Abstract
Natural killer (NK) cells were originally identified as lymphocytes capable of killing cancer cells without prior sensitization (1). Further characterization of these cells in both humans and rodent models has expanded their role towards a broad-based immunosurveillance of diseased and healthy peripheral tissues. Among peripheral organs, the lung contains the largest percentage of NK cells. Accordingly, NK cells are implicated in many immunological responses within the lung, including innate effector functions as well as initiation of the adaptive immune response. In this article, we review the characteristics of NK cells, current models of NK maturation and cell activation, migration of NKs to the lung, and effector functions of NKs in cancer and infection in the airways. Specific emphasis is placed on the functional significance of NKs in cancer immunosurveillance. Therapeutic modulation of NK cells appears to be a challenging but promising approach to limit cancer, inflammation, and infection in the lung.
Keywords: Immunosurveillance, Natural killer cell, Lung cancer, Tumor immunology, Review
2. Introduction: Functional and Molecular Characterization of NK Cells
Natural killer cells were originally described in 1975 as lymphocytes capable of spontaneously and specifically killing tumor targets (1-3). Kiessling and colleagues showed that in the mouse there exists a population of cells that is able to spontaneously kill Moloney leukemia cells. Using the methods of characterization available in 1975, the investigators further defined them as lymphocytes, based upon morphology, but could purify and separate them from T and B lymphocyte populations. Furthermore their development did not require the thymus, which is a critical site of T lymphocyte development (1). Subsequently, a similar population of cells was isolated from human peripheral blood (3). Today, it is clear that the range of target cells extends to some infected and/or stressed cells, and NK cells are more generously defined as large granular lymphocytes with an intrinsic capacity to kill a wide variety of target cells without prior sensitization (4). A critical element to their proper function is the ability of NK cells to distinguish pathologic from healthy cells. Target cell recognition involves a complex interplay of multiple receptor-ligand interactions with activating, inhibiting, co-stimulatory, and adhesion functions. NK function and activation is determined by the sum of activating and inhibitory receptors engaged during an encounter with a target cell. For each encounter, the NK cell can mediate several effector functions, including exocytosis of cytotoxic granules containing perforin and granzyme B and synthesis of cytokines such as interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα), and granulocyte-macrophage colony stimulating factor (GM-CSF) (4-6).
Despite major scientific advances in the 40 years since the initial identification of this cell population, a complete molecular definition of NK cells remains elusive, and the NK cell is still identified predominately by its natural killing function (4). This intrinsic killing function is independent of prior exposure to the target cell, thus defining the NK cell population as a separate entity from cytolytic T lymphocytes. NK cells share some developmental requirements with other lymphocytes, such as a requirement for transcription programs mediated by Ikaros, PU.1, Ets-1, and Runx (4, 7), but previous research using genetically deficient mouse models indicates that it is possible to reduce T and B lymphocyte function without affecting NK cell activity and vice versa. For example, athymic nu/nu (NUDE) mice have stunted T lymphocyte development, and transgenic Rag1-/- and Rag2-/- mice have stunted T, B, and NKT lymphocyte development, but these mutations have no detected affect on NK cell development (8). In contrast, mice deficient in the common gamma chain (γC) subunit of many cytokine receptors, including IL-2 and IL-15, lack NK cells but produce small numbers of T lymphocytes and normal numbers of B lymphocytes (9, 10). Several transcription factors that drive NK cell development from common lymphocyte precursors have been identified. For example, E4bp4 is expressed in developing and mature NK cells, but not B or T cells. E4bp4-deficient mice have normal numbers of B and T cells but development of mature NK cells from bone marrow precursors is arrested at an NK precursor stage (7). Similarly, mice deficient in the transcription factors MEF, Id2, or IRF-2 have a reduced number of NK cells but normal numbers of B and T cells. The importance of Id2 has been confirmed in developing human NK cells (7). Using another transgenic mouse model, deficiency of T-bet was shown to prevent NK cell development by blocking the migration of NK cells from the bone marrow to peripheral tissues. Migratory defects are also apparent in mice with reduced expression of Eomes, CBFβ, and Gata-3 (7). These mouse models demonstrate that although NK cells share many developmental requirements with other lymphocyte populations, they can be identified as a unique population of cells with distinct transcriptional programs. On a cellular level, NK cells are distinguished from T lymphocytes based upon the absence of the T-cell receptor (surface CD3ε expression) in combination with the presence of surface receptors NK1.1 (in C57BL/6 strain only), NKp46, or DX5 in mice, or CD16 (FcγRIII) and CD56 in humans. More recently, NK cells have been classified into a family of innate lymphoid cells (ILCs). ILCs are defined as lymphocytes which lack rearranged antigen receptors. NK cells are distinguished from other subsets of ILCs, namely Rorγt+ and type 2 ILCs, by both their stimulating cytokines, including IL-18, IL-2, IL-12, and IL-15, and their production of IFNγ (11, 12).
3. NK Cell Activation
As a means of immunosurveillance, NK cells temporarily synapse with other cells which allows for the engagement of surface NK receptors with the ligands present on the surface of the target cell. NK cells simultaneously express multiple receptors that act to inhibit or activate their effector functions. The unique combination of receptor expression and receptor-ligand interaction dictates the overall cellular activity. NK cell activation must be precisely controlled since NK cells have a strong cytotoxic capacity and the potential for deleterious self-reactivity. Thus, the molecular identification of NK cell surface receptors and their function has been extensively studied.
3.1. Human KIRs and LIRs and their functional counterparts in mice
Most NK cell receptors can be grouped into just a few gene families based on structural and functional similarity and location within the genome. Specifically the leukocyte receptor complex (LCR), encoded on chromosome 19 in man encodes multiple receptors belonging to the immunoglobulin superfamily. These receptors are expressed broadly on a wide variety of haematopoietic cells. Another set of genes critical to NK function is encoded in the natural killer gene complex (NKC) located on chromosome 12 in man and 6 in the mouse. These receptors are expressed primarily on NK cells but can also be detected on other subsets of cells with lytic capacity, such as activated CD8+ cytotoxic T lymphocytes (13). Genes encoded in these regions are members of several gene families including killer immunoglobulin-like receptors (KIRs), leukocyte immunoglobulin-like receptors (LIRs), and C-type lectin-like family of receptors. KIR and LIR family genes encode receptors with extracellular immunoglobulin domains that bind to class I major histocompatibility complex (MHC I) molecules and either short or long cytoplasmic tails which determine if the receptor inhibits or potentiates NK cell activation. Proteins with a long cytoplasmic tail have an immunoreceptor tyrosine-based inhibition motif (ITIM) and are associated with inhibitory signaling. In contrast, a short cytoplasmic tail mediates activation of NK cells and is either dependent or independent of an immunoreceptor tyrosine-based activation motif (ITAM) (14). In mice, through a convergent evolution process, the Ly49 gene family located in the NKC has evolved functions similar to those of human KIRs and LIRs located in the LCR. Ly49 genes encode type II lectin-like molecules as opposed to their functional counterparts in humans that are immunoglobulin-like receptors. However, unlike most lectin or lectin-like receptors which bind carbohydrate oieties in a calcium-dependent manner, the Ly49 molecules recognize an epitope of MHC I in a carbohydrate-independent manner (14). The Ly49 gene family is comprised of 15 genes in the C57BL/6 strain of mice but demonstrates extreme quantitative and qualitative polymorphisms in other strains (15). As with KIRs and LIRs in humans, the Ly49 receptor family in mice is comprised of both inhibitory and activating receptors (14). Genes belonging to two other families, killer cell lectin-like receptor (KLR) and NKG2 are also present within the NKC locus in mice and a similar region of chromosome 12 in humans. These family members are also C type lectin-like receptors that bind MHC I and mediate either inhibitory or activating signaling pathways within the NK cell (Table 1) (14).
Table 1. NK receptor gene families in human and mouse genomes.
| Gene Family | Human | Mouse | Function |
|---|---|---|---|
| KIRs | 15 genes clustered in LRC on Chr. 19 | 2 genes on Chr. X | Ig-like receptor family members 9 inhibitory, 6 activating receptors in humans |
| LIRs | 12 genes clustered in LRC on Chr. 19 | none known | Ig-like receptor family members 5 inhibitory, 7 activating receptors in humans |
| Ly49 | Psuedogene on Chr. 12 | 15 genes (C57BL/6 mice) clustered in NKC locus on Chr. 6 | Type II lectin-like molecules 9 inhibitory, 2 activating receptors in mice |
| KLR (eg. CD94) and NKG2 families | 4 genes in NKC region of Chr. 12 | 4 genes clustered in NKC locus on Chr. 6 | C-type lectin-like receptors 4 inhibitory receptors in humans and mice |
KIRs, killer immunoglobulin-like receptors; LIRs, Leukocyte Ig-like receptors; KLR, killer cell lectin-like receptor; LRC, leukocyte receptor complex; NKC, natural killer gene complex
3.2. Detection of MHC I and the licensing hypothesis
Detection of class I major histocompatibility complex (MHC I) molecules by NK cell receptors is a key component of NK cell function. MHC I proteins are critical mediators of the initiation of the adaptive immune response, as they present molecular moieties from intracellular pathogens or transformed cells to cytolytic T cells (CTLs). CTLs kill the infected or transformed target cell. Therefore, infected or malignant cells undergo selection towards the downregulation of MHC I antigens due to the destruction of MHC I+ cells. Through what was likely to be a co-evolutionary process, host NK cells have developed the capability of detecting expression of the MHC I molecule itself, rather than the antigen presented by the MHC I protein. In a homeostatic state, cells express autologous MHC I molecules on their surface at normal levels that inhibit NK cell-mediated lysis of healthy host cells. Since ligation of MHC I with NK receptors suppresses NK cell function, reduced MHC I expression relieves the inhibitory signaling of NK cells, and infected cells become vulnerable to NK cell mediated killing. Thus, opposing functions by T cells and NK cells provide a coordinated system of immunosurveillance. This two-pronged approach by the immune system to detect antigens presented by MHC I and MHC I molecules themselves limits the viability of infected or malignant cells within the host (16).
The original concept of NK cell activation proposed that a lack of engagement of MHC I receptors was necessary and sufficient for target cell lysis. The importance of self-MHC expression in the regulation of NK cells was demonstrated by Karre, et al. in 1986. Murine lymphoma cells with a loss of H-2 expression were less malignant than WT cells, suggesting that an immunological defense system was aimed at detecting and eliminating tumor cells with deleted or reduced expression of self-MHC molecules (17). In another seminal study, a β 2-microglobulin knock-in transgene was used to restore H-2 expression in an H-2 deficient lymphoma cell line, A.H.2-YAC-1. Concomitant with induced expression of MHC I molecules, the transgenic cancer cells were protected from NK cell-mediated lysis (18). The interaction of MHC I and inhibitory receptors fueled a concept referred to as the “missing-self hypothesis.” However, NK cell function cannot be fully described by this simplistic model. Mice with a targeted deletion of H-2K and H-2D MHC class I heavy chains or the β2-microglobulin subunit lack surface MHC I expression and MHC I-mediated inhibitory receptor engagement, but, surprisingly, NK cells from these mice are not hyperactive and instead are hyporesponsive to target cell killing (19). This phenomenon fueled the “licensing hypothesis,” which states that some MHC I expression is required for NK cell activation. Inhibitory receptor engagement with self-specific MHC I molecules expressed by autologous cells will permit, or license, the NK cell to respond during an encounter with a target cell (20). Recent studies suggest that the level of MHC I expression on the target cell influences the responsiveness of NK cells to activating signals on the target cell. Detection of high levels of MHC I, or paradoxically, no detection of self-specific MHC I molecules turns down the reactivity of NK cells, while a normal or low level of MHC I promotes activation of cytolytic function, even in response to weaker activation signals (21). In summary, both the downregulation of MHC class I inhibitory signals and the presence of activating signals influence the function of NK cells.
3.3. Activating receptors bind stress ligands
In contrast to inhibitory receptors that suppress NK cell function, activating receptors stimulate effector responses. Activating receptors engage ligands on target cells that are either derived from an intracellular pathogen or endogenous ligands that are overexpressed in malignant or stressed cells. NKG2D and NKp46 in both humans and mice, and NKp44 and NKp30 in humans, have defined ligands of endogenous or viral origin (Figure 1). Engagement of these receptors activates cytotoxic responses directed at the cell expressing the ligand, i.e. the target cell. Members of another gene family in mouse, NKPR, encode lectin-like molecules and appear to function as activating receptors. The human genome contains at least one known homology, NKR-P1A. Their ligands are not well-defined, but these receptors are believed to recognize self-molecules (22). NK cells are also capable of detecting cells against which a humoral antibody response has been elicited. CD16 (Fc receptor) can bind the Fc portion of antibodies and lyse cells in a phenomenon termed antibody-dependent cellular cytotoxicity (ADCC). Receptors that modulate NK cell function by acting as co-stimulatory receptors or adhesion receptors have been described, including CD2, CD28, and Nectin (23, 24). Continued research to identify more ligands expressed by target cells will undoubtedly further our understanding of NK cell activation.
Figure 1.
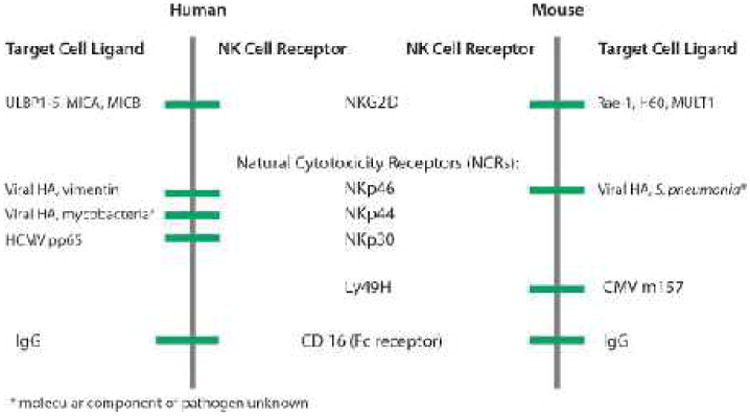
Pathogen and stress-induced ligands bind to activating NK cell receptors. Activating ligands, described to date, are glycoproteins that are derived from either the pathogen itself or from the host cell upon infection, malignant transformation, or cellular stress. UL16 binding protein 1-5 (ULBP1-5) and MHC class I-like polypeptide-related sequences MICA and MICB in humans and RAE-1 family members, H60, and MULT1 in mice are endogenous stress-induced ligands that bind to NKG2D. Pathogen-derived components including influenza hemagglutinin (HA), Mycobacterium tuberculosis vimentin, and human cytomegalovirus (HCMV) pp65 in humans and HA in mice are recognized by natural cytotoxicity receptors (NCRs). Cytomegalovirus (CMV) m157 binds to the Ly49H expressed on mouse NK cells.
3.4. Priming of NK cells
While the effector mechanisms of NK cells are ultimately dependent upon surface receptor engagement with pathogen and/or stress-induced ligands, other components of the immune system, such as cytokines, also affect NK cell activity. Cytokines including interleukins 2, 12, 15, and 18, and type I interferons modulate the activity of NK cells (6, 25, 26). In vitro exposure of human or mouse NK cells to IL-15 or IL-2 “primes” NK cells. Priming can be depicted as NK cells having a lower threshold of activation. Activated NK cells display increased sensitivity to target cells and kill a broader range of target cells (5, 27). Furthermore, recombinant IL-15 and IL-2, which both bind to the IL-2 receptor of NK cells, can induce proliferation of both human and mouse NK cells in vitro and in vivo (5, 26). Longer, overnight exposure (13 – 15h) to IL-12 and low-dose IL-15 has also been shown to induce a memory NK cell phenotype, even in daughter cells, following transfer of memory cells into syngeneic mouse recipients. These memory cells show increased reactivity to tumor cell targets, cytokine exposure, and stimulation by antibodies directed against NK cell receptors (28). Prolonged exposure to IL-2 for 5 days leads to a phenotypic change of NK cells into a new cell type referred to as lymphocyte activated killer cells (LAKs) (29). NK cells also express Toll-like receptors, including TLRs 2, 3, 4, 7, and 8 (30), and accordingly, polyI:C is commonly used in experimental systems to prime NK cells (31). However, other studies indicate that the effect of TLR agonists to prime NK cells may be an indirect effect mediated through Type I IFN released by accessory cells such as dendritic cells and macrophages (32, 33). More experimental work will help to dissect the importance of accessory cells to priming of NK cells upon exposure to TLR agonists.
4. Mechanisms of Effector Functions
Activation of NK cells leads to several effector mechanisms, including: 1) release of cytotoxic granules that lyse target cells, 2) upregulation of death receptor ligand expression and the engagement of cognate death receptors on target cells, which can lead to apoptosis of target cells, 3) release of chemokines and cytokines that promote recruitment and activation of NKs and other immune cells, and 4) release of other soluble mediators, such as PGE2, which shape responses of the immune system. Effector functions will be described in further detail later in this review in the context of immunosurveillance in the lung.
5. Genetic Influences on NK Cellfunction in Mice and Man
Unlike the T and B cell receptors of the adaptive immune system, which undergo somatic cell gene rearrangement, NK receptor diversity is dictated solely by inheritance through the germ line. A link between genetic inheritance and NK cell function has been demonstrated in family studies. In one study, for example, two male siblings almost completely lacked natural killer activity against human melanoma target cells. The functional defect is likely a result of a common genetic mutation(s), because stimulation with IL-2 or IFNα failed to rescue NK cell mediated killing in both siblings (34). Furthermore, analysis of the human genome was used to identify two groups of KIR haplotypes within the human population. In haplotype A, several inhibitory but only one activating KIR gene is present. In haplotype B groups, several activating and inhibitory KIRs are encoded in the germ-line. Thus, an individual homozygous for the A haplotype is likely to have hyporesponsive NK cells in comparison to an individual homozygous for the B haplotype (35). The clinical implications of such polymorphisms are unknown. The chromosomal regions encoding the NK cell receptor families contain polymorphisms reflected in allelic variation and gene copy number in humans as well as mouse models. Polymorphisms in the NKC locus in mice are being used to elucidate the complex interplay between proteins encoded within the gene-rich NKC locus. Among different inbred mouse strains, different haplotypes of the NCR emerge, and to a large extent, these correspond to functional differences in NK cells (15). An additional level of regulation occurs during gene processing. An alternatively-splice isoform of NKp30, NKp30c, which has immunosuppressive function, is more prevalent in patients with gastrointestinal sarcoma compared to healthy controls. Among patients, its expression level relative to NKp30a and NKp30b is associated with clinical outcome (36). Future studies to evaluate how genetic polymorphisms influence NK cell receptor expression on an individual cell basis will advance our understanding of how NK cell receptors work synergistically to promote and tailor NK cell responses to be appropriate to the pathological stimuli.
6. Maturation, Migration, Peripheral Seeding, and the Influence of the Environmental Milieu on NK Cells
The development, maturation, and activation potential of subsets of NK cells continues to be unraveled. NK cells emerge from hematopoietic stem cells that differentiate to common lymphoid progenitor cells, then to bipotential T/NK progenitor cells, and finally to lineage-committed NK precursor cells. Subsequent maturation of NK precursor cells occurs in the bone marrow and can be distinguished by ordered acquisition of NK cell receptors involved in the activation or inhibition of NK cell effector functions (4). In both mice and humans, the bone marrow microenvironment is critical for the generation of at least the majority NK cells in vivo, as bone marrow ablation or congenital bone marrow defects lead to an abnormal or absent NK cell population (27, 37, 38). However, an infrequent subset of NK cells with a distinct transcriptional program and NK receptor profile was shown to develop in the mouse thymus (39). Analogously, in humans, production of a distinct subset of NKs by lymph nodes during a stress response has been described (40). Recently, another investigator used a flow cytometry-based approach to identify seven maturation stages of NK cells in humans, and cells in the first two stages were detected in only the bone marrow (41). Thus, while traditional NK cell development almost always depends on the bone marrow environment, alternative sites of NK cell development do occur under certain inflammatory states. The production of NK cells is an ongoing process, as turnover of NK cells occurs in about two weeks in both humans and mice (42, 43).
NK cells are present in peripheral organs, even in the absence of inflammation, compatible with their established role in surveillance for infected, malignant, stressed, or damaged cells. The frequency of NK cells is much higher in peripheral organs such as the lung and liver compared to blood or the central immune organs, including the spleen, bone marrow, lymph nodes, and thymus of untreated mice (44). NK cells comprise about 10% of the lymphocytes in the lungs of mice (6), which is consistent with their important role in pulmonary immune responses. More recent studies in mice and humans have identified NK cells in the gut, skin, uterus, pancreas, joints, and brain (45).
As evidence emerges that there are NK cell subsets with different surface receptor expression and functional capacities, it is becoming more apparent that the function of NK cells is influenced by their maturity and environmental milieu. The immune profile varies between different organs and influences the local immune response. Soluble mediators released from DCs, macrophages, and T cells, and cell-cell contact between these cells and NKs influences the effector function(s) of NK cells (44). Subsets of NK cells differ in their natural killing potential and cytokine production. For example, the surface antigen CD56 is an isoform of neural-cell adhesion molecule (NCAM) and has unknown function in NK cells. However, due to initial hypotheses that it mediated cell-cell interactions with target cells, CD56 has been traditionally used and can successfully differentiate subsets of human NK cells. Specifically CD56dim NK cells release cytotoxic granules, whereas CD56bright NK cells mediate an effector function predominantly characterized by cytokine production (5). It is unclear if these subsets reflect different states of maturation or if there is bidirectional transition between these subsets which is controlled by the environmental milieu. Such experimental questions are inherently difficult to answer in man. In mice, however, the maturation state of NK cells can be defined by the co-expression of surface CD27 and CD11b. CD27 is a member of the TNF receptor superfamily, and engagement with its ligand, CD70, induces proliferation and production of IFN-γ by NK cells (46). CD11b (Mac-1/CD18) is a β2 integrin that has been implicated in development, differentiation, and survival of NK cells (47). Hayakawa and Smyth found that CD11bhigh NK cells can be separated into two subsets with categorical tissue distribution and activation thresholds, based upon their expression of CD27 (48). Chiossone, et al. proposed a model of murine NK cell maturation with four sequential stages: CD11blowCD27low → CD11blowCD27high → CD11bhighCD27high → CD11bhighCD27low. The stages of developmental were associated with progressive acquisition of effector functions. CD27+CD11b+ NK cells seem to parallel CD56bright human NK cells which produce high levels of IFNγ, and CD27+CD11b- are more similar to CD56dim NK cells which have high cytolytic function (49). Further studies using mouse models will help to clarify how the local environment influences the development and differentiation of NK cell subsets with unique effector functions.
7. The Role of NK Cells in Pulmonary Immunosurveillance
The lungs represent a unique immunological environment, as their constant exposure to a vast array of exogenous particles requires precise mechanisms to distinguish the harmful from innocuous stimuli. Tight control over the immune response is necessary to both eliminate harmful materials and prevent unnecessary inflammation and damage to normal pulmonary tissue, which may compromise the normal function of the lung (50, 51). NK cells account for 10% of the resident lymphocytes in the lung, suggesting that they may play a role in immunosurveillance for pathogens or transformed cells. Additional NK cells are recruited just hours after the induction of inflammation. In the following sections, we discuss the importance of NK cells in the immunosurveillance of cancer and the response to infection in the lungs. Many parallels are seen in the mechanisms used by NK cells to increase the organism's resistance to tumors and infections (14), and an understanding of their role across multiple immune challenges will be useful for developing therapeutics aimed at modulating NK cell functions.
7.1. Primary lung cancer
Pulmonary malignancies are the leading cause of cancer-related deaths, and the second most frequent type of newly diagnosed cancer in both men and women (52). Unlike other malignancies, such as colorectal cancer, the number of newly diagnosed cases of lung cancer has nearly paralleled the number of lung cancer-related deaths since 1975 (52), which reflects the inadequacy of current treatment options. Thus, new therapeutic strategies to complement surgical resection, irradiation, and chemotherapy are necessary. Modulation of the immune system may be a promising future therapeutic option for lung cancer.
Immunotherapy is already a promising treatment option for certain types of malignancies, such as melanoma, renal cell carcinoma, and ovarian cancer (53). For example, ipilimumab, which is an antibody that block human cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), increased antitumor T-cell response, and in phase 3 clinical trials, improved the overall survival of patients previously treated for metastatic melanoma (54). For patients with metastatic renal cell carcinoma a successful therapy for those with widely metastatic disease involves systemic administration of high-dose IL-2. Although its side effects can be severe a complete and durable pathologic response can be evident in a fraction of patients (55). In a pilot study, the poxviral-based vaccine PANVAC was beneficial to some patients with metastatic breast or ovarian cancer. PANVAC contains transgenes that encode 2 tumor-associated antigens, MUC-1 and CEA, and 3 T-cell costimulatory molecules (56).
The immune system can eliminate transformed cells that express a number of unique surface antigens. These include tumor-associated antigens, which represent mutated or ectopically-expressed proteins that are recognized by T lymphocytes, or stress-associated antigens recognized by cells of the innate immune system (56, 57). Supporting this idea, referred to as “tumor immunosurveillance,” previous studies demonstrate that mice with complete or partial deficiencies in certain immune effector pathways have an earlier onset and increased penetrance of cancer (57, 58). Immunosurveillance is a combined effort from both the innate and adaptive immune systems and NK cells, NKT cells, DCs, and macrophages have all been implicated in the detection and elimination of tumor cells during the initial immune response to developing tumors (57, 59). Tumor-antigen specific T cells mediate a second and adaptive immune response that leads to further elimination of tumor cells. The majority of studies to define the contribution of individual immune components to tumor resistance have used models of carcinogen-induced fibrosarcoma (8), so the mechanisms of immunosurveillance for other malignancies, including lung cancer, are less clear.
Immunosurveillance of the human lung is evidenced by an increased risk of lung cancer in immunocompromised patients. Serraino, P., et al. found that the number of observed cases of lung cancer was greater than the number expected, when patients were immunocompromised due to either human immunodeficiency virus (HIV) or therapeutic immunosuppression following an organ transplantation (60). Surprisingly, previous studies using a primary lung carcinogenesis murine model seem to indicate that the adaptive immune system does not significantly contribute to immunosurveillance for lung cancer. For example, in a urethane-induced model of lung cancer, the latency period, penetrance, and total number of tumors was similar between athymic nude (nu/nu) mice, which lack a thymus and thus thymically-derived T cells (61, 62), and euthymic (nu/+) littermate controls (63). Since the thymus is involved in the development of the majority of T cells (61, 62), these studies suggest that T cells do not significantly contribute to tumor immunosurveillance in the lung. Furthermore, nu/nu and nu/+ mice responded similarly to ionizing radiation treatment of urethane-induced lung cancer (64).
NK cells are implicated in immunosurveillance for tumor cells within the lung. A clear correlation between NK cell activity in the lung and tumor cell clearance from the lung has been reported in both mouse and human studies (35, 59, 65). NK cells activated ex vivo with IL-2 and adoptively transferred by systemic i.v. injection to allogeneic mice were able to localize to the lungs of recipient mice, infiltrate tumors, and significantly reduce tumor size (65). Similarly, localization of NK cells to the lung tumor microenvironment has been visualized in human patients with non-small cell lung cancer (NSCLC). Using an antibody to NKp46, a common marker used to identify NK cell populations, NK cells were detected at the invasive margin of NSCLC tumor samples (59). Interestingly, in another study, the NK cell populations that were found infiltrating NSCLC tumors were CD56bright, which is the NK cell subset associated with cytokine production rather than cytotoxic function (35). At the molecular level, the expression of activation markers on NK cells is significantly different in patients with non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) compared to healthy donors (35, 59). In one study, the percentage of peripheral blood NK cells expressing the activating receptor NKp46 was significantly reduced in patients with NSCLC or SCLC compared to healthy donors (35). Additionally, the percentage of NKp46+ cells was lower in patients with stage IV disease than in patients with stage III disease, and mean expression levels of NKp46 were reduced in correlation with advancing stage (35). This recent study extended beyond NK cell receptor expression to look at NK cell function as well. PBMCs harvested from lung cancer patients and healthy controls were tested ex vivo for their ability to kill tumor target cells and produce IFNγ. PBMCs of NSCLC and SCLC patients had a reduced ability to kill K562 myelogenous leukemia cells in a standard 51Cr release assay that is used to detect cancer cell death. Additionally, the percentage of NK cells stimulated to produce IFNγ, by ex vivo treatment with IL-12 or IL-18, was reduced in PBMC samples from lung cancer patients compared to healthy donors. Similarly, the expression of several NK receptors was altered for tumor-infiltrating NK cells compared to peripheral blood NK cells from the same patient. The overall pattern of receptor expression suggests that the function of tumor-infiltrating NK cells is reduced compared to peripheral blood NK cells. Indeed, CD107a and IFNγ assays showed reduced function as expected (59). Together, these data suggest that NK cell effector functions, including cell-specific lysis and cytokine production, can at least partially dictate the detection of tumors, the rate of growth, and the metastatic potential of lung cancer in humans. Cancer progression, therefore, may depend on local impairment of NK cell activity.
Environmental and genetic components may influence the incidence and outcome of lung cancer. Human population studies indicate that smoking is an independent risk factor for cancer (66). Interestingly, smoking can reduce the natural killing activity of human peripheral blood leukocytes. NK cells of patients who smoked had a reduced ability to kill MM200 melanoma target cells (67). Melanoma cancer cells are typically used in these studies, so further studies are necessary to determine if the effect of smoking on NK cells also inhibits their ability to kill lung cancer cells. Previous research has identified eleven genes associated with increased risk for lung cancer, and the majority of patients have mutations in epidermal growth factor receptor (EGFR) or v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) (68). However, to date, none of these genes appear to be especially important in NK cell function or even immune system function at all. Further studies are necessary to clarify whether or not genetic risk factors are consistent between different types of lung cancer and how they are influenced by environmental risk factors. It will also be enlightening to identify how various cell types, including NK cells, are affected by these environmental and genetic risk factors.
7.2. Metastatic cancer to the lung
The number of studies using a primary model of carcinogenesis to evaluate immune pathways involved in the control of lung cancer is limited. However, some information can be gleaned from studies using tumors metastatic to the lung. In mice, for example, in vivo exposure to cigarette smoke increases the number of metastatic melanoma tumors in the lung (69). The contributions of the adaptive immune system and of NK cells in cancer immunosurveillance were tested using Rag2-/- and Rag2-/-γC-/- mice in this model. Rag2-/- mice had low tumor burdens in mice that were not exposed to cigarette smoke, and smoking exacerbated the tumor burden, indicating that even in the absence of T and B lymphocytes, smoking can further impair lung immunosurveillance mechanisms. Rag2-/-γC-/- mice displayed high tumor burdens, even without exposure to cigarette smoke, suggesting that NK cells limit the number of metastatic melanoma lung tumors. Adoptive transfer experiments were used to further investigate the effect of cigarette smoke on NK cell function. Donors were exposed to cigarette smoke or sham, and isolated NK cells were transferred to recipients challenged concurrently with melanoma. Tumor burden was measured in the recipients and was higher in recipients receiving NK cells from cigarette smoke-exposed donors compared to sham-exposed donors (69). Whether or not the effect of smoking on immunosurveillance mechanisms is similar in primary lung cancer is unclear and warrants further investigation. Previous studies indicate that the cellular components involved in tumor immunosurveillance are partially dependent upon the origin of the tumor, the local tumor environment, the cause of malignant transformation, and the aggressiveness of the tumor (57). The efficacy of immunomodulation in the lung is likely to be complicated by the strong tolerogenic environment of the lung (70). Further studies utilizing mouse models of primary lung cancer and analysis of tumor samples collected from lung cancer patients will be a crucial step in understanding how immunosurveillance mechanisms differ between pulmonary malignancies and those, such as fibrosarcoma, that arise in other systemic sites.
7.3. The role of NK cells in pulmonary infection
While much of our knowledge regarding the physiology of NK cells stems from tumor biology, further work has identified the role of this cell population in a multitude of pulmonary inflammatory responses. In the next sections, the contribution of NK cells to immune function in the lung following certain viral and bacterial infections, including tuberculosis (TB), will be discussed. Some elements of NK cell responses are conserved following exposure to multiple types of infection. Other activities which differ may be the result of a tailored response to the pathogen, or alternatively, may be the consequence of functional impairment induced by the pathogen.
7.3.1. Viral infections
As previously mentioned, NK cells likely evolved into an independent cell population due to their role in viral clearance. Individuals with NK cells have a selective advantage in the detection of viruses which are capable of reducing MHC I expression to avoid detection by T cells (16). Thus, patients with genetic deficiencies that lead to the loss of NK cell function suffer from recurrent viral infections (71, 72). In 2006, a novel primary human immunodeficiency, predominantly characterized by an NK cell deficit, was identified in four children belonging to a large Irish, nomadic, inbred population. One child developed lymphoproliferative disorder due to lytic activation of the Epstein-Barr virus. Two other children suffered from severe respiratory illnesses likely caused by viruses. A genome-wide scan, guided by the assumption that the emergence of this disorder in an inbred population represents recessive inheritance of the underlying genetic defect, led to the identification of a 12 Mb region on chromosome 8p1 1.23-q1 1.21 that was not previously associated with NK cell development or function. Further fine mapping and molecular studies were used to identify the underlying genetic cause to be a hypomorphic variant of minichromosome maintenance-deficient 4 (MCM4) (73, 74). These studies provide substantial support for the hypothesis that genetic components are involved in NK cell-mediated resistance to multiple pathologies, including viral infections (75). While the effect of NK cells to control some viruses, such as lymphocytic choriomeningitis virus (LCMV), appears to be at least partially due to modulation of T cell function by NK cells (76), there is evidence to suggest that influenza viral burden may be limited by a direct effect of NK cells.
Infection by influenza viruses are a current medical burden and also present a serious future pandemic risk. NK cells are recruited within the first few days after influenza virus infection (77) and display increased killing activity and IFNγ production at the site of infection (78). Using in vivo mouse and hamster models, NK cell depletion with anti-asialo GM1 caused increased morbity and mortality after influenza infection (79). These physiological observations are supported by molecular studies which indicate that haemagglutinins (HAs) present on virus-infected cells are recognized by the activating NK receptors NKp46 and NKp44 in humans and NKp46 in mice (80, 81). Furthermore, expression level of HAs by different influenza viruses is likely to affect the potential for NK cell activation. In addition to direct NCR-mediated detection of infected cells, NK cells also respond to stress signals from influenza-infected monocytes and dendritic cells. The latter effect is through both a NKG2D-mediated contact-dependent mechanism as well as through a response to cytokines such as IFNα and IL-12, which are released from infected cells. Several effector functions of NK cells have been shown to limit influenza infection, including target cell killing, IFNγ production, and ADCC (6).
7.3.2. Bacterial infections
Traditionally, NK cells are thought to contribute to tumor and virus resistance, but recent work has identified functional roles during bacterial infections as well. Streptococcus pneumoniae can survive in the human respiratory tract as part of the commensal flora, however, it is also responsible of severe infections including pneumoniae, meningitis, and sepsis. It is the leading cause of death of children in the world (82). While the role of NK cells in bacterial infection is less well defined than in viral infections, NK cells are recruited to the lung 6 hours post-infection (hpi), indicating that they may play a role in limiting early bacterial dissemination. Mice with a targeted disruption of NCR1 (NKp46) had higher bacterial loads at 6 hpi. The impairment of viral clearance was likely due to suppressed activation of NK cells and reduced IFNγ production in the lungs of NCR1-deficient mice. NCR1 was not identified as a direct sensor of S. pneumoniae. Instead, macrophages and DCs were shown to mediate the activation of NK cells by S. pneumoniae (83). NK cell function is also modulated by pertussis toxin (PT) of Bordetella pertussis bacteria, further linking NK cells to bacterial clearance (84).
7.3.3. Mycobacterial infection
Mycobacterium tuberculosis (TB) is present in one-third of the human population (82). While TB maintains a chronic latent state in most individuals, active disease can become apparent and accounts for nearly 2 million deaths each year (82). Several investigators have demonstrated that TB is associated with altered NK cell function. NKp46 and NKG2D mediate NK cell function by interacting with the stress-related glycoproteins vimentin and ULBP-1 expressed on TB infected monocytes and macrophages (85, 86). Additionally, NKp44 can bind directly to the mycobacterial cell wall through an as of yet undefined ligand. MICA expression is also upregulated in TB-infected cells, suggesting that this ligand may also influence viral pathogenesis through the NKG2D NK cell receptor (6). Interestingly, NK cells from the peripheral blood of patients with active TB have decreased cytotoxic function (87). This perhaps provides evidence that TB influences NK cell responses to cause an active infection. So far, studies using in vivo exposure of mice have not corroborated an effect of NK cells on TB burden (6). It is possible that NK cell responses help mediate early innate responses but that redundant mechanisms, likely within the adaptive immune system, compensate for NK cell deficiency during the later stages of TB infection, at least in the mouse.
8. Conclusion and Perspectives
NK cells represent a unique class of immune cells. They are derived from lymphocyte precursors, yet clearly function in an innate capacity. Our current knowledge is based upon studies using human patient samples, predominately NK cells isolated from human peripheral blood, and from studies of wild-type and transgenic mice. While species differences are apparent, the general principles of NK cell biology are conserved between mouse and man. Their activation is a rapid process, dictated by a combination of signaling through several germ-line encoded receptors that bind to extracellular ligands. However, it is now apparent that NK cell function is modulated by the local tissue environment. Therefore, we have focused our attention here specifically on NK cell functions within the lung. Modern techniques such as molecular imaging and detection of NK cell receptor expression at the cellular level will undoubtedly further our understanding of the mechanisms regulating NK cell effector functions. Armed with this knowledge, NK cell-based therapeutics may be a promising avenue for the treatment of cancer and inflammation. The short life-span and continuous production of NK cells means that therapeutic strategies could be used to dramatically alter the immune response in a quick and reversible way.
References
- 1.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5(2):117–21. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 2.Pross HF, Jondal M. Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol. 1975;21(2):226–35. [PMC free article] [PubMed] [Google Scholar]
- 3.West WH, Cannon GB, Kay HD, Bonnard GD, Herberman RB. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977;118(1):355–61. [PubMed] [Google Scholar]
- 4.Yokoyama WM. Natural Killer Cells. In: Paul WE, editor. Fundamental Immunology. 2008. [Google Scholar]
- 5.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 6.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128(2):151–63. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesslein DG, Lanier LL. Transcriptional control of natural killer cell development and function. Adv Immunol. 2011;109:45–85. doi: 10.1016/B978-0-12-387664-5.00002-9. [DOI] [PubMed] [Google Scholar]
- 8.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 9.Meazza R, Azzarone B, Orengo AM, Ferrini S. Role of common-gamma chain cytokines in NK cell development and function: perspectives for immunotherapy. J Biomed Biotechnol. 2011;2011:861920. doi: 10.1155/2011/861920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recher M, Berglund LJ, Avery DT, Cowan MJ, Gennery AR, Smart J, Peake J, Wong M, Pai SY, Baxi S, Walter JE, Palendira U, Tangye GA, Rice M, Brothers S, Al-Herz W, Oettgen H, Eibel H, Puck JM, Cattaneo F, Ziegler JB, Giliani S, Tangye SG, Notarangelo LD. IL-21 is the primary common gamma chain-binding cytokine required for human B-cell differentiation in vivo. Blood. 2011;118(26):6824–35. doi: 10.1182/blood-2011-06-362533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 12.Cherrier M, Ohnmacht C, Cording S, Eberl G. Development and function of intestinal innate lymphoid cells. Curr Opin Immunol. 2012;24(3):277–83. doi: 10.1016/j.coi.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16(5):626–33. doi: 10.1016/j.coi.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3(4):304–16. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi DA, Cahan P, Gao J, Ferris ST, Poursine-Laurent J, Graubert TA, Yokoyama WM. Structural variation of the mouse natural killer gene complex. Genes Immun. 2010;11(8):637–48. doi: 10.1038/gene.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195(3):346–55. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 17.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 18.Ljunggren HG, Sturmhofel K, Wolpert E, Hammerling GJ, Karre K. Transfection of beta 2-microglobulin restores IFN-mediated protection from natural killer cell lysis in YAC-1 lymphoma variants. J Immunol. 1990;145(1):380–6. [PubMed] [Google Scholar]
- 19.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 20.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89(2):216–24. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 21.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182(8):4572–80. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 23.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs A, Colonna M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16(5):359–66. doi: 10.1016/j.semcancer.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26(6):798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7(9):703–14. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 27.Puzanov IJ, Bennett M, Kumar V. IL-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. J Immunol. 1996;157(10):4282–5. [PubMed] [Google Scholar]
- 28.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–9. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hook GR, Greenwood MA, Barba D, Ikejiri B, Chen SN, Oldfield EH, Weber RJ, Muul LM. Morphology of interleukin-2-stimulated human peripheral blood mononuclear effector cells killing glioma-derived tumor cells in vitro. J Natl Cancer Inst. 1988;80(3):171–7. doi: 10.1093/jnci/80.3.171. [DOI] [PubMed] [Google Scholar]
- 30.Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6(3):e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004;101(27):10116–21. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart OM, Athie-Morales V, O'Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175(3):1636–42. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 33.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, Murphy KM, Colonna M. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206(13):2967–76. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komiyama A, Kawai H, Yabuhara A, Yanagisawa M, Miyagawa Y, Ota M, Hasekura H, Akabane T. Natural killer cell immunodeficiency in siblings: defective killing in the absence of natural killer cytotoxic factor activity in natural killer and lymphokine-activated killer cytotoxicities. Pediatrics. 1990;85(3):323–30. [PubMed] [Google Scholar]
- 35.Al Omar SY, Marshall E, Middleton D, Christmas SE. Increased killer immunoglobulin-like receptor expression and functional defects in natural killer cells in lung cancer. Immunology. 2011;133(1):94–104. doi: 10.1111/j.1365-2567.2011.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M, Minard-Colin V, Poirier-Colame V, Chaba K, Flament C, Baud V, Authier H, Kerdine-Romer S, Pallardy M, Cremer I, Peaudecerf L, Rocha B, Valteau-Couanet D, Gutierrez JC, Nunes JA, Commo F, Bonvalot S, Ibrahim N, Terrier P, Opolon P, Bottino C, Moretta A, Tavernier J, Rihet P, Coindre JM, Blay JY, Isambert N, Emile JF, Vivier E, Lecesne A, Kroemer G, Zitvogel L. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17(6):700–7. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 37.Ito A, Kataoka TR, Kim DK, Koma Y, Lee YM, Kitamura Y. Inhibitory effect on natural killer activity of microphthalmia transcription factor encoded by the mutant mi allele of mice. Blood. 2001;97(7):2075–83. doi: 10.1182/blood.v97.7.2075. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Ben-Ezra J, Bennett M, Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunol. 1979;123(4):1832–8. [PubMed] [Google Scholar]
- 39.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, Di Santo JP. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7(11):1217–24. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 40.Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, Hughes TL, Marburger TB, Sung J, Baiocchi RA, Guimond M, Caligiuri MA. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22(3):295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Eissens DN, Spanholtz J, van der Meer A, van Cranenbroek B, Dolstra H, Kwekkeboom J, Preijers FW, Joosten I. Defining early human NK cell developmental stages in primary and secondary lymphoid tissues. PLoS One. 2012;7(2):e30930. doi: 10.1371/journal.pone.0030930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, Griffin GE, Taylor GP, Tough DF, Beverley PC, Macallan DC. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–65. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172(2):864–70. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 44.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11(10):658–71. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeda K, Oshima H, Hayakawa Y, Akiba H, Atsuta M, Kobata T, Kobayashi K, Ito M, Yagita H, Okumura K. CD27-mediated activation of murine NK cells. J Immunol. 2000;164(4):1741–5. doi: 10.4049/jimmunol.164.4.1741. [DOI] [PubMed] [Google Scholar]
- 47.Zhang T, Liu S, Yang P, Han C, Wang J, Liu J, Han Y, Yu Y, Cao X. Fibronectin maintains survival of mouse natural killer (NK) cells via CD11b/Src/beta-catenin pathway. Blood. 2009;114(19):4081–8. doi: 10.1182/blood-2009-05-219881. [DOI] [PubMed] [Google Scholar]
- 48.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 49.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113(22):5488–96. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 50.Downey GP, Dong Q, Kruger J, Dedhar S, Cherapanov V. Regulation of neutrophil activation in acute lung injury. Chest. 1999;116(1 Suppl):46S–54S. [PubMed] [Google Scholar]
- 51.Li F, Zhu H, Sun R, Wei H, Tian Z. Natural killer cells are involved in acute lung immune injury caused by respiratory syncytial virus infection. J Virol. 2012;86(4):2251–8. doi: 10.1128/JVI.06209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 53.Murala S, Alli V, Kreisel D, Gelman AE, Krupnick AS. Current status of immunotherapy for the treatment of lung cancer. J Thorac Dis. 2010;2(4):237–44. doi: 10.3978/j.issn.2072-1439.2010.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23(1):133–41. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 56.Mohebtash M, Tsang KY, Madan RA, Huen NY, Poole DJ, Jochems C, Jones J, Ferrara T, Heery CR, Arlen PM, Steinberg SM, Pazdur M, Rauckhorst M, Jones EC, Dahut WL, Schlom J, Gulley JL. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2011;17(22):7164–73. doi: 10.1158/1078-0432.CCR-11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 58.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 59.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, Fridman WH, Sautes-Fridman C, Cremer I. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71(16):5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 60.Serraino D, Piselli P, Busnach G, Burra P, Citterio F, Arbustini E, Baccarani U, De Juli E, Pozzetto U, Bellelli S, Polesel J, Pradier C, Dal Maso L, Angeletti C, Carrieri MP, Rezza G, Franceschi S. Risk of cancer following immunosuppression in organ transplant recipients and in HIV-positive individuals in southern Europe. Eur J Cancer. 2007;43(14):2117–23. doi: 10.1016/j.ejca.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 61.Ikehara S, Pahwa RN, Fernandes G, Hansen CT, Good RA. Functional T cells in athymic nude mice. Proc Natl Acad Sci U S A. 1984;81(3):886–8. doi: 10.1073/pnas.81.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunig T, Bevan MJ. Specificity of cytotoxic T cells from athymic mice. J Exp Med. 1980;152(3):688–702. doi: 10.1084/jem.152.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi S, Otsu H, Noda Y. Comparison of lung tumorigenesis induced by urethan in athymic nude mice and euthymic littermates. Jikken Dobutsu. 1994;43(3):351–6. doi: 10.1538/expanim1978.43.3_351. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi S, Otsu H, Noda Y, Ogiu T. Comparison of dose-dependent enhancing effects of gamma-ray irradiation on urethan-induced lung tumorigenesis in athymic nude (nu/nu) mice ac (nu/+) littermates. J Cancer Res Clin Oncol. 1996;122(4):231–6. doi: 10.1007/BF01209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Q, Goding SR, Hokland ME, Basse PH. Antitumor activity of NK cells. Immunol Res. 2006;36(1-3):13–25. doi: 10.1385/IR:36:1:13. [DOI] [PubMed] [Google Scholar]
- 66.Shaw HM, Milton GW, McCarthy WH, Farago GA, Dilworth P. Effect of smoking on the recurrence of malignant melanoma. Med J Aust. 1979;1(6):208–9. [PubMed] [Google Scholar]
- 67.Ferson M, Edwards A, Lind A, Milton GW, Hersey P. Low natural killer-cell activity and immunoglobulin levels associated with smoking in human subjects. Int J Cancer. 1979;23(5):603–9. doi: 10.1002/ijc.2910230504. [DOI] [PubMed] [Google Scholar]
- 68.Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat Med. 2012;18(3):349–51. doi: 10.1038/nm.2697. [DOI] [PubMed] [Google Scholar]
- 69.Lu LM, Zavitz CC, Chen B, Kianpour S, Wan Y, Stampfli MR. Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J Immunol. 2007;178(2):936–43. doi: 10.4049/jimmunol.178.2.936. [DOI] [PubMed] [Google Scholar]
- 70.Chen L, He Z, Qin L, Li Q, Shi X, Zhao S, Zhong N, Chen X. Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PLoS One. 2011;6(9):e24407. doi: 10.1371/journal.pone.0024407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–58. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 72.Orange JS, Brodeur SR, Jain A, Bonilla FA, Schneider LC, Kretschmer R, Nurko S, Rasmussen WL, Kohler JR, Gellis SE, Ferguson BM, Strominger JL, Zonana J, Ramesh N, Ballas ZK, Geha RS. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109(11):1501–9. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, Picard C, Trouillet C, Eidenschenk C, Aoufouchi S, Alcais A, Smith O, Geissmann F, Feighery C, Abel L, Smogorzewska A, Stillman B, Vivier E, Casanova JL, Jouanguy E. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122(3):821–32. doi: 10.1172/JCI61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, Costigan C, Clark AJ, Metherell LA. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122(3):814–20. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eidenschenk C, Dunne J, Jouanguy E, Fourlinnie C, Gineau L, Bacq D, McMahon C, Smith O, Casanova JL, Abel L, Feighery C. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet. 2006;78(4):721–7. doi: 10.1086/503269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481(7381):394–8. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein-Streilein J, Guffee J, Fan W. Locally and systemically derived natural killer cells participate in defense against intranasally inoculated influenza virus. Reg Immunol. 1988;1(2):100–5. [PubMed] [Google Scholar]
- 78.Ennis FA, Meager A, Beare AS, Qi YH, Riley D, Schwarz G, Schild GC, Rook AH. Interferon induction and increased natural killer-cell activity in influenza infections in man. Lancet. 1981;2(8252):891–3. doi: 10.1016/s0140-6736(81)91390-8. [DOI] [PubMed] [Google Scholar]
- 79.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136(4):1435–41. [PubMed] [Google Scholar]
- 80.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–60. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 81.Ho JW, Hershkovitz O, Peiris M, Zilka A, Bar-Ilan A, Nal B, Chu K, Kudelko M, Kam YW, Achdout H, Mandelboim M, Altmeyer R, Mandelboim O, Bruzzone R, Porgador A. H5-type influenza virus hemagglutinin is functionally recognized by the natural killer-activating receptor NKp44. J Virol. 2008;82(4):2028–32. doi: 10.1128/JVI.02065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.WHO. Acute Respiratory Infections. 2009 Update September 2009. [Google Scholar]
- 83.Elhaik-Goldman S, Kafka D, Yossef R, Hadad U, Elkabets M, Vallon-Eberhard A, Hulihel L, Jung S, Ghadially H, Braiman A, Apte RN, Mandelboim O, Dagan R, Mizrachi-Nebenzahl Y, Porgador A. The natural cytotoxicity receptor 1 contribution to early clearance of Streptococcus pneumoniae and to natural killer-macrophage cross talk. PLoS One. 2011;6(8):e23472. doi: 10.1371/journal.pone.0023472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ayala VI, Teijaro JR, Farber DL, Dorsey SG, Carbonetti NH. Bordetella pertussis infection exacerbates influenza virus infection through pertussis toxin-mediated suppression of innate immunity. PLoS One. 2011;6(4):e19016. doi: 10.1371/journal.pone.0019016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vankayalapati R, Wizel B, Weis SE, Safi H, Lakey DL, Mandelboim O, Samten B, Porgador A, Barnes PF. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168(7):3451–7. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 86.Garg A, Barnes PF, Porgador A, Roy S, Wu S, Nanda JS, Griffith DE, Girard WM, Rawal N, Shetty S, Vankayalapati R. Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J Immunol. 2006;177(9):6192–8. doi: 10.4049/jimmunol.177.9.6192. [DOI] [PubMed] [Google Scholar]
- 87.Schierloh P, Yokobori N, Aleman M, Musella RM, Beigier-Bompadre M, Saab MA, Alves L, Abbate E, de la Barrera SS, Sasiain MC. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J Immunol. 2005;175(10):6852–60. doi: 10.4049/jimmunol.175.10.6852. [DOI] [PubMed] [Google Scholar]


