Abstract
AIM
To evaluate the neuroprotective activity of systemically administered edaravone in early and late stage of experimental glaucoma in rats.
METHODS
In this study, 60 Wistar albino rats were used. Experimental glaucoma model was created by injecting hyaluronic acid to the anterior chamber once a week for 6wk in 46 of 60 subjects. Fourteen subjects without any medication were included as control group. Edaravone administered intraperitoneally 3 mg/kg/d to the 15 of 30 subjects starting at the onset of glaucoma induction and also administered intraperitoneally 3 mg/kg/d to the other 15 subjects starting at three weeks after the onset of glaucoma induction. The other 16 subjects who underwent glaucoma induction was administered any therapy. Retinal ganglion cells (RGCs) have been marked with dextran tetramethylrhodamine (DTMR) retrograde at the end of the sixth week and after 48h, subjects were sacrificed by the method of cardiac perfusion. Alive RGC density was assessed in the whole-mount retina. Whole-mount retinal tissues homogenized and nitric oxide (NO), malondialdehyde (MDA) and total antioxidant capacity (TAC) values were measured biochemically.
RESULTS
RGCs counted with Image-Pro Plus program, in the treatment group were found to be statistically significantly protected, compared to the glaucoma group (Bonferroni, P<0.05). The neuroprotective activity of edaravone was found to be more influential by administration at the start of the glaucoma process. Statistically significant lower NO levels were determined in the glaucoma group comparing treatment groups (Bonferroni, P<0.05). MDA levels were found to be highest in untreated glaucoma group, TAC levels were found to be lower in the glaucoma induction groups than the control group (Bonferroni, P<0.05).
CONCLUSION
Systemic administration of Edaravone in experimental glaucoma showed potent neuroprotective activity. The role of oxidative stress causing RGC damage in glaucoma was supported by this study results.
Keywords: antioxidant, edaravone, glaucoma, neuroprotection
INTRODUCTION
Glaucoma is a progressive, multifactorial optic neuropathy involving optic nerve head cupping and visual field defects caused by the damage of retinal ganglion cells (RGC) bodies and axons and death due to apoptosis. Sixty million people have been affected by the glaucoma in worldwide, and this number is expected to reach 80 million in 2020[1]. Even when lowering intraocular pressure (IOP), the most important risk factor in the development of glaucoma, the disease can be progress[2]. Therefore, it is necessary that reveal the underlying pathology of glaucoma, to determine an ideal treatment strategy.
The term of neuroprotection is a common pathway developed against excitotoxicity of free radicals, oxidative stress, mitochondrial dysfunction and apoptosis that the influential factors on pathophysiological mechanisms of Alzheimer's disease, stroke, glaucoma and many neurodegenerative diseases[3]. Astrocytes, glial cells are also as well as other targets of neuroprotection. However, the final common pathway of glaucomatous optic neuropathy is the loss of RGCs, thus priority of neuroprotection must preserve these cells[4]. Neuronal loss occurs within minutes in the process of stroke whereas glaucomatous optic neuropathy progress in over the years. In both disease use of neuroprotective agents are known to be effective in preventing neuronal loss and delaying the process leading to cell death. Also it is obvious that early initiation of neuroprotective therapy prolongs the process of cell death[5].
Edaravone (3-methyl-1-phenyl-2-pyrazoline-5-one, MC-186, EDA) have been used to treat acute ischemic stroke as a free radical scavenger in Japan, since 2001. Neuroprotective, free radical scavenger, anti-apoptotic and anti-inflammatory effects of edaravone have been shown in recent studies. With the use of edaravone therapeutically effective results have been obtained in cerebral infarction, aneurysmal subarachnoid hemorrhage and acute stroke[6],[7]. Edaravone protects the brain and heart from reperfusion injury by suppressing the production of reactive oxygen radicals[8].
The effect of edaravone has been demonstrated in many acute and chronic neurodegenerative diseases. Glaucoma is a neurodegenerative disease, involving free radical cell damage and oxidative stress in its pathogenesis. Therefore, we have tried to show the effectiveness of edaravone as a new neuroprotectant in the experimental glaucoma model for the first time.
MATERIALS AND METHODS
This work was conducted in Kocaeli University animal experiment research laboratory after obtaining approval from the local ethics committee and in accordance with the Declaration of Helsinki.
In this study, a total of 60 adult male Wistar albino rats were used. Wistar rats (male), in active condition, 12-16 weeks old and weight is about (350-400 g) are included in the study. Rats showing eating and behavior disorder, died before the completion of the experiment were excluded from the study.
The mean weight of the subjects was 383±21 g. Subjects were housed in standard cages with in groups of seven and were fed with standard food and water, in temperature (21±2 °C) and humidity controlled rooms. Room lighting was provided by fluorescent lights, and every 12h opening and closing cycles were performed. The subjects were divided into two groups, glaucoma induction groups (46 subjects) and control group (14 subjects).
Experimental Glaucoma Model
Hyaluronic acid (Sigma catalog no. H1751, Sigma Chemical Co., St. Louis, MO, USA) was injected to both anterior chambers of subjects with 30 gauge needle under general anesthesia after administration of intramuscular ketamine hydrochloride (25 mg/kg, Ketalar®, Pfizer) and xylazine hydrochloride (10 mg/kg, Rompun®, Bayer). Injections was performed at corneoscleral limbus by blocking the needle tip in contact with the iris and the lens, one week intervals for 5wk moving towards from 12 o'clock to 6 o'clock[9]. The same amount of hyaluronic acid, balanced salt solution was injected into the anterior chamber of rats in the control group with 30 gauge needle.
Edaravone Administration and Groups
Single dose of 3 mg/kg/d edarovone was injected intraperitoneally, dissolving with 0.5% dimethylsulfoxide (DMSO) in saline with beginning simultaneously of onset glaucoma induction on the same day time (10:00 to 12:00) daily. This group was identified as early edaravone group (EE, 15 subjects). During the study, a total of 42 doses Edarovone were applied to this group of subjects.
Single dose of 3 mg/kg/d Edarovone was injected intraperitoneally dissolving with 0.5% DMSO in saline with beginning 3wk after onset glaucoma induction on the same day time (10:00 to 12:00) daily. This group was identified as late edaravone group (LE, 15 subjects). During the study a total of 21 doses edarovone were applied to this group of subjects.
DMSO (0.5%) in 0.9% physiological saline were injected intraperitoneally on the same day time (10:00 to 12:00) daily with beginning simultaneously of onset glaucoma induction to other 16 subjects (glaucoma group-G) as control solution. Any drugs were administered to the control group (control group-C).
Intraocular Pressure Measurement
All subjects IOP were measured and recorded following anesthesia with 10 mg/kg ketamine hydrochloride and administration of topical 0.4% proparacaine hydrochloride 0.5% (Alcaine®, Alcon) with Tono-Pen (Medtronic Solan XL) before and after each hyaluronic acid injection weekly, lasted for five weeks. All injections and measurements were performed on the same day time (08:00 to 10:00). At the end of six weeks the subjects were sacrificed by cardiac paraformaldehyde perfusion method and fixed globes were enucleated.
Biochemical Analysis
After extraction by the method of whole mount, retina tissues were homogenized, nitric oxide (NO), malondialdehyde (MDA) levels were measured with cadmium method. To evaluation of total antioxidant capacity (TAC), hydrogen peroxide solution was prepared in accordance with kit catalog (Antioxidant Assay Kit, Cayman) for the measurement of antioxidant buffer, antioxidant chromogen, antioxidant metmyoglobin, antioxidant Trolox and antioxidant. The measurement was carried out by the ELISA device. The data was converted to concentration after forming trolox standard curve as noted in kit prospectus.
Statistical analysis of data was performed with IBM SPSS package program for windows version 19 software. In the statistical analysis for comparison of variables, multivariate variance analyzes test (ANOVA) was used for all groups and in the comparison groups among themselves, the post-hoc evaluation test (Bonferroni test) was used. Limit of statistical significance was considered P<0.05. Results were expressed as mean±standart deviation (SD).
RESULTS
Demographic Characteristics of Rats
At the beginning of the study, mean weights were 367±14 g in group C, 360±9 g in group G, 364±16 g in group EE and 368±7 g in group LE. At the end of the study, the average weights in the groups were 398±14, 379±7, 382±14 and 386±6 g, respectively. Any behavioral disorders and loss of appetite were not detected with the rats.
Intraocular Pressure Values
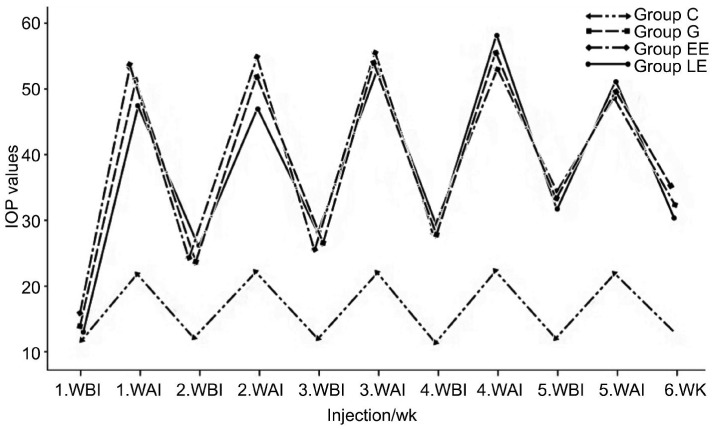
IOP elevation was observed in all subjects, after the glaucoma induction. Adequate IOP values were achieved in all groups for the duration of six weeks. There was a statistically significant difference between the IOP values in G, EE, LE groups compared with group C (P<0.05). Besides there was no statistically significant difference in terms of mean IOP between each other G, EE and LE groups (P>0.05, Figure 1 )
Figure 1. IOP values of the subjects.
WBI: Week before injection; WAI: Week after injection.
Retinal Ganglion Cell Count
RGC cell count was performed with Image Pro Plus (IPP 5.1 for Windows®; Media Cybernetics, Silver Spring, MD, USA) program. DTMR marked average RGC density (average number in mm2±SD), and the differences between the groups are presented in the Table 1. A significant reduction was determined in number of RGC in the subjects with glaucoma induction. The average number of RGC of the groups G, EE and LE were found to be significantly lower compared to group C (Bonferroni, P<0.000, P<0.033, P<0.000). RGC counts were significantly higher in groups LE and EE compared to group G (Bonferroni, P<0.000, P<0.000). Furthermore, average RGC numbers in group EE were significantly higher compared to the group LE (Bonferroni, P<0.000).
Table 1. DTMR marked retinal ganglion cells count in the subjects.
| Groups | No. of cells in mm2 | Total number |
| Group C | 1520±95 | 10856±684 |
| Group G | 822±110 | 5869±789 |
| Group EE | 1366±79b | 9753±567b |
| Group LE | 1125±74b | 8033±534b |
bP<0.01. Comparison of treatment groups with glaucoma group (ANOVA, P<0.05).
Six weeks after glaucoma induction, average RGC reduction was 45%±5% in group G, 10%±3% in group EE and 25%±4% in group LE compared with the group C. Considering parameters of total retinal cell number, cell number in mm2 and percentage reduction in cell number, RGCs were found to be significantly protected in group EE and LE compared to group G. DTMR marked RGC immunofluorescence images of the groups are presented in Figure 2.
Figure 2. DTMR marked immunofluorescence images of RGCs.
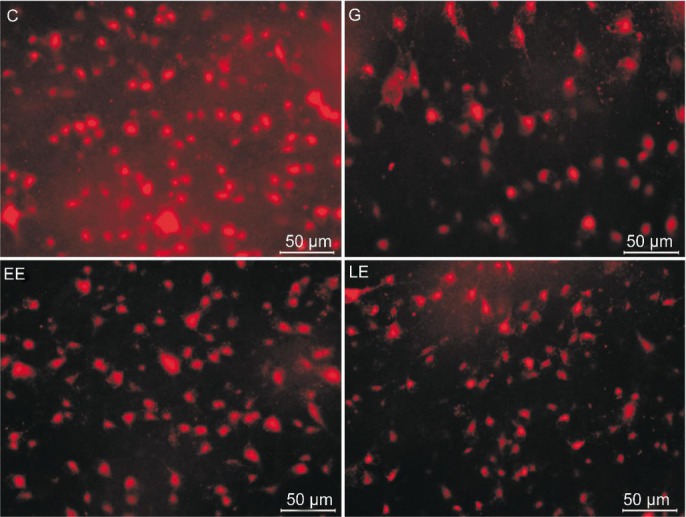
Group abbreviations was placed in picture.
Biochemical Analysis of Retinal Tissue
After excising Whole mount average wet weight of retina tissues were 18.08±5.25 mg in group C, 15.80±4.12 mg in group G, 14.56±3.52 mg in group EE and 15.28±5.71 mg in group LE, respectively. There was no statistically significant difference between the groups in terms of wet tissue weight. TAC, MDA and NO levels measured in all groups are presented in Table 2.
Table 2. Biochemical analysis of TAC, MDA and NO levels in the retinal tissue of subjects.
| Groups | TAC (µmol/g) | MDA (nmol/g) | NO (nmol/g) |
| Group C | 0.31±0.12 | 4.15±0.6 | 25.73±5.3 |
| Groupg G | 0.14±0.04 | 7.95±1.2 | 18.39±6.0 |
| Group EE | 0.19±0.05 | 5.82±0.5 | 28.03±7.2 |
| Group LE | 0.18±0.05 | 7.93±0.2 | 27.61±7.5 |
Total Antioxidant Capacity Measurement
After the glaucoma induction, a significant decrease was observed in TAC values of retinal tissue. Mean TAC values of the groups G, EE and LE were found to be significantly lower compared to the group C (Bonferroni, P<0.000, P<0.013, P<0.009). Mean TAC values of the groups EE and LE treated with EDA, were found to be higher compared to the untreated group G (Bonferroni, P>0.1, P>0.1), otherwise group EE values were found to be higher than group LE but all these differences were not statistically significant (Bonferroni, P>0.1).
Malondialdehyde Measurement
As a result of glaucoma induction a significant increase in MDA levels were detected in retinal tissue of subjects. Mean MDA values were found to be significantly higher in group G, EE and LE compared to group C (Bonferroni, P<0.000, P<0.02, P<0.000). Mean MDA values of the groups G and LE, were found to be significantly higher compared to the group EE (Bonferroni, P<0.000, P<0.000), otherwise group EE values were found to be significantly lower than group LE (Bonferroni, P<0.000).
Nitric Oxide Measurement
The lowest NO levels were measured in the group G. Mean NO values of the groups EE and LE, were found to be significantly higher compared to the group G (Bonferroni, P<0.025, P<0.047), otherwise group EE values were found to be higher than group LE but this difference was not statistically significant (Bonferroni, P>0.1).
DISCUSSION
In this study neuroprotective efficacy of systemically administered edaravone have been attempted to show with RGC count on an experimental rat glaucoma model. In addition, NO, MDA and TAC values were measured in retinal tissue to show antioxidative activity of edaravone in glaucoma. Already the neuroprotective efficacy of edaravone has been shown in many studies, although there is no published study relevant to its effects in glaucoma[10]–[12].
The target effect of glaucomatous damage on RGCs and their axons is well known. RGCs and optic nerve are the integral parts of central nervous system. Previous studies have been suggested that glaucoma as a neurodegenerative disease like Alzheimer disease due to similar cell death mechanisms[13],[14]. Therefore, we wonder edaravone to be effective in glaucoma. Neuroprotective efficacy of edaravone treatment has been evaluated in retinal ischemia and phototoxicity models, but DTMR marked RGC evaluation has not been performed previously[15],[16].
In our study number of RGCs of the groups treated with edaravone were significantly preserved, comparing glaucoma group, on the other hand, average RGCs number were higher in group EE than group LE. This can be explained by the edaravone and similar drugs to be less effective on apoptotic death of ganglion cells in the chronic glaucoma model.
Edaravone provides neuroprotective activity by different mechanisms. Munakata et al[7] demonstrated a marked remission of late ischemic neurological deficits and cerebral infarction due to vasospasm in patients with aneurysmal subarachnoid hemorrhage treated with edaravone. Yasuoka et al[11] showed that edaravone inhibits cytochrome c release and caspase-3 activation in the hippocampus and cortex after ischemia. Edaravone prevents neural cell degeneration and death induced by free radicals after cerebral infarction[17].
Detoxification of increased oxygen metabolites due to glaucoma is dependent ocular antioxidants. Oxidative stress is involved in the pathogenesis of many neurodegenerative diseases[18]–[20]. Suppression of the formation of free oxygen radicals with edarovone therapy in Parkinson model has been shown in previous studies[21],[22]. With edarovone therapy, a marked retardation was determined in progressive motor neuron injury in the experimental amyotrophic lateral sclerosis model in mice[23] 19 amyotrophic lateral sclerosis patients reported by Yoshino et al[24] treated with edaravone also showed retardation in progressive deterioration of motor neuron functions. In an another experimental spinal cord injury rat model, inhibition of lipid peroxidation, reduction in MDA levels and better functional recovery was observed in the group treated with edaravone, thus secondary neuronal damage was prevented by edaravone[25]. Free radical scavenger, antioxidant and neuroprotective effects of edaravone are demonstrated by these studies.
Neurodegeneration and oxidative stress in the pathogenesis of glaucoma are well known. So in the light of previous studies we could imagine aderavone as a neuroprotective and antioxidant agent in the treatment of glaucoma. In our study, oxidative stress parameters were also evaluated. MDA is a product of lipid peroxidation occurring in the oxidative process. Elevated MDA levels were determined in vitreous and retina in experimental glaucoma model of rats[26],[27]. Yildirim et al[28], reported higher plasma MDA levels in patients with primary open angle glaucoma (POAG) compared healthy control group. In an experimental rat retinal ischemia model performed by Song et al[29] lower MDA levels were determined in the retina of the group treated with edaravone compared to the untreated control group. In our work, MDA levels were significantly increased with the glaucoma induction. Furthermore, significant lower MDA levels were detected in group EE comparing groups G and LE.
TAC has evaluated as an indicator of retinal antioxidant activity. In our study protective antioxidant activity reduced due to oxidative stress after the glaucoma induction. Consequently highest TAC values were measured in the control group and lowest values in the glaucoma group. As well as higher TAC values were obtained in the groups treated with edarovone comparing untreared glaucoma group, but this difference was not statistically significant. Özgiray et al[30] compared methylprednisolone and edarovone treatment on experimental spinal cord injury model in rats. NO, MDA, TAC levels, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity were evaluated in blood and tissue. In comparison with the control group lower tissue NO and MDA levels, higher tissue TAC levels and increased SOD, GSH-Px activities were detected in the group treated with edaravone. Additionally lower NO levels, similar SOD and GSH-Px activities, higher MDA levels were detected in the blood.
Increased NO synthase (NOS), glutamin synthase levels and decreased glutathione transferase (GST), SOD levels were found in aqueous humor of patients with POAG in a study conducted by Bagnis et al[31]. In another study performed by Cho et al[32] inducible NOS (iNOS) levels were found to be significantly higher in the ischemia-reperfusion model of rats created by increasing IOP than the control group. Along with increased IOP, Park et al[33] demonstrated elevatedneuronal NOS (nNOS) and NO levels in the chronic glaucoma model in rats.
In our study, NO levels were found statistically significantly higher in the groups treated with edaravone than the glaucoma group. Unlike the previous studies, we found decreased NO levels in glaucoma. A reduction in the levels of NO may be due to NOS insufficiency as a result of apoptotic loss of cells, including NOS, and inhibition of NOS by the dimethyl arginine derivates during the glaucoma process[34],[35]. Decreased NO levels in the aqueous humor of patients with POAG was emphasized by Doğanay et al[36] in a study conducted in 2002 for the first time. Following this study, Galassi et al[37] found a significant decrease in NO2, cGMP levelswhich the markers of NO, in the plasma and aqueous humor of patients with POAG, comparing normal eyes. NO makes vasodilatation and enhances vascular contractility on trabecular meshwork thus act an IOP lowering and antiapoptotic effect. After trabeculectomy NOS levels was found to be lower in trabecular meshwork fragments of the patients with POAG[38]–[40]. In this case, we can say that edaravone shows an antioxidant activity in glaucoma.
There were limited studies on topical use of edaravone in the literature. Hironaka et al[41] have achieved significant results with the intravitreal administration of submicron-sized liposomes (ssLips) containing edaravone against oxidative stress-induced retinal damage. Also Shimazaki et al[15] demonstrated neuroprotective effect of eye drops containing liposomes loaded with edaravone in light-induced retinal damage model in mice. In addition to our work, further studies are needed on the use of topical edarovone in glaucoma.
For today, IOP is the single factor can be controlled that slows progression of glaucoma. But despite the effective IOP control the disease can be progress. Priority in current researches focus on neuroprotection. Neuroprotective agents for treating glaucoma should slow down the process of retinal ganglion cell death. At this point, primarily it is necessary to know how the RGCs die. The purpose of neuroprotection studies conducted so far, both to find an appropriate agent for treatment and clarify the pathogenesis of glaucoma. The main objective of this study to present edarovone as a new neuroprotectan and antioxidan in the treatment of glaucoma. Considering our results edarovone showed a significant antioxidant and neuroprotective activity on the experimental glaucoma model. Therefore results of our work support the presence of oxidative stress in the pathogenesis of glaucoma. Researches in the literature are intended for using edaravone on acut and subacute deficits of neurodegenerative diseases. But glaucoma is a slowly progressing, chronic optic neuropathy compared to these diseases. Consequently, although short term experimental effectiveness of edaravone in our study, large-scale, randomized, long term clinical and experimental studies are needed to the clinical use of edaravone in glaucoma.
Acknowledgments
We thank Mustafa Çekmen for his excellent technical assistance.
Foundation: Supported by Kocaeli University Scientific Research Projects Unit (KOU-BAP: project No.2012/002).
Conflicts of Interest: Toruk AK, None, Yuksel N, None, Gok M, None, Cekmen M, None, CağlarY, None
REFERENCES
- 1.Dahlmann-Noor AH, Vijay S, Limb GA, Khaw PT. Strategies for optic nerve rescue and regeneration in glaucoma and other optic neuropathies. Drug Discov Today. 2010;15(7–8):287–299. doi: 10.1016/j.drudis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115(7):1123–1129. doi: 10.1016/j.ophtha.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 2011;22(2):78–86. doi: 10.1097/ICU.0b013e32834372ec. [DOI] [PubMed] [Google Scholar]
- 4.Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119(5):979–986. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casson RJ, Chidlow G, Ebneter A, Wood JP, Crowston J, Goldberg I. Translational neuroprotectio research in glaucoma: a review of definitions and principles. Clin Experiment Ophthalmol. 2012;40(4):350–357. doi: 10.1111/j.1442-9071.2011.02563.x. [DOI] [PubMed] [Google Scholar]
- 6.Edaravone Acute Brain Infarction Study Group Effect of novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Cerebrovasc Dis. 2003;15(3):222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 7.Munakata A, Ohkuma H, Nakano T, Shimamura N, Asano K, Naraoka M. Effect of a free radical scavenger, edaravone, in the treatment of patients with aneurismal subarachnoid hemorrhage. Neurosurgery. 2009;64(3):423–428; discussion 428–429. doi: 10.1227/01.NEU.0000338067.83059.EB. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe T, Tanaka M, Watanabe K, Takamatsu Y, Tobe A. Research and development of the free radical scavenger edaravone as a neuroprotectant. Yakugaku Zasshi. 2004;124(3):99–111. doi: 10.1248/yakushi.124.99. [DOI] [PubMed] [Google Scholar]
- 9.Moreno MC, Marcos HJ, Oscar Croxatto J, Sande PH, Campanelli J, Jaliffa CO, Benozzi J, Rosenstein RE. A new experimental model of glaucoma in rats through intracameral injections of hyaluronic acid. Exp Eye Res. 2005;81(1):71–80. doi: 10.1016/j.exer.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Noor JI, Ikeda T, Mishima K, Aoo N, Ohta S, Egashira N, Iwasaki K, Fujiwara M, Ikenoue T. Short-term administration of a new free radical scavenger, edaravone, is more effective than its long-termadministration for the treatment of neonatal hypoxic-ischemic encephalopathy. Stroke. 2005;36(11):2468–2474. doi: 10.1161/01.STR.0000185653.49740.c6. [DOI] [PubMed] [Google Scholar]
- 11.Yasuoka N, Nakajima W, Ishida A, Takada G. Neuroprotection of edaravone on hypoxic-ischemic brain injury in neonatal rats. Dev Brain Res. 2004;151(1–2):129–139. doi: 10.1016/j.devbrainres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Itoh T, Satou T, Nishida S, Tsubaki M, Hashimoto S, Ito H. The novel free radical scavenger, edaravone, increases neural stem cell number around the area of damage following rat traumatic brain injury. Neurotox Res. 2009;16(4):378–389. doi: 10.1007/s12640-009-9081-6. [DOI] [PubMed] [Google Scholar]
- 13.Murphy JA, Clarke DB. Target-derived neurotrophins may influence the survival of adult retinal ganglion cells when local neurotrophic support is disrupted: Implications for glaucoma. Med Hypotheses. 2006;67(5):1208–1212. doi: 10.1016/j.mehy.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 14.Guo L, Salt TE, Maass A, Luong V, Moss SE, Fitzke FW, Cordeiro MF. Assessment of neuroprotective effects of glutamate modulation on glaucoma related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47(2):626–633. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimazaki H, Hironaka K, Fujisawa T, Tsuruma K, Tozuka Y, Shimazawa M, Takeuchi H, Hara H. Edaravone-loaded liposome eyedrops protect against light-induced retinal damage in mice. Invest Ophthalmol Vis Sci. 2011;52(10):7289–7297. doi: 10.1167/iovs.11-7983. [DOI] [PubMed] [Google Scholar]
- 16.Inokuchi Y, Imai S, Nakajima Y, Shimazawa M, Aihara M, Araie M, Hara H. Edaravone, a free radical scavenger, protects against retinal damage in vitro and in vivo. J Pharmacol Exp Ther. 2009;329(2):687–698. doi: 10.1124/jpet.108.148676. [DOI] [PubMed] [Google Scholar]
- 17.Niyaz M, Numakawa T, Matsuki Y, Kumamaru E, Adachi N, Kitazawa H, Kunugi H, Kudo M. MCI-186 prevents brain tissue from neuronal damage in cerebral infarction through the activation of intracellular signaling. J Neurosci Res. 2007;85(13):2933–2942. doi: 10.1002/jnr.21412. [DOI] [PubMed] [Google Scholar]
- 18.Sakata Y, Zhuang H, Kwansa H, Koehler RC, Dore S. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol. 2010;224(1):325–329. doi: 10.1016/j.expneurol.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potashkin JA, Meredith GE. The role of oxidative stress in the dysregulation of gene expression and protein metabolism in neurodegenerative disease. Antioxid Redox Signal. 2006;8(1–2):144–151. doi: 10.1089/ars.2006.8.144. [DOI] [PubMed] [Google Scholar]
- 20.Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22(1):76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Xiong N, Xiong J, Khare G, Chen C, Huang J, Zhao Y, Zhang Z, Qiao X, Feng Y, Reesaul H, Zhang Y, Sun S, Lin Z, Wang T. Edaravone guard dopamine neurons in a rotenone model for Parkinson's disease. PLoS ONE. 2011;6(6):1–11. doi: 10.1371/journal.pone.0020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan WJ, Yasuhara T, Shingo T, Muraoka K, Agari T, Kameda M, Uozumi T, Tajiri N, Morimoto T, Jing M, Baba T, Wang F, Leung H, Matsui T, Miyoshi Y, Date I. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008;9:75. doi: 10.1186/1471-2202-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi R. Edaravone in ALS. Exp Neurol. 2009;217(2):235–236. doi: 10.1016/j.expneurol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study) Amyotroph Lateral Scler. 2006;7(4):241–245. doi: 10.1080/17482960600881870. [DOI] [PubMed] [Google Scholar]
- 25.Ohta S, Iwashita Y, Takada H, Kuno S, Nakamura T. Neuroprotection and enhanced recovery with edaravone after acute spinal cord injury in rats. Spine. 2005;30(10):1154–1158. doi: 10.1097/01.brs.0000162402.79482.fd. [DOI] [PubMed] [Google Scholar]
- 26.Ko ML, Peng PH, Ma MC, Ritch R, Chen CF. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. 2005;39(3):365–373. doi: 10.1016/j.freeradbiomed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Yücel I, Akar Y, Yücel G, Ciftçioğlu MA, Keleş N, Aslan M. Effect of hypercholesterolemia on inducible nitric oxide synthase expression in a rat model of elevated intraocular pressure. Vision Res. 2005;45(9):1107–1114. doi: 10.1016/j.visres.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Yildirim O, Ateş NA, Ercan B, Muşlu N, Unlü A, Tamer L, Atik U, Kanik A. Role of oxidative stress enzymes in open-angle glaucoma. Eye (Lond) 2005;19(5):580–583. doi: 10.1038/sj.eye.6701565. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, Gong YY, Xie ZG, Li CH, Gu Q, Wu XW. Edaravone (MCI-186), a free radical scavenger, attenuates retinal ischemia/reperfusion injury in rats. Acta Pharmacol Sin. 2008;29(7):823–882. doi: 10.1111/j.1745-7254.2008.00822.x. [DOI] [PubMed] [Google Scholar]
- 30.Ozgiray E, Serarslan Y, Oztürk OH, Altaş M, Aras M, Söğüt S, Yurtseven T, Oran I, Zileli M. Protective effects of edaravone on experimental spinal cord injury in rats. Pediatr Neurosurg. 2011;47(4):254–260. doi: 10.1159/000335400. [DOI] [PubMed] [Google Scholar]
- 31.Bagnis A, Izzotti A, Centofanti M, Saccà SC. Aqueous humor oxidative stress proteomic levels in primary open angle glaucoma. Exp Eye Res. 2012;103:55–62. doi: 10.1016/j.exer.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Cho KJ, Kim JH, Park HY, Park CK. Glial cell response and iNOS expression in the optic nerve head and retina of the rat following acute high IOP ischemia-reperfusion. Brain Res. 2011;1403:67–77. doi: 10.1016/j.brainres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Park SH, Kim JH, Kim YH, Park CK. Expression of neuronal nitric oxide synthase in the retina of a rat model of chronic glaucoma. Vision Res. 2007;47(21):2732–2740. doi: 10.1016/j.visres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Resch H, Garhofer G, Fuchsijager-Maryl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87(1):4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 35.Javadiyan S, Burdon KP, Whiting MJ, Abhary S, Straga T, Hewitt AW, Mills RA, Craig JE. Elevation of serum asymmetrical and symmetrical dimethylarginine in patients with advanced glaucoma. Invest Ophthalmol Vis Sci. 2012;53(4):1923–1927. doi: 10.1167/iovs.11-8420. [DOI] [PubMed] [Google Scholar]
- 36.Doganay S, Evereklioglu C, Turkoz Y, Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002;12(1):44–48. doi: 10.1177/112067210201200109. [DOI] [PubMed] [Google Scholar]
- 37.Galassi F, Renieri G, Sodi A, Ucci F, Vannozzi L, Masini E. Nitric oxide proxides and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004;88(6):757–760. doi: 10.1136/bjo.2003.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefan C, Dumitrica DM, Ardeleanu C. The future started: nitric oxide in glaucoma. Oftalmologia. 2007;51(4):89–94. [PubMed] [Google Scholar]
- 39.Cleary C, Buckley CH, Henry E, McLoughlin P, O'Brien C, Hadoke PW. Enhanced endothelium derived hyperpolarising factor activity in resistance arteries from normal pressure glaucoma patients: implications for vascular function in the eye. Br J Ophthalmol. 2005;89(2):223–238. doi: 10.1136/bjo.2004.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol. 2005;16(2):79–83. doi: 10.1097/01.icu.0000156134.38495.0b. [DOI] [PubMed] [Google Scholar]
- 41.Hironaka K, Inokuchi Y, Fujisawa T, Shimazaki H, Akane M, Tozuka Y, Tsuruma K, Shimazawa M, Hara H, Takeuchi H. Edaravone-loaded liposomes for retinal protection against oxidative stress-induced retinal damage. Eur J Pharm Biopharm. 2011;79(1):119–125. doi: 10.1016/j.ejpb.2011.01.019. [DOI] [PubMed] [Google Scholar]