Abstract
Objective
To assess the efficacy of increasing the number of fast left repetitive transcranial magnetic stimulations (rTMS) (10 Hz @ 120% of motor threshold (MT) over the left dorsolateral prefrontal cortex (DLPFC)) needed to achieve remission in treatment resistant depression (TRD). And, to determine if patients who do not remit to fast left will remit using slow right rTMS (1 Hz @ 120% MT over the right DLPFC).
Method
Patients were part of a multicenter sham controlled trial investigating the efficacy of fast left rTMS 1. Patients who failed to meet minimal response criteria in the sham controlled study could enroll in this open fast left rTMS study for an additional 3- 6 weeks. Patients who failed to remit to fast left could switch to slow right rTMS for up to four additional weeks. The final outcome measure was remission, defined as a HAM-D score of ≤ 3 or two consecutive HAM-D scores less than 10.
Results
Forty-three of 141 (30.5%) patients who enrolled in the open phase study eventually met criteria for remission. Patients who remitted during fast left treatment received a mean of 26 active treatments (90,000 pulses). 26% of patients who failed fast left remitted during slow right treatment.
Conclusion
The total number of rTMS stimulations needed to achieve remission in TRD may be higher than is used in most studies. TRD patients who do not respond to fast left rTMS may remit to slow right rTMS or additional rTMS stimulations.
Keywords: transcranial magnetic stimulation, rTMS, high frequency rTMS, low frequency rTMS, treatment resistant depression
Introduction
In two recent multicenter trials, repetitive transcranial magnetic stimulation (rTMS) has been significantly more effective than sham in treating depressive symptoms, however the magnitude of the effect has been modest and most remitters had low levels of treatment resistance 1,2. This study examines strategies for improving the response to rTMS especially in treatment resistant patients by evaluating two important treatment parameters: the total number of stimulations and the site and frequency of stimulation. Increasing the total number of stimulations has previously been demonstrated to improve response to rTMS 3,4 . However, the studies used in these comparisons have generally come from trials of different lengths or weeks of rTMS treatment and not comparisons of the effect of increasing the number of active rTMS stimulations. The largest multicenter sham controlled trials to date have studied high frequency (10 Hz) stimulation over the left dorsolateral prefrontal cortex (DLPFC) or fast left rTMS and evaluated the primary depression outcome measure after 45,000 pulses over 3 weeks 1 and 60,000 pulses over 4 weeks 2. The present study uses a duration-adaptive treatment design 5 that allows patients to receive up to 12 weeks of fast left rTMS and a maximum of 180,000 pulses. Response to treatment is assessed weekly to determine the number of weeks of treatment (or total pulses) that correspond to a maximal treatment effect.
The present study also examines the efficacy of switching fast left rTMS nonresponders to low frequency stimulation (1 Hz) over the right DLPFC or slow right rTMS. Both fast left and slow right rTMS are effective in the treatment of major depression 3,4,6,7. However, these studies have randomized patients to either fast left or slow right. This study will compare the therapeutic efficacy of switching from fast left to slow right rTMS in the same patient to determine if some patients may respond preferentially to slow right rTMS.
Methods
Patients were part of a larger sham controlled trial of the efficacy of daily left DLPFC stimulation in the treatment resistant depression (TRD). This study was conducted at four sites (Medical University of South Carolina [MUSC], Columbia University/New York State Psychiatric Institute, University of Washington, and Emory University), with active enrollment taking place from October 15, 2004 through March 31, 2009. The institutional review board at each center approved the protocol, and all the participants provided written informed consent.
All patients met DSM IV criteria for major depression with current episode duration of ≤ five years and no evidence of active substance abuse or previous exposure to rTMS. To qualify patients had to meet severity of illness criteria including a Hamilton Depression Rating Scale8 24-item score (HAM-D) ≥ 20 and a moderate level of treatment resistance as defined by the Antidepressant Treatment History Form (ATHF 9; insufficient clinical benefit to 1–4 adequate medication trials or intolerant to ≥ 3 trials)1.
The study included three phases: Phase 1 was a sham controlled randomized trial of fast left rTMS and is reported elsewhere1; Phase 2 was an open label fast left rTMS trial of patients who did not remit to Phase 1 and is the subject of this paper. Patients in Phase 2 received up to 6 weeks of open label fast left rTMS. Patients who did not remit to fast left rTMS were switched to slow right rTMS; Phase 3 was the long term follow up of patients who remitted in Phase 1 or 2 and will be the subject of a future paper.
In Phase 1, patients underwent a two week lead-in period off of all antidepressant (5 weeks for fluoxetine), anticonvulsant and antipsychotic medication. Using an always active coil, left and right hemisphere motor threshold (MT) was determined weekly by electromyographic measurement (3 sites) or visual monitoring (Emory University) of the resting right thumb (abductor pollicus brevis). The standardized treatment location was over the left or right DLPFC, determined by moving the TMS coil 5 cm anterior to the MT location along a left superior oblique plane with a rotation point about the tip of the patient’s nose. Prior to the first treatment session, patients underwent head magnetic resonance image (MRI), with fiducials (vitamin E capsules) attached to a swim cap over the left and right motor cortex regions identified during the threshold determination and the putative prefrontal brain region. Scans were digitally transferred to MUSC, where a trained observer determined whether the intended coil placement location was over the left or right premotor and prefrontal cortex. In Phase 1, 190 patients were randomized to either sham or active treatment @ 120% magnetic field intensity relative to the patient’s resting MT for 10 pulses per second (10 Hz) for 4 seconds, with an intertrain interval of 26 seconds over the left DLPFC. Treatment sessions lasted for 37.5 minutes (75 trains) with 3000 pulses delivered per session.
At the end of three weeks in Phase 1, patients were assessed to be either in remission, improving but not remitted or not improving. Remission was defined as a HAM-D score of ≤ 3 or two consecutive weekly HAM-D scores less than 1010. Patients who were assessed to be in remission stopped rTMS treatment and entered Phase 3 for long term follow-up. Patients who improved but did not remit (defined as having at least a 30% reduction in HAM-D score from baseline but without an absolute score meeting remission criteria) continued treatment in Phase 1 for up to 3 additional weeks using a duration-adapted design5, with HAM-D assessments performed twice weekly to monitor for continued improvement. If these patients continued to show improvement (defined as at least a two point drop in the HAM-D per week) then they continued to receive treatment in Phase 1 for up to an additional 3 weeks. If they failed to show continued improvement they were given the option of entering the open label Phase 2 trial or being followed naturalistically. If they remitted they entered Phase 3. Patients who did not show sufficient improvement at the end of the fixed 3-week period (defined as a ≤ 30% drop from baseline in HAM-D score) were discontinued from Phase 1 and given the option of crossing over to open treatment (Phase 2) or being followed naturalistically.
Therefore, the patients in Phase 2 were patients who had failed to achieve remission in Phase 1 and who consented to open label treatment. The initial treatment parameters in Phase 2 were identical to the active treatment in Phase 1 (i.e., fast left @ 120% MT for 3000 pulses over the left DLPFC). At the end of three weeks of open label treatment, patients were assessed to be either in remission, improving but not remitted or not improving. Patients in remission were moved to Phase 3. Patients who were improving but not remitted continued treatment for up to an additional three weeks using the duration adaptive design. Patients who did not show sufficient improvement in Phase 2 were given the option of moving to a four week open label trial of slow frequency rTMS over the right DLPFC. The treatment parameters for slow right rTMS were 1 Hz and 120% MT for 30 minutes or 1800 pulses per session for up to 4 weeks. The positioning of the coil was determined by moving the TMS coil 5 cm anterior to the MT location along a right superior oblique plane with a rotation point about the tip of the patient’s nose.
Statistical analyses
Data were analyzed using a logistic regression model in SAS Version 9.2 (SAS, Cary, NC) to determine if the probability of remission or response during Phase 2 differed between the two treatment arms (active/sham) when controlling for treatment resistance (high/low). Remission, as defined above was an absolute a HAM-D score of ≤ 3 or two consecutive weekly HAM-D scores less than 10. Response was defined as a ≤ 50% decrease in the HAM-D score from the baseline HAM-D in Phase 1. Low medication resistance was defined as failing ≤ 1 adequate medication trial in the present episode by ATHF criteria. The primary analysis was conducted using the intent-to-treat population defined as all randomized patients who entered Phase 2 of the study. Baseline demographics were compared using Student’s t-test for continuous measures and a chi-square test for categorical measures. All tests were two-sided and assessed at a significance level of 0.05.
Results
The baseline demographic and clinical variables of the 141 patients entering Phase 2 of the study are outlined in Table 1. Of the patients who agreed to participate in Phase 2, eighty patients had been randomized to sham treatment in Phase 1 and 61 patients randomized to active treatment in Phase 1. Overall the demographics of the patients who entered Phase 2 were not significantly different from those of the patients in Phase 1. Phase 2 patients were predominantly female (55%), were an average age of 47.2 years old with a current depression episode duration of approximately 80 weeks and a mean HAM-D of 26.3 at the start of Phase 1. Phase 2 patients had failed 1.6 adequate antidepressant trials in the present episode and an average of almost 4 antidepressant trials in their lifetime. There were slightly more Phase 2 patients with low antidepressant resistance.
Table 1.
Clinical and demographic data for patients entering phase 2.
| Active (N=61) |
Sham (N=80) |
Phase II (N=141) |
p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 22 (36%) | 41 (51%) | 63 (45%) | 0.07 |
| Age | ||||
| Mean (SD) | 47.5 (11.3) | 46.9 (12.3) | 47.2 (11.8) | 0.76 |
| Range | 22–69 | 23–69 | 22–69 | |
| Current Episode Duration (weeks) | ||||
| Mean, Median (SD) | 77.2, 56 (62.8) | 81.0, 60.5 (66.9) | 79.3, 56 (65.0) | 0.73 |
| Range | 8–260 | 4–260 | 4–260 | |
| Baseline HAM-D | ||||
| Mean (SD) | 26.1 (5.3) | 26.5 (4.6) | 26.3 (4.9) | 0.62 |
| Range | 20–43 | 20–42 | 20–43 | |
| Initial Phase II HAM-D | ||||
| Mean (SD) | 24.6 (5.9) | 24.5 (6.0) | 24.6 (6.0) | 0.92 |
| Range | 13–40 | 11– 39 | 11–40 | |
| Baseline MADRS | ||||
| Mean (SD) | 29.9 (6.9) | 30.2 (6.2) | 30.1 (6.5) | 0.76 |
| Range | 16–43 | 12–44 | 12–44 | |
| Initial Phase II MADRS | ||||
| Mean (SD) | 29.0 (6.7) | 30.4 (6.2) | 29.8 (6.4) | 0.25 |
| Range | 16–47 | 17–43 | 16–47 | |
| Baseline IDS | ||||
| Mean (SD) | 41.7 (9.2) | 39.7 (9.4) | 40.5 (9.3) | 0.22 |
| Range | 26–63 | 18–64 | 18–64 | |
| Initial Phase II IDS | ||||
| Mean (SD) | 38.1 (11.4) | 38.4 (12.0) | 38.3 (11.7) | 0.87 |
| Range | 16–70 | 11–76 | 11–76 | |
| Failed Antidepressant Trials | ||||
| Current | ||||
| Mean, Median (SD) | 1.75, 1 (1.39) | 1.48, 1 (0.98) | 1.60, 1 (1.18) | 0.18 |
| Range | 0–6 | 0–4 | 0–6 | |
| Lifetime | ||||
| Mean, Median (SD) | 3.70, 3 (2.97) | 3.50, 3 (2.10) | 3.59, 3 (2.51) | 0.65 |
| Range | 0–14 | 0–9 | 0–14 | |
| ATHF | ||||
| Low Antidepressant Resistance | 31 (50.8%) | 54 (67.5%) | 85 (60.3%) | 0.05 |
| High Antidepressant Resistance | 30 (49.2%) | 26 (32.5%) | 56 (39.7%) | |
| Right Motor Threshold | n=60 | n=78 | n=138 | |
| Mean (SD) | 56.3 (12.4) | 57.9 (11.7) | 57.2 (12.0) | 0.44 |
| Range | 36–83 | 39–85 | 36–85 | |
| Left Motor Threshold | n=61 | n=79 | n=140 | |
| Mean (SD) | 58.2 (10.7) | 57.2 (10.0) | 57.6 (10.3) | 0.55 |
| Range | 36–80 | 35–76 | 35–80 |
The columns compare those patients entering Phase 2 that were randomized to active vs. sham treatment in Phase 1. All numbers are mean values (± standard deviation). Baseline values for the measures of psychiatric symptoms (HAM-D or 21 item Hamilton Depression Rating Scale (Hamilton 1967); MADRS or Montgomery-Åsberg Depression Rating Scale; IDS 30-item Inventory of Depressive Symptoms). Failed antidepressant trials were assessed using the Antidepressant Treatment History Form (Sackeim 2001) and low antidepressant resistance was failing one or fewer antidepressant trials in the present episode of depression. Motor threshold was assessed at baseline and is the mean percent of maximal output over the premotor cortex.
Hamilton, M. (1967). "Development of a rating scale for primary depressive illness." Br J Soc Clin Psychol 6(4): 278–96.
Sackeim, H. A. (2001). "The definition and meaning of treatment-resistant depression." J Clin Psychiatry 62 Suppl 16 10–7.
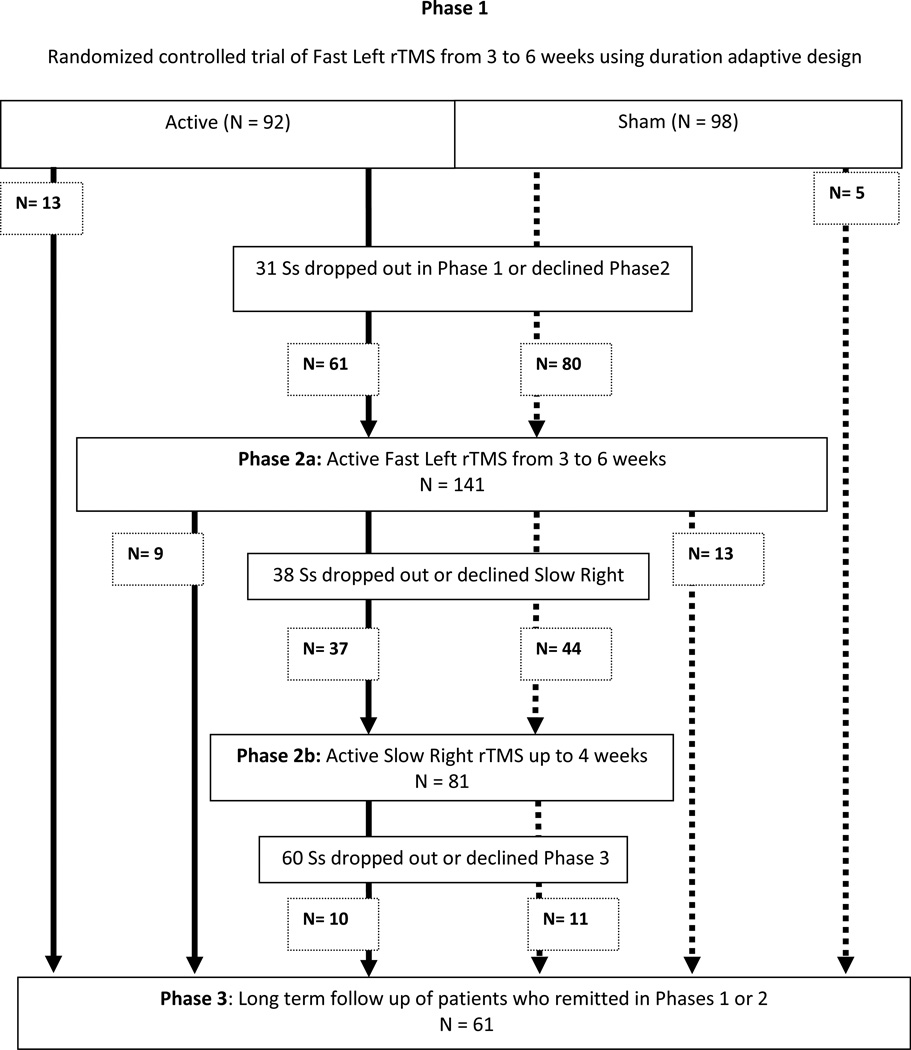
A flow chart of each phase of the study is outlined in Figure 1. Of the original 190 patients enrolled in Phase 1, 92 were in the active arm and 98 were in the sham arm. Thirteen of the patients in the active arm remitted in Phase 1 and moved to Phase 3 and 5 patients in the sham arm remitted and moved to Phase 3. Thirty-one patients declined to continue into Phase 2 and 141 patients consented to Phase 2a: 61 patients consented to Phase 2a who were originally in the active arm of Phase 1 and 80 patients consented to Phase 2a from the sham arm of Phase 2.
Figure 1.
Flow Diagram
Flow diagram of patients participating in OPT TMS trial. rTMS is repetitive transcranial magnetic stimulation. Phase 1 has been described in a previous paper1. Phase 2 is the subject of this paper and includes both an open label active trial of Fast Left rTMS trial (Phase 2a) and an open label Slow Right rTMS trial (Phase 2b). Patients who remitted in each phase are shown moving to Phase 3. Other patients are either included as drop outs or shown moving to the next phase. For example, in Phase 2a (Active Fast Left rTMS) nine patients who were in the active arm in Phase 1 and 13 patients who were in the sham arm of Phase 1 remitted and moved to Phase 3. Thirty-eight patients dropped out or declined further treatment and 37 patients who were originally in the active arm of Phase 1 and 44 patients who were in the sham arm of Phase 1 consented to start Phase 2b (Active Slow Right rTMS). Phase 3 will be detailed in a manuscript under review and is the long term follow up of patients on a standardized medication trial to evaluate relapse rates after rTMS. Of the patients who remitted in either Phase 1, Phase 2a or Phase 2b sixty-one patients consented to participate in Phase 3.
Forty-three of 141 (30.5%) patients in Phase 2 met criteria for remission. Fifty-eight patients (41%) in Phase 2 met criteria for treatment response. Of those patients who remitted in Phase 2, 19/43 (44%) were from the active arm of Phase 1 and 24/43 (56%) were from the sham arm. Treatment assignment (active/sham) in Phase 1 was not statistically associated with remission in Phase 2, for either fast left or slow right treatment (Wald chi-square 0.02; p = 0.89 and 0.08; p =0.78, respectively). A similar result was found for response in Phase 2 to fast left and slow right (Wald chi-square 0.01; p = 0.92 and 0.60; p = 0.44, respectively).
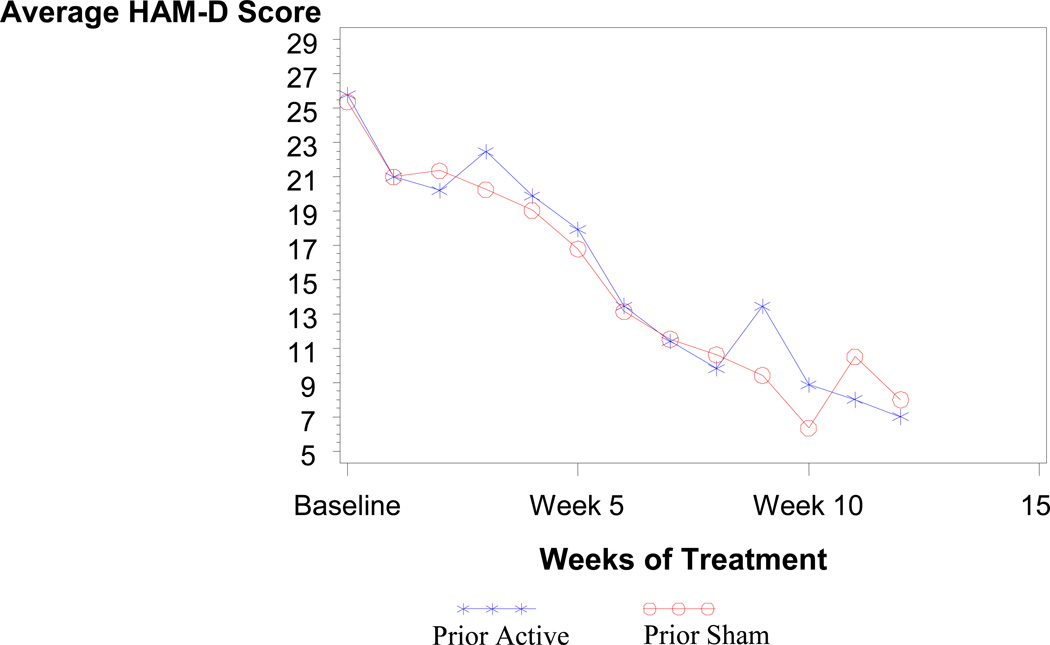
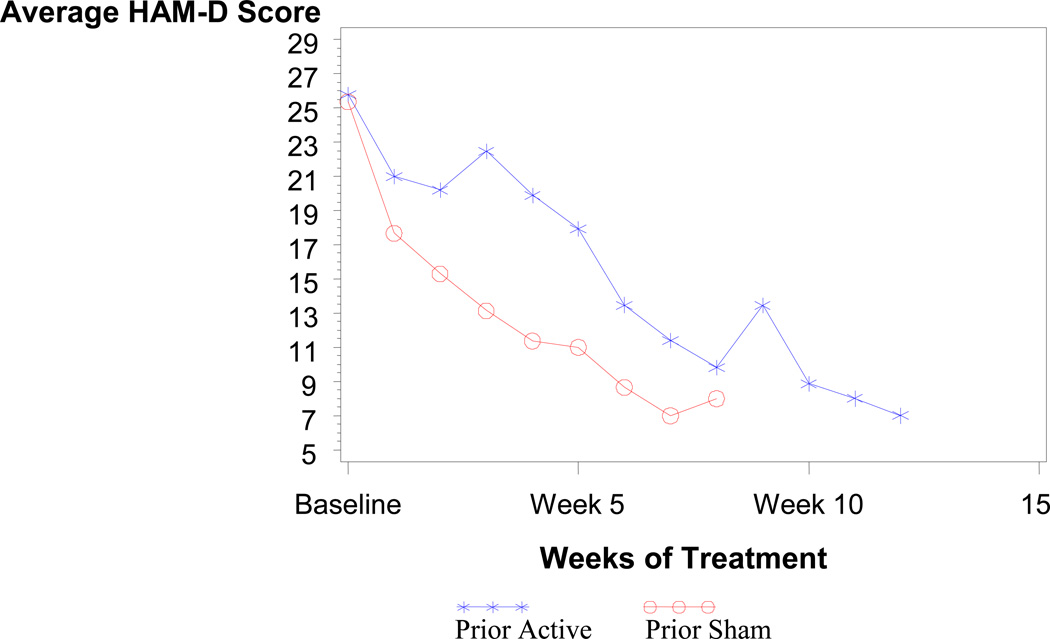
Average HAM-D scores by weeks of treatment and randomization assignment for patients that remitted in Phase 2 are shown in Figure 2. There appears to be no difference in the average scores for patients who were assigned to active or sham treatment in Phase 1. Figure 2 shows the average HAM-D scores by weeks of active treatment (i.e., all weeks of treatment minus weeks of sham treatment equals weeks of active treatment). As shown in Figure 3, the average scores appear to have similar slopes except for the first three weeks in the active treatment group which is relatively flat. This initially flat response in the active treatment group is to be expected since patients entering Phase 2 from Phase 1 were enrolled because they showed no improvement in the first three weeks of Phase 1.
Figure 2.
Remitters average HAM-D score by weeks of treatment
The graph depicts the number of weeks of treatment in the study including Phase 1 and Phase 2 and showing the average Hamilton Depression Scale Score (HAM-D) only for patients who were remained in the study. The x-x-x line depicts patients who received active treatment in Phase 1 (Prior Active) and the o-o-o line depicts patients who received sham treatment in Phase 1 (Prior Sham).
Figure 3.
Average HAM-D score by weeks of active treatment
The graph depicts the number of weeks of treatment in the study including Phase 1 and Phase 2 and showing the average Hamilton Depression Scale Score (HAM-D) only for patients who stayed in the study for the entire 12 weeks. The x-x-x line depicts patients who received active treatment in Phase 1 (Prior Active) and the o-o-o line depicts patients who received sham treatment in Phase 1 (Prior Sham). Real treatment is defined as the total number of weeks of treatment minus the number of weeks of sham treatment (or four weeks during Phase 1 for those patients randomized to sham).
Sixteen percent (n=22/141) of Phase 2 patients who were administered open label fast left treatments remitted. Active vs. sham treatment in Phase 1 was not associated with remission in Phase 2 fast left. The total number of weeks of fast left treatment (combining Phase 1 and Phase 2) did differ between remitters and non-remitters. Remitters received a mean of 5.2 (± 1.9) weeks of active fast left treatment in Phase I and II (median = 5.8 weeks) and nonremitters received a mean of 4.4 (± 1.8) weeks of active treatment (median = 3.8 weeks). Remitters had a slightly lower HAM-D score when they started Phase 2 (HAMD for remitters=22.8 (+ 5.4); HAMD for non-remitters = 24.9 (+6)) but the relationship between remission and initial Phase 2 HAM-D was not significant.
Approximately 40% of the patients who enrolled in Phase 2 had a high level of medication resistance yet only 18% (4/22) of patients who remitted in Phase 2 fast left had a high level of medication resistance. Low medication resistance was associated with remission in Phase 2 fast left (Wald chi-square = 4.59; p =0.03). The odds ratio (95% confidence interval) of a low medication resistance patient remitting was 3.5 (1.1, 11.2) times more likely than a high medication resistance patient remitting.
Twenty-six percent (21/81) of patients who were administered open label slow right treatments remitted. Again active vs. sham treatment in Phase 1 was not associated with remission in Phase 2 slow right (Wald chi-square = 0.08; p = 0.78) but the average number of active weeks of treatments in Phase 1 and 2 was again slightly higher in patients who remitted (7.9 (±3.0) weeks of treatment vs. 7.4 (± 2.1) weeks of treatment for nonremitters). Similar to fast left patients, the patients who remitted during Phase 2 slow right had a lower absolute HAM-D score entering this phase than the nonremitters (HAMD for remitters =17.9 (+7.2); HAMD for nonremitters=22.9 (+7.9)). Unlike the patients in Phase 1 and Phase 2 fast left, the Phase 2 slow right patients did not show a relationship between medication resistance and remission (Wald chi-square = 0.02; p = 0.88)
Adverse events
There were no seizures or suicides in the open label treatment phase of the study. One patient had increased suicidal ideation in fast left treatment. This patient had received sham treatment in Phase 1. One other patient in fast left treatment had a significant worsening of depression and three patients in slow right treatment reported significant worsening of depression. Many of these patients would have been in the study and off of all mood stabilizers for weeks.
The most common spontaneous adverse events are reported in Table 2. The spontaneous adverse events were very similar to those reported for active TMS in the Phase 1 study. For example, in Phase 1 the most common adverse events in the active arm were headache, discomfort at the stimulation site and insomnia which were reported by 32%, 18% and 7.6% of patients, respectively.
Table 2.
Spontaneous adverse events in open label fast left and slow right rTMS
| Adverse Event | Number of unique subjects |
Percent of total subjects |
|---|---|---|
| Headache | 36 | 26% |
| Discomfort at stimulation site | 26 | 18% |
| Insomnia | 15 | 11% |
| Worsening of depression | 14 | 10% |
| Muscle aches | 9 | 6% |
| Gastrointestinal | 7 | 5% |
| Fatigue | 6 | 4% |
| Other | 44 | 31% |
rTMS is repetitive transcranial magnetic stimulation. Adverse events were code in a MedDRA-modified manner similar to a recent rTMS depression trial (reference 2)
2. O'Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. Dec 1 2007;62(11):1208–1216.
Table 3 reports the number of subjects in each arm who reported spontaneous adverse events. None of these patients had any sequelae (e.g., development of chronic headaches or pain on their scalp) that was noted when they were in the study or in our follow up phases.
Table 3.
Spontaneous adverse events in open label fast left rTMS vs. slow right rTMS
| Fixed fast left (n = 141) |
Variable fast left (n = 27) |
Slow right (n = 81) |
|
|---|---|---|---|
| Adverse Event | |||
| Headache | 24 (17%) | 2 (7%) | 10 (12%) |
| Discomfort at stimulation site | 20 (14%) | 2 (7%) | 4 (5%) |
| Insomnia | 11 (8%) | 1 (4%) | 3 (4%) |
| Worsening of depression | 11 (8%) | 0 | 3 (4%) |
| Muscle aches | 6 (4%) | 0 | 3 (4%) |
| Gastrointestinal | 4 (3%) | 0 | 3 (4%) |
| Fatigue | 5 (4%) | 0 | 1 (1%) |
| Other | 27 (19%) | 4 (15%) | 12 (15%) |
rTMS is repetitive transcranial magnetic stimulation. Adverse events are reported as percent of unique number of subjects reporting adverse event in each group with the percent of these subjects in each group reported in parentheses. Adverse events were code in a MedDRA-modified manner similar to a recent rTMS depression trial (reference 2)
Discussion
This paper reports on an open phase extension of a multicenter sham controlled trial of fast left rTMS in TRD 1. The remission rate of patients receiving open label rTMS in this study (30.5%) was more than double the remission rate of patients receiving active treatment in the sham controlled trial (14.1% active rTMS vs. 5.1% sham; p< .02). These remission rates are encouraging given the fact that patients enrolled in this study had failed an average of 1.6 medication trials in their present episode of depression. In patients who have failed two medication trials, open-label studies of pharmacotherapy have shown that less than <20% of patients remit with another medication trial or augmentation. In patients with three failed medication trials (the lifetime average of this group), remission rates are between 10–20%11–13. The safety data is also encouraging given the fact that some patients may have received multiple weeks of active rTMS with few significant adverse events, no reported seizures and no long term sequelae. This study supports rTMS as an important therapy for TRD.
This study examined two variables that are potentially related to this treatment response: optimizing the number of rTMS treatments and changing the treatment site. In the multicenter sham controlled trial (Phase 1)1, no patient received more than 5 weeks of rTMS treatment because patients who did not meet minimal response criteria (e.g., a 30% decrease in the baseline HAM-D at three weeks with continued response in weeks 3–5) were dropped from Phase 1. Yet the average weeks of treatment for remitters in the open phase extension of active fast left rTMS (i.e., Phase 2) was 5.2 weeks of active treatment. Clearly some patients needed more than five weeks of active rTMS and the criteria for patients to remain in Phase 1 and receive additional treatment (i.e., a decrease of ≥ 30% in the HAM-D at three weeks with continued improvement on biweekly HAM-D measurements) may have been overly restrictive and artificially decreased the remission rate for active treatment.
These results are congruent with a previous multicenter sham controlled trial of fast left rTMS which did not find a significant difference in remission rates between active fast left rTMS and sham treatment until week 62. In the open phase extension of that trial, Avery et al. analyzed patients who received active treatment in the blinded randomized trial separately from patients who initially received sham treatments14. The patients who were treated with active treatment in the randomized blinded phase then open label active treatment had a 20% remission which was significantly higher than those who received sham then open label active (14.2% remission rate).The results of the present study are however limited by the fact that the patients were in an open label trial. Although the active sham coil used in Phase 1 was effective in masking patients and treaters from determining active from sham treatments in Phase 11, the more difficult task would be for patients who had experienced sham stimulus to change to active stimulus (i.e., within-as opposed to between- subject blinding) and not notice a qualitative difference. If a patient could distinguish a difference in sensation in the sham and active conditions (e.g., the active conditions was qualitatively different with more scalp sensations than the sham condition) then patients switching from sham to active conditions would “expect” to have a higher rate of response because they experienced a more tactically intense treatment. In this study there were no differences in response or remission rates in Phase II for patients who had received sham or active treatment in Phase 1.
The patients entering phases 1 and 2 were similar in their demographic and clinical variables and the differences in remission rates do not appear to be due to a difference in patient characteristics. 82% of eligible Phase 1 patients (141/172) consented to Phase 2 and the demographic and clinical data of the patients entering Phase 1 and 2 were not significantly different. Therefore the patients who eventually remitted in Phase 2 may have been showing some response to Phase 1 treatment although not enough to meet the minimal response criteria to remain in Phase 1.
Finally, medication resistance was an important clinical variable in remission in both Phase 1 and Phase 2 fast left with medication resistant patients remitting at lower rates. The relationship of treatment response to medication resistance has been found in other randomized rTMS trials2. In Phase 2 the remission rate for patients with high medication resistance on the ATHF was low (only 18% of remitters had high medication resistance) as it was in Phase 1 (16.7% of patients who remitted in Phase 1 and were assessed as having a high level of medication resistance1.).
The remission rate to slow right rTMS (26%) was higher than would have been expected since the patients in this group had already failed fast left rTMS. Unlike responders to fast left, the slow right remitters did not show a relationship of medication resistance to remission rate1,2. Patients who do not remit with fast left rTMS, particularly TRD patients, therefore may benefit from changing to slow right.
The other possibility is that these TRD patients may have benefitted from additional rTMS pulses. In the open label extension by Avery et al.14, patients continued to have improvement in their depression scores over the entire 9 weeks which included a 6 week open phase and the 3 week taper. Avery et al. also found that the more active treatments a patient received, the less important treatment resistance was in determining response.
Slow right remitters received an average of 7.9 weeks of active treatment. In fact, Phase 1 baseline HAM-D scores were similar for remitters and nonremitters to slow right but the remitters had lower HAM-D scores prior to starting slow right supporting the hypothesis that remitters to slow right may have been starting to respond and needed additional treatments. The difference was not statistically significant and future research is needed to determine the clinical variables that should be used to continue treatment in patients who do not remit.
The results of the present study are limited by the open label design and practical considerations. The finding that some patients may need 6- 9 weeks (~ 90,000 or more total pulses) is a practical problem in both study design and clinical practice. Recent advances in rTMS administration demonstrate the safety and efficacy of providing accelerated treatments (i.e., 15,000 pulses administered over two days) 15 and future studies should focus on delivering more treatments and evaluating the efficacy of slow right rTMS particularly in TRD.
Acknowledgments
Funding/Support: The present study was supported by the National Institute of Mental Health (NIMH) as the Optimization of TMS for the Treatment of Depression Study (OPT-TMS) involving grants 5R01MH069929 (Dr. Avery), 5R01MH069887 (Dr. George), 5R01MH069896 (Dr. George), 5R01MH069895 (Dr. Lisanby), and 5R01MH069886 (Dr. McDonald).
Footnotes
Trial Registration: clinicaltrials.gov
Conflict of Interest Statement: Following a competitive bid and request involving all TMS manufacturers at the time of trial initiation, Neuronetics Inc. was selected and loaned the TMS devices, head holders, and coils for the trial and allowed use of the safety Investigational Device Exemption for their device. Neuronetics did not provide any financial support for the study which was funded by the NIMH.
Potential conflicts are reported over the past three years. Dr. McDonald is presently an unpaid consultant to NeoStim. Dr. Holtzheimer reports consulting fees from St. Jude Medical Neuromodulation, AvaCat Consulting and Tetragenex and is an unpaid consultant to NeoStim. Drs. McDonald and Holtzheimer are faculty at Emory University which holds a patent on TMS technology. Neither received royalties or is involved in the development or promotion of this patent. Dr. Lisanby reports research grants, speaking fees, or advisory board work with Neuronetics, Advanced Neuromodulatory Systems, Brainsway and Cyberonics. Dr. Lisanby has a patent application with Columbia University on neuromodulation technology for which she receives no royalties or compensation. Dr. Avery has received research support from Neuronetics and Takeda and has been on the speaker’s bureau for Eli Lilly, Takeda and Forest Pharmaceuticals. Dr. Nahas reports past and current research grants, speaking fees or consulting work with Cyberonics, Neuronetics, MECTA Corporation and Medtronic. Dr. Nahas has acted as an unpaid consultant for Neuropace. Dr. Sackeim received consultant fees from MECTA Corporation and Cyberonics. Dr. George received consultant fees from PureTech Ventures and reports research grants, speaking fees or advisory work with Brainsway, Mecta Corporation, Neuronetics, Cephos, NeoStim and NeoSync, Brainsonix, Cyberonics and Cephos. Dr. George is faculty at MUSC which has 2 patent applications in Dr. George’s name combining MRI and TMS.
Previous Presentations: This study was presented in abstract form at the American Psychiatric Association annual meeting; May 23, 2010; New Orleans, Louisiana.
Additional Contributions: We thank the following, who were either compensated or uncompensated (u): Minnie Dobbins, MEd, MUSC (general administrative support), Judith E. Kiersky, PhD, Columbia University (external expert rater), and Elaine M. Dillingham, BA, Columbia University (coordinated the expert rater tapes/ratings and the neuropsychological administration and scoring); Wenle Zhao, PhD and Catherine Dillon from the MUSC Data Coordinating Center; data and safety monitoring board members: Scott L. Rauch, MD, chairman, McLean Hospital, Belmont, Massachusetts; Eric Wassermann, MD(u), National Institute of Neurological Disorders and Stroke, Bethesda, Maryland; Cynthia Wainscott, Robert G. Robinson, MD, The University of Iowa; and Hongbin Gu, PhD, University of North Carolina, Chapel Hill; MUSC site investigators, raters, and coordinators, including Xingbao Li, MD, Samet Kose, MD, Jeffrey J. Borckardt, PhD, Kevin Johnson, RN, PhD; Columbia University site investigators, raters, and coordinators, including Antonio Mantovani, MD, PhD, Linda Fitzsimons, MS, RNC, Nancy Turret, LCSW, Seth Disner, BA, Austin Harrison, BA, Matthew Truesdale, BS, and Teresa Ngyuen, BS; Emory University site investigators, raters, and coordinators, including Sinéad Quinn, Mustafa A. Mufti, MD, Adriana P. Hermida, MD, Boadie Dunlop, MD, Charles M. Epstein, MD, Ronald Chismar, RN, Kimberly McWhorter, JD, MPH, and Halima N. Garba; and University of Washington site investigators, raters, and coordinators, including Chandra Wajdik, BS, Daniel Krashin, MD, Tobias Dang, MD, Chul Jin Shin, MD, Rita Navarro, MD, Wang-Ku Rho, MD, Susan Bentley, MD, David R. Haynor, MD, Emily Rosenberger, BA, Angela Ghesquiere, MSW, and Peter Roy-Byrne, MD.
See 1. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. May 2010;67(5):507–516. for details.
Bibliography
- 1.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010 May;67(5):507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 2.O'Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007 Dec 1;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Brunelin J, Poulet E, Boeuve C, Zeroug-vial H, d'Amato T, Saoud M. Efficacy of repetitive transcranial magnetic stimulation (rTMS) in major depression: a review. Encephale. 2007 Mar-Apr;33(2):126–134. doi: 10.1016/s0013-7006(07)91542-0. [DOI] [PubMed] [Google Scholar]
- 4.Holtzheimer PE, 3rd, Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull. 2001 Autumn;35(4):149–169. [PubMed] [Google Scholar]
- 5.Sackeim HA, Roose SP, Lavori PW. Determining the duration of antidepressant treatment: application of signal detection methodology and the need for duration adaptive designs (DAD) Biol Psychiatry. 2006 Mar 15;59(6):483–492. doi: 10.1016/j.biopsych.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, De Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003 Oct;60(10):1002–1008. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- 7.Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract. 2002 Sep;8(5):270–275. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 9.Sackeim HA, Prudic J, Devanand DP, Decina P, Kerr B, Malitz S. The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. Journal of Clinical Psychopharmacology. 1990;10(2):96–104. doi: 10.1097/00004714-199004000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Rush AJ, Kraemer HC, Sackeim HA, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006 Sep;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 11.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006 Nov;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 12.Fava M, Rush AJ, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006 Jul;163(7):1161–1172. doi: 10.1176/ajp.2006.163.7.1161. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006 Mar 23;354(12):1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 14.Avery DH, Isenberg KE, Sampson SM, et al. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry. 2008 Mar;69(3):441–451. doi: 10.4088/jcp.v69n0315. [DOI] [PubMed] [Google Scholar]
- 15.Holtzheimer PEI, McDonald WM, Mufti M, et al. Accelerated Repetitive Transcranial Magnetic Stimulation Treatment-Resistant Depression. Depress Anxiety. 2010;10:960–963. doi: 10.1002/da.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]