Abstract
AIM
To investigate the effect of pregnancy on subfoveal choroidal thickness (SFCT) and macular thickness in both pregnant and not pregnant healthy women.
METHODS
Twenty-nine healthy pregnant women in their third trimester and 36 age-matched healthy women were enrolled in a prospective, cross-sectional study. Foveal and parafoveal thickness in the four quadrants and SFCT were measured by optical coherence tomography (OCT) in the healthy pregnant women (i.e. study group) and healthy women (i.e. control group). OCT measurements were again measured 3mo after delivery in the study group.
RESULTS
Mean SFCT measurements in the control group, pregnant women of the study group, and after delivery of the study group were 320.86±59.18 µm, 387.97±59.91 µm, and 332.40±26.03 µm, respectively. There was a statistically significant difference in the mean SFCT values between pregnant women of the study group and the control group (P=0.000). Foveal and parafoveal thickness values were not statistically significant in either the study or control group.
CONCLUSION
SFCT increases during pregnancy and returns to normal range in the three months after delivery. Macular thickness does not show any change during pregnancy.
Keywords: enhanced depth imaging optical coherence tomography, pregnancy, choroidal thickness, foveal thickness
INTRODUCTION
Pregnancy is a process that physiologically changes almost all organs due to changes in the vascular, immunologic, metabolic, and hormonal systems. One of the most important organs affected by these changes is the eye[1]. Previous studies have reported the physiological effects of pregnancy on the eye and eyesight to include decreased intraocular pressure (IOP), loss in the visual field, increased corneal thickness, and altered corneal curvature[2]–[5]. Furthermore, pathological changes during pregnancy that did not otherwise exist have also been reported, including central serous chorioretinopathy (CSC), for which pregnancy was reported to be a risk factor. The increased frequency of CSC has been attributed to the hyperpermeability of choroid vessels during pregnancy[6]–[8].
Optical coherence tomography (OCT), primarily used to view retinal pathologies, is currently being used for choroid screening due to advances in its technology. Studies using enhanced depth imaging OCT (EDI-OCT) have shown the thickening of the choroid in patients with CSC[9],[10]. This finding suggests that the increased frequency of CSC during pregnancy is caused by physiological and pathological changes in the choroid.
To date, a few studies have used the EDI-OCT technique to measure choroidal thickness in pregnant women compared to that of nonpregnant women[11],[12]. No studies, however, have examined choroidal and macular thickness in pregnant women during and after pregnancy.
Our study thus aims to evaluate the effect of pregnancy on choroidal and macular thickness by comparing choroidal and macular thickness in women during and after pregnancy.
SUBJECTS AND METHODS
Study Population and Design
This prospective comparative study was performed in the Departments of Obstetrics, Gynecology, and Ophthalmology at Kayseri Education and Research Hospital. The study followed the tenets of the Declaration of Helsinki and was approved by the local ethics committee. All participants received both oral and written information about the study, and each participant provided written informed consent.
Participants were recruited into two groups; the study group consisted of 29 healthy pregnant women in their third trimester, while the control group consisted of 36 healthy nonpregnant women of reproductive age.
Ocular exclusion criteria for this study included prior history of significant ocular disease, a refractive error of either less than -2 diopters (D) or more than +2 D, a best corrected visual acuity (BCVA) worse than 20/20, amblyopia, IOP readings greater than 21 mm Hg, glaucoma, history of uveitis, retinal disease, ocular trauma or tumor, poor image quality, and dense media opacities.
Extraocular exclusion criteria included a history of systemic disease, such as hypertension or diabetes mellitus, and the development of complications such as gestational diabetes mellitus, preeclampsia, and pregnancy-induced hypertension in pregnant women.
Examination Protocol and Study Measurements
All participants in both groups underwent a complete examination that included a Snellen BCVA, biomicroscopy, IOP measured by Goldmann applanation tonometry, and dilated fundus examination. The axial length (AL) was measured with the IOL Master 500 (Carl Zeiss Meditec Inc, Jena, Germany). Before taking OCT measurements, systolic blood pressures (SBP) and diastolic blood pressures (DBP) were measured using a sphygmomanometer (Omron Inc, Japan) with participants in the sitting position. Readings for SBP and DBP were obtained after each participant had sat for 10min. The mean arterial blood pressure (MABP) was calculated according to the formula MABP=2/3*DBP+1/3*SBP, while the ocular perfusion pressure (OPP) was calculated with OPP=2/3*MABP–IOP[13],[14]. These measurements were retaken 3mo after delivery in the study group. Because of diurnal fluctuation, all examinations were performed between 9 a.m. and 11 a.m.
OCT Measurements
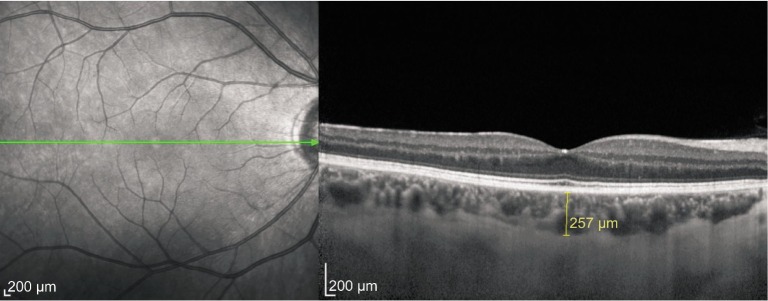
Following this detailed ophthalmologic examination, the third-generation Spectralis OCT device (software version 5.6.3.0; Spectralis OCT, Heidelberg Engineering, Dossenheim, Germany) was used for assessment. The method of obtaining EDI-OCT images has been previously reported[15],[16]. Subfoveal choroidal thickness (SFCT) was determined as the vertical distance from the hyperreflective line of the hyperreflective retinal pigment epithelium to the line of the inner surface of the sclera centered on the fovea, which was taken using a tool with built-in linear measuring. A representative EDI-OCT choroidal image in a pregnant woman is seen in Figure 1. Images were captured by one experienced clinician and assessed by another experienced clinician. Group identities remained anonymous to both clinicians. Macular thickness was determined automatically and analyzed by OCT software. A macular thickness map was used for macular measurements. During assessments, macular thickness and volume analysis were analyzed. We selected the retinal thickness map analysis protocol on the Spectralis device to display numeric averages of the measurements for each of the five subfields, which were the central subfield (in 1 mm), inferior inner macula, nasal inner macula, superior inner macula, and temporal inner macula (in 3 mm). OCT measurements were again measured 3mo after delivery in the study group.
Figure 1. A typical choroidal image and the measurement points in pregnant women.
Statistical Analysis
All statistical tests were performed using SPSS (Statistical Package for the Social Sciences) version 16. The normality of the data was confirmed using the Kolmogorov-Smirnov test (P>0.05). An independent t-test was used to compare variables among study and control groups. Comparison of variables between pregnant women of the study group and after delivery of the study group were performed by a paired t-test. The categorical variables between the groups were analysed by using χ2 test. Pearson's correlation was used to examine the relationships among the measured variables. A P value of less than 0.05 was considered significant.
RESULTS
Table 1 shows the demographic characteristics in the study and control groups. Table 2 shows the results of macular thickness and SFCT measurements in the control group and study group. Mean SFCT measurements differed with statistical significance between the pregnant women of the study group and control group (P=0.000). However, the mean foveal and parafoveal macular thickness values did not differ with any statistical significance between the pregnant women of the study group and control group (P>0.05). There was also a statistically significant difference in the mean SFCT values between those taken during pregnancy and those taken 3mo after delivery (P=0.000). There was, however, no significant difference in the mean foveal and parafoveal macular thickness measured during pregnancy and those measured 3mo after delivery (P>0.05). Table 3 shows the results of the other clinical measurements including AL, IOP, MABP, OPP, and BCVA in the study and control groups. Table 4 shows the correlation analyses between SFCT and other clinical and demographic factors in both groups. The SFCT value was not significantly associated with AL, IOP, MABP, OPP, BCVA or age in either the study or control group. SFCT was also not significantly associated with gestational age during pregnancy.
Table 1. Demographic characteristics in healthy women and pregnant women.
| Variables | Healthy women (control group) | Pregnant women (study group) | Pb |
| Number of eyes /patients | 72/36 | 58/29 | |
| Age (a), mean±SD (range) | 28.00±5.27, (19-40) | 27.41±5.48, (18-38) | 0.536 |
| Gestational age (wk), mean±SD (range) | - | 31.97±3.77, (28-39) |
SD: Standart deviation. bIndependent Student's t-test
Table 2. Evaluation of macular and choroidal thickness in control group and study group.
| Variables | Healthy women (control group) (n=72) | Pregnant women (study group) (n=58) | After pregnancy (study group) (n=58) |
P |
||
| Healthy women vs pregnant womena | Healthy women vs after pregnancya | Pregnant women vs after pregnancyb | ||||
| SFCT | 320.86±59.18 | 387.97±59.91 | 332.40±26.03 | 0.000 | 0.170 | 0.000 |
| Fovealc | 257.24±17.88 | 255.03±17.87 | 252.47±21.37 | 0.486 | 0.168 | 0.363 |
| Superior quadrantd | 343.67±13.41 | 342.78±11.37 | 343.40±13.02 | 0.688 | 0.908 | 0.751 |
| Temporal quadrantd | 335.26±13.07 | 332.57±14.71 | 336.47±16.70 | 0.271 | 0.646 | 0.094 |
| Inferior quadrantd | 337.51±13.82 | 337.10±12.13 | 338.57±12.64 | 0.859 | 0.654 | 0.379 |
| Nasal quadrantd | 333.32±14.84 | 331.79±15.11 | 329.90±14.04 | 0.564 | 0.183 | 0.456 |
SFCT: Subfoveal choroidal thickness. aİndependent Student's t-test; bPaired t-test; cMacular thickness in 1 mm field; dMacular thickness in 3 mm field
Table 3. Other clinical measurements in the control group and study group.
| Variables | Healthy women (control group) (n=72) | During pregnancy (study group) (n=58) | After pregnancy (study group) (n=58) |
P |
||
| Healthy women vs pregnant womena | Healthy women vs after pregnancya | Pregnant women vs after pregnancyb | ||||
| AL (mm) | 22.92±0.90 | 23.04±0.73 | 23.05±0.73 | 0.418 | 0.387 | 0.549 |
| IOP (mm Hg) | 15.92±2.83 | 13.34±2.91 | 15.28±2.75 | 0.000 | 0.195 | 0.000 |
| MABP (mm Hg) | 80.17±8.64 | 74.79±8.39 | 80.34±8.14 | 0.001 | 0.905 | 0.000 |
| OPP (mm Hg) | 37.80±6.64 | 36.77±6.96 | 38.56±6.34 | 0.392 | 0.509 | 0.000 |
| BCVAc | 0.95±0.14 | 0.94±0.09 | 0.94±0.08 | 0.495 | 0.650 | 0.410 |
AL: Axial length; IOP: Intraocular pressure; MABP: Mean arterial blood pressure; OPP: Ocular perfusion pressure; BCVA: Best corrected visual acuity. aİndependent Student's t- test; bPaired t-test; cAccording to Snellen chart
Table 4. Correlation analyses between SFCT and other clinical and demographic factors in all group.
| Variables | SFCT |
|||||
| Healthy women (control group) |
During pregnancy (study group) |
After pregnancy (study group) |
||||
| r | Pa | r | Pa | r | Pa | |
| AL | -0.123 | 0.304 | -0.074 | 0.579 | 0.257 | 0.052 |
| IOP | 0.077 | 0.521 | 0.227 | 0.086 | 0.014 | 0.917 |
| MABP | -0.081 | 0.500 | -0.116 | 0.386 | 0.028 | 0.833 |
| OPP | -0.103 | 0.389 | -0.189 | 0.156 | 0.018 | 0.892 |
| BCVA | 0.74 | 0.538 | -0.48 | 0.723 | -0.86 | 0.523 |
| Age | 0.038 | 0.752 | -0.101 | 0.453 | 0.228 | 0.085 |
| Gestational age | - | - | -0.035 | 0.792 | - | - |
SFCT: Subfoveal choroidal thickness; AL:Axial length; IOP: Intraocular pressure; MABP: Mean arterial blood pressure; OPP: Ocular perfusion pressure; BCVA: Best corrected visual acuity. aPearson correlation test.
DISCUSSION
Pregnancy causes several physiological changes to the human body. Very important cardiovascular changes develop before, during, and after the delivery. Blood volume and erythrocyte counts increase, physiological anemia occurs, and blood pressure drops. There is also a slight increase in both the total fluid volume of the body and the intracellular fluid volume[17]. Increased cardiac flow and volume cause an increase in ocular blood flow during pregnancy, as studies have reported[18],[19]. A high volume, from 65% to 85%, of ocular blood flows through the choroid, thus a structurally and physiologically normal choroid is important for retinal function. Thermoregulation of ocular tissues, uveoscleral aqueous drainage, regulation of intraocular pressure, and removal of waste from the eye are also performed by the choroid[20]. The structure and thickness of the choroid can be affected by different ocular pathologies as well as systemic diseases[21]–[25], hence the importance of choroidal structure assessment.
Various techniques, such as indocyanine green angiography, magnetic resonance imaging, and Doppler ultrasonography, have previously been used with various success rates while studying choroidal structures. However, none of these techniques have been able to measure choroid thickness in detail for morphological structure assessment[26]–[28].
The recent development of OCT technologies has allowed more detailed choroid assessment. With a new technique called EDI-OCT, the evaluation of choroidal thickness, and morphology, studies have reported choroidal thickness to be affected by several factors. One study found that as age increases, choroidal thickness decreases[29]. Other studies have shown that errors of refraction affect choroidal thickness, especially in case of myopia, as well as that, as the refraction degree and axial length increase, choroidal thickness decreases[30]–[32].
Choroidal thickness can also be affected by ocular pathologies such as choroidal neovascular membrane, uveal effusion syndrome, CSC, Vogt-Koyanagi-Harada disease, angioid streaks, and polypoidal choroidal vascularopathy, as well as systemic diseases such as diabetes[21]–[25]. However, there are a few studies about choroidal thickness in pregnant women. Takahashi et al's[11] study on 30 healthy and 30 pregnant women compared subfoveal and parafoveal choroidal thickness in both groups and found no statistically significant difference between those two groups. On the contrary, Kara et al's[12] study found statistically significant difference in SFCT between the pregnant and non-pregnant women.
By contrast, no studies have compared choroidal thickness during and after pregnancy. To the best of our knowledge, our study alone has investigated the changes in SFCT during and after pregnancy. In this study, when healthy and pregnant women were compared in terms of choroidal thickness, the choroid was significantly thicker in pregnant women. Moreover, the same pregnant women's choroidal thickness was closer to normal range in the third month after delivery.
In other studies, choroidal thickness was found to be significantly correlated with age and axial length[29]–[32]. However, in our study, there was no statistically significant correlation between choroidal thickness and age and axial length, which runs counter to the results of previous studies. This discrepancy is likely due to the fact that the age range in the study was narrow. In addition, we excluded women with high refractive errors in our study, which explains the lack of correlation between choroidal thickness and axial length. In studies using SFCT and performed on healthy individuals, IOP and OPP correlations were assessed but no significant correlations were found[33]. In this regard our results were similar to previous studies. There were also no statistically significant correlations found between SFCT, gestational age, and MABP.
A few studies have investigated macular thickness during pregnancy. Demir et al[34] have shown an increase of macular thickness in superior, inferior, and temporal quadrants, while Cankaya et al[35] have reported an increase in macular thickness and volume values during the second and third trimesters.
In addition to our study's review of macular thickness, the same pregnant women were assessed 3mo after delivery in the foveal area (1 mm) and parafoveal area's (3 mm) superior, temporal, inferior, and nasal quadrants' macular thickness. There was no statistically significant difference in the foveal and parafoveal thickness in all quadrants' macular thickness when data taken during pregnancy and 3mo after delivery were compared.
In conclusion, SFCT increases in the third trimester of the pregnancy and returns to normal range in the 3mo after delivery. Otherwise, the 1 mm foveal and 3 mm parafoveal thickness in all quadrants do not show any change during pregnancy. In future studies, all changes in choroid should be assessed by EDI-OCT during the whole pregnancy period (such as 1st, 2nd and 3rd trimester) and these changes should be monitored closely for frequently seen diseases during pregnancy such as CSC.
Acknowledgments
Conflicts of Interest: Ulusoy DM, None; Duru N, None; Ataş M, None; Altınkaynak H, None; Duru Z, None; Açmaz G, None.
REFERENCES
- 1.Kubica-Trzaska A, Karska-Basta I, Kobylarz J, Romanowska-Dixon B. Pregnancy and the eye. Klin Oczna. 2008;110(10–12):401–404. [PubMed] [Google Scholar]
- 2.Qureshi IA. Measurements of intraocular pressure throughout the pregnancy in Pakistani women. Chin Med Sci J. 1997;12(1):53–56. [PubMed] [Google Scholar]
- 3.Sunness JS. The pregnant woman's eye. Surv Ophthalmol. 1988;32(4):219–238. doi: 10.1016/0039-6257(88)90172-5. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb RN, Lu A, Beeson C. Maternal corneal thickness during pregnancy. Am J Ophthalmol. 1988;105(3):258–260. doi: 10.1016/0002-9394(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 5.Park SB, Lindahl KJ, Temnycky GO, Aquavella JV. The effect of pregnancy on corneal curvature. CLAO J. 1992;18(4):256–259. [PubMed] [Google Scholar]
- 6.Haimovici R, Koh S, Gaqnon DR, Lehrfeld T, Wellik S, Central Serous Chorioretinopathy Case-Control Study Group Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244–249. doi: 10.1016/j.ophtha.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Experiment Ophthalmol. 2013;41(2):201–214. doi: 10.1111/j.1442-9071.2012.02848.x. [DOI] [PubMed] [Google Scholar]
- 8.Said-Ahmed K, Moustafa G, Fawzy M. Incidence and natural course of symptomatic central serous chorioretinopathy in pregnant women in a maternity hospital in Kuwait. Middle East Afr J Ophthalmol. 2012;19(3):273–276. doi: 10.4103/0974-9233.97920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31(8):1603–1608. doi: 10.1097/IAE.0b013e31820f4b39. [DOI] [PubMed] [Google Scholar]
- 10.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi J, Kado M, Mizumoto K, Igarashi S, Kojo T. Choroidal thickness in pregnant women measured by enhanced depth imaging optical coherence tomography. Jpn J Ophthalmol. 2013;57(5):435–439. doi: 10.1007/s10384-013-0265-5. [DOI] [PubMed] [Google Scholar]
- 12.Kara N, Sayin N, Pirhan D, Vural AD, Araz-Ersan HB, Tekirdag AI, Yildirim GY, Gulac B, Yilmaz G. Evaluation of subfoveal choroidal thickness in pregnant women using enhanced depth imaging optical coherence tomography. Curr Eye Res. 2014;39(6):642–647. doi: 10.3109/02713683.2013.855236. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa CP, Stefanini FR, Penha F, Góes MÂ, Draibe SA, Canziani ME, Paranhos Junior A. Intraocular pressure and ocular perfusion during hemodialysis. Arq Bras Oftalmol. 2011;74(2):106–109. doi: 10.1590/s0004-27492011000200007. [DOI] [PubMed] [Google Scholar]
- 14.Schmidl D, Weigert G, Dorner GT, Resch H, Kolodjaschna J, Wolzt M, Garhofer G, Schmetterer L. Role of adenosine in the control of choroidal blood flow during changes in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2011;52(8):6035–6039. doi: 10.1167/iovs.11-7491. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Larciprete G, Valensise H, Vasapollo B, Altomare F, Sorge R, Casalino B, De Lorenzo A, Arduini D. Body composition during normal pregnancy: reference ranges. Acta Diabetol. 2003;40(1):225–232. doi: 10.1007/s00592-003-0072-4. [DOI] [PubMed] [Google Scholar]
- 18.Centofanti M, Miqliardi R, Bonini S, Manni G, Bucci MG, Pesavento CB, Amin CS, Harris A. Pulsatile ocular blood flow during pregnancy. Eur J Ophthalmol. 2002;12(4):276–280. doi: 10.1177/112067210201200404. [DOI] [PubMed] [Google Scholar]
- 19.Chen HC, Newsom RS, Patel V, Cassar J, Mather H, Kohner EM. Retinal blood flow changes during pregnancy in women with diabetes. Invest Ophthalmol Vis Sci. 1994;35(8):3199–3208. [PubMed] [Google Scholar]
- 20.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manjunath V, Goren J, Fujimoto JG, Duker JS. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152(4):663–668. doi: 10.1016/j.ajo.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31(9):1904–1911. doi: 10.1097/IAE.0b013e31821801c5. [DOI] [PubMed] [Google Scholar]
- 23.Harada T, Machida S, Fujiwara T, Nishida Y, Kurosaka D. Choroidal findings in idiopathic uveal effusion syndrome. Clin Ophthalmol. 2011;5:1599–1601. doi: 10.2147/OPTH.S26324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakai K, Gomi F, Ikuno Y, Yasuno Y, Nouchi T, Ohguro N, Nishida K. Choroidal observations in Vogt-Koyanagi-Harada disease using high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2012;250(7):1089–1095. doi: 10.1007/s00417-011-1910-7. [DOI] [PubMed] [Google Scholar]
- 25.Esmaeelpour M, Považay B, Hermann B, Hofer B, Kajic V, Hale SL, North RV, Drexler W, Sheen NJ. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5311–5316. doi: 10.1167/iovs.10-6875. [DOI] [PubMed] [Google Scholar]
- 26.Lieb WE, Cohen SM, Merton DA, Shields JA, Mitchell DG, Goldberg BB. Color Doppler imaging of the eye and orbit. Technique and normal vascular anatomy. Arch Ophthalmol. 1991;109(4):527–531. doi: 10.1001/archopht.1991.01080040095036. [DOI] [PubMed] [Google Scholar]
- 27.Quaranta M, Arnold J, Coscas G, Français C, Quentel G, Kuhn D, Soubrane G. Indocyanine green angiographic features of pathologic myopia. Am J Ophthalmol. 1996;122(5):663–671. doi: 10.1016/s0002-9394(14)70484-2. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Nair G, Walker TA, Kim MK, Pardue MT, Thulé PM, Olson DE, Duong TQ. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci U S A. 2006;103(46):17525–17530. doi: 10.1073/pnas.0605790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikuno Y, Kawaquchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51(4):2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 30.Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013;155(2):314–319. doi: 10.1016/j.ajo.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Goldenberg D, Moisseiev E, Goldstein M, Loewenstein A, Barak A. Enhanced depth imaging optical coherence tomography: choroidal thickness and correlations with age, refractive error, and axial length. Ophthalmic Surg Lasers Imaging. 2012;43(4):296–301. doi: 10.3928/15428877-20120426-02. [DOI] [PubMed] [Google Scholar]
- 32.Nishida Y, Fujiwara T, Imamura Y, Lima LH, Kurosaka D, Spaide RF. Choroidal thickness and visual acuity in highly myopic eyes. Retina. 2012;32(7):1229–1236. doi: 10.1097/IAE.0b013e318242b990. [DOI] [PubMed] [Google Scholar]
- 33.Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, Chen CX, Xu J, Wang YX, Zhou JQ, You QS. Subfoveal Choroidal Thickness: the Beijing Eye Study. Ophthalmology. 2013;120(1):175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 34.Demir M, Oba E, Can E, Odabasi M, Tiryaki S, Ozdal E, Sensoz H. Foveal and parafoveal retinal thickness in healthy pregnant women in their last trimester. Clin Ophthalmol. 2011;5:1397–1400. doi: 10.2147/OPTH.S23944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cankaya C, Bozkurt M, Ulutas O. Total macular volume and foveal retinal thickness alterations in healthy pregnant women. Semin Ophthalmol. 2013;28(2):103–111. doi: 10.3109/08820538.2012.760628. [DOI] [PubMed] [Google Scholar]


