Abstract
Cholangiocytes, the epithelial cells lining the biliary tree, represent only a small portion of the total liver cell population (3–5%), but they are responsible for the secretion of up to 40% of total daily bile volume. In addition, cholangiocytes are the target of a diverse group of liver diseases affecting the biliary tract, the cholangiopathies; for most of these conditions, the pathological mechanisms are unclear. MicroRNAs (miRNAs) are small, noncoding RNAs that posttranscriptionally regulate gene expression. Thus, it is not surprising that altered miRNA profiles underlie the dysregulation of several proteins involved in the pathobiology of the cholangiopathies, as well as showing promise as diagnostic and prognostic tools. Here the authors review recent work relevant to the role of miRNAs in the etiopathogenesis of several of the cholangiopathies (i.e., fibroinflammatory cholangiopathies and polycystic liver diseases), discuss their value as prognostic and diagnostic tools, and provide suggestions for further research.
Keywords: cholangiocytes, microRNAs, cholangiopathies, cholangiocarcinoma, polycystic liver disease, primary biliary cirrhosis, primary sclerosing cholangitis, biliary atresia
Cholangiocytes, the epithelial cells lining the biliary tree, represent only a small portion of the total liver cell population, but are responsible for the secretion of up to 40% of the total daily bile volume. Cholangiocytes are the target of a diverse group of diseases now known as the cholangiopathies.1 The cholangiopathies can be subdivided into malignant, immune-mediated, drug- or toxin-induced, infectious, genetic, ischemic, and idiopathic. Except for cholangiocarcinoma (CCA), a malignant tumor of the biliary tree, the common outcome for most cholangiopathies is the destruction of the bile ducts (i.e., ductopenia), with features of cholestasis, inflammation, and ultimately fibrosis and cirrhosis. In general, the pathological mechanisms underlying the cholangiopathies remain unclear and new insights into their etiopathogenesis are needed.
MicroRNAs (miRNAs) are short noncoding RNAs (20–22 nucleotides) that have a critical role in the posttranscriptional regulation of gene expression. MiRNAs are transcribed as primary miRNAs that are recognized and processed inside the nucleus by the RNase III endonuclease, Drosha. The resultant precursor miRNA (60–90 nucleotides) is transported from the nucleus to the cell cytoplasm predominantly by a mechanism involving Exportin-5, in a RAN-GTP dependent manner. This precursor is further processed in the cytoplasm by the RNase III endonuclease, Dicer, resulting in a RNA duplex molecule of 20–23 nucleotides in length. To exert its regulatory effects, the miRNA duplex is loaded into the miRNA-associated RNA-induced silencing complex (miRISC) and separated into a functional guide strand and passenger strand. The guide strand or mature miRNA directs the RISC complex to the target mRNA by base complementarity, mainly between its 5′ seed region and the 3′-UTR region of the target messenger. The end result of this interaction is either the transcriptional suppression or the degradation of the target mRNA.2,3 Liver specific dicer1 knockout illustrates the regulatory role of miRNA in liver function. Even though the hepatic function is preserved in the absence of mature miRNAs, disruption of dicer1 affects proper liver zonation and promotes hepatocarcinogenesis. Even more, the knockout mice showed significant ductular proliferation and inflammation suggesting the potential role of miRNAs in the development of biliary tract diseases.4–6
Therefore, miRNAs are regulatory molecules that directly and precisely modulate gene expression, and it is not surprising that altered miRNA profiles underlie the dysregulation of several proteins involved in the pathobiology of the cholangiopathies including polycystic liver diseases, fibroinflammatory cholangiopathies, and CCA.
The Cholangiopathies and miRNAs
Polycystic Liver Disease
Polycystic liver disease (PLD) is a group of genetic disorders characterized by the presence of multiple cysts derived from cholangiocytes. Polycystic liver disease can be inherited as an isolated entity (i.e., autosomal dominant polycystic liver disease [ADPLD]), but most frequently is associated with autosomal dominant (AD-) or autosomal recessive (AR-) polycystic kidney disease (PKD).7–9 Formation of hepatic cysts is initiated by mutations in disease-related genes: (1) SEC63 and PRKCSH (ADPLD), (2) PKD1 and PKD2 (ADPKD), and (3) PKHD1 (ARPKD). Once formed, cysts continue to grow involving many intracellular signaling pathways.7–10 Recent evidence suggests that cystic cholangiocytes and renal epithelial cells are characterized by global changes in miRNA patterns suggesting a novel regulatory mechanism of cyst progression.11–14 Several studies showed that miRNAs contribute to cystogenesis by regulating the dosage of PLD-related genes.15 Experimental manipulations with two miRNA families (miR-17–92 and miR-200) that target Pkd1 and Pkd2 genes result in cyst development in both liver and kidney.16,17 The role of miRNAs in the regulation of PKHD1, PRKSCH, and SEC63 has yet to be demonstrated. However, by in silico analysis, we detected that miR-1, -17, -20, -23, -31, -106, -130, -150, -194, -218, and -342 are predicted to target the PKHD1, PRKSCH, and SEC63 transcripts. All of these miRNAs are aberrantly expressed in cystic cholangiocytes.13,14 In addition, the aforementioned miRNAs are predicted to bind to mRNAs of proteins involved in cell-cycle progression, cAMP and calcium signaling, cell proliferation, MAPK/ERK pathway, fluid secretion, and cell-matrix interactions (see below) further emphasizing the emerging role of miRNAs in the regulation of network of molecules involved in cystogenesis.9,13,18
Fibroinflammatory Cholangiopathies
Several cholangiopathies are characterized by chronic inflammation, cholestasis, and biliary fibrosis. Two examples, primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC), follow a course that generally progresses to cirrhosis, portal hypertension, and liver failure. Biliary atresia (BA), unlike PBC and PSC, is a disorder exclusively diagnosed in the neonatal period and is the leading indication for pediatric liver transplantation worldwide.19
Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis
The autoimmune nature of PBC is fairly well established and supported by antimitochondrial antibodies (AMAs) and autoreactive T-cells; yet the specific cellular mechanisms that result in the initiation and progress of PBC still remain unclear. A recent miRNA microarray identified 35 differentially expressed miRNAs (11 upregulated and 24 down-regulated) in PBC compared with normal tissue.20 Furthermore, a bioinformatics approach demonstrated that the predicted upregulated genes (i.e., predicted targets of downregulated miRNAs) clustered into the biological processes of inflammatory response, calcium ion homeostasis, and negative regulation of hormone secretion. Further investigations are needed to validate altered target gene expression and identify cell types involved.
There is currently no effective pharmacotherapy for PSC, which exhibits a median liver transplantation- (LT-) free survival of 12 years.21,22 A feared complication of this disease is CCA, which occurs in approximately 10% of patients within 10 years of diagnosis.23,24 The diagnostic potential of miRNAs to monitor disease progression in PSC patients is discussed below.
Biliary Atresia
Biliary atresia progresses to fibro-obliteration of the extrahepatic bile ducts.25,26 Early diagnosis of BA is essential for good outcomes. Following diagnosis, the Kasai procedure (hepatoportoenterostomy) should be performed promptly to restore bile flow.26 Moreover, patients must be carefully monitored as nearly half will gradually develop chronic liver disease and require liver transplantation. In an animal model of BA (i.e., the Rhesus rotavirus- (RRV-) BALB/c model), multiple miR-NAs exhibited altered expression, including upregulated miR-29a and miR-29b1.27
Similarly, a miRNA expression array using RNA isolated from extrahepatic bile ducts (EHBDs) of RRV-BALB/c mice revealed a similar overall expression pattern of miRNA.28 However, miR-29b, but not miR-29a was elevated in the EHBDs. Despite the discrepancy, which may be due to the tissue source of RNAs, the results support a possible functional role of miR-29 family members in the etiopathogenesis of biliary atresia. Intriguingly, decreased miR-29 expression has been implicated in rodent models of fibrosis including carbon tetrachloride- treated and bile duct-ligated mice,29 which correlated with decreased miR-29 expression in livers from patients with advanced liver fibrosis. Decreased miR-29 in hepatic stellate cells in the hepatic fibrosis models was mediated by TGFb and NFkB and associated with increased expression of extracellular matrix molecules. These results suggest that individual miRNAs may have different functional roles depending on the cell type where they are expressed. Whether manipulation of miR-29 in the RRV model of BA modifies disease course has yet to be determined.
MiRNAs in Animal Models of Cholestasis
A recent study has demonstrated that serum levels of several miRNAs are altered following hepatocellular injury, cholestasis, and steatosis in rats.30
These studies reinforced that miRNAs make ideal potential candidate biomarkers due to high tissue specificity and stability in sera. Moreover, the expression profile of plasma miRNAs differed depending on the insult, suggesting that miRNAs could eventually be used as specific and sensitive biomarkers for several types of liver injury. The two cholestatic models assessed were a-naphthyl isothiocyanate (ANIT) treatment and bile duct ligation (BDL) representing both intrahepatic and extrahepatic cholestatic models. None of the upregulated miRNAs were specific to the models of cholestasis, whereas two miRNAs, miR-190 and miR-743b, were specifically downregulated in both of these models. In a functional analysis of miRNAs in cholestasis using the BDL model, it was determined that miR-125b and Let-7a were decreased in cholangiocytes, in a secretin-dependent manner.31 The translation of these promising findings to the human disease remains to be determined.
Cholangiocarcinoma
Cholangiocarcinoma is a lethal malignancy with limited therapeutic options; the cancer is derived from cholangiocytes, and its incidence and mortality have increased in recent decades.32 There are several seminal studies profiling miRNAs expression in CCA33–35; the dysregulation of miR-NAs, as in other tumors, has been linked to the repression of tumor suppressor genes (oncomiRs) and the upregulation of oncogenes (tumor suppressor miRs). The dysregulation of miRNAs in CCA is discussed in detail in the article, “Micro-RNAs in Cholangiocarcinoma” in this issue of Seminars.
MicroRNAs in the Pathobiology of the Cholangiopathies
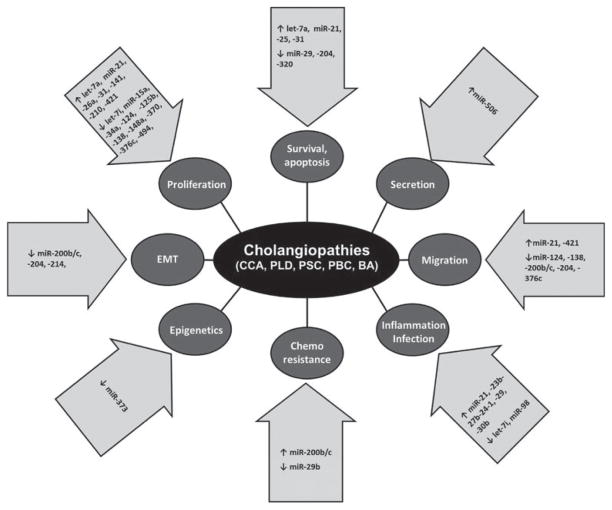
Despite the heterogeneity among the cholangiopathies, they share several fundamental pathogenetic mechanisms, including altered proliferation, secretion, epithelial–mesenchymal transition, and apoptosis among others, even though the contribution of these processes may vary between the different cholangiopathies. We next present recent discoveries regarding the role of miRNAs in the regulation of the common basic cellular mechanisms altered in several cholangiopathies (Fig. 1 and Table 1).
Fig. 1.
MicroRNAs in the cholangiopathies. Dysregulation of specific microRNAs induce abnormal expression of a myriad of targets that contribute to the different pathophysiological processes underlying the development of the cholangiopathies. BA, biliary atresia; CCA, cholangiocarcinoma; PBC, primary biliary cirrhosis; PLD, polycystic liver disease; PSC, primary sclerosing cholangitis.
Table 1.
MicroRNAs dysregulation with confirmed targets in biliary tract diseases
| miRNA | Expression | Function | Target | Reference |
|---|---|---|---|---|
| Cholangiocarcinoma | ||||
| let-7a | Up | Cell survival | NF2 | 36 |
| miR-21 | Up | Apoptosis, proliferation, invasion, metastasis | MBD2, 15-PGDH/HPGD, PTEN, PDCD4, TIMP3 | 33,37–40 |
| miR-25 | Up | Apoptosis | DR4 | 41 |
| miR-26a | Up | Proliferation, colony formation, tumor growth | GSK-3b | 42 |
| miR-29b | Down | Gemcitabine sensitivity, apoptosis | PIK3R1, MMP-2, Mcl1 | 43,44 |
| miR-31 | Up | Proliferation, apoptosis | RASA1 | 45 |
| miR-34a | Down | Cell-cycle, proliferation | c-Myc | 46 |
| miR-124 | Down | Migration, invasion | SMYD3 | 47 |
| miR-138 | Down | Proliferation, cell cycle, migration, invasion | RhoC | 48 |
| miR-141 | Up | Proliferation, circadian rhythm | CLOCK | 33 |
| miR-148a | Down | Proliferation | DNMT-1 | 49 |
| miR-200b | Up | Chemoresistance | PTPN12 | 33 |
| miR-200b/c | Down | Migration, invasion | rho-kinase2, SUZ12 | 50 |
| miR-204 | Down | EMT, migration, invasion, apoptosis | Slug, Bcl-2 | 35,51 |
| miR-210 | Up | proliferation | Mnt | 46 |
| miR-214 | Down | EMT, metastasis | Twist | 52 |
| miR-320 | Down | Apoptosis | Mcl-1 | 35 |
| miR-370 | Down | Proliferation | MAP3K8 | 53 |
| miR-373 | Down | Epigenetics | MBD2 | 54 |
| miR-376c | Down | Migration, proliferation | GRB2 | 55 |
| miR-421 | Up | Proliferation, migration, colony formation | FXR | 56 |
| miR-494 | Down | Proliferation, cell cycle | CDK6 | 57 |
| Polycystic liver diseases | ||||
| miR-15a | Down | Proliferation, cell cycle | Cdc25a | 12 |
| miR-17 | Down | Cyst development | Pkd2 | 11 |
| Fibro-obliterative cholangiopathies | ||||
| Primary biliary cirrhosis | ||||
| miR-506 | Up | Secretion | AE2 | 58 |
| Biliary atresia | ||||
| miR-29 | Up | Epigenetics, cell survival, inflammation | Dnmt3a, Dnmt3b, Igf1, Igf2bp2 | 27 |
| Cholestatic/pathogen induced | ||||
| Let-7a, -7i | Down | Pathogen recognition, inflammation, proliferation | TLR4, NGF | 31,59 |
| miR-98 | Down | Pathogen recognition, inflammation | CIS, SOCS4 | 60,61 |
| miR-125b | Down | Proliferation | VEGFA | 31 |
Abbreviations: EMT, epithelial–mesenchymal transition.
Cholangiocyte Proliferation
During the past decades, several miRNAs have been described to modulate cholangiocyte proliferation, like let-7a, miR-21, -26a, -34a, -421, and -494.62–64 Some recent reports reveal that miRNAs likely promote cell growth via regulation of receptor tyrosine kinase and MAPK signaling, particularly in CCA. One of these miRNAs, miR-376c, was found to be significantly downregulated in CCA cell lines compared with a normal bile duct epithelial cell line.55 Utilizing proteomics analysis of control and pre-miR-376c transfected HuCCT1 cells and further in silico analysis, GRB2, an essential adaptor for epidermal growth factor receptor signaling and Ras/MAPK activation, was identified and validated as the potential mediator of miR-376c downregulation effect in CCA cell phenotype, even though the significance of these observations needs to be validated in vivo using human CCA samples.
Additionally, the expression level of miR-138 in CCA is reduced compared with adjacent nontumor tissues, and the lower expression of this miRNA correlates with the malignant progression of the diseases.48 The mRNA of “Ras-like” superfamily member, RhoC, was found as a direct target of miR-138, suggesting that this miRNA function is a repressor of RhoC expression. Furthermore, in vitro manipulations of miR-138 regulate cell proliferation, likely through RhoC and its downstream effector ERK, but no evidence has been provided beyond the direct binding of miR-138 to the 3′UTR region of RhoC messenger.
As mentioned before, in a cholestatic model, very recent findings demonstrate that bile duct ligation induces the down-regulation of let-7i and miR-125b. The downregulation of these miRNAs is mediated by the increased hormone secretin and is secretin receptor-dependent. Moreover, let-7i and miR-125b directly target the 3′-UTR region of the messengers for nerve growth factor (NGF) and vascular endothelial growth factor (VEGF), respectively, important mediators of cholangiocyte proliferation.31 Hence, secretin-mediated miRNA suppression appears to be an essential component of the molecular network activated in cholestasis-induced hepatobiliary reparative mechanisms. Whether this signaling has direct relevance to human disease remains to be determined.
Cholangiocyte Cell-Cycle Regulation
Cell-cycle dysregulation is a common feature of several cholangiopathies. For example, we found by miRNA micro-array that the majority of miRNAs are downregulated in cystic cholangiocytes from the PCK (polycystic kidney) rat (an animal model of ARPKD) compared with normal rats, and experimentally proved that manipulations with one of the most downregulated miRNAs, miR-15a, affect hepatic cystogenesis in vitro.12 This miRNA promotes cell-cycle progression and cyst expansion through increased expression of cell-division cycle 25A (Cdc25A), an important cell-cycle regulator.12 Using a similar approach, substantial changes in miRNA profiles were described in renal epithelia of PKD/mhm (cy/+) rats, a model of ADPKD.14 Importantly, despite the differences between these two animal models and tissue analyzed, several miRNAs (miR-21, -31, -125, and 196a) were identically downregulated in both renal and hepatic epithelia, suggesting either shared regulation by disease-associated signaling or a common role for these miRNAs in cell-cycle regulation of renal and hepatic epithelia.
Cholangiocyte Secretion
Bile is modified by cholangiocytes via absorptive and secretory processes. Bile flows through the intrahepatic bile ducts lumen, where cholangiocytes reabsorb solutes, mainly bile salts and glucose, and secrete ions, such us Cl− and HCO3−, leading to water secretion through aquaporin water channels. A key biological feature of PBC is the decreased biliary expression of anion exchanger 2 (AE2/SLC4A2), which in turn induces a reduced secretin-stimulated bicarbonate secretion.65,66 AE2 is a Cl−/HCO3− exchanger mainly located in the apical domain of cholangiocytes. This exchanger participates in the regulation of intracellular pH and the alkalinization of bile secretion.67,68 Two different groups reported that miR-506 is upregulated in PBC cholangiocytes.20,58 Interestingly, in silico analysis identified the AE2 transcript as a target of miR-506, and in vitro functional analyses demonstrated that this miRNA targets the 3′UTR of AE2, decreases AE2 protein expression, and modulates bicarbonate secretion. Moreover, isolated human PBC cholangiocytes exhibit increased miR-506 expression and diminished AE2 activity, and the transfection of these cells with a miR-506 antagomir rescues AE2 activity.58 These data suggest an etiopathogenetic role of miR-506 in the downregulation of AE2 and the repression of bicarbonate secretion into bile in PBC.
Ductal Plate Formation
Development of hepatic cysts is linked to ductal plate malformation—embryological arrest of ductal plate development.7,8,69–73 During development, cholangiocyte precursor cells form a single layered sheath of cells, the ductal plate; each ductal plate originates usually a couple of bile ducts per portal tract. But only the minority of these cells is involved in the generation of the bile ducts, and the rest of the ductal plate precursors regress by apoptosis or may generate peri-portal hepatocytes and adult liver progenitor cells.69
MiRNAs have recently emerged as critical regulators of liver development.74 Comprehensive gene and miRNA profiling of human liver reveals miRNAs enriched in embryonic liver (i.e., miR-106a, miR-18a, miR17–92, and miR-574–3p) and in adult liver (i.e., let-7a and c, miR-23b, and miR-22). Moreover, the expression patterns of these miRNAs negatively correlate with levels of their predicted target genes.74 Though miRNA profiling was performed on whole liver tissue, it is important to emphasize that the aforementioned miRNAs are significantly downregulated in cystic cholangiocytes of animal model of PLD, the PCK rats,13 and in patients with ADPKD (unpublished observation). Arising evidence shows that miR-30a plays an important role in ductal plate formation. Indeed, abnormal bile duct development was detected in zebrafish due to specific depletion of miR-30a.75 This observation is of particular interest because miR-30a is decreased in human cystic cholangiocytes.13,14 Moreover, multiple transcriptional factors known to regulate ductal plate remodeling are predicted targets of miRNAs that are negatively expressed in cystic cholangiocytes.13 Collectively, these studies suggest that miRNAs are involved in the maintenance of bile duct integrity, and aberrant miRNA expression contributes to cyst formation and cyst growth.
Cholangiocyte Apoptosis
Apoptosis is the process of programmed cell death characterized by a series of morphologic changes that plays an important role in the development and tissue homeostasis of the biliary tract. Disturbances in apoptotic pathways may lead to uncontrolled cell proliferation or abnormal cell death. The tight control of cholangiocyte apoptosis by miRNAs has been suggested in several reports. For example, a role for miR-21 has been postulated in cholangiocarcinogenesis by suppressing the expression of programmed cell death 4 (PDCD4).37
Furthermore, miR-31 was involved in the pathogenesis of cholangiocarcinoma by directly inhibiting the protein expression of RAS p21 GTPase-activating protein 1 (RASA1).
Downregulation of RASA1 by miR-31 inhibited cellular apoptosis, partially due to upregulation of RAS-mitogen-activated protein kinase (MARK) signaling pathway activity in CCA.45 Another miRNA overexpressed in CCA, miR-25, shows an anti-apoptotic role by protecting cholangiocytes from TNF-related apoptosis-inducing ligand- (TRAIL-) induced apoptosis by decreasing the expression of death receptor 4.41 Conversely, decreased expression of miR-29 in CCA promotes the overexpression of cellular Mcl-1 protein levels, a key antiapoptotic protein, and helps cells evade cell death.76 Likewise, miR-320 and miR-204 are downregulated in CCA, which could fully explain the overexpression of their targets Mcl-1 or Bcl-2, respectively, raising possible mechanisms by which malignant cholangiocyte cells resist apoptosis.35
Cholangiocyte Pathogen Recognition and Inflammatory Response
Cholangiocytes form a simple epithelial layer separating the bile duct lumen from the liver parenchyma. Although under normal conditions microorganisms are undetected in bile by conventional culture methods, cholangiocytes are periodically exposed to potentially pathogenic organisms or products derived from these microbes.77–80 Indeed, the liver is a major organ for lipopolysaccharide (LPS) clearance, and though LPS undergoes metabolism in Kupffer cells and hepatocytes, it is excreted in bile where it remains bioactive.81,82 Moreover, in cholestatic liver diseases, cholangiocytes are exposed to elevated levels of LPS.82 Cholangiocytes express a variety of pathogen pattern recognition receptors (PRRs), such as the Toll-like receptors (TLRs) and nucleotide binding and oligomerization domain-like receptors (NLRs), which recognize pathogens or pathogen-associated molecular patterns (PAMPs). Activation of these receptors in cholangiocytes has been demonstrated in response to bacterial, viral, and parasitic infections.77,83 NF-kB (nuclear factor kappa beta) signal pathway activation via TLRs/NLRs is a common response of epithelial cells following detection of microbial products and NF-kB induces the expression of proinflammatory and antimicrobial molecules.77,84 We focus here on the role of miRNAs in the cholangiocyte response to microbial pathogens.
The role of miRNAs in NF-kB-dependent innate immune responses was first demonstrated in human THP-1 monocytes.85 In response to a variety of TLR agonists, miR-146a/b was induced in an NF-kB dependent manner. It was further demonstrated that miR-146 targets both TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1) genes. In this instance, miRNAs function to repress the innate immune response through the targeting of central mediators of TLR-dependent NF-kB signaling. It is now recognized that multiple miRNAs are induced in response to TLR activation in a variety of cell types, including cholangiocytes. Human cholangiocytes express numerous endogenous miR-NAs.33,59 Activation of cultured cholangiocyte TLR4 via LPS treatment or in vitro infection with the parasitic protozoan, Cryptosporidium parvum activates NF-kB in a Myd88-dependent manner84 and promotes the expression of several miRNAs including miR-125b, miR-21, miR-23b-27b-24–1, and miR-30b.86 The precise function of these miRNAs is not known, yet molecular inhibition of these resulted in increased parasite burden in vitro, suggesting a potential role in antimicrobial defense. In contrast, the expression of several miRNAs is also repressed following NF-kB activation. Using a cell culture model of the cholangiocyte response to microbial pathogens, it was demonstrated that following LPS treatment or C. parvum infection, transcription of the let-7i gene is suppressed through NF-kB p50 subunit and C/EBPβ interaction with the Let-7i promoter.87 Decreased let-7i expression was associated with an upregulation of TLR4 in C. parvum infected cells, increased NF-kB signaling, and diminished parasite numbers.59 Moreover, functional manipulation of NF-kB responsive miRNAs (e.g., let-7i and mir-27b) influenced C. parvum infection burden in vitro.59,86 Further investigations of let-7 miRNAs, including mir-98, revealed miRNA regulation of the cytokine-inducible Src homology 2-containing protein (CIS) and suppressor of cytokine signaling 4 (SOCS4), both members of the SOCS family of proteins.60,61 Again, using a human cultured cholangiocyte model of response to microbial insult (LPS or C. parvum), it was demonstrated, in contrast to the classical negative feedback regulation of cytokine signaling by SOCS family members, that decreased let-7 and miR-98 expression promoted the upregulation of CIS and enhanced NF-kB signaling through CIS-dependent IkBa degradation. The data raise the possibility that miRNA-mediated posttranscriptional pathways may contribute to host-cell responses to microbial infection by increasing inflammatory signaling in response to pathogens59–61 or attenuation of the inflammatory response.85 It is likely that miRNAs fine-tune the TLR/NF-kB signaling cascade through regulation of both positive- and negative-feedback loops to ensure an appropriate epithelial response to microbial insult. As demonstrated here, miRNA regulation of cellular processes involves subtle manipulations of signaling circuitry. How the miRNAs function to fine-tune the inflammatory response in the cholangiopathies or in biliary repair processes needs to be explored further in both cell-culture models of infection and repair and in animal models of disease.
Diagnostic, Prognostic, and Therapeutic Potential of miRNAs in the Cholangiopathies
Early detection of CCA remains challenging; few are detected while still amenable to curative surgical intervention. Hence, more effective screening tools are desired as are reliable prognostic markers. Cholangiocarcinoma miRNA expression profiles have been investigated for utility as prognostic tools.88–90 In a retrospective study, utilizing paraffin-embedded samples, the expression of two miRNAs (overexpression of miR-151–3p or the downregulation of miR-126) showed promise as potential prognostic markers for CCA. Additionally, and similar to what has been shown in colon cancer and pancreatic cancer, miR-21over-expression was associated with poor survival of CCA patients.88 The prognostic value of these miRNAs should be explored further as they may be utilized for patient stratification for clinical trials and in identifying patients that may benefit from adjuvant therapies.90
Cholangiocarcinoma is a dreaded outcome of PSC: Early detection is critical to patient outcome.91,92 The current surveillance modality is serum carbohydrate antigen 19–9 (CA 19–9) coupled with imaging techniques (i.e., magnetic resonance imaging with magnetic resonance cholangiopancreatography). Establishing an accurate diagnosis of cancer using CA 19–9 in the clinical setting is often difficult—frequently resulting in delayed diagnosis and compromising therapeutic options and patient outcome.91,92 Improved diagnostic accuracy for CCA is needed and miRNAs obtained from bile have shown promise.93,94 Initially, small RNA library sequencing and reverse transcription polymerase chain reaction-based array identified an increase in biliary miRNAs in CCA patients.93 One of these, miR-9, demonstrated the most reliable diagnostic specificity and sensitivity for biliary tract cancer. However, a recent analysis demonstrated that miRNAs derived from extracellular vesicles (i.e., exosomes) exhibit greater quality and quantity.94 Using stringent RNA isolation methods from a patient cohort of 46 CCA and 50 control patients (including 13 with PSC but no CCA), it was determined that the combinatorial use of five miRNAs (miR-16, - 486–3p, -484, -1274b, and -191) had the best predictive value. Ultimately, patient bile miRNAs and serum CA-19–9 may allow more reliable, earlier detection of CCA, particularly for those patients at high risk of CCA, such as PSC patients.
As with CCA, the development of a specific, feasible, noninvasive diagnostic marker is still needed for BA. Recently, serum miRNAs were assessed for their utility as a diagnostic tool for BA.95 A miRNA array was performed on sera from BA patients and age- and sex- matched indeterminate cholestasis controls. The miR-200b/429 cluster of miRNAs could differentiate between BA and controls with sensitivity and specificity values ranging from 71% to 92%, comparable to serum γ-glutamyl transpeptidase. Though not improving on the current diagnostic methods, this study serves as a proof-of-principle and ultimately may complement the current serum biochemical parameters for early detection, intervention, and improved patient outcome. The current diagnostic approaches for PBC, PSC, and polycystic liver disease are accurate and efficient; hence, the utility of miRNA analysis for diagnosis is less clear. The utility of miRNA analyses in these cholangiopathies lies in their potential to serve as prognostic tools to detect more aggressive forms of the disease or those that will favorably respond to therapy; this is an area lacking published data.
The cholangiopathies represent a class of diseases with unique obstacles for effective, novel therapeutic strategies including drug delivery and enigmatic etiopathogenesis. While remaining an intensive area of research, curative therapies remain drastic (i.e., transplant) with modest, incremental advances in pharmacological therapies. The manipulation of miR-NAs is a promising approach. An attractive feature of miRNA therapy is the potential to target multiple mediators of pathways that concertedly regulate cellular processes. Ideally, chemically modified miRNA mimics would restore aberrant expression of a diminished miRNA (replacement therapy), whereas antisense-modified oligonucleotides would inhibit an upregulated miRNA (miRNA inhibition therapy), restore cellular homeostasis, and delay progression of disease. RNA-based therapies are now feasible with the use of stable, chemically modified oligonucleotides96,97; however, the critical hurdle of targeted delivery of these oligonucleotides remains an issue. Nonetheless, the delivery of molecules to the liver and “first-pass” metabolism may ultimately prove to be an advantage for RNA-based therapies. Many advances in oligonucleotide delivery have been realized since the discovery of RNA interference (RNAi),98 yet whether any of these delivery methods can be utilized to specifically target the cells contributing to the cholangiopathies remains to be investigated.
Conclusions
In summary, miRNAs are promising as diagnostic, prognostic, and therapeutic tools, but their true value in these areas requires further study, including the validation of previous findings in larger cohorts of patients and the standardization of miRNA isolation, purification, and amplification protocols, as well as established normalization controls.
Furthermore, although there are many studies showing alterations in miRNAs in the cholangiopathies, the mechanisms underlying the modifications in these miRNAs remain obscure. More specifically, alterations of miRNA expression could be happening at gene expression levels, at the degradation level, and/or by alterations in the miRNA biogenesis machinery or nuclear transport. Understanding the mechanisms of miRNA dysregulation in the cholangiopathies is an area of research that definitely needs attention and may uncover novel therapeutic targets for biliary tract diseases.
Acknowledgments
This work was supported by National Institutes of Health Grants CA166635 (to S.A.G), AI089713 (to S.P.O.), DK57993 (to N.F.L), the Mayo Foundation, PSC Partners Seeking a Cure, and the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567).
Abbreviations
- AD
autosomal dominant
- ADPKD
autosomal dominant polycystic kidney disease
- ADPLD
autosomal dominant polycystic liver disease
- AE2
anion exchanger 2
- AMAs
antimitochondrial antibodies
- ANIT
a-naphthyl isothiocyanate
- AR
autosomal recessive
- ARPKD
autosomal recessive polycystic kidney disease
- BA
biliary atresia
- BDL
bile duct ligation
- CCA
cholangiocarcinoma
- Cdc25A
cell division cycle 25A
- CIS
cytokine-inducible src homology 2-containing protein
- EHBD
extrahepatic bile ducts
- HCV
hepatitis C virus
- IRAK1
IL-1 receptor-associated kinase 1
- LPS
lipopolysaccharide
- LT
liver transplantation
- MARK
mitogen-activated protein kinase
- miRISC
miRNA-associated RNA-induced silencing complex
- miR also miRNA
microRNA
- mRNA
messenger RNA
- NF-kB
nuclear factor kappa beta
- NGF
nerve growth factor
- PBC
primary biliary cirrhosis
- PKD
polycystic kidney disease
- PLD
polycystic liver disease
- PRRs
pattern recognition receptors
- PSC
primary sclerosing cholangitis
- RISC
RNA-induced silencing complex
- RNAs
ribonucleic acids
- RNase
ribonuclease
- RRV
Rhesus rotavirus
- SOCS4
suppressor of cytokine signaling 4
- TLRs
Toll-like receptors
- TRAF6
TNF receptor-associated factor 6
- TRAIL
TNF-related apoptosis-inducing ligand
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
References
- 1.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127(5):1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9(10):775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hand NJ, Master ZR, Le Lay J, Friedman JR. Hepatic function is preserved in the absence of mature microRNAs. Hepatology. 2009;49(2):618–626. doi: 10.1002/hep.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekine S, Ogawa R, Ito R, et al. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009;136(7):2304–2315. e1, 4. doi: 10.1053/j.gastro.2009.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekine S, Ogawa R, Mcmanus MT, Kanai Y, Hebrok M. Dicer is required for proper liver zonation. J Pathol. 2009;219(3):365–372. doi: 10.1002/path.2606. [DOI] [PubMed] [Google Scholar]
- 7.Chandok N. Polycystic liver disease: a clinical review. Ann Hepatol. 2012;11(6):819–826. [PubMed] [Google Scholar]
- 8.Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol. 2013;10(2):101–108. doi: 10.1038/nrgastro.2012.254. [DOI] [PubMed] [Google Scholar]
- 9.Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol. 2009;25(3):265–271. doi: 10.1097/MOG.0b013e328328f4ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strazzabosco M, Somlo S. Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology. 2011;140(7):1855–1859. 1859.e1. doi: 10.1053/j.gastro.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt K, Mi QS, Dong Z. microRNAs in kidneys: biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol. 2011;300(3):F602–F610. doi: 10.1152/ajprenal.00727.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SO, Masyuk T, Splinter P, et al. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J Clin Invest. 2008;118(11):3714–3724. doi: 10.1172/JCI34922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masyuk T, Masyuk A, LaRusso N. MicroRNAs in cholangiociliopathies. Cell Cycle. 2009;8(9):1324–1328. doi: 10.4161/cc.8.9.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey P, Brors B, Srivastava PK, et al. Microarray-based approach identifies microRNAs and their target functional patterns in polycystic kidney disease. BMC Genomics. 2008;9(1):624. doi: 10.1186/1471-2164-9-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gresh L, Fischer E, Reimann A, et al. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23(7):1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang E, Hsieh-Li HM, Chiou YY, et al. Progressive renal distortion by multiple cysts in transgenic mice expressing artificial micro-RNAs against Pkd1. J Pathol. 2010;222(3):238–248. doi: 10.1002/path.2765. [DOI] [PubMed] [Google Scholar]
- 17.Schena FP, Serino G, Sallustio F. MicroRNAs in kidney diseases: new promising biomarkers for diagnosis and monitoring. Nephrol Dial Transplant. 2014;29(4):755–763. doi: 10.1093/ndt/gft223. [DOI] [PubMed] [Google Scholar]
- 18.Tan YC, Blumenfeld J, Rennert H. Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochim Biophys Acta. 2011;1812(10):1202–1212. doi: 10.1016/j.bbadis.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoof-nagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46(2):566–581. doi: 10.1002/hep.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padgett KA, Lan RY, Leung PC, et al. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J Auto-immun. 2009;32(3–4):246–253. doi: 10.1016/j.jaut.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aron JH, Bowlus CL. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol. 2009;31(3):383–397. doi: 10.1007/s00281-009-0154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiesner RH, Grambsch PM, Dickson ER, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10(4):430–436. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 23.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36(3):321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 24.Boberg KM, Bergquist A, Mitchell S, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37(10):1205–1211. doi: 10.1080/003655202760373434. [DOI] [PubMed] [Google Scholar]
- 25.Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant. 2005;9(5):646–651. doi: 10.1111/j.1399-3046.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 26.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374(9702):1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 27.Hand NJ, Horner AM, Master ZR, et al. MicroRNA profiling identifies miR-29 as a regulator of disease-associated pathways in experimental biliary atresia. J Pediatr Gastroenterol Nutr. 2012;54(2):186–192. doi: 10.1097/MPG.0b013e318244148b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bessho K, Shanmukhappa K, Sheridan R, et al. Integrative genomics identifies candidate microRNAs for pathogenesis of experimental biliary atresia. BMC Syst Biol. 2013;7:104. doi: 10.1186/1752-0509-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53(1):209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 30.Yamaura Y, Nakajima M, Takagi S, Fukami T, Tsuneyama K, Yokoi T. Plasma microRNA profiles in rat models of hepatocellular injury, cholestasis, and steatosis. PLoS ONE. 2012;7(2):e30250. doi: 10.1371/journal.pone.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glaser S, Meng F, Han Y, et al. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146(7):1795–808. e12. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng F, Henson R, Lang M, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130(7):2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 34.Kawahigashi Y, Mishima T, Mizuguchi Y, et al. MicroRNA profiling of human intrahepatic cholangiocarcinoma cell lines reveals biliary epithelial cell-specific microRNAs. J Nippon Med Sch. 2009;76(4):188–197. doi: 10.1272/jnms.76.188. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Yan HX, Yang W, et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol. 2009;50(2):358–369. doi: 10.1016/j.jhep.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Meng F, Henson R, Wehbe-Janek H, Smith H, Ueno Y, Patel T. The MicroRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J Biol Chem. 2007;282(11):8256–8264. doi: 10.1074/jbc.M607712200. [DOI] [PubMed] [Google Scholar]
- 37.Selaru FM, Olaru AV, Kan T, et al. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology. 2009;49(5):1595–1601. doi: 10.1002/hep.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He Q, Cai L, Shuai L, et al. Ars2 is overexpressed in human cholangiocarcinomas and its depletion increases PTEN and PDCD4 by decreasing microRNA-21. Mol Carcinog. 2013;52(4):286–296. doi: 10.1002/mc.21859. [DOI] [PubMed] [Google Scholar]
- 39.Lu L, Byrnes K, Han C, Wang Y, Wu T. miR-21 targets 15-PGDH and promotes cholangiocarcinoma growth. Mol Cancer Res. 2014;12(6):890–900. doi: 10.1158/1541-7786.MCR-13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chusorn P, Namwat N, Loilome W, et al. Overexpression of micro-RNA-21 regulating PDCD4 during tumorigenesis of liver fluke-associated cholangiocarcinoma contributes to tumor growth and metastasis. Tumour Biol. 2013;34(3):1579–1588. doi: 10.1007/s13277-013-0688-0. [DOI] [PubMed] [Google Scholar]
- 41.Razumilava N, Bronk SF, Smoot RL, et al. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology. 2012;55(2):465–475. doi: 10.1002/hep.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Han C, Wu T. MicroRNA-26a promotes cholangiocarcinoma growth by activating β-catenin. Gastroenterology. 2012;143(1):246–56. e8. doi: 10.1053/j.gastro.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26(42):6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto K, Miyoshi K, Murawaki Y. miR-29b, miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells. PLoS ONE. 2013;8(10):e77623. doi: 10.1371/journal.pone.0077623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu C, Huang F, Deng G, Nie W, Huang W, Zeng X. miR-31 promotes oncogenesis in intrahepatic cholangiocarcinoma cells via the direct suppression of RASA1. Exp Ther Med. 2013;6(5):1265–1270. doi: 10.3892/etm.2013.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, Li TW, Peng J, et al. A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involved in progression. Gastroenterology. 2011;141(1):378–388. 388.e1–388.e4. doi: 10.1053/j.gastro.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng B, Li Z, Chen R, et al. Epigenetic regulation of miR-124 by hepatitis C virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett. 2012;586(19):3271–3278. doi: 10.1016/j.febslet.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Tang H, Yin S, Dong C. Downregulation of microRNA-138 enhances the proliferation, migration and invasion of cholangiocarcinoma cells through the upregulation of RhoC/p-ERK/MMP-2/MMP-9. Oncol Rep. 2013;29(5):2046–2052. doi: 10.3892/or.2013.2304. [DOI] [PubMed] [Google Scholar]
- 49.Braconi C, Huang N, Patel T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology. 2010;51(3):881–890. doi: 10.1002/hep.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng F, Jiang J, Yu Y, et al. Direct targeting of SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma tumourigenesis and metastasis. Br J Cancer. 2013;109(12):3092–3104. doi: 10.1038/bjc.2013.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu YH, Wei YP, Shen NJ, et al. miR-204 inhibits epithelial to mesenchymal transition by targeting slug in intrahepatic cholangiocarcinoma cells. Cell Physiol Biochem. 2013;32(5):1331–1341. doi: 10.1159/000354531. [DOI] [PubMed] [Google Scholar]
- 52.Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J. 2012;279(13):2393–2398. doi: 10.1111/j.1742-4658.2012.08618.x. [DOI] [PubMed] [Google Scholar]
- 53.Meng F, Wehbe-Janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene. 2008;27(3):378–386. doi: 10.1038/sj.onc.1210648. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Luo J, Tian R, Sun H, Zou S. miR-373 negatively regulates methyl-CpG-binding domain protein 2 (MBD2) in hilar cholangiocarcinoma. Dig Dis Sci. 2011;56(6):1693–1701. doi: 10.1007/s10620-010-1481-1. [DOI] [PubMed] [Google Scholar]
- 55.Iwaki J, Kikuchi K, Mizuguchi Y, et al. MiR-376c down-regulation accelerates EGF-dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line. PLoS ONE. 2013;8(7):e69496. doi: 10.1371/journal.pone.0069496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong XY, Yu JH, Zhang WG, et al. MicroRNA-421 functions as an oncogenic miRNA in biliary tract cancer through down-regulating farnesoid X receptor expression. Gene. 2012;493(1):44–51. doi: 10.1016/j.gene.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 57.Olaru AV, Ghiaur G, Yamanaka S, et al. MicroRNA down-regulated in human cholangiocarcinoma control cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology. 2011;54(6):2089–2098. doi: 10.1002/hep.24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banales JM, Sáez E, Uriz M, et al. Up-regulation of microRNA 506 leads to decreased Cl−/HCO3− anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology. 2012;56(2):687–697. doi: 10.1002/hep.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen XM, Splinter PL, O’Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. 2007;282(39):28929–28938. doi: 10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu G, Zhou R, Liu J, et al. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J Immunol. 2009;183(3):1617–1624. doi: 10.4049/jimmunol.0804362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu G, Zhou R, Liu J, Gong AY, Chen XM. MicroRNA-98 and let-7 regulate expression of suppressor of cytokine signaling 4 in biliary epithelial cells in response to Cryptosporidium parvum infection. J Infect Dis. 2010;202(1):125–135. doi: 10.1086/653212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munoz-Garrido P, García-Fernández de Barrena M, Hijona E, et al. MicroRNAs in biliary diseases. World J Gastroenterol. 2012;18(43):6189–6196. doi: 10.3748/wjg.v18.i43.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haga H, Yan I, Takahashi K, Wood J, Patel T. Emerging insights into the role of microRNAs in the pathogenesis of cholangiocarcinoma. Gene Expr. 2014;16(2):93–99. doi: 10.3727/105221614X13919976902174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Natarajan MA, Wehrkamp CJ, Mohr AM, Mott JL. MicroRNA function in human diseases. Med Epigenet. 2013;1:106–115. [Google Scholar]
- 65.Medina JF, Martínez-Ansó, Vazquez JJ, Prieto J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology. 1997;25(1):12–17. doi: 10.1002/hep.510250104. [DOI] [PubMed] [Google Scholar]
- 66.Melero S, Spirlì C, Zsembery A, et al. Defective regulation of cholangiocyte Cl−/HCO3(−) and Na+/H+ exchanger activities in primary biliary cirrhosis. Hepatology. 2002;35(6):1513–1521. doi: 10.1053/jhep.2002.33634. [DOI] [PubMed] [Google Scholar]
- 67.Banales JM, Arenas F, Rodríguez-Ortigosa CM, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43(2):266–275. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- 68.Banales JM, Prieto J, Medina JF. Cholangiocyte anion exchange and biliary bicarbonate excretion. World J Gastroenterol. 2006;12(22):3496–3511. doi: 10.3748/wjg.v12.i22.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raynaud P, Carpentier R, Antoniou A, Lemaigre FP. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2011;43(2):245–256. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Wills ES, Roepman R, Drenth JP. Polycystic liver disease: ductal plate malformation and the primary cilium. Trends Mol Med. 2014;20(5):261–270. doi: 10.1016/j.molmed.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 71.Temmerman F, Missiaen L, Bammens B, et al. Systematic review: the pathophysiology and management of polycystic liver disease. Aliment Pharmacol Ther. 2011;34(7):702–713. doi: 10.1111/j.1365-2036.2011.04783.x. [DOI] [PubMed] [Google Scholar]
- 72.Gunay-Aygun M. Liver and kidney disease in ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C(4):296–306. doi: 10.1002/ajmg.c.30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desmet VJ. Ludwig symposium on biliary disorders—part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc. 1998;73(1):80–89. doi: 10.4065/73.1.80. [DOI] [PubMed] [Google Scholar]
- 74.Tzur G, Israel A, Levy A, et al. Comprehensive gene and microRNA expression profiling reveals a role for microRNAs in human liver development. PLoS ONE. 2009;4(10):e7511. doi: 10.1371/journal.pone.0007511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hand NJ, Master ZR, Eauclaire SF, Weinblatt DE, Matthews RP, Friedman JR. The microRNA-30 family is required for vertebrate hepatobiliary development. Gastroenterology. 2009;136(3):1081–1090. doi: 10.1053/j.gastro.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 2010;110(5):1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen XM, O’Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunol Cell Biol. 2008;86(6):497–505. doi: 10.1038/icb.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157(2):261–270. doi: 10.1111/j.1365-2249.2009.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jafri M, Donnelly B, Bondoc A, Allen S, Tiao G. Cholangiocyte secretion of chemokines in experimental biliary atresia. J Pediatr Surg. 2009;44(3):500–507. doi: 10.1016/j.jpedsurg.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karrar A, Broomé U, Södergren T, et al. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology. 2007;132(4):1504–1514. doi: 10.1053/j.gastro.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 81.Mimura Y, Sakisaka S, Harada M, Sata M, Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109(6):1969–1976. doi: 10.1016/0016-5085(95)90765-3. [DOI] [PubMed] [Google Scholar]
- 82.Sasatomi K, Noguchi K, Sakisaka S, Sata M, Tanikawa K. Abnormal accumulation of endotoxin in biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 1998;29(3):409–416. doi: 10.1016/s0168-8278(98)80058-5. [DOI] [PubMed] [Google Scholar]
- 83.Harada K, Nakanuma Y. Cholangiopathy with respect to biliary innate immunity. Int J Hepatol. 2012;2012:793569. doi: 10.1155/2012/793569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen XM, O’Hara SP, Nelson JB, et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J Immunol. 2005;175(11):7447–7456. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- 85.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou R, Hu G, Gong AY, Chen XM. Binding of NF-kappaB p65 subunit to the promoter elements is involved in LPS-induced transactivation of miRNA genes in human biliary epithelial cells. Nucleic Acids Res. 2010;38(10):3222–3232. doi: 10.1093/nar/gkq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Hara SP, Splinter PL, Gajdos GB, et al. NFkappaB p50-CCAAT/enhancer-binding protein beta (C/EBPbeta)-mediated transcriptional repression of microRNA let-7i following microbial infection. J Biol Chem. 2010;285(1):216–225. doi: 10.1074/jbc.M109.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Q, Liu L, Liu CH, et al. MicroRNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potential biomarker for cancer prognosis. Asian Pac J Cancer Prev. 2013;14(2):829–834. doi: 10.7314/apjcp.2013.14.2.829. [DOI] [PubMed] [Google Scholar]
- 89.Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52(4):297–303. doi: 10.1002/mc.21864. [DOI] [PubMed] [Google Scholar]
- 90.McNally ME, Collins A, Wojcik SE, et al. Concomitant dysregulation of microRNAs miR-151-3p and miR-126 correlates with improved survival in resected cholangiocarcinoma. HPB (Oxford) 2013;15(4):260–264. doi: 10.1111/j.1477-2574.2012.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khan SA, Davidson BR, Goldin R, et al. British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):VI1–VI9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990–2009. World J Gastroenterol. 2009;15(34):4240–4262. doi: 10.3748/wjg.15.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shigehara K, Yokomuro S, Ishibashi O, et al. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLoS ONE. 2011;6(8):e23584. doi: 10.1371/journal.pone.0023584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li L, Masica D, Ishida M, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60(3):896–907. doi: 10.1002/hep.27050. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zahm AM, Hand NJ, Boateng LA, Friedman JR. Circulating micro-RNA is a biomarker of biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55(4):366–369. doi: 10.1097/MPG.0b013e318264e648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krützfeldt J, Kuwajima S, Braich R, et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35(9):2885–2892. doi: 10.1093/nar/gkm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krützfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172(3):962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]