Abstract
AIM
To determine the optimal concentration for inducing the differentiation of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) into neuron-like cells, although it is understood that all-trans retinoic acid (ATRA) regulates cell proliferation in the nervous system by modulating the balance between mitosis and apoptosis.
METHODS
The abilities of ATRA to promote apoptosis as well as neural differentiation were assessed in cultured hUC-MSCs by morphological observation, MTT assay, annexin V-FITC/PI flow cytometry and immunocytochemistry.
RESULTS
The data showed that low concentrations of ATRA (0.5 µmol, 0.25 µmol) had no effect on the number of cells. However, treatment with 1.0 µmol or 2.0 µmol ATRA induced a 24.16% and 52.67% reduction in cell number, respectively, compared with vehicle-treated cultures. Further, 4.0 µmol ATRA had a potent effect on cell number, with almost no adherent cells recovered after 24h. We further showed that 0.5 µmol ATRA caused these cells to express characteristic markers of neuronal progenitor cells.
CONCLUSION
Taken together, we conclude that ATRA has a dose-dependent influence on the neural differentiation and apoptosis of hUC-MSCs. These findings have implications on the use of ATRA-differentiated hUC-MSCs for the study of neural degeneration diseases.
Keywords: human umbilical cord mesenchymal stem cells, differentiation, apoptosis, neuron-like cells
INTRODUCTION
Human embryonic stem cell transplantation is a promising therapeutic approach for the replacement of degenerated retinal cells in patients with age-related macular degeneration, retinitis pigmentosa, Stargardt's disease, and other retinal degenerative disorders. Prior to subretinal transplantation of stem cells, it is important to induce them to differentiate into neuron-like cell lines. To date, the most common source of mesenchymal stem cells (MSCs) has been bone marrow[1],[2]. However, aspirating bone marrow from the patient is an invasive procedure; in addition, it has been demonstrated that the proliferation and differentiation potential of human bone marrow-derived MSCs (hBM-MSCs) decreases with age. For these reasons, much effort has been expended searching for alternative sources of MSCs, with a particular focus on identifying tissues containing cells with a higher proliferative potential and differentiation capacity as well as a lower risk of contamination. Recently, several groups succeeded in isolating MSCs from umbilical cord[3],[4]. The procedure for their isolation is not invasive, and since the MSCs are of fetal origin, their proliferative and differentiation potential may be superior to than that of MSCs derived from other sources[5],[6]. Thus, the umbilical cord is thought to be a promising source of MSCs. Meanwhile, the possible neuroprotective effect of MSC transplantation has received increased attention because of its therapeutic potential[7],[8].
All-trans retinoic acid (ATRA) is a metabolite of vitamin A and has several physiological functions, including roles in cell differentiation, neurite outgrowth and cell survival[9],[10]. Previous experimental studies on ATRA demonstrate that it regulates cell proliferation in the nervous system and other tissues by modulating the balance between mitosis and apoptosis[11],[12]. These observations collectively indicate that ATRA plays an important role in cell differentiation, proliferation and apoptosis. Until now, it has remained undetermined what concentrations are optimal to induce cell differentiation or to initiate apoptosis in different cell lines. Thus, this raises the question of whether researchers should choose to use high or low concentration of ATRA in neuron differentiation research. Further, there is still controversy regarding the feasibility of isolation of MSCs from umbilical cord.
In the current study, we initially attempted to establish a method for isolating MSCs from human umbilical cord. Then, to address the questions highlighted above, we treated the isolated human umbilical cord-derived MSCs (hUC-MSCs) with different concentrations of ATRA to observe the drug's effects on apoptosis, necrosis and proliferation, and to identify the optimal concentration for inducing differentiation into neuron-like cells. We compared the expression of different neuronal markers in undifferentiated and ATRA-differentiated hUC-MSCs by immunocytochemical analysis. Our results have implications for the use of ATRA-differentiated hUC-MSCs in vivo studies of neural degeneration diseases.
SUBJECTS AND METHODS
The protocol was approved by our Institutional Reviews Board, and the research followed the tenets of the Declaration of Helsinki.
Isolation, Culture and Identification of Human Umbilical Cord-derived Mesenchymal Stem Cells
Fresh umbilical cords (n=28, gestational age: 39-40wk) were collected from informed, consenting mothers and processed as soon as possible. Moreover, the fresh umbilical cords were processed within the optimal processing period of 6h. The cords were rinsed twice with phosphate-buffered saline (PBS) containing penicillin and streptomycin to remove the cord blood. The washed cords were cut into 1-mm3 pieces and incubated for 2-3h using 2.5% collagenase (Sigma) in D-hanks' balanced salt solution (PAA, Austria). Following this step, the suspension was filtered, collected and centrifuged for 10min at 2000 rpm. After counting the number of cells, the cell suspension was seeded in 35-mm 6-well plates at a density of approximately 103 cells/cm2; cells were cultured in Dulbecco's modified Eagle's medium/F12 (DMEM/F12) containing 10% FBS, 4 mmol glutamine, 105 U/L penicillin and 100 mg/L streptomycin in a 37°C incubator with a 5% CO2 atmosphere. Non-adherent cells were removed by washing. The culture medium was half-changed every 3d after the initial plating. When well-developed colonies of fibroblast-like cells appeared after 7d, the cultures were trypsinized and passaged (without dilution) into a new flask for further expansion; the medium changed every 3d. Cells from the 3rd to 4th passage were used in our experiments. Cell cultures were examined using an inverted light microscope (Olympus CKX-41; Japan). Samples were photographed with a digital camera at 100-200×magnification.
To analyze growth curve, the culture-expanded cells were seeded at 1×104 cells/well in a 24-well culture plate (Nunc, Roskilde, Denmark) following each subculture. The cells from each well were harvested and counted by hemocytometer. Three sets of cultures were set up at P1 and P3 passage, and the mean of the counts was calculated. The cell cycle statues of MSCs at P3 were analyzed by flow cytometry (FACScan, BD Biosciences). Briefly, the MSC at P3 were released by 0.25% trypsin-EDTA. A total of 1×106 cells were resuspended in 200 µL PBS and were incubated with fluorescein isothiocyanate-conjugated antibodies for 30min at room temperature. The following monoclonal antibodies were used: CD29, CD34, CD44, CD45, CD90, HLA-DR (BD, Phar Mingen, San Diego, CA, USA). After being washed, the cells were analyzed with a flow cytometer.
MTT Assay for Cell Proliferation Measurements
For cell growth and proliferation studies, the colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was employed. For cell culture assays, a freshly prepared solution of ATRA solubilized in dimethyl sulfoxide (DMSO) was diluted with culture medium to a final concentration of 4.0 µmol, 2.0 µmol, 1.0 µmol, 0.5 µmol, 0.25 µmol, and 0 µmol. Each cell line was plated in five 24-well culture plates. After 24h, the medium was replaced with the ATRA solutions. After a further 24h, an aliquot of the MTT solution (Sigma) was incubated with each sample for 4h. The metabolically active cells cleaved the yellow tetrazolium salt, MTT, to yield purple formazan crystals. The formazan produced was solubilized and the absorbance at 563 nm was measured by a multiplate reader. All MTT experiments were performed four times.
Annexin V Assay for Apoptotic Cell Measurements
Staining with propidium iodide (PI) and Annexin V (Molecular Probes) was performed to detect apoptotic cells. Annexin V binds to externalized phosphatidylserine, thereby allowing detection of apoptosis cells. PI stains cells with broken nuclear membranes and binds to free DNA molecules. Non-stimulated cells were used as negative controls. In these sets of experiments, 3×105 cells from the control and ATRA groups (4.0 µmol, 2.0 µmol, 1.0 µmol, 0.5 µmol, and 0.25 µmol) were harvested by centrifugation, washed twice with ice-cold PBS and resuspended in 500 µL binding buffer. Both adherent and floating cells were harvested for use in the apoptosis assay. Annexin V-FITC (5 µL) and PI (5 µL) (Sigma; USA) were added to individual samples and incubated for 15min in a dark environment. The stained cells were then analyzed with a flow cytometer (BECKMAN Coulter). The results were analyzed with CELLQuest software (BD Biosciences). All measurements were carried out with the same instrument and under the same experimental conditions.
Immunocytochemistry
Cells were treated with DMSO (the control group) or 0.5 µmol ATRA (the determined optimal concentration) for 28-30d. Cells for immunocytochemical and flow cytometric analyses were harvested with 0.25% trypsin, washed twice with PBS and fixed for 30min 4°C with phosphate-buffered 2% paraformaldehyde. The fixed samples were washed twice in PBS and permeabilized with 0.6% saponin (Sigma; USA) in PBS solution for 30min at room temperature. The samples were then washed twice with PBS containing 1% FCS and incubated for 24h at 4°C with the following primary anti-human antibodies (Sigma; USA): anti-nestin (1:500), anti-glial fibrillary acidic protein (GFAP, 1:500), and anti-neuron specific enolase (NSE, 1:500). Each sample was washed twice with PBS and incubated for 1h at 4°C with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Sigma; USA). Following this step, samples were washed and fixed in 2% paraformaldehyde solution. For fluorescence microscopy, cells were grown on glass slides in 6-well culture plates. These images were used as raw controls for the flow cytometry experiments.
Statistical Analysis
The results are expressed as mean±SEM and are derived from at least three independent experiments. Differences between the ATRA- and vehicle-treated cells were analyzed by the Student's t-test. For FACS data, two-way analysis of variance (ANOVA) was used for significance tests. Significance was defined as P<0.01.
RESULTS
Isolation, Culture and Morphology of Human Umbilical Cord-derived Mesenchymal Stem Cells
As soon as the collected umbilical cords were delivered to the laboratory, they were processed as described above; the time from delivery to processing in the laboratory did not exceed 3-4h. After the initial 3d of primary culture, the medium was half-changed for the first time once the cells adhered to the plastic surface. Two types of adherent cells were observed: a population of small flattened cells morphologically similar to endothelial cells, and a population of spindle-shaped fibroblast-like cells. Since no growth factors that would stimulate the growth of endothelial-like cells were present in the culture medium, these cells died in about 7-10d. The fibroblast-like cells were the only cell type left growing under these conditions after 2wk of culture. After the 2nd passage, the culture appeared to be homogeneous and a monolayer formed. The cells displayed a long spindle-shaped fibroblastic morphology, began to form colonies and became confluent (Figure 1). Thus, a population of clonogenic cells with fibroblast-like morphology and proliferative potential was selected from an initially heterogeneous cell population.
Figure 1. Morphological appearance of hUC-MSCs.
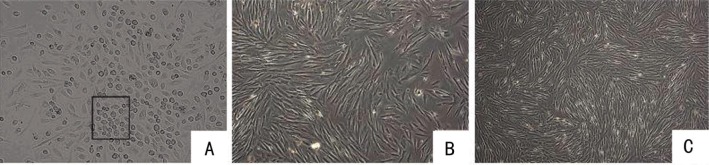
A: After 4d of culture, two types of adherent cells were observed: a population of small flattened cells morphologically similar to the endothelial cells (black rectangle), and a population of spindle-shaped fibroblast-like cells (magnification×100); B: On 7 to 10d after initial plating, the cells began to appear as long spindle-shaped fibroblastic cells, began to form colonies and became confluent (magnification×100); C: The appearance of hUC-MSCs during the 10th passage. The cells retained their proliferative potential with long passages (magnification×100).
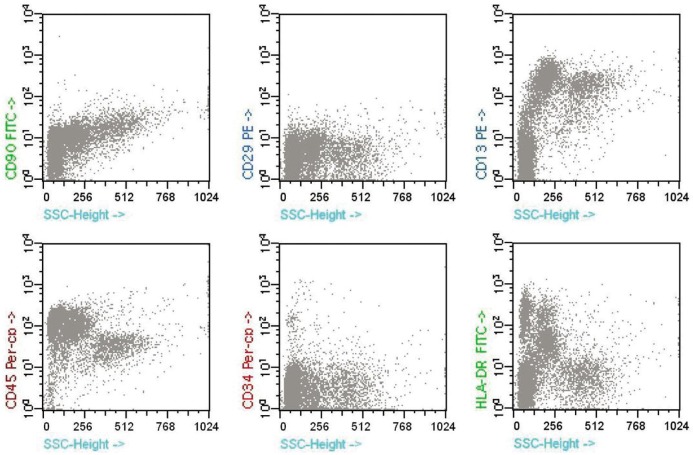
The growth characteristics of MSC were compared between P1 and P3. There were significant differences in cell number and growth kinetics during the culture period. After 5d, a plateau of P3 in the growth was reached while that of P1 occurred after 12d of culture (Figure 2). In the cytometric analysis, MSCs did not present labeling for the hematopoietic lines CD34, CD45, HLA-DR and were positive for the following adhesion molecules: CD90 (Thy 1), CD29 (integrin β1), and CD44 (H-CAM) (Figure 3).
Figure 2. Growth curves of hUC-MSCs.
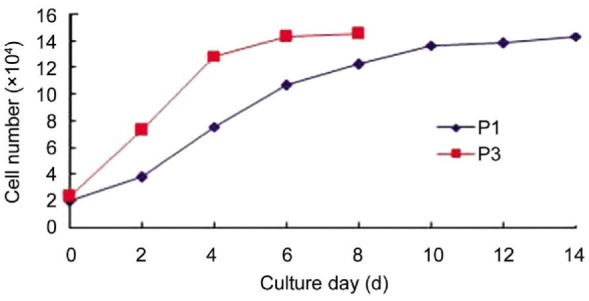
The growth characteristics of hUC-MSCs were compared between passage 1 (P1) and passage 3 (P3). There were significant differences in growth kinetics during the culture period. After 5d, a plateau of P3 in the growth was reached while that of P1 occurred after 12d of culture.
Figure 3. Immunophenotyping results of human hUC-MSCs.
Cells at passage 3 (P3) were examined by flow cytometry. hUC-MSCs were strongly positive for MSC-specific markers, such as CD90, CD29, and CD13, while negative for the hematopoietic line CD34, CD45 and HLA-DR.
All-trans Retinoic Acid Induces a Dose-dependent Reduction in Human Umbilical Cord-derived Mesenchymal Stem Cells Number
To investigate if ATRA has an effect on hUC-MSCs number, we treated hUC-MSCs cultures with different concentrations of ATRA for 24h, trypsinized and counted the adherent cells. As a control, cells were also treated with DMSO, which has previously been shown to have no effect on the proliferation of cultured cells. The data showed that low concentrations of ATRA (0.5 µmol, 0.25 µmol) had no effect on the number of cells. However, treatment with 1.0 µmol or 2.0 µmol ATRA induced a 24.16% and 52.67% reduction in cell number, respectively, compared with vehicle-treated cultures. Further, 4.0 µmol ATRA had a potent effect on cell number, with almost no adherent cells recovered after 24h (Figure 4).
Figure 4. ATRA-induced apoptosis in hUC-MSCs.
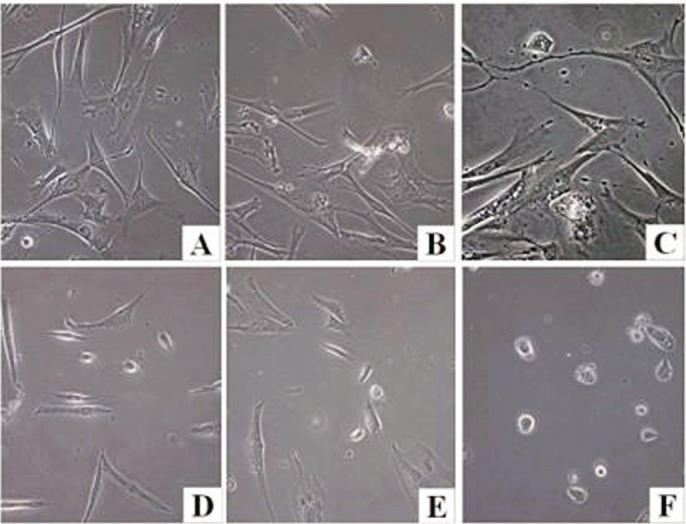
Morphological changes (A-F) in hUC-MSCs during the neural differentiation induced by ATRA. Panels present phase-contrast images of hUC-MSCs cultured with either DMSO (A) or with 0.25 µmol (B), 0.5 µmol (C), 1.0 µmol (D), 2.0 µmol (E), and 4.0 µmol (F) of ATRA (magnification×200).
All-trans Retinoic Acid Affect Human Umbilical Cord-derived Mesenchymal Stem Cells Viability
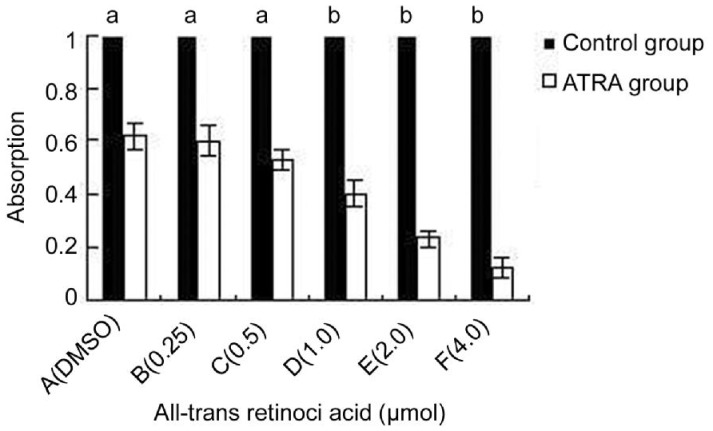
We attempted to study the influence of ATRA on the incidence of apoptosis as well as the proliferation of hUC-MSCs. ATRA was diluted into 10% FCS culture medium at various concentrations (4.0 µmol, 2.0 µmol, 1.0 µmol, 0.5 µmol, 0.25 µmol) and the cells in each group were analyzed both visually and by flow cytometry. For cell growth and proliferation studies, both the colorimetric MTT and Annexin V-FITC/PI flow cytometric assays were performed. As ATRA concentration was raised, we observed a corresponding decrease in cell viability as measured by the MTT assay. Following ATRA treatment, MTT analysis found that there was a 35.48% (1.0 µmol), 62.90% (2.0 µmol) and 19.35% (4.0 µmol) reduction in absorbance compared to DMSO-treated cells (Figure 5). To confirm these results, we employed the Annexin V-FITC/PI assay. Cells stimulated with less than 0.5 µmol ATRA showed nearly no apoptosis or necrosis. Importantly, an increase (2.69-fold) in the number of apoptotic cells was observed for 1.0 µmol ATRA-treated cells. In comparison to the control, incubation with 4 µmol ATRA induces a high degree of apoptosis and necrosis in hUC-MSCs cultures (Figure 4). This result was in line with results of the MTT assay. Together, we conclude that 0.5 µmol ATRA is the optimal concentration for inducing the differentiation of hUC-MSCs without significant cell death.
Figure 5. MTT proliferation assay.
ATRA concentrations above 1 µmol inhibit the proliferation of hUC-MSCs significantly (aP>0.05, bP<0.01).
All-trans Retinoic Acid Promotes the Differentiation of Human Umbilical Cord-derived Mesenchymal Stem Cells into Neuron-like Cells
To address the question whether ATRA can induce the neural differentiation of hUC-MSCs, the cultured hUC-MSCs were cultured in basic differentiation medium containing 0.5 µmol ATRA for another 28-30d. DMSO-treated hUC-MSCs served as negative controls. In the ATRA treatment group, 79.8%±7.6% of surviving cells differentiated into NSE-positive neurons compared to 8.7%±3.4% in the non-treated culture (P<0.01), 35.2%±7.4% of the surviving ATRA-treated cells differentiated into GFAP-positive cells compared to 4.7%±3.3% in the non-treated culture (P<0.01) and 14.2%±6.1% of the surviving ATRA-treated cells differentiated into nestin-positive cells compared to 5.3%±2.1% in the non-treated culture (P<0.01). These percentages are significantly higher than that observed with DMSO-treated hUC-MSCs, indicating that ATRA can improve neural differentiation compared with the control group. Typical immunofluorescence images of cells stained following treatment with ATRA are shown in Figure 6.
Figure 6. In vitro differentiated neural cells derived from hUC-MSCs.
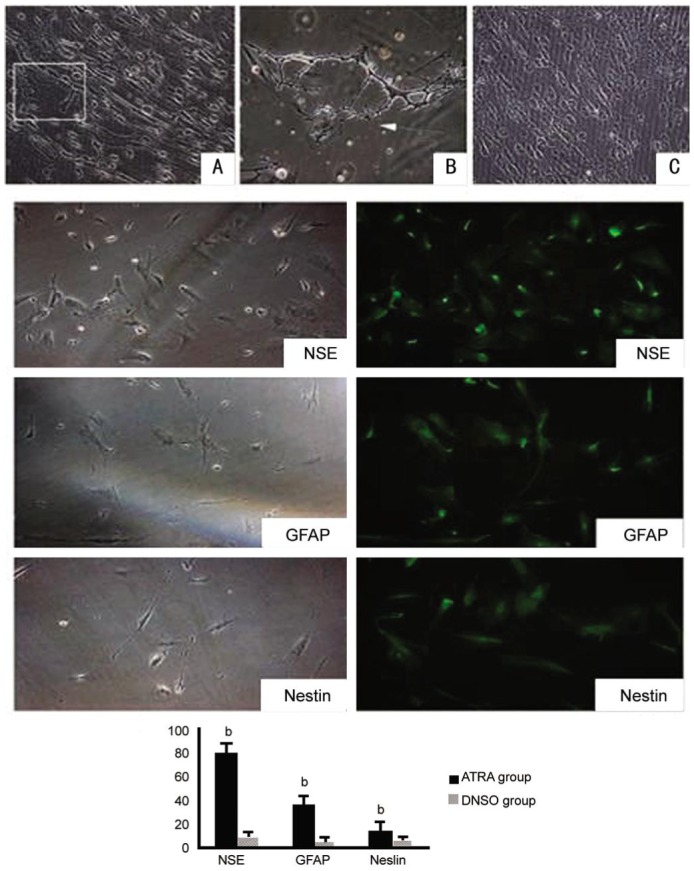
Some differentiated cells formed intercellular bridges (A, B, C) (magnification×200). Fluorescence microscopy of in vitro-differentiated neural cells derived from hUC-MSCs. At the 28thd after the induction of differentiation, many inoculated hUC-MSC expressed neuronal cell antigens NSE, GFAP, nestin, and also show long neurite growth (magnification×200), bP<0.01.
DISCUSSION
Apoptosis is a fundamental biological process of programmed cell death and plays essential roles in both the development of the organism as well as the maintenance of cellular homeostasis; additionally, it participates in various pathological processes[13]. It is well understood that the exposure of whole mouse embryos to low doses of retinoic acid (RA) during early development results in microphthalmia and anophthalmia[14],[15]. Similarly, ATRA induces apoptosis of cultured embryonic rat or murine cerebral neurons, hepatocytes and thymocytes[16],[17]. Taken together, these studies show that ATRA helps regulate cell proliferation in the nervous system and other tissues by modulating the balance between mitosis and apoptosis.
Recent studies indicate that adherent stem cells isolated from umbilical cords, termed “unrestricted somatic stem cells” (USSCs), can be expanded in vitro so that they give rise to multiple lineages and differentiate into adipocytes, osteocytes, cardiomyocytes and neurons[18]–[20]. Animal transplantations involving multi-organ engraftments also demonstrate that these cells contribute to all three germ layers, including ectodermal derivatives such as the nervous system[21],[22]. Yang et al[7] used tricyclodecan-9-yl-xanthogenate (D609) to induce hUC-MSCs to differentiate into neuron-like cells (HUMSC-NCs), and transplanted the HUMSC-NCs into an AβPP/PS1 transgenic AD mouse model. They found that HUMSC-NC transplantation decreased Aβ deposition and improved memory in AβPP/PS1 mice by a mechanism associated with activating M2-like microglia and modulating neuroinflammation. Transplantation of neuron-like cells differentiated from MSCs might be a promising cell therapy for Alzheimer disease[7]. Moreover, as both VEGF and HUMSCs displayed limited neuroprotection in Xiong et al's study[8], they investigated whether hUC-MSCs combined with VEGF expression could offer enhanced neuroprotection. They demonstrated that hUC-MSCs differentiated into dopaminergic neuron-like cells on the basis of neuron-specific enolase (NSE) (neuronal marker), GFAP (astrocyte marker), nestin (neural stem cell marker) and tyrosine hydroxylase (TH) (dopaminergic marker) expression. Further, VEGF expression significantly enhanced the dopaminergic differentiation of HUMSCs in vivo. These findings present the suitability of HUC-MSC as a vector for gene therapy and suggest that stem cell engineering may improve the transplantation strategy for the treatment of nuerodegeneration disease.
ATRA, a metabolic product of vitamin A (retinol), is an established signaling molecule essential for the normal development of the central nervous system, particularly the retina. The importance of vitamin A for the development and maintenance of the visual system has been recognized since Dowling's observations of retinal degenerative phenotypes in vitamin A-deprived rats[23]. The fact that RA can inhibit cell growth in cancer cells by binding to the RA receptor (RAR) is well-known[24]. Interestingly, we found RA can also stimulate cell proliferation and differentiation at a particular concentration. The result raises the question of how RA treatment can lead to two opposite outcomes depending on the dose employed. Clearly, the stimulation of cellular growth and differentiation would have to occur by mechanisms that are different from those involved with the inhibition of tumor growth. The first evidence that helped to reconcile these two opposing effects was obtained by Chapellier et al[25]. These authors found that the activation of the orphan receptor PPARβ/δ, a member of an entirely different family of nuclear receptors, resulted in enhanced mitosis of cells of the basal layer of skin keratinocytes, which is necessary for maintaining skin integrity and the epidermal barrier after wounding. To follow up this finding, Schug and colleagues made the important discovery that RA can activate the transcription of two different nuclear receptors, RAR and PPARβ/δ[26]. The activation of RAR, which is by far the most common mechanism, can give rise to inhibition of cell growth. The activation of the other receptor, PPARβ/δ, leads to expression of genes inducing cell proliferation. These findings serve as the basis for a future study of the mechanisms of RA induction of neural differentiation of hUC-MSCs.
This report demonstrates that ATRA can induce the neural differentiation of hUC-MSCs as well as drive apoptosis. It is worth noting that there are some limitations of this study to keep in mind. In spite of our experiments regarding the morphological differentiation and immunoreactivity of the hUC-MSCs, whether or not the hUC-MSC-derived neural-like cells are functionally active remains to be confirmed by an in vivo study. We believe that both functional and morphological evaluations of neurons differentiated from stem cells are necessary for further study.
Acknowledgments
We wish to thank all the volunteers who donated umbilical cord for this study as well as the opening room staff for their help in procuring them. Thanks are due to Dr. Hua-Min Jin and Dr. Ping-An Zhang for significant advice and technical support, respectively.
Foundations: Supported by Natural Science Foundation of Hubei Province of China (No.2012FFB04401); PhD Programs Foundation of Ministry of Education of China (No.20130141120052)
Conflicts of Interest: Jin W, None; Xu YP, None; Yang AH, None; Xing YQ, None.
REFERENCES
- 1.Wulf GG, Chapuy B, Trümper L. Mesenchymal stem cells from bone marrow: Phenotype, aspects of biology, and clinical perspectives. Med Klin (Munich) 2006;101(5):408–413. doi: 10.1007/s00063-006-1052-6. [DOI] [PubMed] [Google Scholar]
- 2.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 3.Salehinejad P, Alitheen NB, Ali AM, Omar AR, Mohit M, Janzamin E, Samani FS, Torshizi Z, Nematollahi-Mahani SN. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton's jelly. In Virto Cell Dev Biol Anim. 2012;48(2):75–83. doi: 10.1007/s11626-011-9480-x. [DOI] [PubMed] [Google Scholar]
- 4.Simões IN, Boura JS, dos Santos F, Andrade PZ, Cardoso CM, Gimble JM, da Silva CL, Cabral JM. Human mesenchymal stem cells from the umbilical cord matrix: successful isolation and ex vivo expansion using serum-/xeno-free culture media. Biotechnol J. 2013;8(4):448–458. doi: 10.1002/biot.201200340. [DOI] [PubMed] [Google Scholar]
- 5.Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cordblood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kestendjieva S, Kyurkchiev D, Tsvetkova G, Mehandjiev T, Dimitrov A, Nikolov A, Kyurkchiev S. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Bio Int. 2008;32(7):724–732. doi: 10.1016/j.cellbi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu Z, Wang P, Zhao C, Bi J. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res Ther. 2013;4(4):76. doi: 10.1186/scrt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong N, Zhang Z, Huang J, Chen C, Zhang Z, Jia M, Xiong J, Liu X, Wang F, Cao X, Liang Z, Sun S, Lin Z, Wang T. VEGF-expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson's disease. Gene Ther. 2011;18(4):394–402. doi: 10.1038/gt.2010.152. [DOI] [PubMed] [Google Scholar]
- 9.Haskell GT, Lamantia AS. Retinoic acid signaling indentifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005;25(33):7636–7647. doi: 10.1523/JNEUROSCI.0485-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chanda B, Ditadi A, Iscove NN, Keller G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell. 2013;155(1):215–227. doi: 10.1016/j.cell.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 11.Goncalves MB, Agudo M, Connor S, Mcmahon S, Minger SL, Maden M, Corcoran JP. Sequential RARbeta and alpha signaling in vivo can induce adult forebrain neural progenitor cells to differentiate into neurons through Shh and FGF signaling pathways. Dev Biol. 2009;326(2):305–313. doi: 10.1016/j.ydbio.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, Chang RC. Effect of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30(1):127–135. doi: 10.1016/j.neuro.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Tsukane M, Yamauchi T. Increase in apoptosis with neural differentiation and shortening of the lifespan of P19 cells overexpressing tau. Neurochem Int. 2006;48(4):243–254. doi: 10.1016/j.neuint.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Matt N, Ghyselinck NB, Pellerin I, Dupe V. Impairing retinoic acid signaling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320(1):140–148. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 15.Duester G. Keeping an eye on retinoic acid signaling during eye development. Chem Biol Interact. 2009;178(1–3):178–181. doi: 10.1016/j.cbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kholodenko R, Kholodenko I, Sorokin V, Tolmazova A, Sazonova O, Buzdin A. Anti-apoptotic effect of retinoic acid on retinal progenitor cells mediated by a protein kinase A-dependent mechanism. Cell Res. 2007;17(2):151–162. doi: 10.1038/sj.cr.7310147. [DOI] [PubMed] [Google Scholar]
- 17.Borracci P, Carratù MR. Rat embryo exposure to all-trans retinoic acid results in long-term cognitive deficits. Eur Rev Med Pharmacol Sci. 2014;18(1):28–33. [PubMed] [Google Scholar]
- 18.Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26(2):215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 19.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12(5):574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 20.Zwart I, Hill AJ, Girdlestone J, Manca MF, Navarrete R, Navarrete C, Jen LS. Analysis of neural potential of human umbilical cord blood-derived multipotent mesenchymal stem cells in response to a range of neurogenic stimuli. J Neurosci Res. 2008;86(9):1902–1915. doi: 10.1002/jnr.21649. [DOI] [PubMed] [Google Scholar]
- 21.West EL, Pearson RA, Tschernutter M, Sowden JC, Maclaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane lead to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86(4):601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low CB, Liou YC, Tang BL. Neural differentiation and potential use of stem cells from the human umbilical cord for central nervous system transplantation therapy. J Neurosci Res. 2008;86(8):1670–1679. doi: 10.1002/jnr.21624. [DOI] [PubMed] [Google Scholar]
- 23.McFadden SA, Howlett MH, Mertz JR, Wallman J. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp Eye Res. 2006;83(4):949–961. doi: 10.1016/j.exer.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Wolf G. Retinoic acid as cause of cell proliferation or cell growth inhibition depending on activation of one of two different nuclear receptors. Nutr Rev. 2008;66(1):55–59. doi: 10.1111/j.1753-4887.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 25.Chapellier B, Mark M, Messaddeq N, Calléja C, Warot X, Brocard J, Gérard C, Li M, Metzger D, Ghyselinck NB, Chambon P. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21(13):3402–3413. doi: 10.1093/emboj/cdf331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129(4):723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]