Abstract
AIM
To observe the effects of intravitreal injections of different concentrations of human umbilical mesenchymal stem cells on retinopathy in rats with diabetes mellitus.
METHODS
Healthy and adult male Sprague-Dawley (SD) rats were randomly assigned to a normal control group (group A), a diabetic retinopathy (DR) blank control group (group B), a high-concentration transplantation group (group C), a low-concentration transplantation group (group D) and a placebo transplantation group (group E). The expression of nerve growth factor (NGF) protein in the retinal layers was detected by immunohistochemical staining at 2, 4, 6 and 8wk.
RESULTS
The expression of NGF was positive in group A and most positive in the retinal ganglion cell layer. In groups B and E, the expression of NGF was positive 2wk after transplantation and showed an increase in all layers. However, the level of expression had decreased in all layers at 4wk and was significantly reduced at 8wk. In groups C and D, the expression of NGF had increased at 2wk and continued to increase up to 8wk. The level of expression in group C was much higher than that in group D.
CONCLUSION
DR can be improved by intravitreal injection of human umbilical mesenchymal stem cells. High concentrations of human umbilical mesenchymal stem cells confer a better protective effect on DR than low concentrations.
Keywords: diabetic retinopathy, human umbilical mesenchymal stem cells, nerve growth factor, stem cell therapy
INTRODUCTION
Diabetic retinopathy (DR) is not only a microvascular disease but also a neurodegenerative disease[1]. The diabetes-associated pathological changes that occur in glial cells and retinal neurons develop earlier than the microvascular disease. In recent years, although intraocular anti-vascular endothelial growth factor (anti-VEGF) therapy has raised awareness of the feasibility of pharmacological treatment for DR, its limited efficacy has evoked the necessity to identify new drug targets for the fundamental treatment of diabetic macular edema and neoangiogenesis[2]–[4]. Damage to the optic nerve has been shown to occur before the clinically visible DR microvascular disease, which may be the main reason for visual impairment in early DR[5]. Thus, neuroprotection has become a valuable therapeutic target for DR. Mesenchymal stem cells (MSCs) have the potential to differentiate into neuron-like cells, retinal ganglion cells, glial cells and photoreceptor cells and to secrete neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) protein, providing new possibilities for the treatment of retinal degenerative diseases[6]–[8]. Diabetes mellitus (DM) can alter the expression of NGF and other neurotrophins in the retina, resulting in retinal nerve damage[9]; thus, NGF has a neuroprotective effect against DR[10]. While various domestic and foreign studies have examined the treatment of eye diseases through various approaches to the transplantation of human umbilical cord MSCs (hUCMSCs)[7], [11], transplantation using different concentrations of hUCMSCs has rarely been reported. In this study, we observed the expression of NGF protein in the streptozotocin (STZ)-induced DM rat by transplanting different concentrations of hUCMSCs, with the aim of finding the most appropriate concentration of hUCMSCs to ameliorate the DR.
MATERIALS AND METHODS
Materials
Experimental animals
We used 48 male adult SD rats (weighing 180-200 g) with no eye diseases, provided by the Experimental Animal Center of Tianjin Medical University. Laboratory animals and experimental conditions were approved by the “Laboratory Animal Management Regulations” of the National Science and Technology Commission, and we adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research in the Methods section of their manuscript.
Main reagents and cells
We used a streptavidin-perosidase (SP) immunohistochemical staining kit, rabbit anti-mouse NGF antibody (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. China), and hUCMSCs (Bio Co., Ltd. Tianjin Amcellgene cells have been identified).
Methods
Diabetic mellitus model
DM was induced by a single intraperitoneal injection of STZ (60 mg/kg body weight) in sodium citrate buffer, pH 4.5 (Sigma-Aldrich, St. Louis, MO, USA). After 1wk, we measured the blood glucose from the tip of the tail (Taiwan Rui Dien glucose meter); rats were considered diabetic if their blood glucose level was higher than 16.7 mmol/L.
Animal grouping and intravitreal injection
After being raised adaptively for 3d, the rats were randomly divided into a normal control group (group A: 10 rats) and a DM group with 38 rats. After 10wk, 2 rats were randomly selected from each of the two groups to carry out ethidium bromide (EB) staining retinal flat sheet to prove the DR model successfully. Among the remaining 36 rats of the DM group, we randomly selected 9 rats as group B, 9 rats for intravitreal injection of 2 µL high-concentration (1×106-7) hUCMSCs as group C, 9 rats for intravitreal injection of 2 µL low-concentration (1×105-6) hUCMSCs as group D, and 9 rats for intravitreal 2 µL placebo as group E. Groups A and B received no treatments. Rats were housed in the Tianjin Medical University animal facility with a 12h light/dark cycle and allowed free access to food and water.
Ethidium bromide-stained retinal flat sheets
These were prepared after anesthesia by injecting the EB (45 mg/kg weight) slowly-within 1min-through the right jugular vein. When the eyes, tail and limbs turned blue, the perfusion was deemed successful. After perfusion, we removed the eye rapidly, using 4% paraformaldehyde to fix, cut along the limbus, isolated the retina, cut the retina radically, and paved it on clean glass. Next, we mounted the retina using 2% gelatin, observed it under a fluorescence microscope, and photographed it.
Intravitreal injection
After anesthesia, using tropicamide eye drops to dilate the eye, Proparacaine eye drops for surface anesthesia, and a 10-µL micro-syringe to pump 2 µL 1×106-7 hUCMSCs, we pierced the vitreous cavity of the rats (group C) 2 mm behind the temporal limbus, then slowly injected the hUCMSCs. After removal of the needle, a sterile cotton tip applicator was used to prevent reflux. Postoperatively, gentamicin eye drops were administered to the rats four times per day. For groups D and E, we injected 2 µL 1×105-6 hUCMSCs and placebo, respectively, to differentiate them; otherwise, the method used was the same as previously described. No vitreous hemorrhage occurred in this experiment.
Immunohistochemical staining
This was used to detect the expression of NGF protein (SP method): 4-µm-thick paraffin sections were prepared, then dewaxed and dehydrated in graded alcohol solutions. Antigen retrieval was performed by intermittent microwave heating for 6min, and then the sections were washed in phosphate buffer saline (PBS). Endogenous peroxides were eliminated with 3% hydrogen peroxide in methanol for 20min, and nonspecific background staining was prevented by incubating the sections for 30min in 10% normal goat serum (Santa Cruz Biotechnology). Subsequently, the sections were incubated overnight with the primary antibody. Optimal working concentration and incubation time for the antibody were determined earlier in pilot experiments. The sections were then incubated for 10-15min with biotinylated conjugated secondary antibody, washed in PBS, and incubated for 10-15min with horseradish peroxidase. To detect the stain, a 3, 3′-diaminobenzidine (DAB) staining system was used, following the manufacturer's instructions. The slides were then faintly counterstained with Harris' hematoxylin, washed in tap water, dehydrated in graded alcohol, and sealed with neutral gum. Control slides included staining of samples that were incubated under identical conditions but without the primary antibody. The NGF expression detected in groups A, B, C, D and E group was recorded at 2, 4, 6 and 8wk after the intervention.
Observation and analysis of the results
The nucleus or cytoplasm was tan or yellow under Light microscope showing the expression of the NGF protein. Two slices were selected from each eye; five horizons (SP×200) were randomly selected from each slice, measuring average integrated optic density (IOD) in their stained area to represent expression intensity (Image-Pro plus 6.0).
Statistical Analysis
Data and statistical analyses were performed using SPSS 17.0 software. Data are presented as mean±SD. We analyzed the differences between the groups using one-way ANOVA, and made further pairwise comparisons between groups using least significant difference (LSD) t-tests. For all statistical tests, P<0.05 was considered statistically significant.
RESULTS
Staining with Ethidium Bromide
EB staining revealed that, in the control rats, the retinal blood vessels were well lined, the lumen was uniform, and the shallow and deep retinal vascular network was clearly visible. By contrast, in the DM rats the retinal vessels were tortuous and expansive, with visible capillary hemangioma, beaded vessels, and fluorescent leakage or peripheral vascular area, indicating that the DR rats modeled successfully (Figure 1A, 1B).
Figure 1. Ethidium bromide staining.
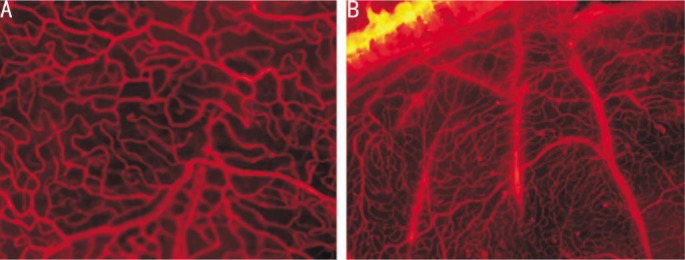
A: Normal rats; B: DM rats.
Immunohistochemical Staining Revealed the Expression of Nerve Growth Factor Protein at Different Time Points in the Retina
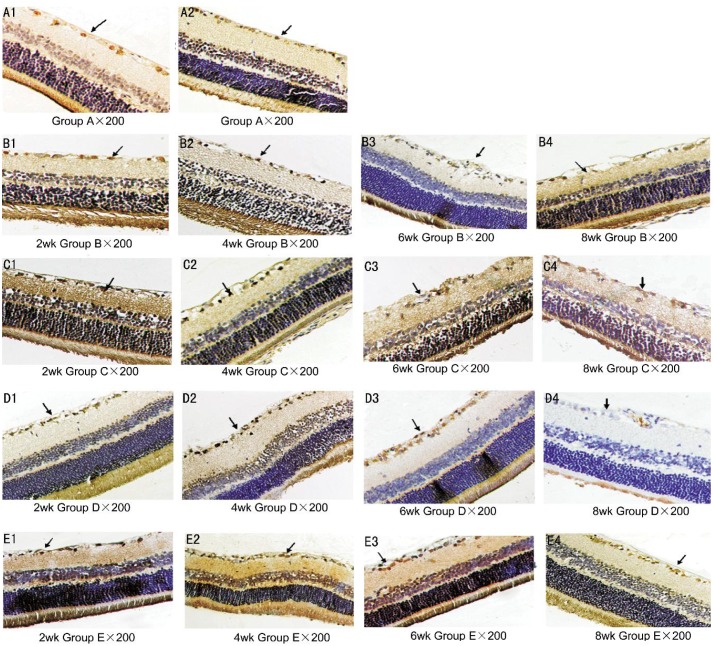
Group A: the expression of NGF protein was positive and occurred mainly in the retinal ganglion cell layer; however, the NGF protein was only slightly expressed in the inner plexiform layer, outer plexiform layer, external membrane, pigment epithelium, outer nuclear layer and inner nuclear layer (Figure 2 A1-A2). Groups B and E: at 2wk, the expression of NGF protein increased in each layer; at 4wk, the expression started to diminish, so the amount of NGF protein in each layer decreased; at 8wk, the expression was significantly reduced (Figure 2 B1-B4, E1-E4). Groups C and D: at 2wk, the expression increased and over time it increased significantly; at 8wk it reached a peak. Expression was more obvious in group C (Figure 2 C1-C4, D1-D4).
Figure 2. The expression of NGF protein in retinal tissue (SP×200).
A1-A2: The boundary of the NGF positive cells was more obvious, the nucleus or cytoplasm was brown or yellow, and the positive expression occurred mainly in the retinal ganglion cells (as indicated by the black arrows); B1-B4 and E1-E4: at 2wk, the expression of each layer increased and the positive staining was darker. Next, the expression began to weaken and the amount of the NGF protein in each layer decreased; at 8wk, the expression was significantly reduced and the positive staining of each layer was lighter (as indicated by the black arrows); C1-C4 and D1-D4: at 2wk there was no obvious difference in expression between groups B and E, but over time the expression increased significantly. The positive cells became deeply stained with the increasing number of cells and the boundary gradually became clear. The positive staining peaked at 8wk. Expression was more obvious in group C (as indicated by the black arrows).
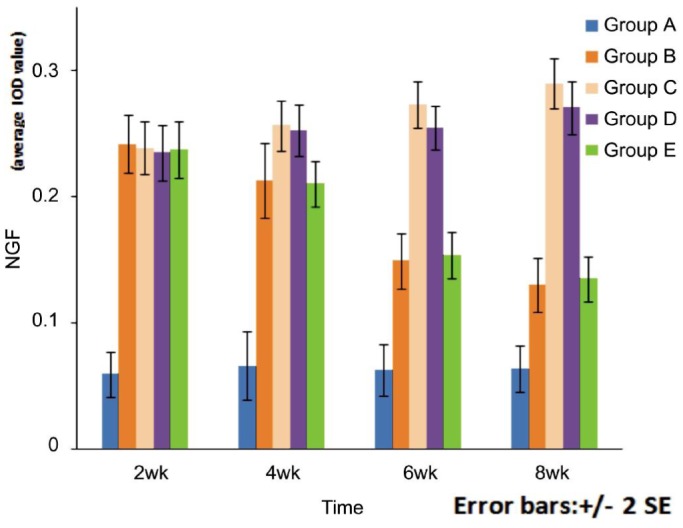
Table 1 and Figure 3 show the level of expression of the retinal NGF protein in each group. At each time point, the expression of the retinal NGF protein was statistically different (F=217.578, 202.414, 295.543, 372.095; P<0.05) among different groups. When comparing group A with the other groups, the difference was statistically significant at each time point (P<0.05). The expression of NGF protein in groups B and E peaked at 2wk, then gradually decreased with time, but the difference between the two groups was not statistically significant at each time point (P=0.572, 0.731, 0.518, 0.510). At 4wk, comparing group B with groups C and D, the difference was not statistically significant (P=0.695, 0.352). Moreover, when group E was compared with groups C and D, there was also no statistically significant difference (P=0.862, 0.714). In group C, the expression of NGF protein increased over time; at each time point the difference was statistically significant (P<0.05). At 2wk, the difference was statistically significant only when compared with group A (P=0.000); when compared with the other DR groups, there was no statistical significance (P=0.695, 0.589, 0.862). At 4wk, the difference was not statistically significant only when compared with group D (P=0.583); when compared with the other DR groups, the difference was statistically significant (P<0.05). At 6 and 8wk, when compared with the other DR groups, the difference was statistically significant (P<0.05). The expression of NGF protein of group D increased over time. At 2wk, the difference was statistically significant only when compared with group A (P=0.000); when compared with the other DR groups, there was no statistical significance (P=0.572, 0.862, 0.714). At 4wk, the difference was not statistically significant only when compared with group C (P=0.583); when compared with the other DR groups, the difference was statistically significant (P<0.01). At 6 and 8wk, when compared with the other DR groups, the difference was statistically significant (P<0.05). The difference in NGF protein expression between the four DR groups was statistically significant at different time points (F=71.187, 19.005, 8.002, 93.528; P=0.000). Further pairwise comparisons revealed the following results. When comparing any two adjacent time points, the differences between groups B, C and E were statistically significant (P<0.05). Comparing time points within group D, the difference was not statistically significant at 4wk and 6wk (P=0.730) but statistically significant at the other time points (P<0.05).
Table 1. the level of NGF protein expression in the retina in each group (IOD value).
| Groups | 2wk | 4wk | 6wk | 8wk | Interclass |
|
| F | P | |||||
| Group A | 0.0591±0.0178 | 0.0659±0.0517 | 0.0625±0.0204 | 0.0637±0.0183 | 0.365 | 0.779 |
| Group B | 0.2417±0.0229 | 0.2129±0.0293 | 0.1489±0.0223 | 0.1299±0.0214 | 71.187 | 0.000 |
| Group C | 0.2388±0.0205 | 0.2564±0.0198 | 0.2736±0.0184 | 0.2899±0.0196 | 19.005 | 0.000 |
| Group D | 0.2348±0.0216 | 0.2523±0.0205 | 0.2549±0.0176 | 0.2707±0.0207 | 8.002 | 0.000 |
| Group E | 0.2375±0.0222 | 0.2103±0.0179 | 0.1537±0.0184 | 0.1348±0.0180 | 93.528 | 0.000 |
| Interblock | ||||||
| F | 217.578 | 202.414 | 295.543 | 372.095 | ||
| P | 0.000 | 0.000 | 0.000 | 0.000 | ||
Figure 3. Bar chart of the NGF protein expression in the retina in each group (IOD value).
DISCUSSION
hUCMSCs are easily and non-invasively available without ethical constraints, and have the potential of self-renewal and multipotent differentiation, which may make their application suited to both scientific research and clinical medicine[12]. In the current study, we used intravenous injection of hUCMSCs as a stem cell therapy for DR in STZ-induced diabetic rats. In addition, human stem cells represent a large reservoir of anti-apoptotic and antineoangiogenic growth factors, and there is growing interest in the ophthalmologic field in which human stem cells are used in the management of a group of retinal diseases induced and controlled by apoptotic and neoangiogenic mechanisms and mediated by proinflammatory growth factors such as cytokines, chemokines and VEGF[13],[14]. Yang et al[15] found that fetal bone marrow stromal cells (BMSCs) were differentiated into neural-like cells expressing special neuronal markers-nestin, glial fibrillary acidic protein (GFAP) and neuron-specific enolase (NSE)-when co-cultured with retinal pigment epithelial (RPE) cells. In our study, compared with controls a single intravitreal injection of hUCMSCs resulted in a significant increase in NGF in rats rendered diabetic by means of a single injection of STZ. The procedure we used allowed control of progression of the chemically induced DR. Furthermore, no deaths, rejection, intolerance phenomena, or postoperative complications were encountered or any adverse reactions on fluorangiographic examination. Moreover, this challenging approach to the treatment of DR using an animal model offered several advantages, in that it was simple to perform, non-dependent on surgeons, portable, relatively inexpensive, and less invasive than other surgical techniques, and produced rapid and very good responses. No disadvantages were identified and no side-effects or rejection phenomena were observed during the study. Our experiment also showed that the NGF protein expression in the retina increased significantly after the injection of hUSMSCs into the vitreous cavity of the DM rats, and that-with the passage of time-the expression of the NGF protein increased, indicating that intravitreal injection of the hUSMSCs could upregulate the level of NGF protein expression in the retina of the DM rats.
DR involves the formation of microvascular lesions and neural degeneration. NGF is one of the most important bioactive molecules of the nervous system and was also the first-discovered neurotrophic factor[16]. NGF plays an extremely important role in the survival of the neuron and the growth, differentiation and regeneration of nerve fibers[17]. NGF may be secreted by the nerve cells and then nourish the target organ from the trailing edge of the axon. In addition, NGF may nourish the surrounding tissue through paracrine or autocrine signaling[18]. Lambiase et al[19] reported that NGF receptor is present in each layer of the retina tissue. In our study, immunochemical (IHC) studies revealed that NGF protein expression was positive in group A and located mainly in retinal ganglion cells. Moreover, in groups B and E, at 2wk, the expression was significantly positive in each layer. However, the level of expression decreased over time and, at 8wk, the level of expression had decreased significantly. The finding that there is a downregulation of NGF in early DM following an initial upregulation is consistent with the findings of Rudzinski et al[20]: that the NGF expression in the retina caused by high intraocular pressure increased only transiently. Therefore, through intravitreal injection, hUCMSCs could significantly increase NGF expression and thus ameliorate DR.
Previous study on the transplantation of MSCs [21] showed that an insufficient number of the stem cells often led to incomplete reparation of the damaged tissue, but if the concentration of the MSCs was too high, the ecological microenvironment of the transplant area would be affected, thereby the survival of transplanted cells and the metabolism would be affected too. Few studies have been conducted on the migration of different concentrations of hUCMSCs. Seeking an appropriate concentration of hUCMSCs for transplantation to treat DR, we used different concentrations of hUCMSCs for the transplantations and IHC staining to detect the NGF protein expression. We then observed the differences in each group. The results showed that, at 2wk and 4wk, the difference between the high-concentration group (group C) and the low-concentration group (group D) was not statistically significant, but at 6wk and 8wk the positive NGF expression of group C was obvious and there was a statistical difference between the two groups, indicating that the high-concentration group with the large number of cells was more suitable for cell survival and could effectively promote reparation and metabolism of the tissue compared with the low-concentration group.
In conclusion, our study confirmed that, when DR rats received different concentrations of stem cell therapy, the hUCMSCs had a therapeutic effect on the DR and could effectively upregulate the level of retinal NGF, thereby ameliorating the DR. In addition, the effect of intravitreal injection with a high concentration of hUCMSCs was even more heightened, laying the foundation for further study of the mechanism of action of hUCMSCs therapeutic for DR. However, this study has some limitations. For example, the study observed the NGF expression and retinal function for only 8wk, limited by the experimental conditions, so the change in the expression of NGF was unclear. Therefore, this study only supported the therapeutic effect of hUCMSCs on DR theoretically; we would need to resolve more problems and gather further data from animal studies before clinical application.
Acknowledgments
Foundation: Supported by Tianjin Science and Technology Project, China (No.13ZCZDSY01500).
Conflicts of Interest: Kong JH, None; Zheng D, None; Chen S, None; Duan HT, None; Wang YX, None; Dong M, None; Song J, None.
REFERENCES
- 1.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS, RISE and RIDE Research Group Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 3.Rauen PI, Ribeiro JA, Almeida FP, Scott IU, Messias A, Jorge R. Intravitreal injection of ranibizumab during cataract surgery in patients with diabetic macular edema. Retina. 2012;32(9):1799–1803. doi: 10.1097/IAE.0b013e31824bebb8. [DOI] [PubMed] [Google Scholar]
- 4.Jain GK, Warsi MH, Nirmal J, Garg V, Pathan SA, Ahmad FJ, Khar RK. Therapeutic stratagems for vascular degenerative disorders of the posterior eye. Drug Discov Today. 2012;17(13–14):748–759. doi: 10.1016/j.drudis.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Wang N, Barile GR, Bao S, Gillies M. Diabetic retinopathy: neuron protection as a therapeutic target. Int J Biochem Cell Biol. 2013;45(7):1525–1529. doi: 10.1016/j.biocel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Wang X, Xu G, Guo J. Light-induced retinal injury enhanced neurotrophins secretion and neurotrophic effect of mesenchymal stem cells in vitro. Arq Bras Oftalmol. 2013;76(2):105–110. doi: 10.1590/s0004-27492013000200010. [DOI] [PubMed] [Google Scholar]
- 7.Scalinci SZ, Scorolli L, Corradetti G, Domanico D, Vingolo EM, Meduri A, Bifani M, Siravo D. Potential role of intravitreal human placental stem cell implants in inhibiting progression of diabetic retinopathy in type 2 diabetes: neuroprotective growth factors in the vitreous. Clin Ophthalmol. 2011;5:691–696. doi: 10.2147/OPTH.S21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, Dilley RJ. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21(12):2189–2203. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valverde AM, Miranda S, García-Ramírez M, González-Rodriguez Á, Hernández C, Simó R. Proapoptotic and survival signaling in the neuroretina at early stages of diabetic retinopathy. Mol Vis. 2013;19:47–53. [PMC free article] [PubMed] [Google Scholar]
- 10.Whitmire W, Al-Gayyar MM, Abdelsaid M, Yousufzai BK, El-Remessy AB. Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol Vis. 2011;17:300–308. [PMC free article] [PubMed] [Google Scholar]
- 11.Bi X, Chen S, Lin JY, Wang YC. Systemic and ocular transplantation of human umbilical cord mesenchymal stem cells into rats with diabetic retinopathy. Chin J Ocul Fundus Dis. 2014;30(2):209–211. [Google Scholar]
- 12.Li DR, Cai JH. Methods of isolation, expansion, differentiating, induction and preservation of human umbilical cord mesenchymal stem cells. Chin Med J (Engl) 2012;125(24):4504–4510. [PubMed] [Google Scholar]
- 13.Joe AW, Gregory-Evans K. Mesenchymal stem cells and potential applications in treating ocular disease. Curr Eye Res. 2010;35(11):941–952. doi: 10.3109/02713683.2010.516466. [DOI] [PubMed] [Google Scholar]
- 14.Jeganathan VS, Palanisamy M. Treatment viability of stem cells in ophthalmology. Curr Opin Ophthalmol. 2010;21(3):213–217. doi: 10.1097/ICU.0b013e32833867ad. [DOI] [PubMed] [Google Scholar]
- 15.Yang LL, Zhou QJ, Wang Y, Wang YQ. Differentiation of human bone marrow-derived mesenchymal stem cells into neural-like cells by co-culture with retinal pigmented epithelial cells. Int J Ophthalmol. 2010;3(1):23–27. doi: 10.3980/j.issn.2222-3959.2010.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebendal T. Function and evolution in the NGF family and its receptors. J Neurosci Res. 1992;32(4):461–470. doi: 10.1002/jnr.490320402. [DOI] [PubMed] [Google Scholar]
- 17.Aloe L, Rocco ML, Bianchi P, Manni L. Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichim G, Tauszig-Delamasure S, Mehlen P. Neurotrophins and cell death. Exp Cell Res. 2012;318(11):1221–1228. doi: 10.1016/j.yexcr.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Lambiase A, Mantelli F, Sacchetti M, Rossi S, Aloe L, Bonini S. Clinical applications of NGF in ocular diseases. Arch Ital Biol. 2011;149(2):283–292. doi: 10.4449/aib.v149i2.1363. [DOI] [PubMed] [Google Scholar]
- 20.Rudzinski M, Wong TP, Saragovi HU. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J Neurobiol. 2004;58(3):341–354. doi: 10.1002/neu.10293. [DOI] [PubMed] [Google Scholar]
- 21.Kang QJ, Liu CA, Wang JY. Therapeutic effects of different concentrations of BMSCs transplantation on ANFH in rabbits. Progress of Anatomical Sciences. 2010;16(1):54–57. [Google Scholar]