Abstract
Introduction:
The optimal timing for repeat evaluation of a cytologically benign thyroid nodule greater than 1 cm is uncertain. Arguably, the most important determinant is the disease-specific mortality resulting from an undetected thyroid cancer. Presently there exist no data that evaluate this important end point.
Methods:
We studied the long-term status of all patients evaluated in our thyroid nodule clinic between 1995 and 2003 with initially benign fine-needle aspiration (FNA) cytology. The follow-up interval was defined from the time of the initial benign FNA to any one of the following factors: thyroidectomy, death, or the most recent clinic visit documented anywhere in our health care system. We sought to determine the optimal timing for repeat assessment based on the identification of falsely benign malignancy and, most important, disease-related mortality due to a missed diagnosis.
Results:
One thousand three hundred sixty-nine patients with 2010 cytologically benign nodules were followed up for an average of 8.5 years (range 0.25–18 y). Thirty deaths were documented, although zero were attributed to thyroid cancer. Eighteen false-negative thyroid malignancies were identified and removed at a mean 4.5 years (range 0.3–10 y) after the initial benign aspiration. None had distant metastasis, and all are alive presently at an average of 11 years after the initial falsely benign FNA. Separate analysis demonstrates that patients with initially benign nodules who subsequently sought thyroidectomy for compressive symptoms did so an average of 4.5 years later.
Conclusions:
An initially benign FNA confers negligable mortality risk during long-term follow-up despite a low risk of identifying several such nodules as thyroid cancer. Because such malignancies appear adequately treated despite detection at a mean 4.5 years after falsely benign cytology, these data support a recommendation for repeat thyroid nodule evaluation 2–4 years after the initial benign FNA.
Thyroid nodules are common (1, 2). Once identified, evaluation of a clinically relevant nodule is performed with the intention of excluding thyroid cancer. In patients with normal or elevated serum TSH, evaluation most often includes ultrasound-guided fine-needle aspiration (FNA) with cytological assessment (3, 4). Numerous published series of consecutive aspirates confirm that FNA cytology most often demonstrates no evidence of malignant cells, and nodules are deemed benign (5). This diagnosis allows recommendation for conservative management.
However, cytological interpretation is not flawless, and false-negative (ie, falsely benign) aspirates may occur. Because most benign nodules are not removed surgically, the precise proportion is difficult to define, although most studies document a rate of 1%–10% (6). Although modest, this false-negative rate is not negligible and has formed the basis for generalized recommendations that patients with cytologically benign nodules undergo repeat, follow-up assessment. The American Thyroid Association recommends serial ultrasound examinations 6–18 months after an initial benign FNA (4). Others recommend consideration of repeat FNA at 6–18 months (7), even if growth is not apparent. Nearly all recommendations are based on expert opinion. At present, it remains uncertain how long cytologically benign nodules should be followed and whether any true clinical benefit is achieved from such monitoring. Importantly, all follow-up strategies primarily seek detection of thyroid nodule growth, with the assumption that this variable is a surrogate for malignancy. When growth is detected, repeat nodule evaluation is recommended. This recommendation, however, may be flawed because studies also confirm that nearly all benign nodules also grow over time (8).
Arguably, the principal purpose of surveillance is to identify those falsely benign nodules, which, if otherwise left undetected and untreated, would shorten survival. To date, however, there exist no studies that evaluate this important end point. To accomplish such an analysis, a consistent, large cohort would have to be evaluated over an extended period of time. Furthermore, consistent thyroid nodule assessment using ultrasound guidance and standardized cytological interpretation should be used.
In 1995, we prospectively began cataloging all patients referred to the Brigham and Women's Hospital thyroid nodule clinic (5) This clinic was designed to foster multidisciplinary care because clinical, sonographic, and cytological assessment are all provided during a single visit. Previous analyses confirm that 95% of thyroid nodules evaluated within our hospital system are referred to this clinic (9, 10). Importantly, our electronic medical record (EMR) has also existed since the beginning of the thyroid nodule clinic, providing reliable monitoring of our health care population. Together these platforms provide important tools, allowing us to accurately investigate the follow-up of patients with initially benign thyroid nodules. We hypothesized that an initial benign FNA of a thyroid nodule greater than 1 cm conveyed an outstanding long-term prognosis and sought to perform the first unbiased, long-term assessment of mortality risk in this patient cohort.
Materials and Methods
We reviewed the records of 2283 consecutive patients referred to the Thyroid Nodule Clinic at the Brigham and Women's Hospital between 1995 and 2003, with the goal of identifying all patients with initially benign thyroid nodule FNA cytology. Every patient in this cohort underwent thyroid ultrasonography. Serum TSH was measured, and if normal or elevated, patients with nodules 10 mm or greater in diameter were advised to undergo ultrasound-guided FNA. If serum TSH was less than 0.5 μU/mL, patients were referred for thyroid scintigraphy to identify autonomously functioning nodules and were no longer included in this investigation.
One of five radiologists, each with expertise in thyroid sonography, performed thyroid ultrasonography using a 10- to 17-mHz transducer. FNA was performed by one of four thyroidologists under ultrasound guidance. A 25-gauge needle was used to typically obtain three needle samples per nodule. With rare exception, a maximum of two nodules were aspirated during a single visit. If FNA results were nondiagnostic, a reaspiration was performed one or more times until a cytological diagnosis was successfully obtained or the patient elected surgical resection prior to a cytological diagnosis.
FNA cytology was evaluated by a Brigham and Women's Hospital cytopathologist. Although most of the study predates the Bethesda System for Reporting Thyroid Cytopathology (11, 12), the cytologists at the Brigham and Women's Hospital used identical criteria and terminology later adopted by the Bethesda System for Reporting Thyroid Cytopathology throughout the entire study period. Specifically, all thyroid FNAs were classified into one of the following categories: nondiagnostic, negative for malignant cells (benign), atypical cells of undetermined significance, suggestive of a follicular or Hurthle cell neoplasm, suspicious for malignancy, or positive for malignancy. Patients with indeterminate or malignant cytology were recommended for hemi- or near-total thyroidectomy. In cases that included surgery, the final diagnosis was based on a histopathological analysis of the surgical specimen by a staff pathologist. Although all nodules evaluated were 1 cm or greater sonographically, in rare circumstances the histopathology measurement was less than 1 cm. In such circumstances, the nodule was still included for study analysis so long as the referential integrity of the nodule could be confirmed from ultrasound to histopathology. In patients with more than one nodule, each nodule greater than 1 cm was individually classified as benign or malignant based on the above criteria.
For this investigation, we identified the date of initial benign ultrasound-guided FNA. Thereafter the medical record was searched for all subsequent aspirates, repeat thyroid sonography, surgical thyroidectomy, or other interventions. For each patient, we also used our EMR to identify whether each patient was living or deceased (and if so, the cause of death). We documented the last date of clinical follow-up anywhere throughout our health care system. Subsequent ultrasound follow-up was considered relevant to this study only if occurring at least 3 months or more after the initial nodule assessment with benign cytology.
We defined each patient's follow-up interval beginning with the initial benign FNA occurring between 1995 and 2003, and ending with any one of the following factors: thyroidectomy, death, or the date of most recent clinical follow-up appointment with any provider documented in our EMR system. We note that an average calculation of follow-up time may inappropriately underestimate the mortality risk for some patients, especially those with shorter follow-up intervals. This is because such patients could theoretically die of thyroid disease/cancer between the end of their short follow-up interval and the described average time point above. If occurring, such mortality would not be captured in our analysis. To address this limitation, we separately analyzed our data by subgroups based on the variable defining the completion of follow-up. False-negative results were defined as a thyroid nodule with initial benign cytology but subsequent identification as malignant upon histopathological evaluation. For most false-negative nodules, a secondary FNA was performed at a later time point that identified abnormal cytology. This prompted surgical consultation and most often thyroidectomy. Details depicting repeat assessment and nodule aspiration have been previously published as part of a prior analysis (8).
Permission for this review and analysis was granted by the Investigational Review Board at the Brigham and Women's Hospital. Results are presented according to patient or nodule, respectively, and compared using a t test or a χ2 test, as appropriate. Values of P < .05 are considered significant.
Results
Two thousand two hundred eighty-three patients with 4270 nodules 1 cm or greater were evaluated in the Brigham and Women's Hospital Thyroid Nodule Clinic between 1995 and 2003. One thousand five hundred forty-nine of 2283 (68%) patients were found to have a cytologically benign thyroid nodule. One hundred eighty of these patients (12%) had no subsequent follow-up of any kind documented in our EMR system (presumptively seeking care elsewhere) and were thus not evaluable. Therefore, a total of 1369 patients with 2010 cytologically benign nodules defined our study cohort. Patient demographics and nodule characteristics, including those lost to follow-up and thus excluded, are listed in Table 1. As expected, the population was primarily female (89.9%) with a mean age of 50 years. Thyroid nodule size averaged 2.4 cm.
Table 1.
Patient and Nodule Characteristics
| Study Cohort | Patients With No Available Follow-Up Data (Excluded) | |
|---|---|---|
| Patients | ||
| n | 1369 | 180 |
| Age, y | ||
| Mean (SEM) | 50 (0.4) | 48 (1.1) |
| Range | 16–87 | 13–85 |
| Sex | ||
| Female | 1231 (89.9%) | 149 (82.8%) |
| Male | 138 (10.1%) | 31 (17.2%) |
| Nodules | ||
| n | 2010 | 354 |
| Size, cm | ||
| Mean | 2.4 | 2.3 |
| Range | 1.0–9.4 | 1.0–7.5 |
| Cystic content | ||
| <50% cystic | 1730 (86.1%) | 290 (81.9%) |
| >50% cystic | 280 (13.9%) | 64 (18.1%) |
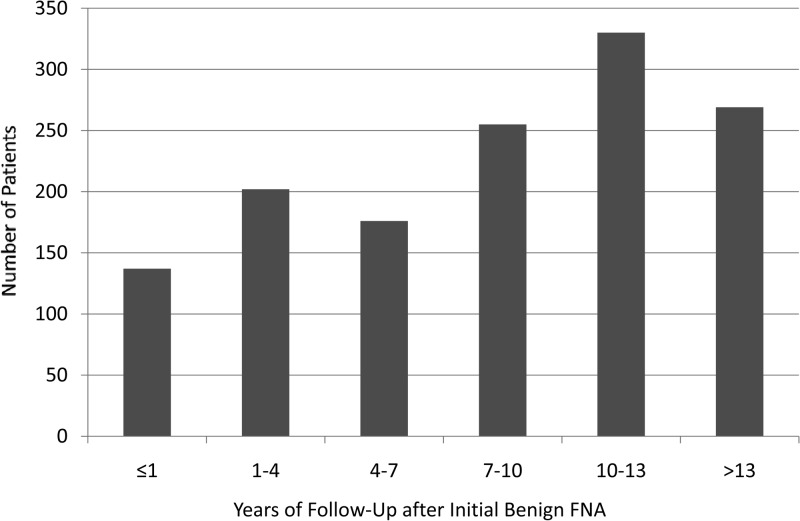
Using our predefined stopping point (thyroidectomy, death, or date of most recent clinical encounter), we assessed our cohort over a mean follow-up interval of 8.5 years after the initial benign FNA cytology. This interval ranged from 0.25 years to 18.1 year, as shown in Figure 1. Four hundred seven of 1369 (30%) had been followed up for less than 5 years, whereas 363 patients (27%) had been followed up for 5–10 years. Five hundred ninety-nine of 1369 patients (44%) had documented follow-up for 10 years or more after their initial benign aspiration. The follow-up interval stopping point was defined by death in 30 of 1369 patients (2%), and due to subsequent thyroidectomy in 325 of 1369 patients (24%). The follow-up interval stopping point for the remaining 1014 patients (74%) was defined by their most recent clinical appointment.
Figure 1.
Distribution of follow-up intervals (years) among 1369 patient with 2010 cytologically benign thyroid nodules. The mean duration of follow-up is 8.5 years.
Among the 325 who underwent subsequent thyroidectomy despite an initial benign cytology, the average time from initial FNA to surgery was 4.2 years (range 0.25–16.5 y). The indication for surgery was large nodule size and/or compressive symptoms in 196 of 325 patients (60%). Abnormal FNA cytology from a separate thyroid nodule prompted the surgery in 84 of 325 patients (26%), whereas abnormal FNA cytology was obtained on a repeat biopsy of the same nodule performed because of growth in 45 of 325 patients (14%). These data are shown in Table 2.
Table 2.
Description of the 325 Patients Who Underwent Thyroidectomy After Initial Thyroid Nodule FNA With Benign Cytology
| Variable | |
|---|---|
| Patients (n = 325) | |
| Age, y | |
| Mean | 49.4 |
| Sex | |
| Female | 292 (90%) |
| Male | 33 (10%) |
| Indication for surgery | |
| Compressive symptoms | 196 (60%) |
| Abnormal cytology in separate nodule | 84 (26%) |
| Abnormal cytology in same nodule | 45 (14%) |
| Timing of surgery, y | |
| Mean | 4.2 |
| Range | 0.25–16.5 |
| Nodules (n = 520) | |
| Size, cm | |
| Mean | 3.0 |
| Range | 1.0–8.9 |
| Cystic content | |
| <50% cystic | 472 (91%) |
| >50% cystic | 48 (9%) |
Mortality assessment
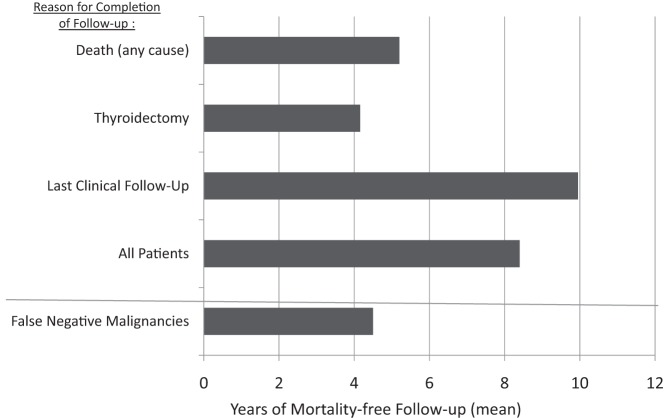
Following initial benign FNA cytology from a clinically relevant (≥1 cm) thyroid nodule, we documented zero deaths (0%) attributable to thyroid disease or thyroid cancer during an average follow up of 8.5 years. However, a total of 30 patients (2% of cohort) died of other medical illness. We separately analyzed our data by subgroups based upon the variable defining the completion of follow up (Figure 2). Among these subgroups, the shortest mean duration of follow-up was 4.2 years in those patients who ultimately underwent thyroidectomy, whereas those who died from any cause were followed up for a mean of 5.3 years. Most importantly, regardless of the stopping point or thyroid nodule size, there were no deaths attributable to thyroid disease or thyroid cancer in any patient.
Figure 2.
Subanalysis of the study cohort. Years of mortality-free follow-up are depicted and grouped by the variable defining the completion of patient follow-up. The bottom row depicts the mean follow-up of the 18 false-negative thyroid cancers.
However, a total of 18 false-negative FNA results (1.3%) were confirmed in our cohort. The mean time from the initial benign cytology to the surgical removal of carcinoma in these 18 patients was 4.5 years (range 0.3–10 y) (Table 3). Ten of the 18 false-negative malignancies (56%) were detected within 5 years of the initial benign aspirate; 16 of 18 (89%) within 8 years of the initial benign aspirate; and all 18 (100%) detected within 10 years of the initial benign aspirate. Details of all 18 false-negative malignancies are shown in Table 3. Sixteen of 18 false-negative nodules (89%) proved to be papillary carcinoma, whereas one was a follicular carcinoma and one was a poorly differentiated carcinoma. None had distant metastatic disease, and none have died of thyroid cancer or thyroid illness over a mean follow-up period of 11 years.
Table 3.
Description of the 18 Subjects With Falsely Benign Thyroid Nodules
| Age, y | Gender | Initial Nodule Size, cm | Nodule Size at Time of Surgery, cm | Reason for Removal | Time From Initial Benign FNA to Surgical Removal, y | Final Pathology | AJCC Staginga | Clinical Status | Total Follow-Up Time From Initial Benign FNA, y | |
|---|---|---|---|---|---|---|---|---|---|---|
| Subject number | ||||||||||
| 1 | 34 | Female | 2.0 | 1.9 | Abnormal repeat cytology | 5.4 | Papillary carcinoma | T1bN0M0 | Alive with no recurrence | 16.7 |
| 2 | 61 | Female | 2.2 | 2.7 | Abnormal repeat cytology | 4.0 | Papillary carcinoma | T2N0M0 | Alive with no recurrence | 10.1 |
| 3 | 38 | Female | 2.0 | 2.3 | Abnormal repeat cytology | 6.5 | Papillary carcinoma | T3N0M0 | Alive with no recurrence | 11.4 |
| 4 | 48 | Female | 1.5 | 1.6 | Abnormal repeat cytology | 7.3 | Follicular carcinoma | T1N0M0 | Alive with no recurrence | 7.4 |
| 5 | 49 | Female | 3.5 | 3.7 | Abnormal repeat cytology | 3.4 | Poorly differentiated carcinoma | T4bN0M0 | Alive with no recurrence | 16.3 |
| 6 | 52 | Female | 2.3 | 2.8 | Abnormal repeat cytology | 6.6 | Papillary carcinoma | T1N0M0 | Alive with no recurrence | 16.9 |
| 7 | 41 | Female | 2.0 | 2.2 | Abnormal repeat cytology | 2.4 | Papillary carcinoma | T1N0M0 | Alive with no recurrence | 2.4 |
| 8 | 51 | Female | 2.4 | 3.8 | Abnormal repeat cytology | 2.0 | Papillary carcinoma | T2N0M0 | Alive with no recurrence | 13.8 |
| 9 | 64 | Female | 3.4 | 4.0 | Abnormal repeat cytology | 1.8 | Papillary carcinoma | T2M0N0 | Alive with no recurrence | 9.9 |
| 10 | 54 | Female | 1.2 | 1.6 | Abnormal repeat cytology | 10.0 | Papillary carcinoma | T1N0M0 | Alive with no recurrence | 17.1 |
| 11 | 50 | Female | 3.0 | 3.9 | Abnormal repeat cytology | 9.8 | Papillary carcinoma | T3N0M0 | Alive with no recurrence | 17.7 |
| 12 | 24 | Female | 2.9 | 4.3 | Compressive symptoms | 2.0 | Papillary carcinoma | T2N0M0 | Alive with no recurrence | 7.1 |
| 13 | 41 | Female | 1.9 | 3.4 | Compressive symptoms | 5.9 | Papillary carcinoma | T3N0M0 | Alive with no recurrence | 8.7 |
| 14 | 61 | Female | 3.0 | 3.0 | Abnormal cytology in separate nodule | 3.2 | Papillary carcinoma | T2N0M0a | Alive with no recurrence | 10.3 |
| 15 | 23 | Female | 4.2 | 3.0 | Compressive symptoms | 0.3 | Papillary carcinoma | T2N0M0 | Alive with no recurrence | 1.0 |
| 16 | 45 | Female | 1.3 | 1.5 | Abnormal cytology in separate nodule | 3.0 | Papillary carcinoma | T1bN0M0a | Alive with no recurrence | 10.7 |
| 17 | 66 | Male | 1.3 | 1.9 | Abnormal cytology in separate nodule | 1.6 | Papillary carcinoma | T4aN0M0a | Alive with no recurrence | 10.3 |
| 18 | 54 | Female | 3.2 | 3.2 | Compressive symptoms | 6.2 | Papillary carcinoma | T2N0M0 | Alive with no recurrence | 11.3 |
Abbreviation: AJCC, American Joint Committee on Cancer.
Staging representative of entire thyroid cancer, including the separate nodule prompting FNA.
If patients who underwent thyroidectomy due to an abnormal cytological result in a separate second nodule or because of compressive symptoms are excluded, then the cohort comprised a total of 11 false-negative FNA results (1.0%), with 5 of 11 (45%) detected within 5 years of initial benign aspirate; 9 of 11 (82%) within 8 years; and all 11 (100%) detected within 10 years. The initial size of such nodules was 2.2 cm, and upon repeat aspiration measured 2.8 cm (mean time between aspirations 3.4 y). Nine of 11 false-negative nodules (82%) were papillary carcinoma, whereas one was a follicular carcinoma and one a poorly differentiated carcinoma. None had distant metastatic disease or died of thyroid cancer over the mean follow-up period of 12.7 years (Table 3).
To investigate whether the detection of false-negative thyroid malignancies would be enhanced by earlier and more frequent ultrasound follow-up, we divided all patients in our cohort who underwent thyroidectomy into two groups based on the time interval between the initial benign FNA and a subsequent ultrasound follow-up. When the ultrasound follow-up was less than 2, 3, or 4 years, 7.5%, 7.8%, and 8.2% of patients, respectively, undergoing thyroidectomy were found to have a false-negative aspirate. When ultrasound follow-up occurred after 2, 3, or 4 years, 7.4%, 6.6%, and 4.5% of patients, respectively, had a false-negative aspirate (P = .97, P = .74, and P = .41, respectively). These limited data suggest minimal benefit to repeat follow-up ultrasound for approximately 2–4 years after an initial benign FNA, although we acknowledge this recommendation should be refined after further prospective analysis.
Discussion
Since the inception of FNA, benign thyroid nodule cytology has brought both the relief of a nonmalignant diagnosis yet the uncertainty of follow-up. Recommendations have been almost universally based on expert opinion, although with variable conclusions (4, 7). Although many studies have investigated rates of false-negative cytology, arguably the most important end point is disease-specific mortality. Our investigation followed up a consecutive cohort of 1369 patients with 2010 initially benign cytology for an average of 8.5 years. Although 30 deaths (2%) were documented in this group, none of them (0%) were attributable to thyroid illness or thyroid cancer. Thus, an initial benign thyroid nodule biopsy conveys an outstanding prognosis and negligible risk of thyroid cancer mortality, even though false-negative thyroid cancer may rarely be detected. A subgroup analysis allows for the estimation of reasonable time intervals for follow-up evaluation. Patients with falsely benign aspirates yet subsequent thyroid malignancy underwent surgery on average 4.5 years after the initial evaluation yet have remained healthy (and are still living) for an average of 11 years with no disease-related mortality. Of these patients, the shortest duration from initial benign FNA to subsequent thyroidectomy was 4 months (Table 3, subject 15), although nearly all others were detected approximately 2 or more years after an initial benign biopsy. Separately, patients with initially benign nodules who subsequently sought thyroidectomy for compressive symptoms did so at an average of 4.5 years later. Together these data support that most benign thyroid nodules in low-risk, asymptomatic individuals can be safely recommended for follow-up in 2–4 years without mortality risk or likelihood of harm.
Many have assumed that the goal of follow-up nodule assessment is to detect malignancy (13). Although partially true, there is a strong argument that the ultimate goal is actually one of detecting those malignancies that pose risk of future harm if not detected and treated. This important distinction is worthy of emphasis because a similar argument has formed the basis of recommending diagnostic FNA of thyroid nodules only when larger than 1–1.5 cm (14, 15). Our data demonstrate that benign cytology alone, when obtained via ultrasound-guided FNA, excludes the most dangerous and harmful of thyroid malignancies. As shown in Table 3, even those false-negative malignancies that are subsequently identified years later appear to be low risk and effectively treated, even at a delayed time point. For example, the T-status as defined by the tumor node metastasis staging system for thyroid cancer (American Joint Committee on Cancer classification system) did not worsen in 17 of 18 patients during the follow-up interval. However, one patient's tumor increased from 1.9 to 3.4 cm 5.9 years later, thus changing from a T1b to T2 tumor.
It is notable that 24% of our cohort with benign nodules ultimately pursued surgery. This may be explained by two factors. First, a portion of this cohort was likely referred to surgery based on the finding of abnormal cytology in a separate, secondary thyroid nodule. This finding also suggests that slow nodule growth is common (8), and symptomatic enlargement may be more frequent than initially assumed (16, 17). This may lead to surgical removal. Although we have previously shown that large nodules do not convey an increased risk of false-negative aspirates (9), large nodules may cause difficulty swallowing and/or tightness in the neck. For many individuals, surgery remains an important therapeutic option, especially when performed by an experienced, high-volume thyroid surgeon (18, 19). We currently query all of our patients with thyroid nodules for such symptoms and counsel them to notify us if they develop such symptoms over time.
Although our data demonstrate 0% mortality for all individuals at a mean of 8.5 years, such data may be better translated into the clinic using a subgroup analysis. For example, an older, asymptomatic individual with comorbid illness may warrant no further recommended sonographic follow-up of their benign nodule. This is because our data depict a negligible mortality risk attributable to thyroid disease/cancer over the decade ahead. In contrast, a younger individual with a 3-cm nodule may benefit from repeat sonographic assessment in 2 years. False-negative malignancy can occur, and nodules can grow or become symptomatic. Intervention in such circumstances can be important, although our data also confirm that a 2- to 4-year delay does not affect prognosis. Overall, it is apparent that the care of patients with benign thyroid nodules must be individualized, although a more conservative approach than that previously believed can be used.
We acknowledge limitations to our study. Our study was retrospective in nature and from a single center. Although the possibility of sampling bias cannot be excluded, our thyroid nodule biopsy clinic was among the first of its kind to provide consistent, multidisciplinary care inclusive of standardized ultrasound and cytology reporting (5). Furthermore, our clinic captures greater than 95% of patients undergoing thyroid evaluation in our health care system, and all have been registered in our database. Together this adds validity to our analysis. We also acknowledge that follow-up evaluation was not standardized, allowing for great variation in follow-up length (range 0.25–18.1 y). However, we chose the principal study end point of mortality for this very reason. Our evidence nonetheless suggests that most patients do indeed remain in our health care system over time while seeking care from other providers. And, importantly, our EMR (in place since the inception of our thyroid nodule clinic) allowed us easy access to all clinical records. We nonetheless acknowledge the possibility that some patients may have left our health care system without our knowledge, and this may theoretically influence our findings.
In summary, our data are the first to assess long-term mortality risk after a benign thyroid nodule biopsy. At a mean of 8.5 years, no thyroid cancer attributable death was detected, regardless of follow-up strategy. False-negative malignancies rarely occur, although most often are low risk and effectively treated, even several years after the initial benign cytology. Together these data provide clinicians with information to individualize follow-up care for patients with benign thyroid nodules. Asymptomatic, low-risk patients with nodules unlikely to cause structural compromise can typically be recommended for repeat assessment in 2–4 years' time. However, older individuals, or those with poor health or concerning comorbidities, may not require any further clinical or sonographic assessment ahead so long as they remain asymptomatic. Future prospective investigations should be designed to better define the risk of even longer intervals between follow-up assessments.
Acknowledgments
Disclosure Summary: The authors reports no disclosures relevant to this work. E.K.A. has served as a consultant to Genzyme, Inc and Veracyte, Inc and is on the Scientific Advisory Board of Asuragen, Inc.
Footnotes
- EMR
- electronic medical record
- FNA
- fine-needle aspiration.
References
- 1. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553–559. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society. Key statistics about thyroid cancer. 2012. http://www.cancer.org/cancer/thyroidcancer/detailedguide/thyroid-cancer-key-statistics Accessed August 13, 2013.
- 3. Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118(4):282–289. [DOI] [PubMed] [Google Scholar]
- 4. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- 5. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111(6):508–516. [DOI] [PubMed] [Google Scholar]
- 6. Wang CC, Friedman L, Kennedy GC, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011;21:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gharib H, Papini E, Pashke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16(suppl 1):1–41. [DOI] [PubMed] [Google Scholar]
- 8. Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003;138(4):315–318. [DOI] [PubMed] [Google Scholar]
- 9. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564–570. [DOI] [PubMed] [Google Scholar]
- 10. Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91(9):3411–3417. [DOI] [PubMed] [Google Scholar]
- 11. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19(11):1159–1165. [DOI] [PubMed] [Google Scholar]
- 12. Ali SZ, Cibas ES, eds. The Bethesda System for Reporting Thyroid Cytopathology. New York: Springer; 2009. [DOI] [PubMed] [Google Scholar]
- 13. Choi YJ, Jung I, Min SJ, et al. Thyroid nodule with benign cytology: is clinical follow-up enough? PLoS One. 2013;8(5):e63834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–428. [DOI] [PubMed] [Google Scholar]
- 15. Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103(11):2269–2273. [DOI] [PubMed] [Google Scholar]
- 16. McHenry CR, Huh ES, Machekano RN. Is nodule size an independent predictor of thyroid malignancy? Surgery. 2008;144(6):1062–1068. [DOI] [PubMed] [Google Scholar]
- 17. McCoy KL, Jabbour N, Ogilvie JB, Ohori NP, Carty SE, Yim JH. The incidence of cancer and rate of false-negative cytology in thyroid nodules greater than or equal to 4 cm in size. Surgery. 2007;142(6):837–844. [DOI] [PubMed] [Google Scholar]
- 18. Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393(5):667–673. [DOI] [PubMed] [Google Scholar]
- 19. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228(3):320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]