentiation of milk secreting cells [6–8] depends on stimulation of phospholipid synthesis and transport [9]. The relatively large amount of lipid that is secreted by most mammals during lactation, and the novel membrane envelopment mechanism by which milk lipids are secreted [10, 11], further increase demands on lipid synthesis and transport processes in milk secreting cells above that of other types of secretory cells.
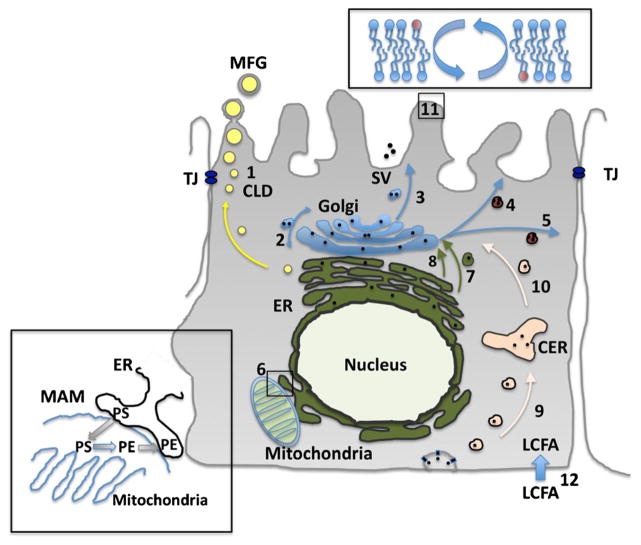
Multiple routes have been identified in polarized epithelial cells, including milk secreting cells of the mammary epithelium, for directed transport of protein, carbohydrate, and lipid cargo from their respective sites of synthesis within the ER, Golgi or mitochondria to apical and basolateral regions of the plasma membrane, and for transferring theses substances within, and among, organelle and endosomal membrane compartments [12, 13] (Fig. 1). Events within the Golgi have long been recognized to play primary roles in determining cargo trafficking patterns within cells, however recent research has uncovered additional diversity and complexity of cellular transport processes, including the presence of multiple, and intersecting routes, between organelles and cargo destination sites [12]. In particular, significant diversity exists in the mechanisms of phospholipid and sphingolipid transport within cells. Phospholipids and sphingolipids are synthesized by enzymatic reactions on membranes of the ER, Golgi, and mitochondria and undergo transport to other cellular compartments by vesicular, carrier-mediated, and direct transfer processes [14]. In milk secreting cells, the diverse vesicular transport network is thought to play a primary role in transferring newly synthesized phospholipids and sphingolipids between ER and Golgi membranes (pathways 7 and 8, Fig. 1), between cis and trans Golgi membranes (pathway 2, Fig. 1), and for transporting phospholipids from Golgi membranes to apical and basolateral plasma membranes for membrane renewal (pathways 4 and 5, Fig. 1), and to the apical membrane during the process of secreting casein, lactose and other soluble milk substances (pathway 3, Fig. 1). However, studies of lipid synthesis and transport mechanisms in the liver and other organs have demonstrated that phospholipid and sphingolipid transport mechanisms depend on the type and physiological functions of the lipid being transported. Although details are lacking, carrier-mediated and direct transfer processes are also expected to contribute to phospholipid transport in the mammary gland. Additional lipid transport pathways used by milk secreting cells include, endosomal transport processes associated with transcytosis and secretion of serum-derived substances into milk (pathways 9 and 10, Fig. 1) [13], and en masse transport of phospholipids, along with triglycerides and cholesterol esters, to the apical plasma membrane in association with milk lipid secretion (pathway 1, Fig. 1) [15].
Fig. 1.
Summary of possible mechanisms contributing to PL transport in milk secreting cells of lactating animals. (1) Milk lipid secretion; (2) Vesicle trafficking within the Golgi; (3) Secretory vesicle transport from the Golgi to the apical plasma membrane; (4) Vesicle trafficking from the Golgi for apical plasma membrane renewal; (5) Vesicle trafficking from the Golgi for basolateral membrane renewal; (6) Intermembrane PL transport between ER and mitochondria at MAM sites; (7) Vesicle trafficking from the endoplasmic reticulum (ER) to the Golgi; (8) Carrier mediated PL transport from the ER to the Golgi; (9) Endocytosis and vesicle transport of basolatral membrane elements to the CER during transcytosis; (10) Vesicle transport of membrane elements from common endosome recycling compartment (CER) to the Golgi during transcytosis; (11) PL translocation to establish membrane PL asymmetry; (12) Transport of LCFA into the cytoplasm TJ tight junction; SV secretory vesicle; CLD cytoplasmic lipid droplet
Phospholipid Transport
Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phoshpatidylinositol (PI), phosphatidylserine (PS) and sphingomyelin (SM) are the major phospholipids of eukaryotic membranes, including the mammary gland. However, differences in the relative abundances of individual PL exist within cellular membrane compartments (Table 1), among tissues, and between membrane bilayer leaflets [16]. Little precise information exists about the membrane distribution, the biosynthesis, or the transport of PL in milk secreting cells. However, mechanisms underlying these functions are being identified in other tissues and cell types that are likely to have applicability to PL transport in milk secreting cells of the mammary gland.
Table 1.
Phospholipid distribution in cellular membrane compartments
| PC | PE | PI | PS | SM/SL | Other | |
|---|---|---|---|---|---|---|
| Plasma membranea | 42 % | 22 % | 4 % | 7 % | 24 % | 1 % |
| Endoplasmic reticuluma | 55 % | 25 % | 10 % | 5 % | NR | 5 % |
| Golgia | 50 % | 18 % | 7 % | 5 % | 13 % | 7 % |
| Endosomesa | 50 % | 22 % | 2 % | 1 % | 10 % | 15 % |
| Cytoplasmic lipid dropletsb | 47 % | 20 % | 8 % | 1 % | 2 % | 22 % |
| Milk fat globulesc | 8–45 % | 26–72 % | 1.4–14 % | 2–16 % | 4–29 % | NR |
Transport of de novo synthesized phospholipids
The endoplasmic reticulum (ER) is the primary, but not the only site of PL synthesis [16, 17], and it is now known that PL biosynthesis enzymes found on Golgi, mitochondrial, nuclear, endosomal, and plasma membranes contribute to overall membrane PL homeostasis and site-specific PL synthesis in eukaryotic cells [16]. In particular, membrane levels of PC, PE and PS, which are metabolically interconvertible [18, 19], are determined by the activities of enzymes located on membranes of the ER, mitochondria and Golgi, and involve mechanisms for exchanging or transporting PL among these compartments [14].
Phosphatidylcholine
PC is synthesized from CDP-choline and diacylglycerol (DAG) by two distinct CDP-choline:1,2-diacylglycerol cholinephosphotransferase enzymes; choline-ethanolamine phosphotransferase (Cept), a bifunctional ER membrane enzyme that catalyzes the synthesis of both PC and PE, and CDP-choline phosphotransferase (Cpt), a Golgi-membrane enzyme that specifically catalyzes the synthesis of PC [18, 20]. PC is also synthesized by methylation of PE, which is catalyzed by phosphatidylethanolamine N-methyltransferase (Pemt) [21]. Under normal dietary conditions, the CDP-choline pathways are responsible for approximately 70 % of de novo synthesized PC and Pemt is responsible for the remaining PC synthesis. PC synthesis from PE by the Pemt pathway is a multi-compartment process. The PE used in this reaction originates at the inner mitochondrial membranes by decarboxylation of PS, and must be transported to external mitochondrial membranes and then to the ER for PC synthesis [22] (Fig. 1). Pemt is expressed in mammary epithelial cells and it has been shown that Pemt activity contributes to PC synthesis [23]. However, observations that Pemt activity levels are low in mammary tissue [24], and that defects in mammary gland function have not been reported for Pemt knockout mice [21], suggest that the CDP-choline pathway may also be the primary pathway for PC synthesis in milk secreting cells.
Phosphatidylethanolamine
As already stated, PE is synthesized by the CDP-ethanolamine pathway in the ER [17]. PE is also synthesized by decarboxylation of phosphatidylserine (PS) in the mitochondria [25]. Disruption of either pathway is embryonically lethal [26], documenting the essential role of PE in membrane synthesis and function. PE is rapidly transported from its sites of synthesis on the ER or the mitochondria to other membrane compartments [27]. Observations that PE, newly synthesized in the mitochondria by decarboxylation of PS, was transported to the ER for PC synthesis more rapidly the existing PE demonstrated the presence of at least two types of PE transport mechanisms between the mitochondrial and ER membranes [28]. Reconstitution experiments, demonstrating that the rapid PE transport process required only mitochondrial and ER membranes and was independent of cytosoloic proteins and ATP, provided evidence that rapid PE transport involved formation of direct membrane connections between mitochondrial and ER membranes at specialized ER domains referred to as mitochondrial associated membranes (MAM) (pathway 6, Fig. 1) [28]. PE transportation from its sites of synthesis in the ER or the mitochondria to the plasma membrane is also insensitive to ATP depletion and cytoskeletal disruption, suggesting that non-vesicular mechanisms also contribute the PE transport to non-mitochondrial compartments [29].
Phosphatidylserine
Synthesis of PS occurs primarily in the ER by base-exchange reactions catalyzed by phosphatidylserine synthase 1 and 2 (Pss1, Pss2) that respectively exchange the choline and ethanolamine head groups of PC and PE with serine [19]. Like other phospholipids, PS is uniformly distributed between inner and outer membrane leaflets of the ER [16]. Pss1 and Pss2 localize to contact sites between the ER and the mitochondria [30] and nascent PS is transported from the ER to the mitochondria during periods of transient connections of ER and mitochondrial membranes [28].
Phosphatidylinositol
PI biosynthesis is catalyzed by phosphatidylinositol synthase (Pis) on the cytoplasmic face of ER membranes [31, 32]. It was thought that PI was rapidly transported from its site of synthesis on the ER to other membrane compartments by PI transfer proteins [33], however more recent studies have identified a highly mobile ER-derived membrane compartment that makes contacts with other membranes as the source of PI synthesis and the mechanism by which it undergoes inter-membrane transport [34].
Sphingolipids (SL)
Sphingolipid synthesis is a two-compartment process. ER membrane enzymes catalyze the synthesis of ceramide, a lipid amide. Ceramide is then transported to the Golgi by vesicular trafficking (pathway 7, Fig. 1), or by the ceramide transport protein (CERT) (pathway 8, Fig. 1) [35], where it is converted into sphingolipids by enzymes within the Golgi lumen that catalyze transfer of phosphoamine, sugar, or carbohydrate groups to the hydroxyl moiety of ceramide [36]. Coupled with the absence of significant SL transmembrane transport mechanisms, the luminal location of the SL synthesis enzymes results in specific incorporation of SL into external facing membrane leaflets [16].
Establishing PL membrane asymmetry
Phospholipids are asymmetrically distributed between leaflets of the Golgi, endosomal and plasma membranes [16]. PE, PS and phosphoinositides are enriched on the cytosolic leaflets of these compartments, whereas PC, SM and glycosphingolipids are enriched on their outward facing leaflets [37]. The basis of this asymmetry is related to inherent differences in the abilities of PL to spontaneously cross membrane bilayers; to differences in PL transport and retention mechanisms; and to the presence of transporters that facilitate movement of PL across membrane bilayers. The processes by which PL bilayers asymmetry is established occur downstream of their ER synthesis. The catalytic sites of the major PL biosynthesis enzymes are located on the cytosolic face of the ER [16, 38, 39]. Newly synthesized PL are inserted into the cytosolic facing leaflet of the ER membrane initially, and undergo transbilayer equilibration with half-times of approximately 20 min [27] through the actions of ER membrane associated proteins with PL flippase activity [14, 40]. Transport of newly synthesized PL from the ER to other membrane compartments occurs at variable rates, which may account for initial PL asymmetry differences in some compartments. For instance, PC and PE are transported from the ER to the plasma membrane with a half-times of 1 min or less [41], whereas PC undergoes transport to the mitochondria with a half-time of approximately 5 min, while PE is transported from the ER to the mitochondria with half-time of 2 h [27]. For SM and glycosphingolipids, their membrane asymmetry results from their synthesis on the luminal facing leaflet of the Golgi membrane and the absence of significant transmembrane transport [16].
PL translocase activities
Although differences in sites of synthesis and rates of transbilayer and transmembrane transport of newly synthesized PL may contribute to membrane asymmetry, energy-dependent transport proteins appear to underlie much of the PL transbilayer asymmetry [42]. Plasma membranes, trans-Golgi membranes and membranes of secretory vesicles have been shown to possess aminophospholipid translocase activities that utilizes ATP hydrolysis to inwardly transport (flip) PE and PS across bilayers to the cytoplasmic leaflet (pathway 11, Fig. 1) [43, 44]. Flippase activity is encoded by a large family of Type IV P-type ATPases (P4-ATPases) [42], and defects in PE or PS transport associated with mutations of P4-ATPase family members have been identified in yeast and mammalian cells [42, 45].
In contrast to inward transport of PE and PS catalyzed by P4-ATPases, members of the ATP-binding cassette (ABC) transporter family catalyze outward transport (flop) of PL, including PC, PS, PE, SM and glycosphingolipids [16, 46]. The ABC transporter family includes multiple drug resistance transporters that catalyze export of a wide range of compounds, including cholesterol, and various xenobiotics [16, 47]. Members of the ABC transporter family have been detected in milk secreting cells of bovine and murine mammary glands [48] and secreted milk fat globules in bovine milk [49]. ABC-transporter dependent efflux of cholesterol has been detected on the apical and basolateral sides of polarized mammary epithelial cells cultured on transwell plates, with significantly greater efflux occurring from the basal membrane side [50]. These observations support the concept that ATP-binding cassettes contribute to the regulation of cholesterol homeostasis in the mammary gland; and along with evidence of the presence of the cholesterol acceptor, ApoA1, in human milk [51], they suggest that ABC transporters may play a role in transporting cholesterol, and perhaps other lipids, into milk [50]. Nevertheless, specific roles of ABC transporters in regulating membrane transport of PL in mammary epithelial cells have not been identified. Thus, their contributions to controlling PL transbilayer asymmetry in the mammary gland remain to be established.
Intermembrane PL transport
PL transport among membrane compartments is known to occur by vesicular and non-vesicular pathways [27]. Vesicular transport largely follows the canonical pathways used in membrane protein transport between the ER, Golgi and plasma membrane (pathways 2–6, Fig. 1) and incorporates elements of the endocytic pathway. In the lactating mammary gland, there is considerable apical membrane trafficking of endocytotic vesicles associated with the transcytosis of serum-derived hormones, proteins, and minerals into milk (pathway 9, Fig. 1) [13, 52, 53]. The mechanisms of transcytosis involve a series of complex sorting events in the basolateral early endosome and the common endosome recycling (CER) compartments that intersect with Golgi and secretory vesicle pathways (pathway10) [54–56]. Consequently, transcytosis provides mechanisms for transferring basolateral membrane phospholipids, which are distinct from apical membrane phospholipids and enriched in PC [5], to multiple internal membrane compartments as well as to the apical plasma membrane. Because there appears to be minimal apical membrane recycling, or trafficking from the apical membrane to the basolateral membrane during lactation [53], it seems likely that PL transport by vesicular pathways involves net basolateral to apical membrane flow during active milk secretion. Few details exist about basal-apical vesicular trafficking during lactation, and the contributions of this pathway to PL transfer or exchange mechanisms between membrane compartments of milk secreting cells are unknown. Nevertheless, tracer experiments demonstrated that transcytosis mediates the transport of serum lipoprotein particles into milk, and have provided evidence that this process contributes to phospholipid secretion into the whey fraction of milk [57].
Despite established mechanisms for vesicular trafficking of PL, longstanding observations that disrupting vesicular trafficking processes does not impair PL lipid transport from the ER to the other membrane compartments [29, 41], have documented the existence of non-vesicular pathways for inter-membrane PL transport. Exchange of individual PL between membrane compartments, mediated by soluble lipid transfer proteins (LTP) has been demonstrated for PC, PI, sphingomyelin and ceramide [58] in mammalian cells, with CERT being the best-characterized transporter [59–62]. In addition, multiprotein complexes at sites of contact between membrane compartments (e.g. MAM complexes) have been demonstrated to mediate inter-membrane transfer of some PL [14].
Milk Lipid Secretion
The amount of lipid secreted during the course of lactation varies by species, but in general the processes involved in milk lipid production and secretion greatly increase lipid transport requirements. During a 6-month period of lactation the typical woman secretes more than 5 kg of lipid, whereas a 30 g mouse dam secretes about 30 g, or the equivalent of her body weight, of lipid into milk over a 21-day period of lactation [63]. The amounts of lipid secreted, and the novel membrane envelopment mechanism by which milk lipid occurs, combine to substantially increase lipid synthesis and transport demands of milk secreting cells over that of other cell types.
Milk lipid secretion is a specialized apocrine process (pathway 1, Fig. 1), in which elements of the apical plasma membrane envelope cytoplasmic lipid droplets (CLD) to produce membrane-bound secreted products referred to as milk fat globules (MFG). CLD in turn are organelle-like structures composed of a core of neutral lipids (predominantly triglycerides) surrounded by a phospholipid (PL) monolayer and selected attached proteins [64, 65]. CLD transport to the apical membrane for secretion is thus an example of an en mass mechanism of neutral and phospholipid trafficking within cells (pathway 1, Fig. 1). Few details exist about the specific nature and regulation of the transport processes mediating milk lipid formation and secretion. However, during lactation, efficient mechanisms must exist for transporting fatty acids to sites of neutral lipid and phospholipid synthesis on the endoplasmic reticulum (ER) membrane, for transporting CLD to the apical membrane for secretion, and for transporting of PL to the apical membrane to replace the loss that occurs during milk lipid secretion.
Transport Processes Involved in Milk Lipid Synthesis
In eukaryotic cells, CLD originate from the ER by processes that are still poorly defined and controversial [66, 67]. The enzymes responsible for glycerol lipid synthesis, including TG and cholesterol esters composing the neutral lipid core, are found on ER membranes, and the available evidence indicates that the composition of the PL monolayer surrounding the neutral lipid core of CLD is similar to that of ER membranes (Table 1). The ability of ER to directly incorporate newly synthesized TG into CLD was originally demonstrated in the lactating mouse mammary gland over 50 years ago. Using EM radioautography, Stein and Stein [68] showed that following tail vein injections of radioactive glycerol or oleic acid the labeled molecules initially localize to ER cisternae and then rapidly accumulate in CLD surrounded by ER membranes.
The rapid incorporation of serum-derived oleic acid into the neutral lipid core of CLD demonstrates that efficient mechanisms exist during lactation for transporting long-chain fatty acids (LCFA) from the circulation into milk secreting cells, and then to sites of glycerol lipid synthesis within these cells. Although it has not been determined directly, similar mechanisms presumably mediate fatty acid transport for PL and SL synthesis. Few details exist about specific fatty acid transport process within milk secreting cells, or the relationship of fatty acid transport to milk lipid secretion. However, the identities of physiologically important mechanisms for uptake and intracellular trafficking of long-chain fatty acids have been identified in other mammalian cells. Transcripts of members of gene families encoding fatty acid transport and/or trafficking proteins have also been detected in mammary glands of cattle [69] and mice [70] (http://biogps.org), and the expression levels of selected members of these families have been shown to correlate with increased milk lipid synthesis during lactation [71].
Fatty acid transport into cells
Long chain fatty acids (16+ carbons) used in the synthesis of milk lipids are obtained from serum triglycerides following hydrolysis by lipoprotein lipase (LPL) activity [72] or from circulating fatty acids bound to albumin [73]. Transport of long-chain fatty acids into cells from the vascular system involves transfer from serum carrier proteins, such as albumin, to the external plasma membrane leaflet, transport across the membrane bilayer, and desorption from the internal membrane leaflet. Evidence from artificial and biologic membrane systems has documented protein-independent rapid flip-flop of LCFA across the membrane bilayers [74]. However, under physiological conditions the available evidence indicates that specific transport proteins mediate LCFA transfer into cells (pathway 12, Fig. 1) [75]. Multiple protein mediators of LCFA transport into cells have been identified in eukaryotic cells [76]. In mammals, LCFA transport across membranes is mediated, or facilitated, by CD36 (Fatty acid translocase, FAT), and members of the fatty acid transport proteins/solute carrier 27 (FATP/SLC27A) and acyl-coA synthetase (ACSL) families.
CD36/FAT
The gene responsible for fatty acid translocation in adipose was identified as being closely related to CD36 by Abumrad [77]. CD36/FAT is as a multifunctional scavenger receptor [78], and multiple lines of evidence have documented its physiological importance in cardiac and intestinal function [79]. CD36/FAT is also identical to PAS IV, a glycoprotein that is highly enriched on the apical membrane of milk secreting cells in the bovine mammary gland [80], and on membranes surrounding MFG [81]. Subsequent studies showed that CD36/FAT transcripts are highly expressed in bovine mammary glands and undergo up-regulation in response to lactation [71]. Although mammary glands of non-pregnant, non-lactating female CD36/FAT-null mice exhibit altered morphological features [82], specific effects of CD36/FAT on fatty acid transport and lactation remain to be determined.
FATP and ACSL
FATP/SLC27A and ACSL families are acyl-CoA synthetases that catalyze fatty acid bioactivation through formation of coenzyme-A thioesters [83]. Bioactivation of FA can indirectly influence FA transport due to metabolic trapping of products, and it remains unclear if members of the FATP/SLC27A and ACSL families are true fatty acid transporters [83]. Mammals have 5 ACSL [84] and 6 FATP [85] family members and each family member possess splice variants [84]. Differences in substrate specificities, reaction kinetics, tissue and cellular distributions patterns of individual family members further suggest that member of these families may possess physiologically distinct FA transport functions [83, 84].
Members of the FATP/SLC27A and ACSL families are expressed in bovine and mouse mammary glands [70, 71]. However transcript levels of individual family members vary widely within each of these species and there is also considerable variability in the relative transcript abundance between the two species. The activities and intracellular localizations of mammary gland FATP/SLC27A and ACSL proteins are unknown and mammary gland defects associated with knockouts of specific members of these families have not been reported. Thus, the physiological importance of members of either family in milk lipid formation or other mammary gland functions remains uncertain.
Transport processes involved in milk lipid secretion
CLD, the immediate precursors of milk lipids, originate from basally located ER and accumulate at the apical membrane prior to being secreted into the lumen (pathway 1, Fig. 1) [11]. Observations that CLD accumulate at the apical border of mammary acini only during lactation [6] suggest that transportation and/or docking processes are regulated by the secretory activity of the mammary gland. Evidence linking apical localization of CLD to lipid secretion has been obtained from transgenic models of impaired lactation and from studies of lactating wild type in which milk secretion is inhibited by forced weaning [86, 87]. At present it is unclear whether apical polarization of CLD during lactation reflects activation of apical membrane docking functions, or alterations in CLD trafficking patterns that lead to net movement of these structures in the apical direction, or both. However, in multiple cell types CLD transport has been shown to be rapid, dependent on an intact microtubules network, and require the actions of plus- and minus-directed motor proteins [88, 89]. Although circumstantial evidence suggests that CLD are also transported to the apical membrane for milk lipid secretion on microtubules [10, 65, 90], there is currently no direct evidence supporting this mechanism.
Summary
The quantities of milk secreted during the course of a normal lactation, the concentration of lipid found in the milk of most species, and the unique mechanism by which milk lipids are secreted, combine to place unique demands on lipid transport processes in milk secreting cells. Despite significant progress in our knowledge of the genes regulated by lactation [91], our understanding of the pathway machinery for lipid transport and secretion, and how diverse types of lipid transport processes are integrated to meet the demands of lactation remains very limited. Nevertheless, numerous mechanistic advances in how lipids are synthesized, trafficked and metabolized in other cell types provide experimental frameworks for understanding the molecular and physiological details of lipid transport in lactating mammary glands.
Acknowledgments
Supported by National Institutes of Health grants 5R01-HD045962, 1R01-HD075285 and P01-HD38129.
Abbreviations
- ABC
ATP-binding cassette
- ACSL
Acyl-coA synthetase
- ApoA1
Apolipoprotein A1
- ATP
Adenosine triphosphate
- Cept
Choline-ethanolamine phosphotransferase
- CER
Common endosome recycling
- CERT
Ceramide transport protein
- CLD
Cytoplasmic lipid droplets
- Cpt
CDP-choline phosphotransferase
- DAG
Diacylglycerol
- FA
Fatty acid
- FAT
Fatty acid translocase
- FATP
Fatty acid transport protein
- ER
Endoplasmic reticulum
- LCFA
Long-chain fatty acids
- LPL
Lipoprotein lipase
- LTP
Lipid transport proteins
- MAM
Mitochondrial associated membranes
- MFG
Milk fat globule
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- Pemt
Phosphatidylethanolamine N-methyltransferase
- PI
Phosphatidylinositol
- PL
Phospholipid
- PS
Phosphatidylserine
- PSS1
Phosphotidyserine synthase 1
- PSS2
Phosphotidyserine synthase 2
- SL
Sphingolipids
- SM
Sphingomyelin
- SV
Secretory vesicle
References
- 1.Federovitch CM, et al. The dynamic ER: experimental approaches and current questions. Curr Opin Cell Biol. 2005;17:409–14. doi: 10.1016/j.ceb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Coleman JA, et al. Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport. Biochim Biophys Acta. 2013;1831:555–74. doi: 10.1016/j.bbalip.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson SM, et al. Key stages in mammary gland development. Secretory activation in the mammary gland: it’s not just about milk protein synthesis! Breast Cancer Res. 2007;9:204. doi: 10.1186/bcr1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mashek DG, et al. Long-chain acyl-CoA synthetases and fatty acid channeling. Futur Lipidol. 2007;2:465–76. doi: 10.2217/17460875.2.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikonen E, Simons K. Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin Cell Dev Biol. 1998;9:503–9. doi: 10.1006/scdb.1998.0258. [DOI] [PubMed] [Google Scholar]
- 6.Hollmann KH. Cytology and fine structure of the mammary gland. In: Larson BL, Smith VR, editors. Lactation. New York: Academic; 1974. pp. 3–95. [Google Scholar]
- 7.Wooding FBP. Comparative mammary fine structure. In: Peaker M, editor. Comparative aspects of lactation. London: Academic; 1977. pp. 1–41. [Google Scholar]
- 8.Clermont Y, et al. Structure of the Golgi apparatus in stimulated and nonstimulated acinar cells of mammary glands of the rat. Anat Rec. 1993;237:308–17. doi: 10.1002/ar.1092370303. [DOI] [PubMed] [Google Scholar]
- 9.Ron D, Hampton RY. Membrane biogenesis and the unfolded protein response. J Cell Biol. 2004;167:23–5. doi: 10.1083/jcb.200408117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mather IH, Keenan TW. Origin and secretion of milk lipids. J Mammary Gland Biol Neoplasia. 1998;3:259–73. doi: 10.1023/a:1018711410270. [DOI] [PubMed] [Google Scholar]
- 11.McManaman JL, et al. Molecular determinants of milk lipid secretion. J Mammary Gland Biol Neoplasia. 2007;12:259–68. doi: 10.1007/s10911-007-9053-5. [DOI] [PubMed] [Google Scholar]
- 12.Folsch H, et al. Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells. Traffic. 2009;10:972–81. doi: 10.1111/j.1600-0854.2009.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monks J, McManaman JL. Secretion and fluid transport mechanisms in the mammary gland. In: Zibadi S, et al., editors. Handbook of dietary and nutritional aspects of human breast milk. Wageningen: Wageningen Academic Publishers; 2013. pp. 35–56. [Google Scholar]
- 14.Holthuis JC, Levine TP. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–20. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 15.McManaman JL. Milk lipid secretion: recent biomolecular aspects. Biomol Concepts. 2012;3:581–91. doi: 10.1515/bmc-2012-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Meer G, et al. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelsema CL, Morre DJ. Distribution of phospholipid biosynthetic enzymes among cell components of rat liver. J Biol Chem. 1978;253:7960–71. [PubMed] [Google Scholar]
- 18.Henneberry AL, et al. The major sites of cellular phospholipid synthesis and molecular determinants of Fatty Acid and lipid head group specificity. Mol Biol Cell. 2002;13:3148–61. doi: 10.1091/mbc.01-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49:1377–87. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 2009;50(Suppl):S311–316. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vance DE. Physiological roles of phosphatidylethanolamine N-methyltransferase. Biochim Biophys Acta. 2013;1831:626–32. doi: 10.1016/j.bbalip.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Osman C, et al. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang EK, et al. Rat and human mammary tissue can synthesize choline moiety via the methylation of phosphatidylethanolamine. Biochem J. 1988;256:821–8. doi: 10.1042/bj2560821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vance DE, de Kruijff B. The possible functional significance of phosphatidylethanolamine methylation. Nature. 1980;288:277–9. doi: 10.1038/288277a0. [DOI] [PubMed] [Google Scholar]
- 25.Zborowski J, et al. Phosphatidylserine decarboxylase is located on the external side of the inner mitochondrial membrane. FEBS Lett. 1983;157:179–82. doi: 10.1016/0014-5793(83)81141-7. [DOI] [PubMed] [Google Scholar]
- 26.Vance JE, Tasseva G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim Biophys Acta. 2013;1831:543–54. doi: 10.1016/j.bbalip.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Voelker DR. Organelle biogenesis and intracellular lipid transport in eukaryotes. Microbiol Rev. 1991;55:543–60. doi: 10.1128/mr.55.4.543-560.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vance JE. Newly made phosphatidylserine and phosphatidylethanolamine are preferentially translocated between rat liver mitochondria and endoplasmic reticulum. J Biol Chem. 1991;266:89–97. [PubMed] [Google Scholar]
- 29.Sleight RG, Pagano RE. Rapid appearance of newly synthesized phosphatidylethanolamine at the plasma membrane. J Biol Chem. 1983;258:9050–8. [PubMed] [Google Scholar]
- 30.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–56. [PubMed] [Google Scholar]
- 31.Agranoff BW, et al. The enzymatic synthesis of inositol phosphatide. J Biol Chem. 1958;233:1077–83. [PubMed] [Google Scholar]
- 32.Bell RM, et al. Lipid topogenesis. J Lipid Res. 1981;22:391–403. [PubMed] [Google Scholar]
- 33.Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771:677–91. doi: 10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Kim YJ, et al. A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev Cell. 2011;21:813–24. doi: 10.1016/j.devcel.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanada K, et al. CERT and intracellular trafficking of ceramide. Biochim Biophys Acta. 2007;1771:644–53. doi: 10.1016/j.bbalip.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Hanada K. Discovery of the molecular machinery CERT for endoplasmic reticulum-to-Golgi trafficking of ceramide. Mol Cell Biochem. 2006;286:23–31. doi: 10.1007/s11010-005-9044-z. [DOI] [PubMed] [Google Scholar]
- 37.Daleke DL. Phospholipid flippases. J Biol Chem. 2007;282:821–5. doi: 10.1074/jbc.R600035200. [DOI] [PubMed] [Google Scholar]
- 38.Lagace TA, Ridgway ND. The role of phospholipids in the biological activity and structure of the endoplasmic reticulum. Biochim Biophys Acta. 2013;1833:2499–510. doi: 10.1016/j.bbamcr.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Pilarska M, et al. Properties and topology of enzymes methylating phosphatidylethanolamine to phosphatidylcholine in sarcoplasmic reticulum. Int J Biochem. 1987;19:705–11. doi: 10.1016/0020-711x(87)90084-x. [DOI] [PubMed] [Google Scholar]
- 40.Bishop WR, Bell RM. Assembly of the endoplasmic reticulum phospholipid bilayer: the phosphatidylcholine transporter. Cell. 1985;42:51–60. doi: 10.1016/s0092-8674(85)80100-8. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan MR, Simoni RD. Intracellular transport of phosphatidylcholine to the plasma membrane. J Cell Biol. 1985;101:441–5. doi: 10.1083/jcb.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muthusamy BP, et al. Linking phospholipid flippases to vesicle-mediated protein transport. Biochim Biophys Acta. 2009;1791:612–9. doi: 10.1016/j.bbalip.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984;81:3751–5. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devaux PF, Morris R. Transmembrane asymmetry and lateral domains in biological membranes. Traffic. 2004;5:241–6. doi: 10.1111/j.1600-0854.2004.0170.x. [DOI] [PubMed] [Google Scholar]
- 45.Yabas M, et al. ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat Immunol. 2011;12:441–9. doi: 10.1038/ni.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pomorski T, et al. Tracking down lipid flippases and their biological functions. J Cell Sci. 2004;117:805–13. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- 47.Gottesman MM, et al. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 48.Mani O, et al. Expression, localization, and functional model of cholesterol transporters in lactating and nonlactating mammary tissues of murine, bovine, and human origin. Am J Physiol Regul Integr Comp Physiol. 2010;299:R642–654. doi: 10.1152/ajpregu.00723.2009. [DOI] [PubMed] [Google Scholar]
- 49.Mani O, et al. Identification of ABCA1 and ABCG1 in milk fat globules and mammary cells–implications for milk cholesterol secretion. J Dairy Sci. 2011;94:1265–76. doi: 10.3168/jds.2010-3521. [DOI] [PubMed] [Google Scholar]
- 50.Ontsouka EC, et al. Characteristics and functional relevance of apolipoprotein-A1 and cholesterol binding in mammary gland tissues and epithelial cells. PLoS One. 2013;8:e70407. doi: 10.1371/journal.pone.0070407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Alessandro A, et al. Human milk proteins: an interactomics and updated functional overview. J Proteome Res. 2010;9:3339–73. doi: 10.1021/pr100123f. [DOI] [PubMed] [Google Scholar]
- 52.Hunziker W, Kraehenbuhl JP. Epithelial transcytosis of immunoglobulins. J Mammary Gland Biol Neoplasia. 1998;3:287–302. doi: 10.1023/a:1018715511178. [DOI] [PubMed] [Google Scholar]
- 53.Monks J, Neville MC. Albumin transcytosis across the epithelium of the lactating mouse mammary gland. J Physiol. 2004;560:267–80. doi: 10.1113/jphysiol.2004.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golachowska MR, et al. Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity. Trends Cell Biol. 2010;20:618–26. doi: 10.1016/j.tcb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Welsch U, et al. Internalization of ferritin-concanavalin A by the lactating mammary cell in vivo. Cell Tissue Res. 1984;235:433–8. doi: 10.1007/BF00217870. [DOI] [PubMed] [Google Scholar]
- 57.Monks J, et al. A lipoprotein-containing particle is transferred from the serum across the mammary epithelium into the milk of lactating mice. J Lipid Res. 2001;42:686–96. [PubMed] [Google Scholar]
- 58.Mousley CJ, et al. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim Biophys Acta. 2007;1771:727–36. doi: 10.1016/j.bbalip.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wirtz KW, et al. Properties and possible function of phosphatidylinositol-transfer proteins. Biotechnol Appl Biochem. 1990;12:485–8. [PubMed] [Google Scholar]
- 60.de Vries KJ, et al. An isoform of the phosphatidylinositol-transfer protein transfers sphingomyelin and is associated with the Golgi system. Biochem J. 1995;310(Pt 2):643–9. doi: 10.1042/bj3100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dickeson SK, et al. Isolation and sequence of cDNA clones encoding rat phosphatidylinositol transfer protein. J Biol Chem. 1989;264:16557–64. [PubMed] [Google Scholar]
- 62.Hanada K. Intracellular trafficking of ceramide by ceramide transfer protein. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:426–37. doi: 10.2183/pjab.86.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwertfeger KL, et al. Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res. 2003;44:1100–12. doi: 10.1194/jlr.M300045-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Tauchi-Sato K, et al. The surface of lipid droplets is a phospholipid monolayer with a unique Fatty Acid composition. J Biol Chem. 2002;277:44507–12. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- 65.Wu CC, et al. Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis. 2000;21:3470–82. doi: 10.1002/1522-2683(20001001)21:16<3470::AID-ELPS3470>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 66.McManaman JL, et al. Secretion and fluid transport mechanisms in the mammary gland: comparisons with the exocrine pancreas and the salivary gland. J Mammary Gland Biol Neoplasia. 2006;11:249–68. doi: 10.1007/s10911-006-9031-3. [DOI] [PubMed] [Google Scholar]
- 67.Walther TC, Farese RV., Jr The life of lipid droplets. Biochim Biophys Acta. 2009;1791:459–66. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stein O, Stein Y. Lipid synthesis, intracellular transport, and secretion. II. Electron microscopic radioautographic study of the mouse lactating mammary gland. J Cell Biol. 1967;34:251–63. doi: 10.1083/jcb.34.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bionaz M, Loor JJ. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation. J Nutr. 2008;138:1019–24. doi: 10.1093/jn/138.6.1019. [DOI] [PubMed] [Google Scholar]
- 70.Han LQ, et al. mRNA abundance and expression of SLC27A, ACC, SCD, FADS, LPIN, INSIG, and PPARGC1 gene isoforms in mouse mammary glands during the lactation cycle. Genet Mol Res. 2010;9:1250–7. doi: 10.4238/vol9-2gmr814. [DOI] [PubMed] [Google Scholar]
- 71.Bionaz M, Loor JJ. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics. 2008;9:366. doi: 10.1186/1471-2164-9-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr. 1997;17:159–83. doi: 10.1146/annurev.nutr.17.1.159. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg IJ, et al. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50(Suppl):S86–90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamilton JA. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot Essent Fat Acids. 2007;77:355–61. doi: 10.1016/j.plefa.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 75.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–7. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schaffer JE. Fatty acid transport: the roads taken. Am J Physiol Endocrinol Metab. 2002;282:E239–246. doi: 10.1152/ajpendo.00462.2001. [DOI] [PubMed] [Google Scholar]
- 77.Abumrad NA, et al. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–8. [PubMed] [Google Scholar]
- 78.Acton SL, et al. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–9. [PubMed] [Google Scholar]
- 79.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39:2012–30. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greenwalt DE, Mather IH. Characterization of an apically derived epithelial membrane glycoprotein from bovine milk, which is expressed in capillary endothelia in diverse tissues. J Cell Biol. 1985;100:397–408. doi: 10.1083/jcb.100.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenwalt DE, et al. PAS IV, an integral membrane protein of mammary epithelial cells, is related to platelet and endothelial cell DC36 (GPIV) Biochemistry. 1990;29:7054–9. doi: 10.1021/bi00482a015. [DOI] [PubMed] [Google Scholar]
- 82.DeFilippis RA, et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012;2:826–39. doi: 10.1158/2159-8290.CD-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watkins PA. Very-long-chain acyl-CoA synthetases. J Biol Chem. 2008;283:1773–7. doi: 10.1074/jbc.R700037200. [DOI] [PubMed] [Google Scholar]
- 84.Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Exp Biol Med (Maywood) 2008;233:507–21. doi: 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stahl A. A current review of fatty acid transport proteins (SLC27) Pflugers Arch. 2004;447:722–7. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- 86.Palmer CA, et al. Transgenic mice expressing recombinant human protein C exhibit defects in lactation and impaired mammary gland development. Transgenic Res. 2003;12:283–92. doi: 10.1023/a:1023398926763. [DOI] [PubMed] [Google Scholar]
- 87.McManaman JL, et al. Regulation of milk lipid formation and secretion in the mouse mammary gland. Adv Exp Med Biol. 2004;554:263–79. doi: 10.1007/978-1-4757-4242-8_22. [DOI] [PubMed] [Google Scholar]
- 88.Welte MA. Fat on the move: intracellular motion of lipid droplets. Biochem Soc Trans. 2009;37:991–6. doi: 10.1042/BST0370991. [DOI] [PubMed] [Google Scholar]
- 89.Orlicky DJ, et al. Dynamics and molecular determinants of cytoplasmic lipid droplet clustering and dispersion. PLoS One. 2013;8:e66837. doi: 10.1371/journal.pone.0066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patton S, et al. The supression of milk fat globule secretion by clochicine: an effect coupled to inhibition of exocytosis. Biochim Biophys Acta. 1977;499:404–10. doi: 10.1016/0304-4165(77)90071-x. [DOI] [PubMed] [Google Scholar]
- 91.Lemay DG, et al. Gene regulatory networks in lactation: identification of global principles using bioinformatics. BMC Syst Biol. 2007;1:56. doi: 10.1186/1752-0509-1-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartz R, et al. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–47. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- 93.Contarini G, Povolo M. Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. 2013;14:2808–31. doi: 10.3390/ijms14022808. [DOI] [PMC free article] [PubMed] [Google Scholar]