Abstract
Background
There is conflicting evidence regarding the benefit of post-mastectomy radiation therapy (PMRT) for pathologic stage T3N0M0 breast cancers. We analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database to investigate the benefit of PMRT in these patients.
Methods
We queried the SEER database for T3N0M0 breast cancer patients diagnosed from 2000–2010 who underwent modified radical mastectomy. We excluded males, patients with unknown radiation timing/type, other primary tumors, or survival <6 months. 2525 patients were included in this analysis. We performed univariate and multivariate statistical analysis using Chi-squared tests, log rank test, and Cox proportional hazards regression. Primary endpoints were overall survival (OS) and breast cancer-specific survival (CSS).
Results
Of the 2525 patients identified, 1063 received PMRT. The median follow-up was 56 months (range: 6–131).
On univariate analysis, PMRT improved OS (76.5% vs. 61.8%, p<0.01) and CSS (85.0% vs. 82.4%, p<0.01) at 8 years. The use of PMRT remained significant on multivariate analysis: PMRT improved OS (HR 0.63, p<0.001) and CSS (HR 0.77, p=0.045). Low tumor grade (p<0.01) and marital status "married" (p=0.01) also predicted for improved CSS on multivariate analysis.
Conclusion(s)
PMRT was associated with significant improvements in both CSS and OS in patients with T3N0M0 breast cancers treated with modified radical mastectomy from 2000 to 2010. PMRT should be strongly considered in T3N0M0 patients.
Keywords: Post-mastectomy, radiation therapy, T3N0, breast cancer
Introduction
Although there are nearly 235,000 new breast cancers diagnosed in the United States each year, patients with T3N0M0 breast cancers account for less than 2% of all breast cancer cases.1, 2 The American Joint Committee on Cancer (AJCC) 7th edition staging manual defines T3 tumors as those which have a greatest dimension of greater than 5cm, N0 as no histologic identification of lymph node metastasis, and M0 as no clinical or radiographic evidence of distant metastasis.3 Patients with T3N0M0 tumors may represent a unique subgroup whose tumor biology is different.4 Based on published data, T3 tumors have a propensity for regional nodal spread; a nomogram predicting the likelihood of lymph node metastasis demonstrates an 88% risk of positive axillary sentinel nodes in these patients.4 Thus, local and regional recurrence in these patients could be high despite the lack of nodal disease at initial presentation, necessitating aggressive local and regional therapy.
Post-mastectomy radiation therapy (PMRT) in patients with stage II or III breast cancer has been shown to improve local-regional failure (LRF) and overall survival (OS) in the Danish Breast Cancer Cooperative Group 82b and 82c trials, which included T3N0M0 patients.5, 6 However, the role of PMRT in T3N0M0 patients remains controversial in light of the fact that the Danish trial included few patients with T3N0M0 disease and the number of axillary nodes dissected was small (median=7). For this reason, we undertook a Surveillance, Epidemiology, and End Results (SEER) analysis to investigate the benefit of PMRT in patients with T3N0M0 breast cancer in a contemporary patient population.
Methods and Materials
A National Cancer Institute SEER database query was performed after completion of a SEER Research Data Agreement. SEER*Stat software, version 8.0.4 was used to access the SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub [1973–2010 varying] database using SEER*Stat’s client-server mode. Search criteria identified women with AJCC stage T3N0M0 breast cancer (C50.0–C50.9) who underwent modified radical mastectomy diagnosed between 2000 and 2010. Patients were excluded if they were male, if they had unknown radiation sequence, unknown type of radiation, if this was not their first cancer, and if death occurred within 6 months of diagnosis.
The primary endpoints were OS and cause-specific survival (CSS). OS was defined as the time from diagnosis to any-cause mortality, with living patients censored at last follow-up date. CSS was defined as the time from diagnosis to death from breast cancer, with patients censored at the time of last follow-up or death from another cause.
We categorized patients according to various patient and tumor factors such as age, ethnicity, tumor grade, and histology. We examined the relationship between these factors and the use of radiation therapy using Chi-squared tests. We then evaluated the association between radiation therapy and survival (both overall and cause-specific) by generating survival curves using the methods of Kaplan and Meier and testing for associations using the log-rank test.7 Multivariable Cox proportional hazards regression was then used to find the covariate-adjusted effect of radiation therapy on OS and CSS.7 Covariates examined, with SEER variable name given in parenthesis, included: year of diagnosis (year of diagnosis), age (age at diagnosis), tumor grade (grade), ethnicity (race recode [White, Black, other]), estrogen receptor (ER) status (ER status recode breast cancer [1990+]), progesterone receptor (PR) status (PR status recode breast cancer [1990+]), SEER Registry subgroup (SEER registry), number of axillary lymph nodes examined (regional nodes examined [1988+]), marital status (marital status at diagnosis), level of education (% < high school education 2000), grouping by patient’s first primary (sequence number), median income (median household income [in tens] 2000), and histology (Histology ICD-O-2). Values were categorized for the variables age (15–34, 35–49, 50–64, 65+), and grade (I–II, III–IV, unknown). Marital status was defined as married for “married (including common law),” and not married for “single,” “divorced,” “separated,” or “widowed.” Median income and education of at least high school (HS) level were categorized by quintile. We tested for violations of the proportional hazards assumption using Schoenfeld residuals,8 and used time-varying covariates7 when violations of the assumptions were present.
Based on findings in the multivariate analysis, we analyzed the differences between the effect of radiation in high grade vs. low grade tumors using a Cox proportional hazards regression with an interaction between radiation and grade. We allowed for time-varying effects of grade to account for violations of the proportional hazards assumption.
This work was performed in accordance with approval from our institutional review board. All statistical analysis was performed using Stata version 12.1 (StataCorp, College Station TX).
Results
A total of 2,525 patients were identified who met our selection criteria, of whom 42.1% received PMRT. The distribution of patients according to clinical and demographic features is detailed in Table 1. 73% were Caucasian and 18% were African American. The median follow-up was 56 months (range: 6–131). The median number of lymph nodes examined was 12 (range: 0–50). The primary was left-sided in 50.5% of cases. Patients receiving radiation were younger (p<0.01), more commonly had ER+ and PR+ tumors (p<0.01), had a different geographic distribution (p<0.01), were more likely to be married (p<0.01), from a county with higher education (p<0.01) and higher income (p<0.01), and have lobular histology (p<0.01) compared to those not receiving radiation. There were no differences between the groups in terms of tumor grade, ethnicity, number of nodes examined, or year of diagnosis.
Table 1.
Supplement: Patient Descriptives
| No RT | PMRT | ||
|---|---|---|---|
| Variable | N (%) | N (%) | p value |
| Year of Diagnosis | 0.057 | ||
| 2000–2002 | 525 (35.9) | 331 (31.1) | |
| 2003–2005 | 384 (26.3) | 311 (29.3) | |
| 2006–2008 | 387 (26.5) | 293 (27.6) | |
| 2009–2010 | 166 (11.4) | 128 (12.0) | |
| Registry | <0.001 | ||
| Alaska Natives | 1 (0.1) | 1 (0.1) | |
| Atlanta | 45 (3.1) | 44 (4.1) | |
| California | 313 (21.4) | 207 (19.5) | |
| Connecticut | 53 (3.6) | 36 (3.4) | |
| Detroit | 100 (6.8) | 111 (10.4) | |
| Greater Georgia | 121 (8.3) | 82 (7.7) | |
| Hawaii | 27 (1.8) | 21 (2.0) | |
| Iowa | 49 (3.4) | 56 (5.3) | |
| Kentucky | 63 (4.3) | 43 (4.0) | |
| Los Angeles | 188 (12.9) | 79 (7.4) | |
| Louisiana | 138 (9.4) | 84 (7.9) | |
| New Jersey | 189 (12.9) | 100 (9.4) | |
| New Mexico | 22 (1.5) | 14 (1.3) | |
| Rural Georgia | 3 (0.2) | 4 (0.4) | |
| San Francisco-Oakland | 55 (3.8) | 61 (5.7) | |
| San Jose-Monterey | 26 (1.8) | 38 (3.6) | |
| Seattle | 32 (2.2) | 53 (5.0) | |
| Utah | 37 (2.5) | 29 (2.7) | |
| Number of Nodes Examined | 0.683 | ||
| 0–5 | 304 (20.8) | 207 (19.5) | |
| 6–10 | 328 (22.4) | 255 (24.0) | |
| 11–50 | 819 (56.0) | 595 (56.0) | |
| Unknown | 11 (0.8) | 6 (0.6) | |
| At Least HS Education (quintiles) | <0.001 | ||
| 1 (Highest educated) | 182 (12.4) | 216 (20.3) | |
| 2 | 235 (16.1) | 195 (18.3) | |
| 3 | 290 (19.8) | 232 (21.8) | |
| 4 | 395 (27.0) | 231 (21.7) | |
| 5 (Least educated) | 360 (24.6) | 189 (17.8) | |
| Median Income (quintiles) | 0.002 | ||
| 1 (Lowest income) | 387 (26.5) | 260 (24.5) | |
| 2 | 349 (23.9) | 195 (18.3) | |
| 3 | 263 (18.0) | 212 (19.9) | |
| 4 | 215 (14.7) | 171 (16.1) | |
| 5 (Highest income) | 248 (17.0) | 225 (21.2) |
ER: Estrogen Receptor, PR: Progesterone Receptor, HS: High School
On univariate analysis, OS at 96 months was improved in patients who received PMRT: 76.5% vs. 61.8% (p<0.01). CSS at 96 months was also improved in patients who received PMRT: 85.0% vs. 82.4% (p<0.01). Kaplan-Meier curves for OS and CSS are shown in Figures 1 and 2, respectively. When cause of death was analyzed by age group, death attributable to breast-cancer accounted for 90.5% of deaths in patients 15–34 years old, 76.2% of deaths in patients 35–49 years old, 62.2% of deaths in patients 50–64 years old, and 34.9% of deaths in patients 65 years and older.
Figure 1.
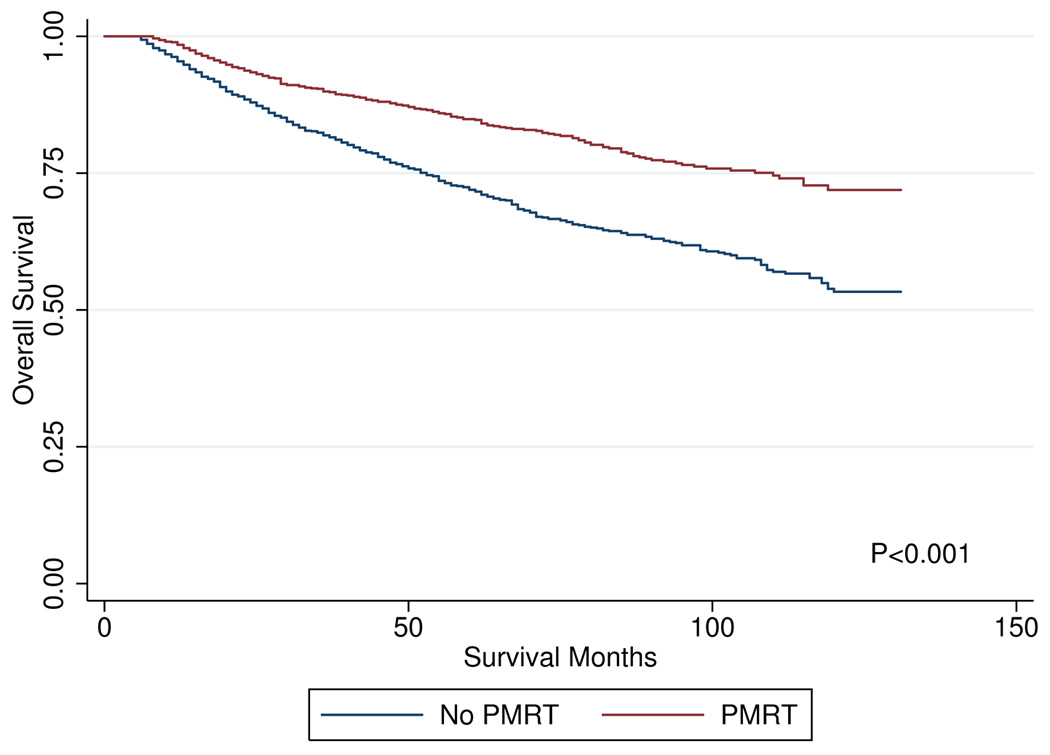
overall survival curves by post-mastectomy radiation
Figure 2.
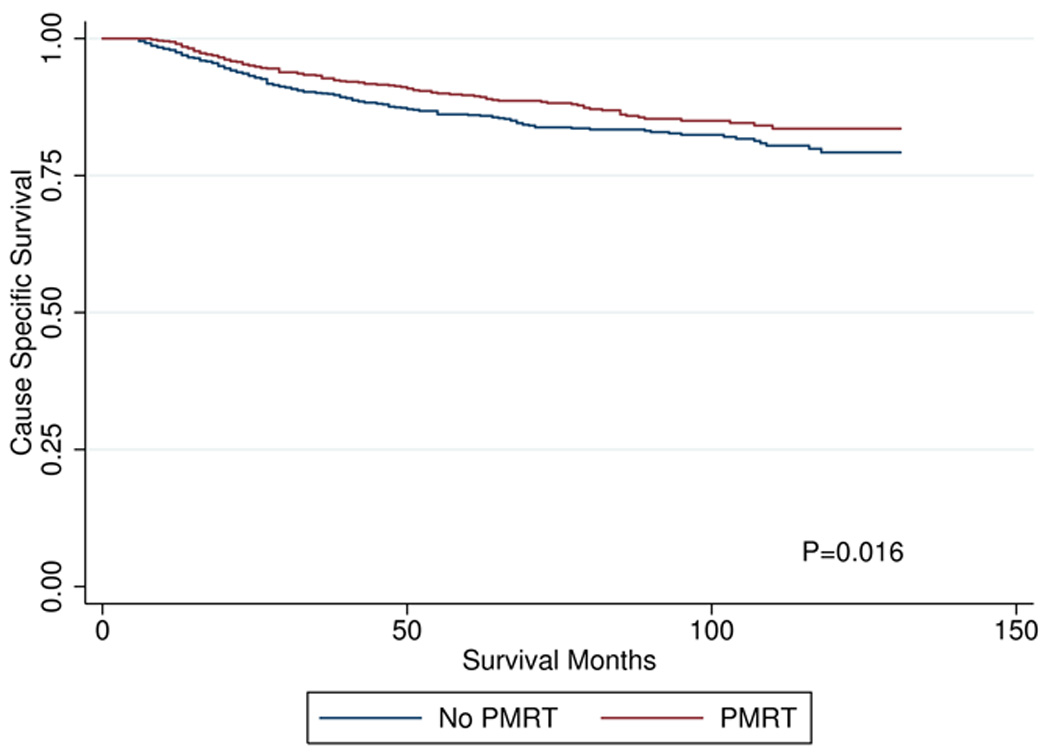
cause specific survival by post-mastectomy radiation
Multivariate models controlling for year of diagnosis, age, grade, ethnicity, ER and PR status, SEER registry location subgroup, number of nodes examined, education, income, and histology demonstrated that PMRT remained a significant predictor of OS (HR 0.63, p<0.001) and CSS (HR 0.77, p=0.045). In addition to PMRT, low tumor grade (p<0.01) and marital status of "married" (p=0.01) were significant predictors of improved CSS. Multivariate analyses are shown in Table 2. We found violations of the proportional hazards assumption for both OS and CSS (p=0.001 and p=0.003, respectively), driven by non-proportional effects of ER+ status. After including ER+ as a time-varying covariate, the proportional hazards assumption was satisfied in both models (p=0.27, p=0.69). The effects of radiation were basically unchanged (OS: HR=0.78, p=0.049; CSS: HR=0.64, p<0.001), leading us to conclude that the main results were robust.
Table 2.
Supplement: Multivariate Cox Model
| Overall Survival | Cause-Specific Survival | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value |
|
| Variable | ||||
| Year of Diagnosis | ||||
| 2000 | 1† | 1† | ||
| 2001 | 1.04 (0.79–1.37) | 0.79 | 0.98 (0.65–1.49) | 0.94 |
| 2002 | 1.16 (0.87–1.55) | 0.31 | 1.19 (0.79–1.80) | 0.41 |
| 2003 | 1.16 (0.86–1.57) | 0.32 | 0.98 (0.63–1.53) | 0.92 |
| 2004 | 0.73 (0.51–1.04) | 0.08 | 0.77 (0.47–1.26) | 0.30 |
| 2005 | 1.12 (0.79–1.59) | 0.52 | 1.21 (0.75–1.95) | 0.43 |
| 2006 | 1.09 (0.77–1.55) | 0.62 | 1.06 (0.66–1.72) | 0.81 |
| 2007 | 1.22 (0.84–1.78) | 0.29 | 1.15 (0.68–1.94) | 0.59 |
| 2008 | 1.12 (0.70–1.82) | 0.48 | 0.92 (0.48–1.78) | 0.82 |
| 2009 | 1.40 (0.79–2.46) | 0.25 | 1.11 (0.51–2.43) | 0.79 |
| 2010 | 0.84 (0.11–6.16) | 0.87 | 1.38 (0.18–10.37) | 0.76 |
| Registry | ||||
| Utah | 1† | 1† | ||
| Atlanta | 1.11 (0.53–2.32) | 0.79 | 0.87 (0.39–3.03) | 0.87 |
| California | 0.91 (0.46–1.81) | 0.80 | 0.94 (0.37–2.53) | 0.94 |
| Connecticut | 0.84 (0.37–1.92) | 0.68 | 0.47 (0.18–2.20) | 0.47 |
| Detroit | 1.34 (0.66–2.73) | 0.42 | 0.44 (0.54–4.02) | 0.44 |
| Greater Georgia | 1.28 (0.63–2.59) | 0.50 | 0.63 (0.47–3.48) | 0.63 |
| Hawaii | 1.62 (0.65–4.04) | 0.30 | 0.97 (0.27–3.91) | 0.97 |
| Iowa | 1.31 (0.67–2.57) | 0.43 | 0.53 (0.53–3.51) | 0.53 |
| Kentucky | 1.14 (0.54–2.40) | 0.73 | 0.94 (0.36–3.05) | 0.94 |
| Los Angeles | 0.90 (0.40–2.04) | 0.80 | 0.94 (0.30–3.06) | 0.94 |
| Louisiana | 1.09 (0.54–2.21) | 0.80 | 0.92 (0.39–2.86) | 0.92 |
| New Jersey | 0.97 (0.48–1.95) | 0.93 | 0.51 (0.26–1.97) | 0.51 |
| New Mexico | 1.80 (0.77–4.16) | 0.17 | 0.30 (0.58–5.94) | 0.30 |
| Rural Georgia | 0.51 (0.06–4.02) | 0.52 | 0.98 (0.11–8.64) | 0.98 |
| San Francisco-Oakland | 1.14 (0.52–2.48) | 0.75 | 0.87 (0.49–4.28) | 0.43 |
| San Jose-Monterey | 1.26 (0.49–3.23) | 0.63 | 0.86 (0.59–6.73) | 0.34 |
| Seattle | 1.60 (0.80–3.21) | 0.19 | 0.99 (0.51–3.75) | 0.52 |
| Number of Nodes Examined | ||||
| 0–5 | 1† | 1† | ||
| 6–10 | 0.95 (0.74–1.21) | 0.66 | 0.87 (0.61–1.24) | 0.43 |
| 11–50 | 0.85 (0.68–1.05) | 0.14 | 0.86 (0.64–1.17) | 0.34 |
| Unknown | 1.29 (0.56–3.00) | 0.55 | 0.99 (0.24–4.14) | 0.99 |
| At Least HS Education (quintiles) | ||||
| 1 (Highest educated) | 1† | 1† | ||
| 2 | 1.06 (0.74–1.51) | 0.76 | 1.12 (0.67–1.87) | 0.65 |
| 3 | 0.89 (0.60–1.31) | 0.55 | 0.76 (0.43–1.33) | 0.33 |
| 4 | 0.94 (0.60–1.49) | 0.81 | 0.89 (0.46–1.73) | 0.74 |
| 5 (Least educated) | 0.95 (0.56–1.61) | 0.85 | 0.96 (0.45–2.03) | 0.91 |
| Median Income (quintiles) | ||||
| 1 (Lowest income) | 1† | 1† | ||
| 2 | 0.87 (0.59–1.28) | 0.47 | 0.75 (0.43–1.31) | 0.32 |
| 3 | 0.99 (0.71–1.39) | 0.97 | 1.03 (0.66–1.65) | 0.89 |
| 4 | 1.01 (0.66–1.52) | 0.98 | 1.08 (0.60–1.94) | 0.80 |
| 5 (Highest income) | 0.98 (0.60–1.59) | 0.93 | 1.05 (0.53–2.08) | 0.89 |
Index Value
ER: Estrogen Receptor, PR: Progesterone Receptor, HS: High Schoo
Analysis of the impact of tumor grade on CSS did not reach significance regarding the effect of PMRT between high and low grade tumors (p=0.55). For low-grade (I–II) tumors, CSS at 96 months was 89.5% with PMRT vs. 88.6% without PMRT (p=0.082). For high-grade (III–IV) tumors, CSS at 96 months was 80.8% with PMRT vs. 76.1% without PMRT (p=0.067). Kaplan-Meier CSS curves by tumor grade can be seen in Figure 3.
Figure 3.
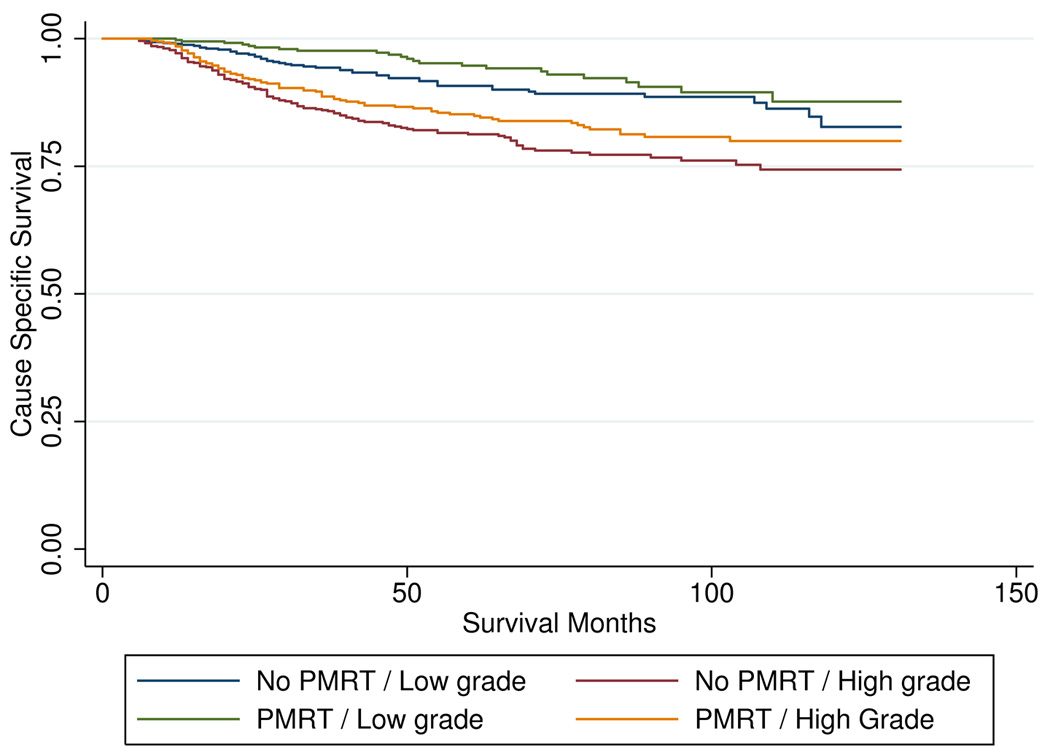
impact of grade on cause specific survival by post-mastectomy radiation
Discussion
Following the publication of the Danish Breast Cancer Cooperative Group 82b and 82c trials in 1997 and 1999, support for PMRT in patients with T3N0M0 breast cancer was established. These prospective trials included high-risk post-mastectomy patients who had one or more of the following: involvement of axillary nodes, tumor size greater than 5cm, or invasion of the skin or pectoralis fascia. Node-negative patients made up 7.9% and 9.6% of patients on 82b and 82c respectively, but the number of node-negative patients without skin or pectoralis fascia invasion was not specified so the absolute number of T3N0 patients enrolled cannot be determined. In addition, the number of lymph nodes dissected in patients enrolled on these trials is fewer than in other trials, implying that some of the patients classified as N0 may have had positive nodes had they undergone a more thorough axillary dissection. Regardless, the Danish trials provided prospective, randomized support for the role of PMRT in T3N0 breast cancer. There are several retrospective studies with conflicting data for the role of PMRT for T3N0M0 cancers. A SEER analysis examining the role of PMRT for T3N0M0 patients treated between 1988 and 2002 showed no significant difference in CSS and an OS benefit only in patients 50 years of age and older.11 Another retrospective series demonstrated a 9% LRF rate in T3N0 patient treated with PMRT.2 There have been several retrospective studies in T3N0 patients not treated with PMRT. One demonstrated a 15% LRF rate in 101 patients,12 while the other demonstrated a 7.6% LRF rate in 70 patients. The latter study included 24 patients with tumors measuring 5.0 cm who were therefore T2.13 One large series of patients who were not treated with PMRT is a retrospective study of 313 patients enrolled onto various National Surgical Adjuvant Breast and Bowel Project (NSABP) trials. This cohort included patients with tumors ≥5cm. Without systemic therapy, the 10-year LRF rate was 16.3%. With systemic therapy (chemotherapy, endocrine therapy or both), the LRF rate was 6 to 7.7%.14
Based on the available prospective and retrospective data, the 2001 American Society of Clinical Oncology practice guidelines suggests stage pT3 as an indication for the routine use of PMRT.9 The acceptance of this recommendation is reflected in a 2004 practice pattern survey, in which nearly 90% of 1,137 radiation oncologists surveyed in the United States and Europe would offer PMRT to T3N0 patients.10 In addition, the National Comprehensive Cancer Network (NCCN) guidelines recommend consideration of radiation therapy to the chest wall and regional lymph nodes for T3N0M0 breast cancer following total mastectomy and axillary staging (2B level of evidence).15
Our data support the role of PMRT in T3N0M0 patients showing improvements in both OS and CSS. This is an important finding, as demonstrating a survival benefit for radiation in any disease site usually requires that several factors be met: 1) a large cohort of patients whose risk of local recurrence is fairly high without radiation 2) a relatively healthy cohort whose competing risks of death are minimal and 3) the development of distant metastatic disease is well controlled by systemic therapy. Our study certainly fulfilled the first criteria as we included over 2,500 patients. It is unknown whether the second factor was met given the lack of SEER data regarding medical comorbidities, although the fact that both OS and CSS were improved is reassuring. The third criteria was likely fulfilled given that systemic therapies have improved over time (especially with the advent of Trastuzamab) and are better at suppressing metastatic spread. This may be the reason why some earlier studies failed to show a survival benefit to PMRT.
We sought to examine the interaction between tumor grade and the benefit of PMRT as high tumor grade is a known risk factor for LRF. A report from the British Columbia Provincial Database of T3N0 patients who were not given PMRT showed a crude LRF rate of 17% for patients with high grade tumors compared to 9% for all grades.16 Our subgroup analysis did not demonstrate that grade impacted CSS. Although the absolute improvement in CSS for PMRT was greater in high-grade tumors, the statistical significance that we found may have been impacted by the small sample size of this subgroup, as 12% of patients did not have grade recorded. Other risk factors for LRF such as lymphovascular invasion and margin status were not anaylzed in our dataset as this information is not available in the SEER database. These factors have been shown to impact LRF in other studies of breast cancer patinet and may have influenced our findings.
At 96 months, the absolute improvement in OS was 14.7%, while the absolute improvement in CSS was 2.4% with PMRT. The larger magnitude of benefit seen in OS may be attributable to selection bias, as patients who are healthier were more likely to be offered radiation. In our dataset there was definite evidence of selection bias (younger patients were more likely to receive radiation), and it is likely that we were not able to adjust for all of these confounding factors. Furthermore, comorbid conditions and functional status are not recorded in the SEER database. These factors are likely to impact death due to other causes, which would be reflected in OS but not CSS. In our data, age was found to be a significant predictor of both OS and CSS on MVA. Our data is consistent with the available literature, showing that younger age is associated with higher grade tumor, both of which predict for inferior CSS.17 In our series, younger women were also more likely to receive PMRT. Despite this proclivity to offer PMRT to younger women, the majority of deaths in women <50 years old remain breast cancer related, while the majority of deaths in women >65 years old were due to other causes or comorbid conditions.
The main strength of our study is that it represents a contemporary cohort of patients treated with modern radiation techniques and contemporary systemic therapies. The main weakness of this study is the non-randomized nature of the dataset and the inherent biases that exist in any retrospective study. Another weakness of this study is that imbalances in patient characteristics may not be completely depicted given the limitations of the SEER dataset, including variables not captured such as systemic therapy regimen and dose, lymphovascular invasion, margin status, and local and regional recurrence data.
Conclusion
PMRT is associated with significant improvements in both CSS and OS in patients with T3N0M0 breast cancers treated with modified radical mastectomy from 2000 to 2010. PMRT radiation should be strongly considered in T3N0M0 patients. Other risk factors including age, tumor grade, LVI, and margin status may be considered as well to better individualize recommendations for PMRT in patients with T3N0 breast cancer until randomized evidence is available.
Acknowledgments
Funding sources: none.
Footnotes
Part of this work has been accepted for oral presentation at the American Radium Society’s Annual meeting, April 26–29, 2014
Conflict of Interest: The authors have no conflict of interest to declare
References
- 1.American Cancer Society. Cancer Facts and Figures 2013. [accessed November 1, 2013]; Available from URL: www.cancer.org. [Google Scholar]
- 2.Helinto M, Blomqvist C, Heikkila P, Joensuu H. Post-mastectomy radiotherapy in pT3N0M0 breast cancer: is it needed? Radiother Oncol. 1999;52:213–217. doi: 10.1016/s0167-8140(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 3.Edge SBB, D R, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2011. [Google Scholar]
- 4.Bevilacqua JL, Kattan MW, Fey JV, Cody HS, 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007;25:3670–3679. doi: 10.1200/JCO.2006.08.8013. [DOI] [PubMed] [Google Scholar]
- 5.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 6.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 7.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. 2nd Edition. New York: Springer; 2005. [Google Scholar]
- 8.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 9.Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 10.Ceilley E, Jagsi R, Goldberg S, et al. Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61:365–373. doi: 10.1016/j.ijrobp.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 11.McCammon R, Finlayson C, Schwer A, Rabinovitch R. Impact of postmastectomy radiotherapy in T3N0 invasive carcinoma of the breast: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2008;113:683–689. doi: 10.1002/cncr.23611. [DOI] [PubMed] [Google Scholar]
- 12.Mignano JE, Gage I, Piantadosi S, Ye X, Henderson G, Dooley WC. Local recurrence after mastectomy in patients with T3pN0 breast carcinoma treated without postoperative radiation therapy. Am J Clin Oncol. 2007;30:466–472. doi: 10.1097/COC.0b013e31805c13ba. [DOI] [PubMed] [Google Scholar]
- 13.Floyd SR, Buchholz TA, Haffty BG, et al. Low local recurrence rate without postmastectomy radiation in node-negative breast cancer patients with tumors 5 cm and larger. Int J Radiat Oncol Biol Phys. 2006;66:358–364. doi: 10.1016/j.ijrobp.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Taghian AG, Jeong JH, Mamounas EP, et al. Low locoregional recurrence rate among node-negative breast cancer patients with tumors 5 cm or larger treated by mastectomy, with or without adjuvant systemic therapy and without radiotherapy: results from five national surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol. 2006;24:3927–3932. doi: 10.1200/JCO.2006.06.9054. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. Invasive Breast Cancer;3. [accessed November 1, 2013];2013 Available from URL: www.nccn.org. [Google Scholar]
- 16.Goulart J, Truong P, Woods R, Speers CH, Kennecke H, Nichol A. Outcomes of node-negative breast cancer 5 centimeters and larger treated with and without postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:758–764. doi: 10.1016/j.ijrobp.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]


