Abstract
AIM
To investigate the effect of bevacizumab treatment on Notch signaling and the induction of epithelial-of-mesenchymal transition (EMT) in human retinal pigment epithelial cells (ARPE-19) in vitro.
METHODS
In vitro cultivated ARPE-19 cells were treated with 0.25 mg/mL bevacizumab for 12, 24, and 48h. Cell morphology changes were observed under an inverted microscope. The expression of zonula occludens-1 (ZO-1), vimentin and Notch-1 intracellular domain (NICD) was examined by immunofluorescence. The mRNA levels of ZO-1, α-SMA, Notch-1, Notch-2, Notch-4, Dll4, Jagged-1, RBP-Jk and Hes-1 expression were evaluated with quantitative real-time polymerase chain reaction (qRT-PCR). The protein levels of α-SMA, NICD, Hes-1 and Dll-4 expression were examined with Western blot.
RESULTS
Bevacizumab stimulation increased the expression of α-SMA and vimentin in ARPE-19 cells which changed into spindle-shaped fibroblast-like cells. Meanwhile, the mRNA expression of Hes-1 increased and the protein expression of Hes-1 and NICD also increased, which Notch signaling was activated. The mRNA expression of Notch-1, Jagged-1 and RBP-Jk increased at 48h, and while Dll4 mRNA and protein expression did not change after bevacizumab treatment.
CONCLUSION
Jagged-1/Notch-1 signaling may play a critical role in bevacizumab-induced EMT in ARPE-19 cells, which provides a novel insight into the pathogenesis of intravitreal bevacizumab-associated complication.
Keywords: bevacizumab, Notch signaling, epithelial-to-mesenchymal transition, retinal pigment epithelial cells
INTRODUCTION
The leading cause of blindness and visual impairment in elderly individuals of developed countries is age-related macular degeneration (AMD) which leads to approximately 90% of severe vision loss because of choroidal neovascularization (CNV) development in the macular region[1]–[3]. Anti-vascular endothelial growth factor (VEGF) therapy (intravitreal ranibizumab or bevacizumab) is an available approach to treatment of retinal or choroidal neovascularization, but scar fibrosis has become the most common side effect during the long-term follow-up[4]–[6]. Approximately half of 1059 eyes enrolled in Age-related Macular Degeneration Treatments Trials (CATT) developed scar by 2y[7]. In eyes with proliferative diabetic retinopathy treated with anti-VEGF injection, there is also increased fibrosis caused by imbalances between connective tissue growth factor (CTGF) and VEGF[8]. The pathogenesis of these complications after intravitreal bevacizumab (IVB) remains unclear. Chen et al[9] reported bevacizumab can induce epithelial-to-mesenchymal transition (EMT) in the retinal pigment epithelial cells. A growing body of literature demonstrates crosstalk between Notch and VEGF pathways in angiogenesis, VEGF can induce Dll4 expression as part of a negative regulatory[10],[11]. Emerging evidence suggests that the Notch signaling pathway regulates EMT, leading to a large range of fibrotic diseases developed in the kidney, liver, and lung[12]–[14]. Little is known about whether the Notch sighaling pathway is involved in fibrosis formation after IVB. In the present study, we investigated expression changes of Notch signaling during the process of bevacizumab-induced EMT of retinal pigment epithelialium (RPE) cell which may contribute to the possible mechanism of fibrosis scar associated with IVB in clinical applications.
MATERIALS AND METHODS
Reagents and Antibodies
The primary antibodies were smooth muscle α-actin (α-SMA; ab7817, Abcam), Hes-1 (ab71559, Abcam), the Notch-1 intracellular domain (NICD; ab8925, Abcam), vimentin (sc-5565; Santa Cruz, CA, USA) and Dll4 (ab7280, Abcam). The secondary antibody against rabbit labeled with fluorescein isothiocyanate (FITC) was purchased from Zhongshan Golden Bridge Biotechnology Co. Ltd. (Beijing, China). Primers were purchased from the Invitrogen Corporation (China). Bevacizumab (Avastin; Genentech, Inc.). All chemical reagents were of analytical grade and were purchased from sigma.
Cell Culture
Human RPE cell line ARPE-19 (ATCC, catalog No.CRL-2302, Rochkefeller, MD, USA) was cultured in Dulbecco's modified Eagle's medium/Nutrient Mixture F-12 (DMEM/F-12; HyClone Company, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco Company, CA, USA) and penicillin/streptomycin. When the experiment was conducted, bevacizumab was mixed in the medium, with a final concentration of 0.25 mg/mL, roughly equal to the concentration used clinically. ARPE-19 cells were serumstarved overnight and then cultured in DMEM/F-12 with 2% FBS and 0.25 mg/mL bevacizumab treatment for 12, 24, and 48h. Cell morphology changes were observed under an inverted microscope (Olympus CKX41, Tokyo, Japan). The expression of ZO-1, vimentin, and NICD was examined using immunofluorescence staining. The mRNA levels of ZO-1, α-SMA, Notch-1, Notch-2, Notch-4, Dll4, Jagged-1, RBP-Jk and Hes-1 expression were evaluated with quantitative real-time polymerase chain reaction (qRT-PCR), and the protein levels of α-SMA, NICD, Hes-1, Dll4 expression were examined using western blot.
Immunofluorescence Staining
ARPE-19 cells were seeded in 48-well tissue culture plates in amounts of 2×104 cells for incubation of 24h and serumstarved overnight and then cultured in 2% FBS with 0.25 mg/mL Bevacizumab treatment for 48h. The expression of epithelial cell maker ZO-1, the fibrosis marker vimentin, and NICD was examined using immunofluorescence staining in the control group and the bevacizumab group. Primary antibodies were ZO-1, vimentin, and NICD; secondary antibody was FITC-labeled goat antirabbit IgG, and nucleus stained with DAPI purchased from Zhongshan Golden Bridge Biotechnology Co. Ltd. (Beijing, China). Finally, the cells were visualized and photographed using a confocal microscope (NIKON TE2000, Tokyo, Japan).
Real-time Polymerase Chain Reaction Analysis
ARPE-19 cells were seeded in 6-well tissue culture plates in amounts of 1×105 cells for incubation of 24h and serumstarved overnight and then cultured in 2% FBS with 0.25 mg/mL bevacizumab treatment for 12, 24, and 48h. The total RNA was extracted with the NucleoSpin RNA II System (Company Macherey-Nagel, Düren, Germany) according to the manufacturer's protocol and was reverse-transcribed using PrimeScript RT reagent Kit (Takara Biotechnology Company, Dalian, Liaoning Province, China). qRT-PCR was performed with SYBR Green RealMasterMix and specific primers (Table 1).
Table 1. Primers for the genes in this study.
| Gene | Oligo | Sequences | Amplicon (bp) |
| α-SMA | F | GGGACATCAAGGAGAAACTGTGT | 150 |
| R | TCTCTGGGCAGCGGAAAC | ||
| ZO-1 | F | TTCCAGATAAAGCCCCAGTTAATG | 150 |
| R | GCAGAAGATTGTGATTGAATTTAGGA | ||
| NOTCH-1 | F | CGCCGTGAACAATGTGGAT | 157 |
| R | GTCCCGGTTGGCAAAGTG | ||
| RBP-JK | F | TTCCACCAGCCTTACCTTTACC | 150 |
| R | GTGCTGGCGTTTGTGTAACTTC | ||
| HES-1 | F | TTTGGATGCTCTGAAGAAAGATAGC | 150 |
| R | CGGTACTTCCCCAGCACACTT | ||
| Jagged-1 | F | GGGAACCCGATCAAGGAAAT | 150 |
| R | GCTCAGCAAGGGAACAAGGA | ||
| NOTCH-2 | F | GGCTCCCTGTTCCCCAAA | 186 |
| R | ATGTAGCTGCCCTGGGTGTT | ||
| NOTCH-4 | F | CCTGGTTGAAGAACTGATTGCA | 150 |
| R | TTGTCCTGGGCATCTTTATCG | ||
| Dll4 | F | TGTGGCAAACAGCAAAACCA | 150 |
| R | CCGACACTCTGGCTTTTCACT |
Western Blot
ARPE-19 cells were seeded in 25 cm2 tissue culture flask in amounts of 4×105 cells for incubation of 24h and serumstarved overnight and then cultured in 2% FBS with 0.25 mg/mL bevacizumab treatment for 48h. Cells were harvested in 120 µL 1.5×loading buffer. The homogenates, which contained 20 µg of protein, were then separated by SDS-PAGE and transferred to nitrocellulose membrane (Thermo Fisher Scientific, Beijing, China). The blots were probed with the following primary antibodies: α-SMA (ab7817, Abcam), Hes-1 (ab71559, Abcam), NICD (ab8925, Abcam). Quantification of the western blot data was performed by measuring the intensity of the hybridization signals using Image J software.
Statistical Analysis
All results were expressed as the means±standard deviations (SD). Data were analyzed with the SPSS 17.0 statistical package. A one-way analysis of independent samples t-test for two-sample comparisons was used to compare the means of two groups. A P value of less than 0.05 was considered statistically significant. The reported results were representative of three independent experiments.
RESULTS
Changes in Endothelial Cell Morphology During Bevacizumab-induced EMT in ARPE-19 Cells
ARPE-19 cells exhibited a cobblestone-like morphology in the absence of bevacizumab, while after exposure to bevacizumab, some ARPE-19 cells changed into spindle-shaped fibroblast-like cells, which were larger and less compact than the untreated cells (Figure 1).
Figure 1. Cell morphological change.
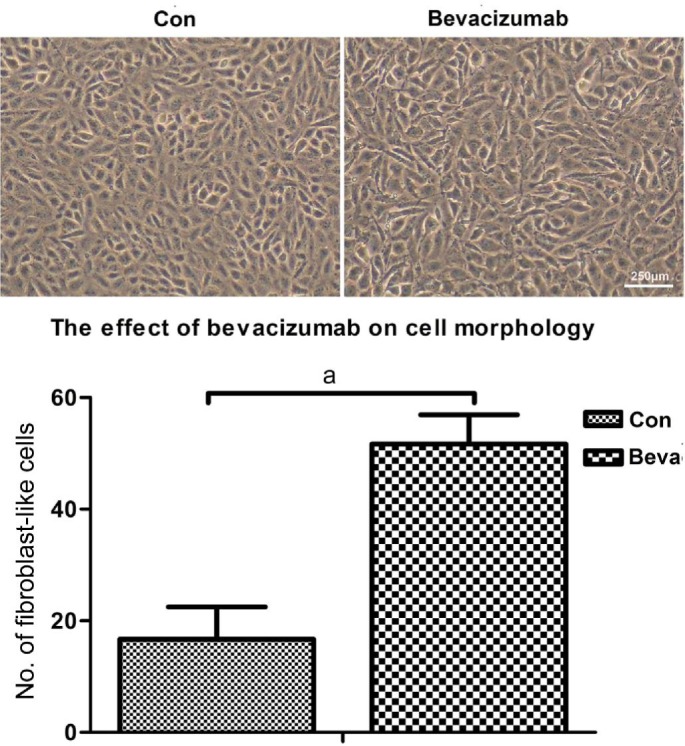
ARPE-19 cells exhibited a cobblestone-like morphology in the absence of bevacizumab, after exposure to bevacizumab for 48h. ARPE-19 cells changed into spindle-shaped fibroblast-like cells, larger and less compact than untreated cells. aP<0.05.
Effect of Bevacizumab on ZO-1, Vimentin and α-SMA Expression in ARPE-19 Cells
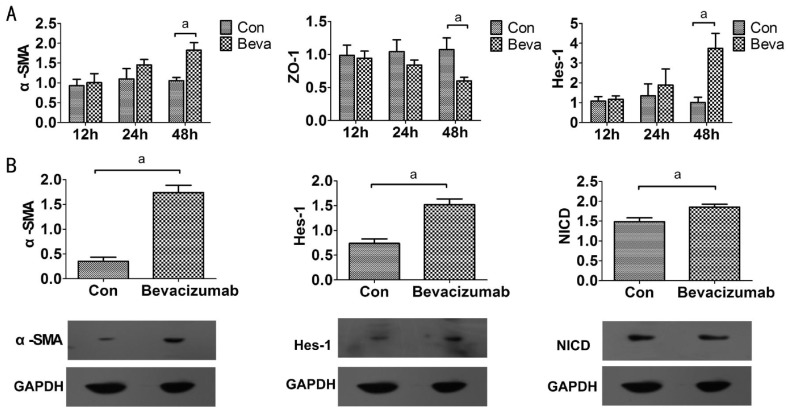
We assessed EMT-related cellular responses in ARPE-19 cells after bevacizumab treatment. Epithelial-mesenchymal transition is characterized by loss of epithelial makers such as ZO-1 and replacement by mesenchymal markers α-SMA and vimentin[15]. REP cells were incubated with 0.25 mg/mL bevacizumab treatment for 48h, and immunofluorescence revealed that bevacizumab treatment decreased the expression of the epithelial phenotype marker ZO-1 at endothelial cell-cell junctions and increased the expression of the mesenchymal marker vimentin in the cytoplasm (Figure 2). We found the mRNA levels of ZO-1 and α-SMA expression were not affected at 12 and 24h. In contrast, when the ARPE-19 cells were exposed to bevacizumab for 48h, ZO-1 mRNA expression decreased and α-SMA mRNA expression increased (Figure 3A), meanwhile, the α-SMA protein expression also increased (Figure 3B). These results indicated that bevacizumab can make ARPE-19 cells transdifferentiate into mesenchymal cells at 48h.
Figure 2. Immunofluorescence staining.
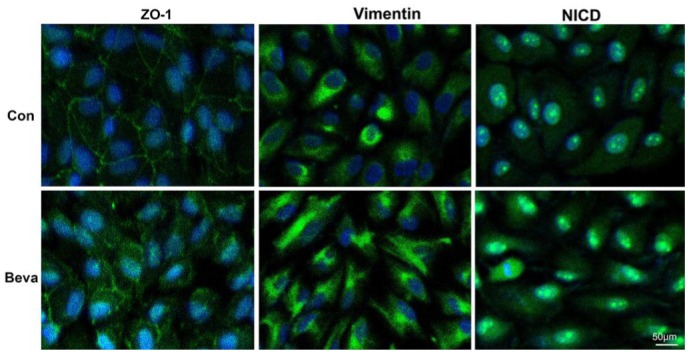
Immunoflurescence staining of ZO-1, vimentin, and NICD in ARPE-19 cells in control group and bevacizumab group for 48h. Nuclei were stained with DAPI (blue).
Figure 3. Bevacizumab can promote EMT and activate Notch signaling in ARPE-19 cells.
A: The mRNA expression of α-SMA, ZO-1 and Hes-1 was examined by real-time PCR after 0.25 mg/mL bevacizumab exposure for 12, 24, and 48h. Data are given as mean±SD (n=4). aP<0.05; B: The protein levels of α-SMA, Hes-1, and NICD were analyzed by western blot at 48h after bevacizumab (0.25mg/mL) treatment. Graphic representation of relative abundance of α-SMA, Hes-1, and NICD normalized to GAPDH. Data are given as mean±SD (n=3). aP<0.05.
Bevacizumab Promoted Hes-1 Expression and Activated Notch Signaling
To investigate whether Notch signaling attended EMT, we observed Notch signaling changes when bevacizumab induced EMT in ARPE-19 cells. Because the cleavage of Notch-1 is an indicator of Notch signaling activation and Hes-1 is Notch downstream target, immunofluorescence revealed that bevacizumab treatment increased the production of NICD (Figure 2). We examined NICD and Hes-1 as markers of activated Notch expression by western blot. Hes-1 expression was not affected at 12 and 24h in mRNA levels after bevacizumab treatment and increased in protein and mRNA levels at 48h (Figure 3); The protein expression of NICD also increased at 48h (Figure 3B) and Notch signaling was activated during bevacizumab induced EMT in ARPE-19 cells.
Jagged-1/Notch-1 Pathway Up-regulated by Bevacizumab
We further examined Notch ligands and its receptors in mRNA levels expression by real time PCR analysis. The mRNA expression of Notch-1, Jagged-1 and RBP-Jk increased (Figure 4), and Notch-2, Notch-4 and Dll4 mRNA expression was not affected at 12, 24, 48h after bevacizumab treatment (data not shown). These results showed that Jagged-1/Notch-1 pathway may play a critical role in bevacizumab-induced EMT in ARPE-19 cells, not Dll4/Notch-1 pathway.
Figure 4. Jagged-1/Notch-1 pathway was up-regulated by bevacizumab.
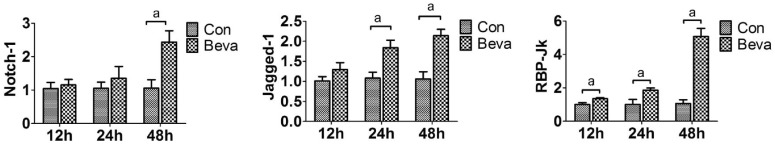
The mRNA expression of Notch-1, Jagged-1, and RBP-Jk was examined by real-time PCR after 0.25 mg/mL bevacizumab exposure for 12, 24, and 48h. Data are given as mean±SD (n=4). aP<0.05
DISCUSSION
AMD is the leading cause of severe irreversible vision loss in patients over the age of 50 in the developed world. Neovascular AMD is responsible for 90% of the cases with severe visual loss[16]. It is known that CNV lesions commonly evolve into scars over time as a consequence of the wound healing process[17]. In addition, subretinal fibrosis contributed to a loss in visual acuity in neovascular AMD. VEGF is a major cytokine involved in angiogenesis, and anti-VEGF therapy (intravitreal ranibizumab or bevacizumab) can make retinal or CNV regress, but the visual outcome was ultimately limited by submacular fibrosis[18]. Bevacizumab can modulate EMT in the RPE cells by up-regulationg CTGF[9]. We also found that bevacizumab at clinical doses (0.25mg/mL) can promote α-SMA expression and cell morphology change in ARPE-19 cells. Greater fibrotic responses after anti-VEGF therapy may result from an imbalance in the complex interactions between angiogenesis and pro-fibrotic signaling pathway.
Recent studies suggest that the VEGF pathway regulates angiogenesis in concert with Notch signaling [19]. Notch signaling plays an important role in CNV[20]. Notch signaling includes four receptors (Notch1-4) and five ligands (Jagged1, Jagged2, Dll1, Dll3, and Dll4) in mammalian cells, with four extremely similar receptors exhibiting subtle differences in certain domains [21]. RBP-Jk, which could recognize and bind specific DNA sequences (RTGGGAA) located in Notch-induced gene promoters, is a key DNA-binding protein in the Notch signaling pathway. The Notch pathway is activated through an interaction of a Notch receptor with a Jagged or Delta-lile ligand leading to proteolytic cleavages of Notch receptor at two distinct sites. The cleavage releases the NICD so that it can enter the nucleus and function as a transcription activator. The second cleavage is mediated by the γ-secretase complex, and an effective inhibition of Notch activation can be achieved by pharmacological inhibition of this protelytic activity. Within the nucleus, NICD interacts with RBP-Jk and activates transcription of downstream target genes, such as those of the Hes and Hey families[22]. Little is known about whether the Notch sighaling pathway is involved in fibrosis formation after IVB. In this study, we found that bevacizumab could promote NICD release and Hes-1 expression. Notch signaling was activated and maybe involved in bavacizumab-induced EMT in ARPE-19 cells.
In laser-injury wound model of CNV in the mouse eye, the α-SMA positive myofibroblastic scaffold covered the entire neovascular area, and neovascularization did not extend beyond this fibrovascular scaffold, which thus served to demarcate the area of neovascularization [23]. The activation of the canonical Notch pathway by Jagged 1 peptides (Jag1) significantly reduces the volume of CNV lesions and inhibition of this pathway by the γ-secretase inhibitor DAPT exacerbates CNV lesions in laser-induced CNV, which was related to strike a balance between the expression changes in proangiogenic and antiangiogenic factors[15]. A growing body of research documented that Notch signalling has involved in fibrotic diseases developedment in the kidney, liver and lung[12]–[14]. Jagged-1/Notch pathway is also involved in the transforming growth factor beta2 (TGF-β2)-mediated EMT of human RPE cells, and blockade of Jagged/Notch pathway abrogates TGF-β2-induced EMT in human retinal pigment epithelium cells[24]. Jagged 1 peptides (Jag1) may be reduce the volume of CNV lesions by promoting fibrovascular scaffold formation. We further examined Notch ligands and its receptors in mRNA levels expression by real time PCR analysis. We showed that Notch signaling was markedly activated, as indicated by the increased expression of Jagged-1, Notch-1 and RBP-Jk during bevacizumab induced EMT in ARPE-19 cells. Therefore, bevacizumab maybe induce EMT of human RPE cells by activating Notch-1/Jagged-1 signaling in ARPE-19 cells.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81170815); the Taishan Scholar Program (No.ts20081148); the Science and Technology Development Foundation of Shinan District of Qingdao City (No.2012-3-004-YY); Youth Foundation of Shandong Academy of Medical Sciences (No.2014-41)
Conflicts of Interest: Zhang JJ, None; Chu SJ, None; Sun XL, None; Zhang T, None; Shi WY, None.
REFERENCES
- 1.Mookiah MR, Acharya UR, Koh JE, Chandran V, Chua CK, Tan JH, Lim CM, Nq EY, Noronha K, Tong L, Laude A. Automated diagnosis of age-related macular degeneration using greyscale features from digital fundus images. Comput Biol Med. 2014;53:55–64. doi: 10.1016/j.compbiomed.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Casaroli-Marano RP, Alforja S, Giralt J, Farah ME. Epimacular brachytherapy for wet AMD: current perspectives. Clin ophthalmol. 2014;8:1661–1670. doi: 10.2147/OPTH.S46068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, Hoyng CB, Hykin P, Staurenghi G, Heldner S, Bogumil T, Heah T, Sivaprasad S. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2014;99(2):220–226. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthelmes D, Walton R, Campain AE, Simpson JM, Arnold JJ, McAllister IL, Guymer RH, Hunyor AP, Essex RW, Morlet N, Gillies MC. Outcomes of persistently active neovascular age-related macular degeneration treated with VEGF inhibitors: observational study data. Br J Ophthalmol. 2015;99(3):359–364. doi: 10.1136/bjophthalmol-2014-305514. [DOI] [PubMed] [Google Scholar]
- 6.Palejwala NV, Lauer AK, Weleber RG. Choroideremia associated with choroidal neovascularization treated with intravitreal bevacizumab. Clin Ophthalmol. 2014;8:1675–1679. doi: 10.2147/OPTH.S68243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, Huang J, Ying GS, Hagstrom SA, Winter K, Maguire MG, Comparison of Age-related Macular Degeneration Treatments Trials Research Group Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(3):656–666. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJ, Klaassen I, Schlingemann RO. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96(4):587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CL, Liang CM, Chen YH, Tai MC, Lu DW, Chen JT. Bevacizumab modulates epithelial-to-mesenchymal transition in the retinal pigment epithelial cells via connective tissue growth factor up-regulation. Acta Ophthalmol. 2012;90(5):e389–398. doi: 10.1111/j.1755-3768.2012.02426.x. [DOI] [PubMed] [Google Scholar]
- 10.Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem Soc Trans. 2009;37(Pt 6):1233–1236. doi: 10.1042/BST0371233. [DOI] [PubMed] [Google Scholar]
- 11.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104(9):3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Cir Res. 2004;94(7):910–917. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- 13.Leask A. Targeting the jagged/notch pathway: a new treatment for fibrosis? J Cell Commun Signal. 2010;4(4):197–198. doi: 10.1007/s12079-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;11(6):745–751. doi: 10.2174/138945010791170860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joussen AM, Bornfeld N. The treatment of wet age-related macular degeneration. Dtsch Arztebl Int. 2009;106(18):312–317. doi: 10.3238/arztebl.2009.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunwald JE, Daniel E, Ying GS, Pistilli M, Maguire MG, Alexander J, Whittock-Martin R, Parker CR, Sepielli K, Blodi BA, Martin DF, CATT Research Group Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119(8):1634–1641. doi: 10.1016/j.ophtha.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloch SB, Lund-Andersen H, Sander B, Larsen M. Subfoveal fibrosis in eyes with neovascular age-related macular degeneration treated with intravitreal ranibizumab. Am J Ophthalmol. 2013;156(1):116–124.e1. doi: 10.1016/j.ajo.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134(15):2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad I, Balasubramanian S, Del Debbio CB, Parameswaran S, Katz AR, Toris C, Fariss RN. Regulation of ocular angiogenesis by Notch signaling: implications in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(6):2868–2878. doi: 10.1167/iovs.10-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107(6):2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 22.Zhu F, Li T, Qiu F, Fan J, Zhou Q, Ding X, Nie J, Yu X. Preventive effect of Notch signaling inhibition by a gamma-secretase inhibitor on peritoneal dialysis fluid-induced peritoneal fibrosis in rats. Am J Pathol. 2010;176(2):650–659. doi: 10.2353/ajpath.2010.090447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He L, Marneros AG. Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am J Pathol. 2013;182(6):2407–2417. doi: 10.1016/j.ajpath.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu M, Ye S, Liu Y. Blockade of Jagged/Notch pathway abrogates transforming growth factor beta2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells. Curr Mol Med. 2014;14(4):523–534. doi: 10.2174/1566524014666140331230411. [DOI] [PubMed] [Google Scholar]