Abstract
Dopamine (DA) signaling in the central nervous system mediates the addictive capacities of multiple commonly abused substances, including cocaine, amphetamine, heroin and nicotine. The firing of DA neurons residing in the ventral tegmental area (VTA), and the release of DA by the projections of these neurons in the nucleus accumbens (NAc), is under tight control by cholinergic signaling mediated by nicotinic acetylcholine (ACh) receptors (nAChRs). The capacity for cholinergic signaling is dictated by the availability and activity of the presynaptic, high-affinity, choline transporter (CHT, SLC5A7) that acquires choline in an activity-dependent matter to sustain ACh synthesis. Here, we present evidence that a constitutive loss of CHT expression, mediated by genetic elimination of one copy of the Slc5a7 gene in mice (CHT+/−), leads to a significant reduction in basal extracellular DA levels in the NAc, as measured by in vivo microdialysis. Moreover, CHT heterozygosity results in blunted DA elevations following systemic nicotine or cocaine administration. These findings reinforce a critical role of ACh signaling capacity in both tonic and drug-modulated DA signaling and argue that genetically-imposed reductions in CHT that lead to diminished DA signaling may lead to poor responses to reinforcing stimuli, possibly contributing to disorders linked to perturbed cholinergic signaling including depression and attention-deficit hyperactivity disorder (ADHD).
Keywords: choline, transporter, dopamine, cocaine, nicotine, microdialysis
1. Introduction
Acetylcholine (ACh) signaling is known to control or modulate a wide range of physiological and behavioral responses, including neuromuscular and gastrointestinal function, cardiac rhythms and output, attention and executive function, arousal and reward [1–6]. In turn, perturbed ACh signaling is associated with myasthenias [7, 8], constipation [9], heart disease [10, 11], attention-deficit hyperactivity disorder (ADHD) [12], and addiction [13, 14]. With respect to addiction, central ACh receptors contribute to both the alerting and reinforcing effects of nicotine, the latter action funneling into the mesoaccumbal dopamine (DA) system to mimic, and when abused, commandeer DA signaling that is engaged normally recognize the novelty and saliency of stimuli [14, 15].
In order to sustain cholinergic signaling, cholinergic nerve terminals must acquire significant quantities of choline to insure ongoing ACh synthesis. The choline acquired derives from dietary sources as well as from recapture of choline following acetylcholinesterase (AChE)-mediated hydrolysis of synaptic ACh [16]. To accomplish choline uptake, cholinergic neurons produce and traffic to the plasma membrane, a high-affinity, hemicholinium-3 (HC-3) sensitive choline transporter (CHT), the gene for which in humans is designated as SLC5A7 [17–19]. Although CHT activity is manifested at the plasma membrane, subcellular fractionation, immunoisolation techniques and immune-electron micrographic studies have revealed that the transporter resides predominantly on cholinergic synaptic vesicles [19, 20]. Localization of CHT on cholinergic synaptic vesicles results in depolarization-induced elevations in presynaptic choline transport capacity and as such, like ACh release, can be prevented if depolarization occurs in preparations where vesicle fusion is prevented with botulinum toxin or by blocking voltage-dependent calcium channels [19]. The vesicular localization of the bulk of CHT protein on cholinergic synaptic vesicles provides an elegant mechanism to insure continued ACh synthesis at high-rates of ACh release [21].
As the KM of choline acetyltransferase (ChAT) is thought to be higher than available cytoplasmic levels of choline, particularly at high rates of ACh utilization, transport of choline by CHT is rate-limiting for sustaining cholinergic signaling [22, 23]. As such, inhibition of CHT with HC-3 leads to a rundown of ACh release that can result, ultimately, in death [24]. However, almost all conclusions related to a dependence of cholinergic signaling on CHT have been based on the use of HC-3, an agent first characterized in an age where the understanding of potential off-target effects was limited [24]. To provide new tools to study requirements for CHT in cholinergic signaling, Ferguson and colleagues generated CHT KO (CHT−/−) mice [25]. Although CHT−/− mice are born at Mendelian ratios when bred from CHT+/− parents, they exhibit paralysis and cyanosis within minutes after birth and die in 30–60 min. Ex vivo neuromuscular junction recordings in these mice demonstrated a progressive rundown of ACh signaling at postsynaptic nAChRs [25], consistent with the idea that asphyxiation is due to a failure to maintain the diaphragm contractions needed for inhalation. Recently, humans with a dominant-negative CHT mutation have been identified and these subjects demonstrate progressive distal neuropathy that can severely compromise motor function [26].
The early postnatal lethality of CHT−/− mice greatly limits the opportunity to study CHT contributions to cholinergic signaling in vivo. Although life can be extended in these animals through motorneuron-specific restoration of CHT expression [27], these animals nonetheless expire within 24 hrs, possibly due to the onset of spinal and brainstem cholinergic signaling to drive and coordinate spinal motor output. Given the limitations imposed by the neonatal lethality of CHT−/− mice, Bazalakova and colleagues initiated studies of partial loss of the transporter using CHT+/− mice [28]. In the original report of CHT−/− mice, heterozygous animals were shown to produce ~50% of WT CHT protein levels, indicating a lack of compensation for diminished CHT mRNA expression by the remaining WT allele. Bazalakova et al extended these studies to demonstrate that the CHT+/− mice produce and store ~50% less ACh in the brain as WT animals, and that they exhibit a reduced locomotor responsiveness to scopolamine, consistent with a diminished capacity for drug-induced ACh release in basal ganglia motor circuits. A critical role of CHT to sustain neuromuscular signaling is evident from findings that CHT+/− mice fail to reach speeds comparable to WT mice on an accelerating treadmill test, nor do they run as long as WT mice on a fixed speed treadmill test. CHT+/− mice also exhibit basal tachycardia and lack the ability of WT mice to reset their heart rates after exercise [29]. Recently, Sarter’s group demonstrated that although CHT+/− mice exhibit basal extracellular levels of ACh no different from WT littermates, they fail to sustain ACh release in frontal cortex with sustained stimulation of cholinergic inputs, and they fail to perform as well as WT mice in tasks that require sustained attention [30]. These findings are consistent with evidence provided by English and colleagues that a common, low functioning coding variant associates with ADHD [12].
Matthies and colleagues pursued another approach to overcome the neonatal lethality of CHT−/− mice in studying the functional consequences of homozygous loss of the transporter through investigations of the C. elegans CHT ortholog CHO-1. Unlike mice and humans, nematodes lacking the capacity for ACh signaling are viable and can reproduce [31]. Nonetheless, homozygous cho-1 mutant animals demonstrate reduced levels of ACh and display activity-dependent, motor fatigue when monitored in thrashing assays, consistent with studies of the CHT+/− model [32]. Future studies may be able to adapt the powerful genetic approaches of the nematode model to identify conserved regulators of CHO-1.
The studies of biochemical and behavioral deficits associated with loss of CHT expression raise the question as to whether elevated CHT levels can augment cholinergic signaling. Possibly, levels of the transporter in WT animals are set to achieve a maximum contribution to ACh synthesis, release and response. Interestingly, this may not be the case, opening a possibility for therapeutics designed to augment CHT levels or activity [21]. Thus, in the same studies where Lund and colleagues extended the lifespan of CHT−/− mice using motorneuron-specific CHT re-expression, animals were also produced where the CHT transgene was carried on a WT background, thus driving CHT levels at the neuromuscular junction above that seen with WT animals [27]. These animals produced larger compound muscle action potentials (CMAPs) and ran longer in the fixed speed treadmill paradigm. Recent studies with constitutive CHT overexpression, produced through BAC transgenic methods (Holmstrand and Blakely, manuscript in preparation), reveals that these mice grow and reproduce normally and exhibit elevated CHT protein and choline uptake levels levels, with transporter expression evident by immunohistochemistry in the same neurons and processes that express CHT in WT animals. These animals also exhibit a 2–3 fold elevation in ACh levels, in contrast to the reduced levels seen in the CHT+/− mice, and they do not display compensatory changes in rates of ACh synthesis or metabolism. Finally, the BAC CHT mice display a similar extension of motor endurance as seen with motorneuron-specific CHT overexpressor. Together, these studies argue for a significant influence of the level of CHT protein in establishing the capacity for cholinergic signaling.
As noted earlier, cholinergic signaling figures prominently in reward pathways and addiction mechanisms. To initiate studies that examine the impact of CHT expression on cholinergic signaling in reward-relevant subcortical pathways, specifically those intersecting with mesoaccumbens DA signaling, we have investigated whether loss of CHT expression impacts basal and drug-modulated levels of extracellular DA in vivo. Our findings demonstrate that reduced CHT expression has functional consequences with respect to extracellular DA levels and their alteration by systemic nicotine and cocaine. We discuss pathways and mechanisms that could support these observations and that should be elaborated in future studies.
2. Materials and Methods
2.1 Animals
The production and characterization of CHT+/− mice has been previously described [25, 28]. These animals are congenic on a C57BL/6J background. Control mice were littermate CHT+/+ (WT) animals. All experiments utilized male animals at 4–5 months of age. All procedures were conducted under an approved protocol reviewed annually by the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC).
2.2 In Vivo Microdialysis and Dopamine Quantification
All biochemical reagents utilized, except where noted, were from Sigma (St. Louis, MO USA) and were of the highest grade possible. Microdialysis guide cannulas (CMA/7) (CMA/Microdialysis, Solna, Sweden) were positioned in the medial nucleus accumbens (NAc) directed toward the shell and were secured with acrylic cement and three screws in the skull [33, 34]. The stereotaxic coordinates (relative to bregma) were anterior-posterior 1.4 mm, lateral 0.55 mm, and dorso-ventral 3.3 mm. After surgery the animals were placed singly into their home cages and allowed to recover for 3–5 days before the experiments. On the day preceding the test session, microdialysis probes (outer diameter = 0.24 mm; length = 1 mm; Cuprophane membrane (CMA/Microdialysis, Solna, Sweden) cutoff = 6,000 Da) were perfused with artificial cerebral spinal fluid (149 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, and 0.25 mM ascorbic acid, 5.4 mM D-glucose). At least 14 hrs before the experiment, probes were lowered slowly into the brain through the guide cannula while the mouse was briefly anesthetized (5–10 min) with 2% isoflurane. The perfusion flow rate was decreased to 0.5 μL/min overnight and then increased to 2.0 μl/min at least 1 hr prior to baseline sampling. Samples were collected every 20 min with the timeline for the dialysis experiment was as follows: 60 min of baseline sampling, saline injection followed by 60 min of sampling, nicotine injection (1 mg/kg, i.p.) followed by 240 min of sampling, cocaine injection (10 mg/kg, i.p.) followed by 60 min of sampling. Each sample vial was manually changed and immediately stored at −80°C until analyzed. After these experiments, mice were sacrificed with an overdose of anesthetics and then transcardially perfused with saline and then fixed with 10% formalin. The accuracy of probe placement was confirmed by histological serial sectioning.
The DA content of microdialysates was determined with an HPLC system using reversed phase chromatography and electrochemical detection. The system included a pump (Model 582; ESA, Inc., Chelmsford, MA, USA), an autosampler (Model 542; ESA, Inc.), and a HR-80 x 3.2 mm column (3-μm particle size; ESA Inc.). A coulometric cell (5014B; ESA, Inc.) was connected to an ESA Coulochem II detector. The mobile phase consisted of citric acid (4.0 mM), sodium dodecyl sulfate (3.3 mM), sodium dihydrogen phosphate (100.0 mM), ethylenediaminetetraacetic acid (EDTA, 0.3 mM), acetonitrile (15%), and methanol (5%). The autosampler mixed 27 μL of the dialysate with ascorbate oxidase (EC 1.10.3.3; 162 units/mg; Sigma-Aldrich Inc., St. Louis, MO, USA) prior to injection. Determination of dialysate DA concentration was carried out by comparing peak height to external standards (0–2 nM). Data are presented as raw DA concentration based on calibration with external standards. Statistical analyses were performed using Prism 6.0 (GraphPad, San Diego, CA USA). Time course DA release was analyzed by a Repeated Measures Analysis of Variance (RMANOVA) followed by post-hoc Bonferroni comparisons at collection time points between genotypes. Cumulative release of DA induced by nicotine and cocaine was established using the area under the curve (AUC) calculator in Prism 6.0 with the baseline set as the level of DA in the fraction collected prior to injection. AUC analyses were performed with a two-way ANOVA with genotype and drug as main effects. Uncorrected Fisher’s LSD tests were used for post-hoc comparisons of drug effects within each genotype. For all statistical tests, P<.05 was taken as a statistically significant finding.
3. Results and Discussion
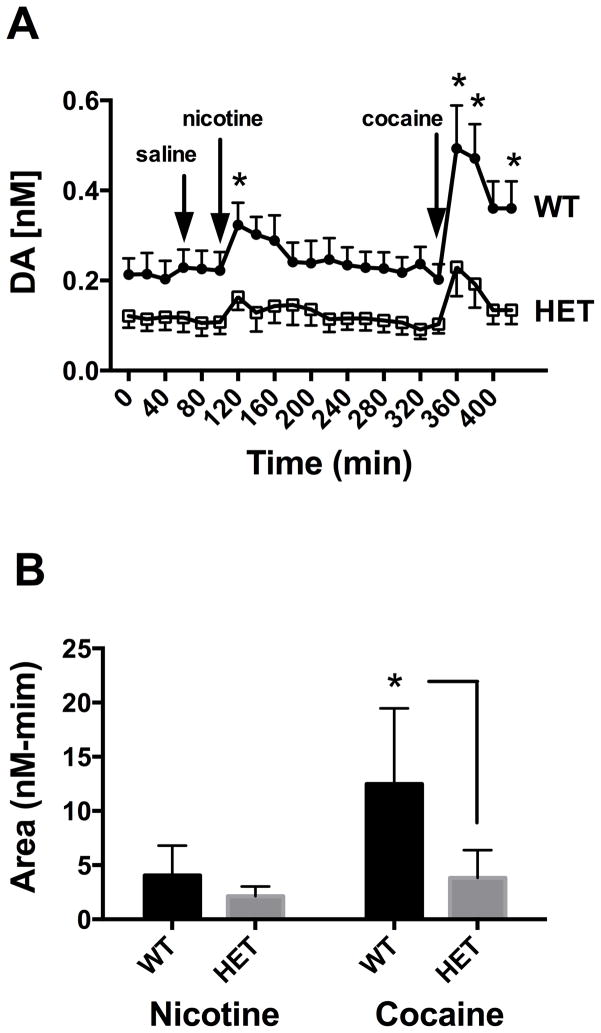
As described in Methods, microdialysis measurements of extracellular DA levels in the NAc shell were obtained in WT and CHT+/− animals under baseline conditions (1st 60 min), following saline injection (subsequent 60 minutes), and following either nicotine or cocaine injections (Figure 1A). Analysis of the time course data from n=9 animals of either genotype revealed significant time (P<0.001), genotype (P=0.013) and interaction effects (P<0.001) using two way repeated measures ANOVA. Genotype effects were driven by changes in the baseline extracellular DA levels, with pre-saline baseline differences remaining relatively stable (CHT+/− ~ 50% of WT DA levels) throughout the post-saline collection period and into the 160 minutes prior to the cocaine injection (Figure 1A). Post-hoc analyses between genotypes at each time point revealed that the interaction effects are due to significant differences in the effects of nicotine and cocaine where, for both drugs, the WT animals demonstrated a greater relative increase in extracellular DA post drug injection than CHT+/− animals (Bonferroni, P<0.01). As a further analysis of genotype effects, we assessed the cumulative response of nicotine and cocaine by genotype (Figure 1B). This assessment again revealed significant genotype (P=.001) and drug (P=.001) main effects as well as a significant genotype x drug interaction (P=.024). In post-hoc analyses by genotype, we found that the difference between genotypes for nicotine did not reach significance (P=.34), whereas a significant effect was detected between genotypes for cocaine (P=.0002). These findings indicate that changes in drug responses, particularly for cocaine, are not totally explained by a shift in the basal extracellular DA level.
Figure 1.
Evaluation of the impact of CHT heterozygosity on basal and drug-evoked elevations in extracellular DA. A) Basal and drug evoked DA levels as measured by microdialysis. Each animal was exposed to saline, nicotine (1mg/kg) and cocaine (10 mg/kg) as designated by the arrows. Data reflects mean +/− SEM from n=9 animals of each genotype. # = significant genotype effect as assessed by a Repeated Measures two-way ANOVA analysis (P<0.05). * = significant genotype differences in DA elevations induced by drug injection over baseline, assessed by Bonferroni post-hoc analysis of time-matched samples (P<0.05). B) Cumulative response to nicotine and cocaine by genotype. Area under the curve (AUC) analysis of data presented in A) was performed for the nicotinic and cocaine responses of each genotypes post injection. Peaks were detected automatically In Prism software. Data were analyzed via two-way ANOVA. Significant genotype, drug and interaction effects were detected (P<.05). Uncorrected Fisher’s LSD post-hoc comparisons between genotypes for each drug condition revealed a non-significant genotype effect for cocaine and a significant (*P=.0002) genotype effect for cocaine.
Cholinergic and dopaminergic pathways intersect directly in three major ways that could result in the reduction in basal extracellular DA levels detected in the nucleus accumbens of the CHT+/− mice. First, ascending cholinergic afferents from the lateral dorsal tegmentum (LDTg) and pedunculopontine tegmentum (PPTg) provide inputs onto DA and GABA neurons in the ventral tegmental area (VTA) [1, 2, 35]. Second, and likely more importantly, intrinsic cholinergic interneurons in the striatum and NAc potently regulate DA release [36–39]. Additional indirect connections also provide for crosstalk between ACh and DA signaling. For example, basal forebrain cholinergic inputs on descending glutamatergic neurons in the PFC provide for “top-down” modulation of VTA DA neurons [40–42]. The midbrain DA areas are also innervated by inputs from the extended amygdala that in turn receive cholinergic inputs [43, 44], providing a pathway by which stressful events can regulate DA projections to the NAc [45].
The microdialysis methods reported here cannot distinguish among the various projections to determine which is key to the diminished extracellular DA of the CHT+/− mice. Because CHT+/− mice exhibit a ~50% reduction in ACh levels throughout the brain [19, 46], reduced ACh signaling capacity is likely, but not yet proven, to be evident within each of the cholinergic projections. We suspect that the DA reductions we detected most likely arise from impaired function of the cholinergic interneurons that regulate DA release in the NAc [36–39]. Ongoing nicotinic activity potentially boosts DA release, especially to tonic DA neuron firing [36, 38, 47]. Inhibiting nAChRs or decreasing ACh release, as we observe in the NAc with the CHT+/− mice, could substantially decrease DA release to a single action potential. Use of cell-specific methods, such as afforded by the use of optogenetics approaches [48] may provide the resolution needed to further address this issue. As the loss of expression from one CHT allele has been present throughout the life of the animal, we must also consider a shift in baseline DA levels as arising from altered burst or phasic firing of DA neurons in response to natural rewards that normally drive DA release across the lifespan, such as maternal interactions or food [49].
In addition to the diminished basal DA levels detected, we also found that systemic nicotine injections produced blunted peak DA elevations in the NAc. One might expect that a chronic hypocholinergic state would upregulate nicotinic ACh receptors (nAChRs) and that nicotine administration would drive greater DA release in the CHT+/− mice. Indeed, Paolone and colleagues, analyzing extracellular levels of ACh while the animals performed a sustained attention task (SAT) have recently reported an upregulation of postsynaptic α4β2* nAChRs in the PFC of CHT+/− mice as measured by radioligand binding, as well as a greater degradation of performance on a sustained attention task (SAT) compared to WT littermates with reverse dialysis of the nAChR antagonist mecamylamine (Paolone et al, submitted). In contrast, loss of postsynaptic nAChRs at preganglionic synapses has been shown to induce a loss of presynaptic CHT [50]. Given the diversity of nAChR subunits, their pre-and postsynaptic localization, and their expression within both excitatory and inhibitory circuits that control DA neuron firing and DA release, it is difficult to predict the direction of drug-induced changes in the CHT+/− mice. It is also possible that the loss of CHT may produce pathway-specific reductions of nAChR expression, possibly as a result of a lack of ACh signaling during development. In this regard, ACh release has been suggested to play a developmental role at the neuromuscular junction, including an influence on the localization of nAChRs [51, 52].
Cocaine-induced elevations of DA are a result of diminished clearance of DA that is released with ongoing excitation of DA terminals. As such, the blunted cocaine response in the CHT+/− mice could arise from either reduced DA neuron excitation or the ability of nicotinic cholinergic activity to assist in translation of this activity into vesicular DA release [36, 37, 47], possibly linking cocaine-induced DA release to reduced basal AD levels. However, we found that cocaine effects were smaller than would be expected simply from a baseline shift. Possibly, the reduction in cocaine-induced DA elevations in CHT+/− mice may result from further compensatory changes in synthesis or release of DA per unit impulse, or could arise from changes in DA transporter (DAT) levels/activity. These mechanisms may still be connected to changes in basal DA levels as, for example, lower DAT levels could arise as a compensation for reduced tonic DA neuron activity, as high rates of clearance of DA would presumably be unnecessary.
Finally, do these findings of reduced basal and elicited DA levels provide insights into disorders for which constitutively reduced CNS ACh signaling is suspected? Specifically, we are drawn to evidence that a common, reduced function coding variant in CHT (Ile89Val) increases risk for ADHD and depression [12]. If the tonically lower extracellular DA levels present in the CHT+/− mice occur in subjects expressing the Ile89Val variant, these individuals might have a diminished ability both to produce the burst firing of DA neurons seen with salient stimuli and/or have difficulty utilizing the transition from tonic to phasic firing of DA neurons that characterizes reinforcing stimuli. Importantly, Parikh and colleagues recently reported that CHT+/− mice perform like WT animals in tasks that do not require sustained attention [30], indicating that these animals can associate cues with outcomes to the degree that they can learn to associate cues with reward. Whether animals differ, however, in their perception of the strength of reinforcers or their willingness to expend significant effort to achieve rewards is not clear, and such factors could contribute to both inattention and impulsivity. As for a role of reduced CHT expression and depression risk, we note recent findings pointing to a role of DA signaling in rodent behaviors that are thought to mimic features of depression [53, 54]. However, it has been shown that systemic treatment of animals with the cholinesterase inhibitor physostigmine as well as viral suppression of AChE levels, elicits depressive-like behavior that can be reversed by the serotonin-selective reuptake inhibitor fluoxetine [55]. One way to reconcile these findings is through a consideration that tonically elevated synaptic ACh could lead to desensitization of nAChRs (and muscarinic AChRs) with the emergence over time of a hypocholinergic state in relevant areas of the brain. Continued use of the CHT+/− mice in paradigms with face and/or predictive validity for ADHD and depression are warranted to understand how the plasticity produced by constitutive reductions in transporter expression/activity can lead to disease risk. The CHT+/− mouse model may also be a useful platform for the development of novel therapeutics that target ACh-dependent dopaminergic hypofunction.
Acknowledgments
We thank the research staff of the Blakely and Dani laboratories for consistently excellent support, and acknowledge the NIH (MH073159, RDB; DA09411 and NS21229, JAD) for support of these studies.
References
- 1.Kasa P. The cholinergic systems in brain and spinal cord. Prog Neurobiol. 1986;26:211–72. doi: 10.1016/0301-0082(86)90016-x. [DOI] [PubMed] [Google Scholar]
- 2.Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- 3.Changeux JP, Edelstein SJ. Nicotinic Acetylcholine Receptors: from molecular biology to cognition. New York, New York: Odile Jacob Publishing Corp; 2005. [Google Scholar]
- 4.De Biasi M. Nicotinic receptor mutant mice in the study of autonomic function. Curr Drug Targets CNS Neurol Disord. 2002;1:331–6. doi: 10.2174/1568007023339148. [DOI] [PubMed] [Google Scholar]
- 5.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 6.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper CM. Congenital myasthenic syndromes. Semin Neurol. 2004;24:111–23. doi: 10.1055/s-2004-829592. [DOI] [PubMed] [Google Scholar]
- 8.Spillane J, Higham E, Kullmann DM. Myasthenia gravis. BMJ. 2012;345:e8497. doi: 10.1136/bmj.e8497. [DOI] [PubMed] [Google Scholar]
- 9.Mitolo-Chieppa D, Mansi G, Rinaldi R, Montagnani M, Potenza MA, Genualdo M, et al. Cholinergic stimulation and nonadrenergic, noncholinergic relaxation of human colonic circular muscle in idiopathic chronic constipation. Dig Dis Sci. 1998;43:2719–26. doi: 10.1023/a:1026615730533. [DOI] [PubMed] [Google Scholar]
- 10.Takase B, Hamabe A, Satomura K, Akima T, Uehata A, Matsui T, et al. Comparable prognostic value of vasodilator response to acetylcholine in brachial and coronary arteries for predicting long-term cardiovascular events in suspected coronary artery disease. Circ J. 2006;70:49–56. doi: 10.1253/circj.70.49. [DOI] [PubMed] [Google Scholar]
- 11.Kaur-Knudsen D, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Nicotinic acetylcholine receptor polymorphism, smoking behavior, and tobacco-related cancer and lung and cardiovascular diseases: a cohort study. J Clin Oncol. 2011;29:2875–82. doi: 10.1200/JCO.2010.32.9870. [DOI] [PubMed] [Google Scholar]
- 12.English BA, Hahn MK, Gizer IR, Mazei-Robison M, Steele A, Kurnik DM, et al. Choline transporter gene variation is associated with attention-deficit hyperactivity disorder. J Neurodev Disord. 2009;1:252–63. doi: 10.1007/s11689-009-9033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–97. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–30. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dani JA, Kosten TR, Benowitz NL. The pharmacology of nicotine and tobacco. In: Ries RK, Fiellin DA, Miller SC, Saitz R, editors. Principles of Addiction Medicine. Philadelphia, PA: Lippincott Williams & Wilkins, Wolters Kluwer; 2009. pp. 179–91. [Google Scholar]
- 16.Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–5. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- 17.Yamamura HI, Snyder SH. Choline: high-affinity uptake by rat brain synaptosomes. Science. 1972;178:626–8. doi: 10.1126/science.178.4061.626. [DOI] [PubMed] [Google Scholar]
- 18.Apparsundaram S, Ferguson SM, George AL, Jr, Blakely RD. Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem Biophys Res Commun. 2000;276:862–7. doi: 10.1006/bbrc.2000.3561. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, et al. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci. 2003;23:9697–709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakata K, Okuda T, Misawa H. Ultrastructural localization of high-affinity choline transporter in the rat neuromuscular junction: enrichment on synaptic vesicles. Synapse. 2004;53:53–6. doi: 10.1002/syn.20029. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson SM, Blakely RD. The choline transporter resurfaces: new roles for synaptic vesicles? Mol Interv. 2004;4:22–37. doi: 10.1124/mi.4.1.22. [DOI] [PubMed] [Google Scholar]
- 22.Birks RI, Macintosh FC, Sastry PB. Pharmacological inhibition of acetylcholine synthesis. Nature. 1956;178:1181. doi: 10.1038/1781181a0. [DOI] [PubMed] [Google Scholar]
- 23.Mulder AH, Yamamura HI, Kuhar MJ, Snyder SH. Release of acetylcholine from hippocampal slices by potassium depolarization: dependence on high affinity choline uptake. Brain Res. 1974;70:372–6. doi: 10.1016/0006-8993(74)90329-1. [DOI] [PubMed] [Google Scholar]
- 24.Schueler FW. A new group of respiratory paralyzants. I. The “hemicholiniums”. J Pharmacol Exp Ther. 1955;115:127–43. [PubMed] [Google Scholar]
- 25.Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, Blakely RD. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc Natl Acad Sci U S A. 2004;101:8762–7. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barwick KE, Wright J, Al-Turki S, McEntagart MM, Nair A, Chioza B, et al. Defective presynaptic choline transport underlies hereditary motor neuropathy. Am J Hum Genet. 2012;91:1103–7. doi: 10.1016/j.ajhg.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund D, Ruggiero AM, Ferguson SM, Wright J, English BA, Reisz PA, et al. Motor neuron-specific overexpression of the presynaptic choline transporter: impact on motor endurance and evoked muscle activity. Neuroscience. 2010;171:1041–53. doi: 10.1016/j.neuroscience.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, Levey AI, et al. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes Brain Behav. 2007;6:411–24. doi: 10.1111/j.1601-183X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 29.English BA, Appalsamy M, Diedrich A, Ruggiero AM, Lund D, Wright J, et al. Tachycardia, reduced vagal capacity, and age-dependent ventricular dysfunction arising from diminished expression of the presynaptic choline transporter. Am J Physiol Heart Circ Physiol. 2010;299:H799–810. doi: 10.1152/ajpheart.00170.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh V, St Peters M, Blakely RD, Sarter M. The presynaptic choline transporter imposes limits on sustained cortical acetylcholine release and attention. J Neurosci. 2013;33:2326–37. doi: 10.1523/JNEUROSCI.4993-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rand JB, Nonet ML. In: Synaptic Transmission. C elegans II. Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. New York: Cold Spring Harbor Press; 1997. pp. 611–43. [PubMed] [Google Scholar]
- 32.Matthies DS, Fleming PA, Wilkes DM, Blakely RD. The Caenorhabditis elegans choline transporter CHO-1 sustains acetylcholine synthesis and motor function in an activity-dependent manner. J Neurosci. 2006;26:6200–12. doi: 10.1523/JNEUROSCI.5036-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Y, Zhang T, Li W, Doyon WM, Dani JA. Route of nicotine administration influences in vivo dopamine neuron activity: habituation, needle injection, and cannula infusion. J Mol Neurosci. 2010;40:164–71. doi: 10.1007/s12031-009-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Dong Y, Doyon WM, Dani JA. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry. 2012;71:184–91. doi: 10.1016/j.biopsych.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Domburg PHMF, ten Donkelaar HJ. The human substantia nigra and ventral tegmental area. Advances in Anatomy Embryology and Cell Biology. 1991 [PubMed] [Google Scholar]
- 36.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–9. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–2. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 38.Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–43. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 40.McCormick DA. Actions of acetylcholine in the cerebral cortex and thalamus and implications for function. Prog Brain Res. 1993;98:303–8. doi: 10.1016/s0079-6123(08)62412-7. [DOI] [PubMed] [Google Scholar]
- 41.Sarter M, Bruno JP, Turchi J. Basal forebrain afferent projections modulating cortical acetylcholine, attention, and implications for neuropsychiatric disorders. Ann N Y Acad Sci. 1999;877:368–82. doi: 10.1111/j.1749-6632.1999.tb09277.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang D, Gao M, Xu D, Shi WX, Gutkin BS, Steffensen SC, et al. Impact of prefrontal cortex in nicotine-induced excitation of ventral tegmental area dopamine neurons in anesthetized rats. J Neurosci. 2012;32:12366–75. doi: 10.1523/JNEUROSCI.5411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hecker S, Mesulam MM. Two types of cholinergic projections to the rat amygdala. Neuroscience. 1994;60:383–97. doi: 10.1016/0306-4522(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 44.Muller JF, Mascagni F, McDonald AJ. Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Comp Neurol. 2011;519:790–805. doi: 10.1002/cne.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandon EP, Mellott T, Pizzo DP, Coufal N, D’Amour KA, Gobeske K, et al. Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency. J Neurosci. 2004;24:5459–66. doi: 10.1523/JNEUROSCI.1106-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–4. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 48.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rassadi S, Krishnaswamy A, Pie B, McConnell R, Jacob MH, Cooper E. A null mutation for the alpha3 nicotinic acetylcholine (ACh) receptor gene abolishes fast synaptic activity in sympathetic ganglia and reveals that ACh output from developing preganglionic terminals is regulated in an activity-dependent retrograde manner. J Neurosci. 2005;25:8555–66. doi: 10.1523/JNEUROSCI.1983-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–48. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 52.de Castro BM, De Jaeger X, Martins-Silva C, Lima RD, Amaral E, Menezes C, et al. The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol Cell Biol. 2009;29:5238–50. doi: 10.1128/MCB.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–41. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A. 2013;110:3573–8. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]